There is a significant reduction in tenofovir drug levels in cervicovaginal fluid and tissue when gel is applied prior to sex compared with no sex, suggesting that before and after sex dosing or sustained drug delivery may be optimal.
Keywords: MTN-011, tenofovir gel, pharmacokinetics, post-coital, HIV
Abstract
Background. Tenofovir (TFV) gel partially protected against human immunodeficiency virus (HIV) in one but not subsequent trials. The disappointing results were attributed largely to poor adherence. However, timing of gel application relative to sex may impact pharmacokinetics and contribute to outcomes. Thus, we conducted a single-dose pharmacokinetic study of TFV gel applied 1 or 24 hours before or 1 hour before and 1 hour after (BAT) sex and compared results with dosing without sex.
Methods. Twenty-four couples were enrolled; cervicovaginal lavage (CVL) and tissue were collected 2 hours after sex with matching timed collections at no sex visits and assayed for drug concentrations and CVL anti-HIV activity.
Results. Compared with dosing without sex, median TFV concentrations after sex decreased 72% and 78% (P < .001) in CVL, 75% and 71% (P < .001) in vaginal tissue, and 75% (P = .06) and 55% (P < .001) in cervical tissue with −1 hour and −24 hour dosing, respectively. Median concentration of TFV–diphosphate also decreased significantly in cervical tissue with −1 hour, dosing. BAT dosing resulted in drug levels at least as great as those in the absence of sex. Percent inhibition of HIV infection by post-coital CVL increased significantly from median (interquartile range) of 55% (54%) in the absence of gel to 99% (7%), 77% (57%), and 100% (0.4%) with −1 hour, −24 hour, or BAT dosing, respectively, and correlated significantly with drug concentration.
Conclusions. Timing of TFV gel application relative to sex significantly impacts drug levels. BAT dosing or sustained delivery may be optimal for preexposure prophylaxis.
Oral and topical preexposure prophylaxis (PrEP) can be effective in reducing human immunodeficiency virus (HIV) transmission. However, clinical trial outcomes have differed substantially, reflecting variable adherence [1–5]. For example, oral tenofovir (TFV) disoproxil fumarate alone or combined with emtricitabine and vaginally applied 1% TFV gel were ineffective in the Microbicide Trials Network (MTN)-003 trial where TFV was detected in the plasma of only 30%, 29%, and 25% of participants randomized to those products, respectively [6]. TFV gel dosed before and after sex was modestly effective (39%) in Center for Aids Programme of Research In South Africa (CAPRISA) 004 but ineffective in the Follow-on African Consortium for Tenofovir Studies 001 trial [7]. Post hoc analyses indicated HIV protection with increased levels of adherence [8].
While more behavioral research is needed to identify strategies to facilitate adherence, biological factors may also contribute to different outcomes. In particular, frequency and timing of dosing relative to sex may modify drug biodistribution and efficacy. For example, a woman who applies a topical formulation shortly before sex may lose a substantial fraction of the product from vaginal leakage or dilution of the drug by seminal and cervicovaginal fluids associated with coitus. In contrast, sex may have less impact on pharmacokinetics (PK) and pharmacodynamics (PD) in the setting of more frequent product application or with sustained delivery formulations. Possibly, timing and frequency of dosing relative to sex contributed to the variability in drug concentrations observed in CAPRISA 004 [9].
