This work demonstrates that cytomegalovirus coinfection is necessary for CD8 T-cell expansion and is associated with higher levels of inflammatory and coagulation indices that are all linked to morbid outcomes of treated human immunodeficiency virus infection.
Keywords: HIV, CMV, coinfection, CD8 T-cell expansion, inflammation
Abstract
Background. Persistent CD8 T-cell expansion, low CD4/CD8 T-cell ratios, and heightened inflammation persist in antiretroviral therapy (ART)-treated human immunodeficiency virus (HIV) infection and are associated with increased risk of morbid outcomes. We explored the role of cytomegalovirus (CMV) infection in CD8 lymphocytosis and inflammation in ART-treated HIV infection.
Methods. Absolute CD4 and CD8 T-cell counts were abstracted from clinical records and compared among 32 HIV-infected CMV-seronegative subjects, 126 age, CD4 and gender-matched HIV-infected CMV-seropositive subjects, and among 21 HIV-uninfected controls (9 CMV-negative, 12 CMV-positive). Plasma inflammatory indices were measured in a subset by ELISA.
Results. Median CD8 counts/µL were higher in HIV-positive/CMV-positive patients (795) than in HIV-positive/CMV-negative subjects (522, P = .006) or in healthy controls (451, P = .0007), whereas CD8 T-cell counts were similar to controls' levels in HIV-positive/CMV-negative subjects. Higher plasma levels of IP-10 (P = .0011), TNF-RII (P = .0002), and D-dimer (P = .0444) were also found in coinfected patients than in HIV-positive/CMV-negative subjects.
Conclusions. CMV infection is associated with higher CD8 T-cell counts, resultant lower CD4/CD8 ratios, and increased systemic inflammation in ART-treated HIV infection. CMV infection may contribute to risk for morbid outcomes in treated HIV infection.
In the era of combination antiretroviral therapy (ART), human immunodeficiency virus (HIV)-infected individuals are living longer and healthier lives. More HIV-infected people than ever are entering old age, but due to increased inflammation and elevated risks of cardiovascular disease that are linked to HIV infection, even younger ART-treated patients are succumbing earlier to many of the same complications that affect the HIV-uninfected elderly [1]. We and others have previously linked persistent CD8 T-cell expansion and inflammatory mediators such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and type 1 interferons to the morbid outcomes of HIV infection [2–5], and more recently, we have specifically implicated the inflammatory mediators interferon (IFN)-α, IL-6, and IL-1β in the pathogenesis of poor CD4 T-cell restoration in the setting of sustained combination ART [6, 7]. In most persons with HIV infection, expansion of the CD8 T-cell pool is demonstrable early in infection as CD4 T-cell numbers progressively fall, and this expansion typically persists even when HIV replication is controlled with ART [8]. In ART-treated patients, inversion of the ratio of CD4 T cells to CD8 T cells is associated with poor clinical outcomes, even in the setting of normal CD4 T-cell counts [5, 9], suggesting that CD8 T-cell expansion is associated with and could be an important driver of increased morbidity and mortality [5]. In ART-treated HIV infection, the determinants of persistent CD8 T-cell expansion are poorly understood.
Like HIV, human cytomegalovirus (CMV) is a lifelong viral pathogen associated with inflammation and cardiovascular risk, particularly in the elderly [10]. CMV infection is especially prevalent in aging populations—increasing in prevalence from 36% in 6–11 year-olds to over 90% in those older than 80 years old [11]—whereas approximately 90% of HIV-infected individuals are coinfected with CMV, independently of age [12]. In immunosuppressed individuals, such as untreated HIV-infected patients and organ transplant recipients, active CMV infections can be particularly devastating, leading to severe end organ disease and death. Most HIV-infected persons experience intermittent bursts of CMV replication (even during suppressive ART) that might contribute to persistent stimulation of the CD8 T-cell population [10].
Here, we sought to determine whether persistent CD8 T-cell expansion and increased inflammation observed in ART-treated HIV infection was associated with CMV coinfection.
