Hepatitis C virus treatment with interferon-free direct-acting antivirals could reduce prevalence by more than 80% within a decade. Even further reductions in prevalence and transmission are achievable but require targeted interventions that expand both screening and treatment of high-risk populations.
Keywords: hepatitis C, people who inject drugs, direct-acting antivirals, screening, treatment
Abstract
Background. The effectiveness of interferon-free direct-acting antivirals (DAA) in treating chronic hepatitis C virus (HCV) is limited by low screening and treatment rates, particularly among people who inject drugs (PWIDs).
Methods. To evaluate the levels of screening and treatment with interferon-free DAAs that are required to control HCV incidence and HCV-associated morbidity and mortality, we developed a transmission model, stratified by age and by injection drug use, and calibrated it to epidemiological data in the United States from 1992 to 2014. We quantified the impact of administration of DAAs at current and at enhanced screening and treatment rates, focusing on outcomes of HCV incidence, prevalence, compensated and decompensated cirrhosis, hepatocellular carcinoma, liver transplants, and mortality from 2015 to 2040.
Results. Increasing annual treatment of patients 4-fold—from the approximately 100 000 treated historically to 400 000—is predicted to prevent 526 084 (95% confidence interval, 466 615–593 347) cases of cirrhosis and 256 315 (201 589–316 114) HCV-associated deaths. By simultaneously increasing treatment capacity and increasing the number of HCV infections diagnosed, total HCV prevalence could fall to as low as 305 599 (222 955–422 110) infections by 2040. Complete elimination of HCV transmission in the United States through treatment with DAAs would require nearly universal screening of PWIDs, with an annual treatment rate of at least 30%.
Conclusions. Interferon-free DAAs are projected to achieve marked reductions in HCV-associated morbidity and mortality. Aggressive expansion in HCV screening and treatment, particularly among PWIDs, would be required to eliminate HCV in the United States.
It is estimated that more than 5 million people in the United States are chronically infected with hepatitis C virus (HCV), the most common blood-borne infection in the United States and the current leading cause of cirrhosis nationwide [1]. More than half of those with chronic HCV are unaware of their status [2], including up to two-thirds of people who inject drugs (PWIDs), the population with the highest HCV incidence [3]. Historically, HCV patients were treated with 24–48 weeks of interferon-based regimens, the adverse effects of which limited their use [4]. Consequently, fewer than 12% of diagnosed patients and fewer than 5% of diagnosed PWIDs underwent therapy [5, 6]. Since 2013, interferon-free direct-acting antivirals (DAAs) have been approved that feature sustained virologic response (SVR) rates greater than 90%, minimal adverse effects, reduced pill burden, and ease of oral administration. These interferon-free DAAs have opened a new frontier in HCV treatment, raising the possibility of using treatment not only to prevent HCV-associated deaths but also to interrupt the transmission chain among PWIDs and potentially eliminate HCV altogether [4, 7].
Birth-cohort screening of those born 1945 through 1965—a demographic with an HCV prevalence 3 times greater than the overall adult population—was recommended in 2012 by the Centers for Disease Control and Prevention [8, 9]. It has been predicted that this birth-cohort screening would prevent 120 000–130 000 HCV-associated deaths compared with interferon-based treatments in the absence of birth-cohort screening [10, 11]. However, these predictions were based on estimates of chronic HCV cases from the National Health and Nutrition Examination Survey (NHANES), which underrepresents high-prevalence populations and may underestimate the actual HCV prevalence by as many as 2 to 4 million cases [1, 8]. Furthermore, current PWID treatment rates are insufficient to substantially reduce HCV prevalence [12], and low screening rates among the estimated 775 000 PWIDs pose a barrier to treating this high-risk group [13, 14]. To quantify the long-term benefit of interferon-free DAAs on HCV prevalence, morbidity, and mortality, we developed a dynamic transmission analysis that captures the impact of treatment on both chronic disease and transmission dynamics, distinguishes screening and treatment as separate but related public health objectives, and accounts for underreporting in NHANES estimates of national HCV prevalence.