The notion that sex and semen impact PK and/or PD is supported by a post-coital study of PRO 2000 gel [10]. There was a significant reduction in drug levels and in anti-HIV–herpes simplex virus (HSV) activity of cervicovaginal lavage (CVL) obtained following a single dose of gel prior to sex compared with no sex. The loss of antiviral activity was attributed to decreased CVL drug concentrations and ability of seminal proteins to competitively block binding of PRO 2000 to the viral envelope [11]. While no reduction in antiviral activity of TFV has been observed in vitro when seminal fluids are added to cultures [12, 13], timing of gel application relative to sex might impact drug biodistribution. Therefore, we conducted a single-arm crossover study (MTN-011) to compare concentrations of TFV and TFV–diphosphate (TFV–DP), the active intracellular metabolite of TFV, in multiple compartments in response to a single dose of 1% TFV gel applied 1 or 24 hours prior to or 1 hour before and 1 hour after (BAT) sex compared with similarly timed no sex dosing. We also compared anti-HIV and anti-HSV-2 activity of CVL as a biomarker of PD. We hypothesized that TFV concentrations and antiviral activity would decrease significantly when applied 1 hour prior to sex compared with no sex because of leakage, displacement, and/or dilution of gel and that a post-coital dose would maintain drug concentration and protective effect. In contrast, we anticipated that sex would have less effect if gel was applied 24 hours before sex, reflecting tissue penetration, intracellular retention, and the prolonged half-life of TFV–DP [14].
METHODS
Study Visits and Participants
The University of Pittsburgh and Case Western Reserve University institutional review boards approved MTN-011, and all participants provided written informed consent. Twenty-four healthy couples were enrolled between December 2012 and February 2014. Inclusion criteria required participants to be in a mutually monogamous relationship for at least 6 months, HIV-uninfected, no sexually transmitted infections (STIs) in the prior 6 months, no nontherapeutic intravenous drug use in the prior 18 months, and currently use effective nonbarrier contraception. Couples must also have agreed to abstain from sexual intercourse, and for women, other vaginal practices, for 48 hours prior to each study visit. Women were excluded if they had a pregnancy within the past 90 days; were breastfeeding; menopausal; had a genitourinary infection; intermenstrual bleeding; abnormal Pap test; abnormal renal or liver function; hepatitis B surface antigen (HBsAg)–positive test; post-exposure prophylaxis for HIV within prior 6 months; participation in other research study involving drugs, devices, or genital products within prior 30 days; history of domestic violence with current partner; systemic or topical antimicrobials within the last 7 days; or currently using or planning to use immune modulator(s). Men were excluded if they reported penile procedures within the prior 42 days or treatment of candidal balanoposthitis/balanitis in the prior 30 days.
Medical history and physical examination were performed at screening (visit 1). Women had urine collected for culture (if indicated), pregnancy testing, and nucleic acid amplification tests for gonorrhea and chlamydia (GC–CT). Blood was obtained for complete blood count (CBC) with platelets, HIV-1 and syphilis serology, and HBsAg. A pelvic exam was performed for vaginal fluid pH, testing for trichomonas, and, if indicated, testing for candidiasis, bacterial vaginosis, and Pap smear. CVL was obtained by washing the cervix and vagina with 10 mL of normal saline. Men had urine obtained for GC–CT and culture (if indicated) and blood obtained for CBC with platelets, HIV-1 and syphilis serology, and HBsAg.
Enrolled couples completed up to 6 additional paired visits (Table 1). Local hotels were used for coital visits to facilitate sampling within the prescribed timeframe. Couples were reassessed for HIV–STI risk by obtaining an interim history and focused physical exam; lab tests were only repeated if clinically indicated. At visit 2 (no gel/sex), women returned to the clinic approximately 2 hours after barrier-unprotected vaginal sex for post-coital sampling. At visit 3, women applied 1% TFV gel 1 hour before sex (−1 hour gel/sex) and returned to the clinic for sampling approximately 2 hours after sex. At visit 4 (−1 hour gel/no sex), women applied 1% TFV gel, and sampling was performed at the same time post-gel as for visit 3. For visits 5 and 6, gel was applied approximately 24 hours prior to sex (−24 hour gel/sex) or no sex (−24 hour gel/no sex), with sampling at similar times as for visits 3 and 4. At visit 7 (BAT), women applied gel approximately 1 hour before and approximately 1 hour after sex, with sampling approximately 2 hours post-coitus. All gel visits were separated by a minimum of 10 days to allow TFV washout, and no visits were scheduled during menses. Plasma, CVL, vaginal and cervical biopsies, cervical cytobrush (Cleveland only), and rectal sponges were collected to measure drug concentrations at each visit. CVL for antiviral activity and protein concentration (BCA protein assay, Pierce Biotechnology, Rockford, Illinois), cervical cytobrush for flow cytometry (Pittsburgh only), and vaginal fluid for pH were also collected.