METHODS
Clinical Indices
This work was approved by the Institutional Review Board at University Hospitals/Case Medical Center. With written informed consent, blood was acquired in EDTA tubes from 21 HIV-uninfected persons (12 CMV-positive, 9 CMV-negative) and 158 HIV-infected patients (126 CMV-positive, 32 CMV-negative) receiving ART (median duration of treatment 3.15 years) with undetectable plasma HIV levels (typically below 50 copies/mL). CD4 and CD8 T-cell counts were determined in the hospital clinical laboratory by flow cytometry. CMV serostatus was determined in the hospital clinical laboratory by IMMULITE 2000 CMV IgG Ab immunoassay (Siemens). HIV-infected CMV-seropositive subjects were age, gender-, and CD4 T cell count-matched 4:1 to HIV-infected CMV-seronegative subjects. Participant characteristics are shown in Table 1.
Table 1.
Participant Characteristics
| HIV-positive |
P Value | Total |
P Value | |||
|---|---|---|---|---|---|---|
| CMV-negative | CMV-positive | HIV-positive | HIV-negative | |||
| N, (male, %) | 32 (84.75%) | 126 (84.13%) | 1.00 | 158 (84.18%) | 21 (52.4%) | .0018 |
| CMV-positive (%) | 0% | 100% | <.0001 | 79.7% | 42.9% | .0281 |
| Age (y), Median (IQR) | 41.5 (35.25–47) | 42 (36–48.25) | >.9999 | 42 (36–48) | 37 (30.5–48.5) | .0526 |
| Time on ART (y), Median (IQR) | 3.29 (2.61–4.71) | 3.14 (1.86–4.69) | .4634 | 3.15 (2.11–4.67) | NA | NA |
| CD4 (cells/µL), Median (IQR) | 437 (275.3–591.8) | 490 (309–640) | .4201 | 467 (300.5–635) | 907 (703.5–1059) | <.0001 |
| CD4 nadir (cells/µL), Median (IQR) | 178.5 (89.3–268.5) | 180 (71–300.1) | .6527 | 180 (72.5–290) | NA | NA |
Significance was determined using Mann–Whitney U test or Fisher exact test. P values <.05 were considered significant.
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable.
ELISA
Whole blood in EDTA was obtained at the time of CMV sero-status determination and after centrifugation, plasmas were frozen at −80°C, then thawed and analyzed in batch by enzyme-linked immunosorbent assay (ELISA) per manufacturer's protocols for levels of D-dimers (Asserachrom), IP-10, IL-6, IL-18, soluble CD14 (sCD14), and TNF-RII (all from R & D Systems).
Statistics
We compared continuous variables using the Mann–Whitney U test or the Kruskal–Wallis test with Dunn's correction for multiple variables. Categorical data were compared using Fisher exact test. Correlations were determined using a nonparametric Spearman test.
RESULTS
Clinical Characteristics
We compared 3 groups: HIV-uninfected controls (n = 21), ART-treated HIV-infected CMV-seronegative individuals (n = 32), and ART-treated HIV-infected CMV-seropositive individuals (n = 126). All 3 groups were similarly aged, and the HIV-infected groups had similar CD4 counts, CD4 nadirs, duration of ART, and proportions of men (Table 1). We did not find significant differences in CD4 or CD8 numbers between the CMV-seropositive (n = 12, median CD4 count = 938/µL, median CD8 count = 501/µL) and the CMV-seronegative HIV-uninfected controls (n = 9, median CD4 count = 876/µL, median CD8 count = 440/µL), so we analyzed them here as one group. Plasma levels of IL-6, D-dimer, and sCD14 among these healthy controls have been reported as a group earlier [13].
Elevated CD8 T-Cell Numbers and Decreased CD4/CD8 Ratio in HIV and CMV Coinfection
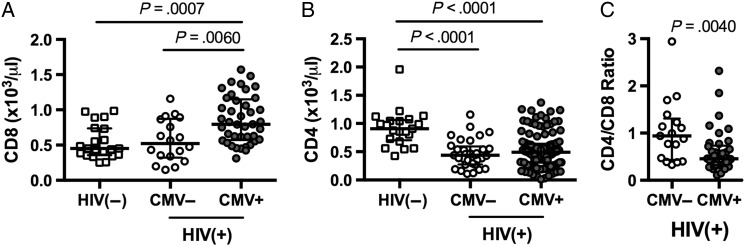
Median circulating CD8 T-cell number was significantly higher in HIV-infected CMV-seropositive patients (795/µL) than in HIV-infected CMV-seronegative subjects (522/µL, P = .006) or HIV-uninfected controls (451/µL, P = .0007). Absolute CD8 T-cell counts among the HIV-infected CMV seronegative subjects were not different from those among healthy controls (P > .99), suggesting that the expansion of circulating CD8 T cells that is a hallmark of ART-treated HIV infection is specifically linked to coinfection with CMV. As the HIV-infected groups were matched for CD4 T-cell counts, CD4 T-cell nadirs, and duration of ART, the observed difference in CD8 T-cell counts was not related to differences in immune restoration. As expected, CD4 T-cell counts in each HIV-infected group were lower than among HIV-negative controls (Figure 1B). Consequently, coinfection with HIV and CMV resulted in a significantly lower CD4/CD8 ratio than was seen among HIV-infected CMV-seronegative subjects (P = .004, Figure 1C). As both increased circulating CD8 T-cell numbers and low CD4/CD8 ratios are associated with poor clinical outcomes in ART-treated HIV infection [5, 9], our findings implicate CMV coinfection as a possible driver of non-AIDS morbidities in treated HIV disease.