METHODS
To determine the levels of screening and treatment that are required to avert both new infections as well as liver disease among existing cases, we built on recent analyses [10, 11, 15] to develop a model that specifically incorporates both acute transmission and chronic disease. We fit model parameters to historical data on HCV incidence, age-stratified prevalence, HCV transmission, and chronic disease progression among both PWIDs and the noninjecting population. We projected the impact through 2040 of current and enhanced treatment with DAAs on total HCV prevalence, morbidity, and mortality. We then assessed the improvements in HCV outcomes that could be achieved by additional screening, beyond existing risk-based and birth-cohort levels. Finally, we quantified the screening and treatment rates among PWIDs that would be necessary to control transmission.
Model Overview
To quantify the impact of screening and treatment with interferon-free DAAs on HCV infection and HCV-associated liver disease, we developed an age-structured compartmental model that incorporated injection drug use, HCV transmission, and chronic liver disease progression. We fit our model to 22 years of historical data from 1992 to 2014, which spans the period following the introduction of blood donor screening for HCV in 1992 [16] through the 2013 approval of interferon-free DAAs [4]. We then projected the epidemiological trajectory of HCV from 2015 to 2040 under a range of screening and DAA treatment scenarios.
Demographics
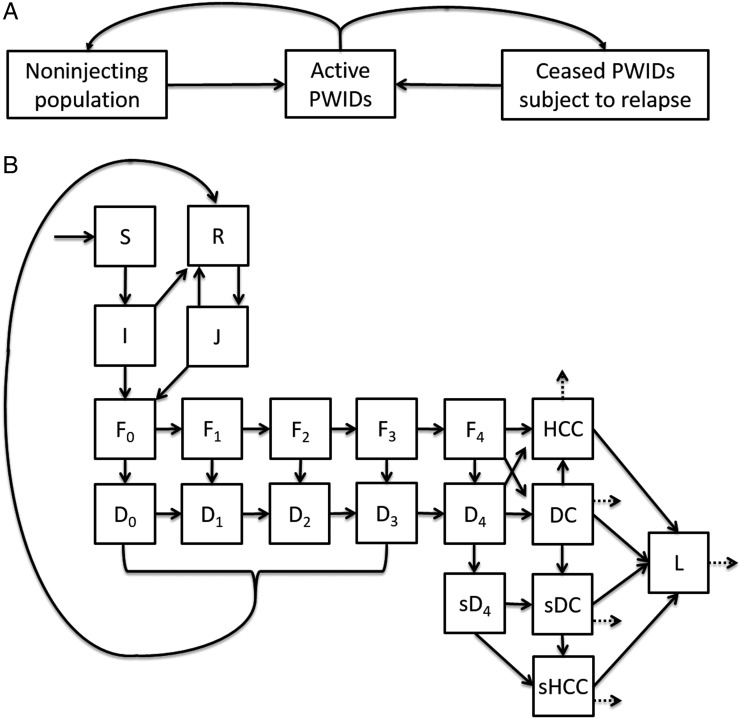
We initialized the model with the US population age distribution in 1992 (Supplementary Materials). Specifically, we differentiated 15 five-year classes spanning ages 11–85 years and fit birth, aging, and age-specific death rates to values from the American Community Survey. We further stratified the model into 3 injection drug use groups (Figure 1A): active PWIDs, PWIDs in temporary cessation and at risk for relapse, and the noninjecting population, including both those who have never injected drugs and former PWIDs who achieve permanent cessation. We calibrated age-specific rates at which individuals became active PWIDs (Supplementary Table 3).
Figure 1.
Compartment diagram of injection drug use, hepatitis C virus (HCV) infection transmission dynamics, and liver disease progression. A, Individuals in the noninjecting population became people who inject drugs (PWIDs) at age-specific rates. Active PWIDs ceased injecting over time, with a fraction relapsing and the remainder returning to the noninjecting population. B, The compartmental model includes susceptible (S), acutely infected (I), recovered (R), acutely reinfected (J), undiagnosed chronic HCV Metavir stages 0–4 (F0–4), diagnosed chronic HCV Metavir stages 0–4 (D0–4), cirrhosis with sustained virologic response (SVR; sD4), decompensated cirrhosis (DC), decompensated cirrhosis with SVR (sDC), hepatocellular carcinoma (HCC), hepatocellular carcinoma with SVR (sHCC), and post-liver transplant (L). HCC, sHCC, DC, sDC, and L experience increased mortality (dashed arrows).