Table 1.
Schema of Study Visits
| Gel | Visit | Visit Name | Targeted Visit Schedule | Sex |
|---|---|---|---|---|
| 1 ♂♀ | Screening no gel/no sex | 30 d screening window | ||
| 2a ♂♀ | Enrollment no gel/sex | Approximately 2–12 d after menses | X | |
| 2b♀ | Post-sex sampling | Approximately 2 h after sex | ||
| −1 h | 3a ♂♀ | −1 h gel/sex | Approximately 3–90 d after visit 2b | X |
| 3b♀ | −1 h gel/sex sampling | Approximately 2 h after sex | ||
| 4a ♀ | −1 h gel/no sex | After a minimum 10-d washout period; not more than 56 d following previously scheduled visit | ||
| 4b ♀ | −1 h gel/no sex sampling | Similar time point relative to sampling at visit 3b | ||
| −24 h | 5a ♂♀ | −24 h gel/sex | After a minimum 10-d washout period, not more than 90 d following previously scheduled visit | X |
| 5b ♀ | −24 h gel/sex sampling | Approximately 2 h after sex | ||
| 6a ♀ | −24 h gel/no sex | After a minimum 10-d washout period, not more than 56 d following previously scheduled visit | ||
| 6b ♀ | −24 h gel/no sex sampling | Similar time point relative to sampling at visit 5b | ||
| BAT24 | 7a ♂♀ | −1 h gel/sex/+1 h gel (BAT) | After a mininmm10-d washout period, not more than 90 d following previously scheduled visit | X |
| 7b ♂♀ | BAT sampling | Approximately 2 h after sex |
♀ = female ♂ = male.
Abbreviation: BAT, 1 dose before and 1 dose after.
Study Drug
TFV 1% gel was applied with prefilled polypropylene applicators capable of administering a 4-g (approximately 4 mL) dose of gel (CONRAD, Arlington, Virginia) [15].
Pharmacokinetics
TFV concentrations were measured in blood plasma, CVL, rectal fluid, and vaginal and cervical tissue homogenates using validated liquid chromatographic–tandem mass spectrometric (LC–MS/MS) methods [12, 16, 17]. The lower limits of quantification (LLOQ) for TFV in plasma, CVL, tissue, and rectal fluid are 0.31 ng/mL, 5 ng/mL, 0.05 ng/sample, and 1.25 ng/sponge, respectively. Final concentrations of TFV in tissue and rectal fluid were corrected for respective net weight and reported as nanograms per milligram. TFV–DP concentrations were measured in tissue homogenates and cervical cytobrushes using validated LC–MS/MS assays [16]. The LLOQ for TFV–DP in tissue and cytobrushes is 50 fmol/sample; final concentrations of TFV–DP in tissue (fmol/mg) and cervical cytobrushes (fmol/million cells) were based on the net tissue weight or cell number, respectively.
Antiviral Activity
TZM-bl cells were infected with 3000 median tissue culture infective dose (TCID50) of HIV-1BaL in the presence of saline or CVL (1:4 final dilution). HIV inhibition was measured as mean percent reduction compared with untreated controls [18]. All samples were tested in triplicate. For anti-HSV activity, Vero cells were infected with 50–1000 pfu of HSV-2(G) mixed 1:1 with each CVL or control buffer [19]. Plaques were counted after 48 hours. All samples were tested in duplicate.