Figure 1.
Elevated CD8 T-cell counts and reduced CD4/CD8 ratio in human immunodeficiency virus (HIV)-positive/cytomegalovirus (CMV)-positive individuals. Absolute CD8 T-cell numbers (A), absolute CD4 T-cell numbers (B), or CD4/CD8 ratios (C) were determined for HIV-uninfected individuals (n = 21); HIV-infected CMV-seronegative subjects (n = 32); and HIV-infected CMV-seropositive subjects (n = 126). (A and B) Significance was determined by Kruskal–Wallis test with Dunn's correction for multiple comparisons; (C) Significance was determined using Mann–Whitney U test.
Elevated Expression of Select Markers of Inflammation
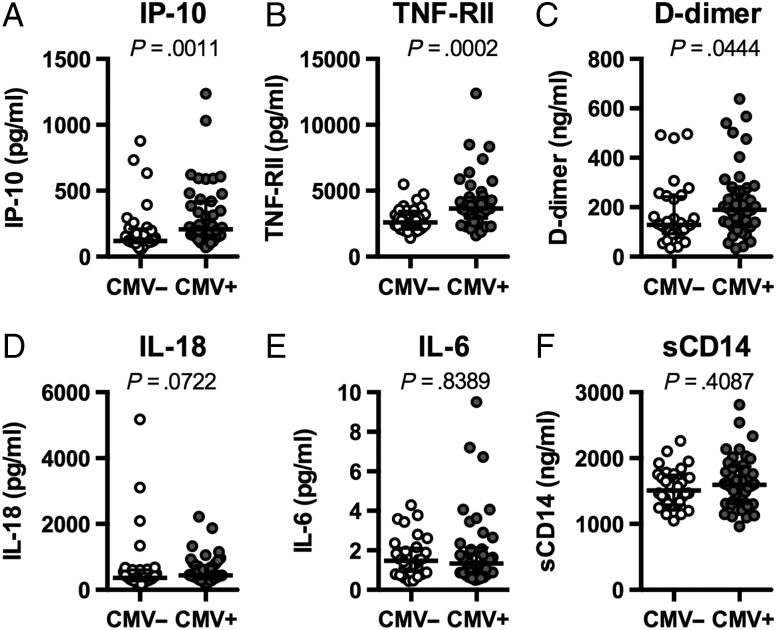
We next asked if the presence of HIV and CMV coinfection was associated with increased plasma levels of the inflammatory and coagulation markers IP-10, TNF-RII, D-dimer, IL-18, IL-6, and sCD14 (Figure 2). Levels of IP-10 (P = .0011), TNF-RII (P = .0002), and D-dimer (P = .0444) were higher in plasmas of HIV, CMV coinfected subjects compared to HIV-infected CMV-seronegatives. Expression of IL-18, a member of the IL-1β family of cytokines, was not significantly elevated (P = .0722). Plasma levels of IL-6, a powerful predictor of morbid outcomes in treated HIV infection [3, 4] were not different (P = .8389) between the CMV-seropositive and seronegative HIV-infected groups, suggesting that the drivers of IL-6 expression might not be related to CMV coinfection in ART-treated HIV infection. The soluble form of the lipopolysaccharide (LPS) coreceptor, sCD14, is elevated in settings of microbial translocation but also can be induced by other monocyte/macrophage-activating stimuli [14]. Similarly, levels of sCD14 were comparable between CMV-seronegative CMV-seropositive ART-treated HIV-infected subjects (P = .4087), suggesting that CMV coinfection is not central to microbial translocation/monocyte activation in ART-treated HIV infection.