Epidemiology
For every age and injection drug use group, we modeled transmission, the progression and treatment of chronic HCV, as well as HCV-associated liver disease (Figure 1B). Individuals who were susceptible (S) could become acutely infected (I), leading to either chronic infection (F0) or spontaneous clearance (R). Chronic HCV then progressed through 5 stages of liver fibrosis (F0–F4), and may be diagnosed (D). For computational convenience, we assumed that patients progress sequentially through fibrosis at stage-specific rates [17] before developing advanced liver disease. Diagnosed chronic HCV may be successfully treated (R) with a time-dependent and Metavir-specific SVR. Rates of screening and treatment were distinct for PWIDs and for the noninjecting population.
Infections that spontaneously cleared or were successfully treated (R) were subject to reinfection (J). Individuals with chronic HCV and liver cirrhosis (F4 and D4) were at risk of developing decompensated cirrhosis (DC). Patients with cirrhosis, compensated (F4 and D4) or decompensated (DC), were at risk of developing hepatocellular carcinoma (HCC). Individuals with DC or HCC could receive liver transplants (L). Individuals with compensated cirrhosis who achieved SVR (sF4) were no longer infectious but were subject to decompensated cirrhosis with SVR (sDC) and hepatocellular carcinoma with SVR (sHCC) at a reduced risk. The mortality of those with DC, HCC, sDC, sHCC, or L was elevated across all years. From 2014 onward, patients with DC received the same treatment as diagnosed precirrhotic patients, reflecting the expansion of treatment options available to these patients made possible by DAAs [4, 18].
Parameters
Initializing our model to the year 1992 (Supplementary Table 1), we set age-specific HCV prevalence among the noninjecting population to data collected as part of NHANES from 1988 to 1994 [19] and corrected for underreporting as estimated from a metaanalysis [1]. For the PWID population, age-specific HCV prevalence was estimated from data gathered in Baltimore, Chicago, Los Angeles, and New York [20]. Based on empirical surveys, we specified that 75% (95% confidence interval [CI], 71%–79%) of HCV antibody-positive individuals had chronic infections [21]. The remaining 25% of those testing positive had cleared infection. All HCV cases were specified as undiagnosed in 1992, when diagnostics were implemented.
Transmission
We incorporated a higher transmission rate among active PWIDs that reflects the high risk of infection arising from the sharing of drug paraphernalia and a lower transmission rate among the entire population that reflects other routes such as accidental exposure by healthcare workers or sexual transmission. Active PWIDs with HCV thus transmit to other active PWIDs at a high transmission rate and to non-PWIDs at the low transmission rate, while non-PWIDs with HCV transmit to both active PWIDs and non-PWIDs at a low transmission rate. We assumed that the transmission rate of infected individuals is independent of HCV infection progression, liver complication, genotype, or diagnosis. Transmissibility among cases is correlated with viral load as determined by RNA levels [22]. However, no correlation has been found between viral load and degree of liver cirrhosis or HCV genotype [23, 24]. Likewise, patient studies suggest comparable viral loads between acutely infected and chronically infected individuals [24, 25]. Cohort studies have also found that the HCV transmission rate among young adult PWIDs remains unchanged following diagnosis [3, 26, 27]. Given the conflicting results of studies comparing the relative risk of reinfection to primary infection [28], we also assumed that the risk of primary infection and reinfection are the same.
A longitudinal PWID study has demonstrated that the viral load of reinfected individuals is approximately 103 lower than during primary infection [29]. Combined with the finding that viral loads below 104 copies/mL result in 11-fold lower transmissibility compared with loads above 106 copies/mL [22], we quantified the transmission rate from reinfected individuals as 1/11 of that from individuals with primary infection.
Calibration
We used Bayesian Markov chain Monte Carlo simulation to calibrate our model to extensive epidemiological data over the period 1992–2014 (Supplementary Methods, Supplementary Table 4). The calibration produced a sample of 1000 parameter sets from their joint posterior distribution (Supplementary Table 3). We then used this ensemble to project uncertainty into the future and to evaluate policy scenarios. Calibrated model outputs were validated by comparison to additional data that were not used for the calibration (Supplementary Table 5).
Screening and Treatment
We assumed that diagnosed HCV cases were treated with interferon from 1992 to 1998; with interferon in combination with ribavirin from 1998 to 2002; with pegylated interferon in combination with ribavirin from 2002 to 2012; with pegylated interferon, ribavirin, and boceprevir or telaprevir from 2012 to 2013; and with interferon-free DAAs from 2014 onward [4]. For each treatment regimen, we estimated the probability of SVR by computing a weighted average of genotype-specific SVR with HCV genotype prevalence, distinguishing between the early stages of fibrosis (F0–F2) and the later stages of fibrosis and cirrhosis (F3–F4) (Supplementary Table 2). For projections from 2014 onward, we assumed a DAA treatment SVR, weighted across genotypes, of 95% [4, 30–32]. We projected the impact of the following 4 policy options from 2015 to 2040: screening of the noninjecting population; DAA treatment of the noninjecting population; screening of PWIDs, both active and temporarily ceased; and DAA treatment of PWIDs, both active and temporarily ceased. To model screening of the noninjecting population, we included both birth-cohort and routine screening. For birth-cohort screening, we specified a 1-time screening of those born in the years 1945–1965 and assumed that 82% of HCV infections in this cohort would be diagnosed, as estimated in previous analyses [10]. For routine screening, we specified a baseline probability of 6.2% (4.6%–8.0%) that an undiagnosed individual is successfully diagnosed annually [2]. In addition to this baseline rate, we also considered universal screening, approximated by a screening rate of 99%, in order to give the upper bound of the maximum health benefits achievable through enhanced screening.
To model treatment of the noninjecting population, we assumed that prior to the availability of DAAs, individuals diagnosed with HCV received treatment at an annual rate of 2.5% (2.4%–2.5%) [5]. Following the availability of DAAs, we assumed that provider willingness and patient demand for treatment will be high, such that the primary constraints to treating patients will be provider capacity and reimbursement limits. Following historical trends, we specified a baseline treatment of 100 000 patients annually [2], which we scaled up to 400 000 diagnosed patients per year.
To model screening of PWIDs, we first specified a baseline screening rate of 4.1% (3.6%–4.6%) annually [3]. We then considered increasing annual screening to 10%, 20%, and 99%. We assumed that active and temporarily ceased PWIDs would not be reached under birth-cohort screening. To model treatment of PWIDs, we first specified a baseline rate of 0.58% (0.42%–0.82%) annually [6]. We then investigated the effects of expanding PWID treatment to 5%, 10%, 20%, and 30% annually.
Policy Scenarios
We first evaluated the impact of the policies considered on total HCV prevalence, incidence, and HCV-associated morbidity and mortality of current and enhanced treatment of the noninjecting population. We varied the risk-based screening of the noninjecting population, while maintaining screening and treatment of PWIDs constant. Likewise, we then evaluated the impact of expanded screening and treatment of PWIDs on the HCV prevalence and incidence specifically among PWIDs. We varied the treatment of the noninjecting population, holding risk-based screening of the noninjecting population unchanged.
RESULTS
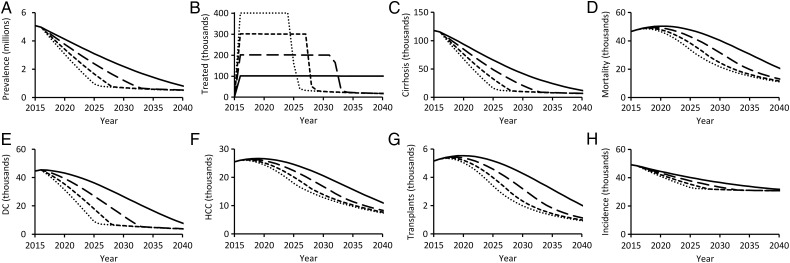
Our model predicts that at current treatment rates, total HCV prevalence in the United States would fall by more than 80% by 2040. If annual treatment increased to 200 000, 300 000, or 400 000 patients, an 80% reduction in HCV prevalence is predicted to be achieved by 2031, 2028, or 2025, respectively (Figure 2A and 2B). Substantial improvements will also be achieved in HCV-associated morbidity and mortality through enhanced treatment of the noninjecting population (Figure 2C–G). Compared with an annual treatment of 100 000 patients, treatment of 200 000 patients is predicted to result in 289 206 (95% CI, 261 092–322 939) fewer cases of cirrhosis and 143 055 (111 361–176 166) fewer deaths through 2040. Treating 400 000 patients annually is predicted to avert 526 084 (466 615–593 347) cases of cirrhosis and 256 315 (201 589–316 114) deaths through 2040 (Table 1).