Figure 2.
Elevated expression of selected markers of inflammation. Donor plasma was acquired from human immunodeficiency virus infected donors who were cytomegalovirus (CMV)-seronegative (CMV-negative; n = 32) or CMV-seropositive (CMV-positive; n = 42) and tested by enzyme-linked immunosorbent assay for expression of inflammatory mediators interferon-inducible protein 10 (A), tumor necrosis factor (TNF)-RII (B), D-dimer (C), interleukin (IL)-18 (D), IL-6 (E), and sCD14 (F). Significance was determined using Mann–Whitney U test.
DISCUSSION
In our age-matched cohorts, we found elevated circulating CD8 T-cell numbers only in individuals coinfected with both CMV and HIV but not in persons infected with HIV alone or CMV alone. Although not associated with IL-6 or soluble CD14 levels, CMV coinfection was associated with lower CD4/CD8 ratios and higher plasma levels of interferon-inducible protein 10 (IP-10), tumor necrosis factor receptor – type II (TNF-RII), and D-dimers, suggesting CMV coinfection in HIV-infected persons is a potential contributor to increased inflammation and coagulation observed in HIV disease [3, 5, 9, 15, 16]. These findings also suggest that the drivers of activation and morbidities in treated HIV infection are likely to be multifactorial [17, 18]. The mechanisms of how CMV coinfection drives circulating CD8 T-cell persistence and increased inflammation in HIV infection and the role of CMV in the morbid outcomes of treated HIV infection merit further study.
CMV infection is linked to CD8 T expansion in the HIV-uninfected elderly but less so among younger CMV-infected adults [19–23]. In our slightly younger HIV-uninfected controls, we saw no significant differences in CD8 T-cell counts between CMV-seronegative and CMV-seropositive individuals. Yet CD8 T-cell expansion was striking in CMV/HIV coinfected subjects but not in those singly infected with HIV, who presented normal levels of circulating CD8 T cells. Barrett, et al reported that among HIV-infected patients on ART but not necessarily with controlled HIV replication, CMV coinfection was associated with both diminished CD4 T-cell restoration and a modest CD8 T-cell expansion that was characterized by increased expression of the senescence marker CD57 and decreased expression of the coreceptor CD28 [24]—both indices that are linked to CD8 T-cell expansion/maturation. That study did not include comparison to HIV-uninfected controls, nor were soluble indices of inflammation measured. In the present study, performed among patients with controlled HIV replication on ART carefully matched for current and nadir CD4 T-cell counts and duration of ART exposure, CMV coinfection was linked to dramatic CD8 T-cell expansion, whereas singly HIV-infected subjects had normal CD8 T-cell counts. Thus, our data suggest that HIV and CMV infections together drive CD8 T-cell expansion—but how does this happen? One possibility is that increased CMV reactivation and shedding in HIV-infected subjects drives activation and proliferation of CMV-specific CD8 T cells. Immunocompromised individuals experience more frequent CMV reactivation and shedding [25], and CMV shedding is associated with increased levels of T-cell activation, proliferation, and exhaustion [10]. As many as half of all CD8 T cells can be CMV-reactive in the CMV-infected elderly [20, 26], and the percentage of CD8 T cells specific for CMV antigens is increased in HIV-infected subjects [27–30]. One potential driver of CD8 T-cell expansion in the setting of coinfection is IL-15, which can be upregulated by herpesvirus-infected cells and is upregulated early in HIV infection [31, 32]. Previous studies have clearly demonstrated that CMV infection favors a fully differentiated, effector memory phenotype [33] and that HIV infection may be characterized by a proliferative block in CD8 T cells [34]. Therefore, it is conceivable that the combined proinflammatory environment of HIV and CMV coinfection drives both T-cell activation and some level of bystander proliferation that augments cognate peptide-driven CD8 T-cell expansion, coupled with a failure to deplete the existing CD8 T-cell pool. Although the precise role for CMV in driving CD8 T-cell expansion has not yet been demonstrated in HIV disease, administration of the anti-CMV drug valganciclovir to ART-treated HIV-infected patients with incomplete CD4 T-cell recovery reduced CD8 T-cell activation [35], suggesting that herpesvirus recrudescence contributes to persistent activation in ART-treated HIV infection.