Figure 2.
Hepatitis C virus (HCV)-associated health outcomes over time, predicted with annual treatment of the noninjecting population at a national treatment rate of 100 000 (solid), 200 000 (long-dashed), 300 000 (short-dashed), or 400 000 (dotted) patients annually. Health outcomes depicted are (A) total HCV prevalence, (B) annual number of patients treated, (C) new cases of compensated cirrhosis, (D) annual mortality, (E) new cases of decompensated cirrhosis (DC), (F) new cases of hepatocellular carcinoma (HCC), (G) annual liver transplants, and (H) new HCV infections. All scenarios assume treatment exclusively with direct-acting antivirals, 1-time birth-cohort screening, and no change to the risk-based screening rate or to people who inject drugs screening or treatment rates.
Table 1.
Hepatitis C Virus Infections, Morbidity, and Mortality Under Current and Enhanced Treatment Rates
| Outcome Summed Over 2015–2040 | No Change (95% CI) | Health Improvement Under Increased Treatment (95% Confidence Interval) |
||
|---|---|---|---|---|
| 200 000 Patients Treated Annually | 300 000 Patients Treated Annually | 400 000 Patients Treated Annually | ||
| New infections | 897 327 (680 510–1 279 320) | 41 114 (25 233–72 673) | 61 035 (37 372–108 014) | 72 419 (44 117–127 673) |
| Cases of cirrhosis | 1 279 469 (1 135 338–1 443,319) | 289 206 (261 092–322 939) | 437 948 (391 603–491 021) | 526 084 (466 615–593 347) |
| Cases of decompensated cirrhosis | 642 205 (521 922–781 651) | 165 539 (131 827–204 560) | 240 296 (191 380–297 878) | 282 064 (221 870–351 205) |
| Cases of hepatocellular carcinoma | 454 965 (355 781–552 351) | 72 373 (41 707–100 710) | 108 151 (65 859–148 246) | 128 536 (79 309–175 569) |
| Liver transplants | 84 319 (22 647–236 478) | 15 010 (3578–48 315) | 22 386 (5342–72 226) | 26 545 (6336–85 461) |
| Deaths | 876 033 (741 794–1 016 798) | 143 055 (111 361–176 166) | 215 626 (168 395–264 983) | 256 315 (201 589–316 114) |
All scenarios assume treatment from 2015 to 2040 exclusively with direct-acting antivirals, birth-cohort screening among the noninjecting population, no change to routine (nonbirth-cohort) screening, and no change to people who inject drugs screening or treatment. Health improvements under increased treatment are calculated by subtracting the cumulative hepatitis C virus infections, morbidity, and mortality under each increased treatment scenario from the corresponding outcomes under the “No change” scenario.
Abbreviation: CI, confidence interval.
Without expansion of HCV screening, at least 462 736 (340 546–620 562) cases would remain untreated through 2040 among undiagnosed individuals and PWIDs. Universal screening among the noninjecting population could further reduce prevalence to 305 599 (222 955–422 110) cases (Table 2). New infections occur principally among PWIDs and therefore will not be appreciably reduced without increasing targeted screening and treatment of this vulnerable population (Figure 2H). Specifically, reduction of HCV prevalence to below 300 000 cases and reduction of incidence by more than 10% requires programs specifically targeted toward identifying HCV-positive PWIDs and improving their access to medication (Table 2).
Table 2.