During inflammation, TNF-RII is shed from the surface of cells upon binding with TNFα and its expression can be used as a surrogate for TNFα activity [36]. In untreated HIV infection, TNF-RII levels correlate with HIV RNA levels and are reduced upon initiation of ART [37]. Serum TNF-RII levels are also increased during CMV disease [38] and, notably, also in untreated HIV-infected patients with CMV disease [39]. Our data suggest that CMV-induced inflammation may be an important driver of TNFα expression during ART-treated HIV infection. Similarly, IP-10 is an important chemokine induced by interferons that is involved in a variety of immune pathways and is a biomarker for disease severity in multiple settings. IP-10 expression is elevated in ART-treated HIV infection and in settings of CMV infection following lung transplantation [40, 41]. Here we show that CMV coinfection increases IP-10 expression more than is seen in ART-treated HIV infection alone. Thus, TNFα and interferons produced during the cellular immune response to CMV could contribute to the increase in TNF-RII and IP-10 levels, seen in treated HIV infection.
Notably, HIV and CMV infection and related inflammation are each associated with increased cardiovascular risk [42]. In particular, levels of the fibrin degradation product D-dimer, a coagulation biomarker, are associated with cardiovascular disease and mortality in HIV-infected patients [2]. Elevated CMV-specific T-cell responses and levels of CMV IgG are correlated with increased carotid artery intima-media thickness in HIV-infected patients [28], making CMV a plausible contributor to risk and an attractive target for therapeutic intervention to prevent HIV-associated cardiovascular complications. Future studies are needed to determine if suppressing CMV replication in HIV coinfection will lead to reductions in inflammatory and coagulation indices and CD8 T-cell numbers, and a diminution in the risk of cardiovascular complications and other morbidities in ART-treated HIV infection.
Notes
Acknowledgments. The authors wish to thank Brian Clagett, Dominic Dorazio, and Janet Robinson for excellent technical assistance, and would like to thank Louis Picker for helpful discussions.
Financial support. This work was supported by the Case Western Reserve University (CWRU) Center for AIDS Research (AI036219) and the CWRU Clinical Trials Unit (AI069501), and by funding from the Richard J. Fasenmyer Foundation.
Potential conflicts of interest. M. M. L., P. W. H., and B. R. have received institutional grant support from the National Institutes of Health (NIH). S. G. has received institutional grant support from the NIH Center for AIDS Research. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Guaraldi G, Orlando G, Zona S et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 2.Kuller LH, Tracy R, Belloso W et al. . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenorio AR, Zheng Y, Bosch RJ et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt PW, Sinclair E, Rodriguez B et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serrano-Villar S, Sainz T, Lee SA et al. . HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TP, Bazdar DA, Mudd JC et al. . Interferon-alpha inhibits CD4 T cell responses to interleukin-7 and interleukin-2 and selectively interferes with Akt signaling. J Leukoc Biol 2015; 97:1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shive CL, Mudd JC, Funderburg NT et al. . Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis 2014; 210:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mudd JC, Lederman MM. CD8 T cell persistence in treated HIV infection. Curr Opin HIV AIDS 2014; 9:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano-Villar S, Gutierrez C, Vallejo A et al. . The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect 2013; 66:57–66. [DOI] [PubMed] [Google Scholar]
- 10.Gianella S, Massanella M, Wertheim JO, Smith DM. The sordid affair between human herpesvirus and HIV. J Infect Dis 2015; 212:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 2006; 43:1143–51. [DOI] [PubMed] [Google Scholar]
- 12.Robain M, Carre N, Dussaix E, Salmon-Ceron D, Meyer L. Incidence and sexual risk factors of cytomegalovirus seroconversion in HIV-infected subjects. The SEROCO Study Group. Sex Transm Dis 1998; 25:476–80. [DOI] [PubMed] [Google Scholar]