Total Hepatitis C Virus Prevalence Achievable in the United States in 2040 at Current Risk-based Screening and Universal Screening of the Noninjecting Population
| Hepatitis C Virus Screening | Patients Treated Annually |
|||
|---|---|---|---|---|
| 100 000 | 200 000 | 300 000 | 400 000 | |
| Current risk-based screening | 561 337 (382 034–829 631) | 474 911 (350 562–636 057) | 466 457 (343 090–625 937) | 462 736 (340 546–620 562) |
| Universal screening | 519 028 (305 732–786 036) | 310 399 (225 471–427 119) | 307 259 (223 906–423 740) | 305 599 (222 955–422 110) |
All scenarios assume treatment after 2015 exclusively with direct-acting antivirals, birth-cohort screening among the noninjecting population, and no change to people who inject drugs screening or treatment. Values in parentheses indicate 95% confidence intervals.
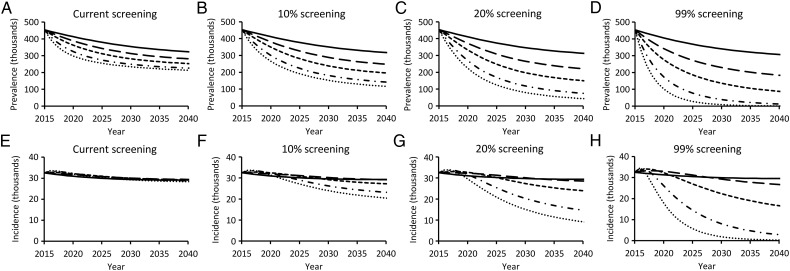
Without increasing PWID screening beyond the current 4.1% baseline rate, by 2040 total HCV prevalence among PWIDs will be reduced by, at most, 53% and incidence will be reduced by, at most, 15%, even under an aggressive 30% annual treatment rate of diagnosed infections among PWIDs (Figure 3A and 3E). A reduction of prevalence among PWIDs of at least 90% would require a 20% screening rate combined with 30% treatment (Figure 3C) or universal screening combined with 20% treatment (Figure 3D). A reduction of incidence of new infections by 90% would require universal screening and at least 20% treatment (Figure 3H).
Figure 3.
Impact of current (solid), 5% (long-dashed), 10% (short-dashed), 20% (dash-dotted), and 30% (dotted) people who inject drugs (PWIDs) treatment rates on total hepatitis C virus (HCV) prevalence among PWIDs (A–D) and annual incidence among PWIDs of new HCV cases (E–H) at current, 10%, 20%, and universal PWID screening rates. All scenarios assume treatment exclusively with direct-acting antivirals, 1-time birth-cohort screening among the noninjecting population, and no change to routine risk-based screening or treatment of the noninjecting population. Results were insensitive to increased treatment of the noninjecting population.
DISCUSSION
We found that HCV prevalence, HCV-associated liver disease, and HCV-associated mortality in the United States can be substantially reduced through widespread treatment with DAAs. Total HCV prevalence is likely to fall by more than 80% within 10–20 years through treatment alone. Up to 150 000 additional cases could be identified by increasing HCV screening, effectively eliminating HCV from the noninjecting population. Further opportunities exist to greatly reduce prevalence and new infections among PWIDs through targeted screening and treatment.
While birth-cohort screening and treatment with DAAs will markedly reduce HCV prevalence in the United States, a number of individuals will remain unidentified and unlinked to care under current policies. Targeted interventions will be necessary to reach these individuals. Screening at emergency room visits has been shown effective in identifying undiagnosed individuals and linking them to care, both among baby boomers as well as among PWIDs [33, 34]. Access to medical care in a nonjudgmental setting and provider-initiated screening have been shown to be effective in increasing HCV screening among PWIDs [35]. The potential to eliminate HCV can be improved through combination interventions that incorporate enhanced screening and treatment with needle and syringe exchange programs to reduce transmission and with opiate substitution therapy to reduce the reservoir of drug users actively transmitting [12].
Our results complement recent model-based predictions of HCV burden [10, 11] by incorporating both chronic disease and ongoing HCV transmission, distinguishing screening and treatment as separate but related public health objectives, and accounting for underreporting of HCV prevalence in NHANES. As with any model, we made a number of simplifying assumptions. First, we did not explicitly account for genotype-specific effects, such as the correlation between liver disease and viral load observed for HCV genotype 3, which could heighten transmission from those with more severe fibrosis [36]. Instead, we represented genotypes implicitly, using population-average parameter sources and calculating effective SVR rates as weighted averages of genotype prevalence. Given that genotype 3 comprises only 8%–12% of HCV cases in the United States [30], such genotype stratification would be unlikely to affect our results. Also, we modeled HCV transmission with homogeneous mixing. As injection drug behavior and the corresponding transmission risk vary across individuals, a strategy that could successfully target treatment toward high-risk individuals would more efficiently reduce incidence than a strategy that targets all individuals with equal probability [37].
To improve the runtime of our analyses, it was necessary to model birth-cohort screening as occurring at one point in time—the year 2015—rather than rolled out over a period of several years. This simplification does not affect our results because most transmission occurs among PWIDs who are not affected by birth-cohort screening and, since we assumed a fixed number of patients treated annually, a partial delay in the roll-out of birth-cohort screening would have minimal impact on the total number of patients actually treated over that period in our model.
We assumed treatment rates to be independent of stage of liver fibrosis. In practice, the prioritization of treatment toward patients with more severe fibrosis might improve overall liver disease outcomes but also might bring about a slightly higher acute HCV incidence than our projections indicate, as such a policy would result in more healthy carriers who are capable of transmitting HCV. Although severity-specific treatment would be important to consider when evaluating the cost effectiveness of treatment [38], such considerations would be unlikely to appreciably impact our overall conclusions of screening and treatment coverage.
We assumed that individuals with acute infection transmit at the same rate as those with chronic infection, based on studies indicating comparable HCV viral load between the acute and chronic infection [24, 25]. Primate studies have indicated that acute infections may be more transmissible than chronic infections, but it is unclear whether these findings can be generalized to humans [15, 39]. Given the transience of acute HCV infection, this assumption should not significantly affect our results.
DAAs hold tremendous promise in terms of improving health outcomes and reducing transmission. It is likely that treatment acceptance will increase following the availability of interferon-free DAAs, for which delivery is tolerable with minimal adverse effects. However, the improvement of health outcomes expected from greater acceptance might be tempered by the high costs of treatment and by limited insurance coverage and willingness to treat PWIDs [4]. Our analysis provides a forecast for the potential impact of DAAs in reducing HCV-associated liver disease, demonstrating that achievable expansion of HCV treatment at current screening rates can substantially reduce morbidity and mortality.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Chizoba Nwankwo and Shannon Ferrante for helpful comments and feedback and Forrest Jones for assistance with data compilation. Simulations were run at the Yale University Biomedical High Performance Computing Center, which is supported by National Institutes of Health grants RR19895 and RR029676-01.
Financial support. This research was funded by the Notsew Orm Sands Foundation and Merck.
Potential conflicts of interest. D. P. D., A. P. G., and J. P. T. have consulted for and received research funding from Sanofi Pasteur and Merck. E. H. E. is a current employee of Merck and holds stocks and stock options. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int 2011; 31:1090–101. [DOI] [PubMed] [Google Scholar]
- 2.Volk ML, Tocco R, Saini S, Lok ASF. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 2009; 50:1750–5. [DOI] [PubMed] [Google Scholar]
- 3.Hagan H, Campbell J, Thiede H et al. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep 2006; 121:710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice CM, Saeed M. Hepatitis C: treatment triumphs. Nature 2014; 510:43–4. [DOI] [PubMed] [Google Scholar]
- 5.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut 2007; 56:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta SH, Genberg BL, Astemborski J et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health 2008; 33:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edlin BR, Winkelstein ER. Can hepatitis C be eradicated in the United States? Antiviral Res 2014; 110C:79–93. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GL. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705. [DOI] [PubMed] [Google Scholar]