- 13.Lederman MM, Calabrese L, Funderburg NT et al. . Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29:1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan RC, Kingsley LA, Gange SJ et al. . Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008; 22:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stylianou E, Aukrust P, Bendtzen K, Muller F, Froland SS. Interferons and interferon (IFN)-inducible protein 10 during highly active anti-retroviral therapy (HAART)-possible immunosuppressive role of IFN-alpha in HIV infection. Clin Exp Immunol 2000; 119:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sylwester AW, Mitchell BL, Edgar JB et al. . Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan N, Shariff N, Cobbold M et al. . Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 2002; 169:1984–92. [DOI] [PubMed] [Google Scholar]
- 21.Khan N, Cobbold M, Cummerson J, Moss PA. Persistent viral infection in humans can drive high frequency low-affinity T-cell expansions. Immunology 2010; 131:537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. Dysfunctional CMV-specific CD8+ T cells accumulate in the elderly. Exp Gerontol 2004; 39:607–13. [DOI] [PubMed] [Google Scholar]
- 23.Chidrawar S, Khan N, Wei W et al. . Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol 2009; 155:423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett L, Stapleton SN, Fudge NJ, Grant MD. Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS 2014; 28:2045–9. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldo CR Jr, Kingsley LA, Ho M, Armstrong JA, Zhou SY. Enhanced shedding of cytomegalovirus in semen of human immunodeficiency virus-seropositive homosexual men. J Clin Microbiol 1992; 30:1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang Q, Wagner WM, Wikby A et al. . Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol 2003; 23:247–57. [DOI] [PubMed] [Google Scholar]
- 27.Naeger DM, Martin JN, Sinclair E et al. . Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsue PY, Hunt PW, Sinclair E et al. . Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006; 20:2275–83. [DOI] [PubMed] [Google Scholar]
- 29.Stone SF, Price P, Khan N, Moss PA, French MA. HIV patients on antiretroviral therapy have high frequencies of CD8 T cells specific for Immediate Early protein-1 of cytomegalovirus. AIDS 2005; 19:555–62. [DOI] [PubMed] [Google Scholar]
- 30.Stone SF, Price P, French MA. Cytomegalovirus (CMV)-specific CD8+ T cells in individuals with HIV infection: correlation with protection from CMV disease. J Antimicrob Chemother 2006; 57:585–8. [DOI] [PubMed] [Google Scholar]
- 31.Fawaz LM, Sharif-Askari E, Menezes J. Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J Immunol 1999; 163:4473–80. [PubMed] [Google Scholar]
- 32.Mueller YM, Katsikis PD. IL-15 in HIV infection: pathogenic or therapeutic potential? Eur Cytokine Netw 2010; 21:219–21. [DOI] [PubMed] [Google Scholar]
- 33.Appay V, Dunbar PR, Callan M et al. . Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002; 8:379–85. [DOI] [PubMed] [Google Scholar]
- 34.Lee SA, Sinclair E, Hatano H et al. . Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One 2014; 9:e89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt PW, Martin JN, Sinclair E et al. . Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valle Y, Ledezma-Lozano IY, Torres-Carrillo N et al. . Circulating TNFRI and TNFRII levels correlated with the disease activity score (DAS28) in rheumatoid arthritis. Scand J Rheumatol 2009; 38:332–5. [DOI] [PubMed] [Google Scholar]
- 37.Nokta M, Rossero R, Loesch K, Pollard RB. Kinetics of tumor necrosis factor alpha and soluble TNFRII in HIV-infected patients treated with a triple combination of stavudine, didanosine, and hydroxyurea. AIDS Res Hum Retroviruses 1997; 13:1633–8. [DOI] [PubMed] [Google Scholar]
- 38.Humbert M, Roux-Lombard P, Cerrina J et al. . Soluble TNF receptors (TNF-sR55 and TNF-sR75) in lung allograft recipients displaying cytomegalovirus pneumonitis. Am J Respir Crit Care Med 1994; 149:1681–5. [DOI] [PubMed] [Google Scholar]
- 39.Jakobsen PH, Dodt KK, Meyer CN, Katzenstein T, Gerstoft J, Skinhoj P. Increased levels of soluble tumour necrosis factor receptor-I (P55) and decreased IgG1 reactivities in HIV-1 patients with cytomegalovirus disease. Scand J Immunol 1998; 47:591–5. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez LA, Arango TA, Thompson E, Naji M, Tebas P, Boyer JD. High IP-10 levels decrease T cell function in HIV-1-infected individuals on ART. J Leukoc Biol 2014; 96:1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weseslindtner L, Nachbagauer R, Kundi M et al. . Human cytomegalovirus infection in lung transplant recipients triggers a CXCL-10 response. Am J Transplant 2011; 11:542–52. [DOI] [PubMed] [Google Scholar]
- 42.Hsue PY, Lo JC, Franklin A et al. . Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004; 109:1603–8. [DOI] [PubMed] [Google Scholar]