- 9.Smith BD, Morgan RL, Beckett GA et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep 2012; 61:1–32. [PubMed] [Google Scholar]
- 10.Rein DB, Smith BD, Wittenborn JS et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med 2012; 156:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med 2014; 161:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57(suppl 2):S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckwith CG, Larney S, Flanigan TP. Editorial commentary: hepatitis C virus testing and drug use in North America; is there more than meets the eye? Clin Infect Dis 2014; 58:762–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansky A, Finlayson T, Johnson C et al. Estimating the number of persons who inject drugs in the United States by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One 2014; 9:e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbasha E. Model for hepatitis C virus transmission. Math Biosci Eng 2013; 10:1045–65. [DOI] [PubMed] [Google Scholar]
- 16.Donahue JG, Muñoz A, Ness PM et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med 1992; 327:369–73. [DOI] [PubMed] [Google Scholar]
- 17.Thein H-H, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418–31. [DOI] [PubMed] [Google Scholar]
- 18.Everson GT, Bhatt A. Treatment of hepatitis C in the patient with decompensated cirrhosis. Curr Hepat Rep 2013; 12:236–45. [Google Scholar]
- 19.Alter MJ, Kruszon-Moran D, Nainan OV et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999; 341:556–62. [DOI] [PubMed] [Google Scholar]
- 20.Amon JJ, Garfein RS, Ahdieh-Grant L et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis 2008; 46:1852–8. [DOI] [PubMed] [Google Scholar]
- 21.Grebely J, Dore GJ, van der Loeff MS et al. Factors associated with spontaneous clearance during acute hepatitis C virus infection. J Hepatol 2010; 52:S411. [Google Scholar]
- 22.Yazdanpanah Y, De Carli G, Migueres B et al. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case-control study. Clin Infect Dis 2005; 41:1423–30. [DOI] [PubMed] [Google Scholar]
- 23.De Moliner L, Pontisso P, De Salvo GL, Cavalletto L, Chemello L, Alberti A. Serum and liver HCV RNA levels in patients with chronic hepatitis C: correlation with clinical and histological features. Gut 1998; 42:856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo AET, Ghany M, Conry-Cantilena C et al. Stability of HCV-RNA level and its lack of correlation with disease severity in asymptomatic chronic hepatitis C virus carriers. J Viral Hepat 2001; 8:256–63. [DOI] [PubMed] [Google Scholar]
- 25.Glynn SA, Wright DJ, Kleinman SH et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005; 45:994–1002. [DOI] [PubMed] [Google Scholar]
- 26.Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, Maryland. Clin Infect Dis 2002; 35:783–8. [DOI] [PubMed] [Google Scholar]
- 27.Spelman T, Morris MD, Zang G et al. A longitudinal study of hepatitis C virus testing and infection status notification on behaviour change in people who inject drugs. J Epidemiol Community Heal 2015; 69:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grebely J, Prins M, Hellard M et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis 2012; 12:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osburn WO, Fisher BE, Dowd KA et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010; 138:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nainan OV, Alter MJ, Kruszon-Moran D et al. Hepatitis C virus genotypes and viral concentrations in participants of a general population survey in the United States. Gastroenterology 2006; 131:478–84. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson IM, Gordon SC, Kowdley KV et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013; 368:1867–77. [DOI] [PubMed] [Google Scholar]
- 32.Sulkowski MS, Gardiner DF, Rodriguez-Torres M et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370:211–21. [DOI] [PubMed] [Google Scholar]
- 33.Ditah I, Al Bawardy B, Gonzalez HC et al. Lack of health insurance limits the benefits of hepatitis C virus screening: insights from the National Health and Nutrition Examination Hepatitis C Follow-Up Study. Am J Gastroenterol 2015; 110:1126–33. [DOI] [PubMed] [Google Scholar]
- 34.Galbraith JW, Franco RA, Donnelly JP et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology 2015; 61:776–82. [DOI] [PubMed] [Google Scholar]
- 35.Barocas JA, Brennan MB, Hull SJ, Stokes S, Fangman JJ, Westergaard RP. Barriers and facilitators of hepatitis C screening among people who inject drugs: a multi-city, mixed-methods study. Harm Reduct J 2014; 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abenavoli L, Masarone M, Peta V et al. Insulin resistance and liver steatosis in chronic hepatitis C infection genotype 3. World J Gastroenterol 2014; 20:15233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn JA, Wylie D, Dill J et al. Potential impact of vaccination on the hepatitis C virus epidemic in injection drug users. Epidemics 2009; 1:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med 2015; 162:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion 2009; 49:2454–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.