Flow Analyses
Cervical cytobrush immune cells were stained with a LIVE/DEAD Fixable Aqua Fluorescent Reactive Dye (Life Technologies, Grand Island, New York) and the following murine anti-human monoclonal antibodies: Pacific Blue anti-CD3, Alexa Fluor 700 anti-CD4, APC-H7 anti- CD8, APC anti- CD20, PE-CF594 anti-CD14, FITC anti-CD177, PerCP anti-CD69, PE-Cy7 anti-CD25, and PE anti-CD195. All antibodies were from BD Biosciences (San Jose, California) except CD177-FITC, which was from BioLegend (San Diego, California). An aliquot of unstained cells was used as a negative gating control. Data acquisition occurred within 48 hours using the Becton-Dickinson LSRFortessa with FACSDiva Software (BD Biosciences). Compensation was performed using anti-mouse Ig, κ and Negative Control Compensation Particles Set (BD Biosciences). Acquired data were analyzed using FlowJo, version 7.6 (TreeStar, Inc, Ashland, Oregon).
Statistical Analyses and Sample Size
The difference in drug concentration or antiviral activity when TFV was applied −1 or −24 hours before sex was compared with the respective no sex visit using the Wilcoxon signed rank test with a null hypothesis that there is no difference in TFV or TFV–DP concentration under sex and no sex conditions. BAT was compared with −1 hour/sex visit. Adjustment of P values for multiple comparisons was made across 32 pairwise comparisons by the false discovery rate method. Similar analyses were conducted for immune cell types, including signed rank tests and adjustment of multiple comparisons. Linear regression and generalized estimating equations were applied to assess whether differences in CVL drug levels were impacted by protein recovered. Based on the variability of drug concentration measurements in CVL in 2 prior studies [10, 12], 20 couples would provide 80% power to detect at least a 25% difference in drug concentration between visits. We recruited 24 couples to account for potential participant drop-out.
RESULTS
Tenofovir Drug Concentrations Are Reduced Following Coitus
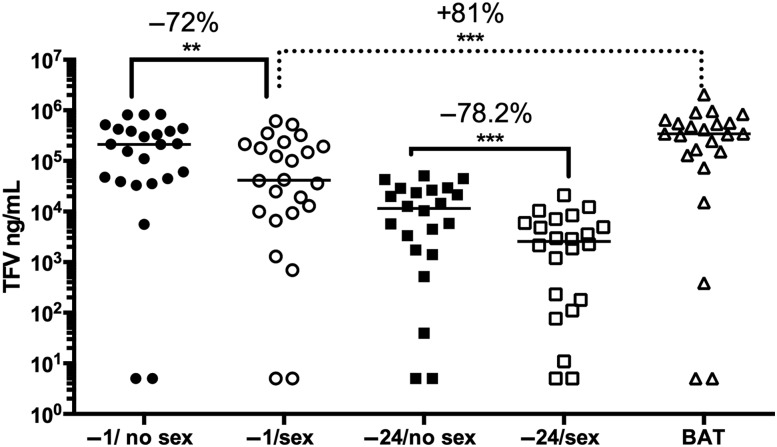
Demographics are summarized in Supplementary Table 1. All 24 couples completed the baseline (no gel) paired sex/no sex visits and the −1 hour gel paired visits; 22 completed the paired −24 hour visits, and 23 completed the BAT visit. The median time between sex and sample collection was 1 hour 52 minutes (range, 1 hour 2 minutes to 3 hours 17 minutes). The CVL TFV drug concentration was 72.2% (37.5%) (median, interquartile range [IQR]) lower when gel was applied 1 hour before sex compared with without sex (P = .0009; Figure 1, Table 2). CVL concentrations were lower when −24 hour dosing was compared with −1 hour dosing and were 78.2% (33%) lower following sex when compared with the −24 hour pair (P < .0001). Applying another dose of gel approximately 1 hour after sex (BAT) resulted in concentrations that were at least as great as those detected without sex. There was a modest increase in CVL protein concentration after sex (Supplementary Table 2), but the differences in drug concentrations remained significant when adjusted for protein.
Figure 1.
Tenofovir (TFV) drug levels are reduced in cervicovaginal lavage (CVL) samples following unprotected sex. The concentration of TFV in CVL at each visit was measured (ng/mL); samples with drug levels below the lower limits of quantification (LLOQ) were set at the LLOQ (n = 2 at each visit). The line indicates the median, and the percentage change in drug levels in paired samples is shown (−1 hour gel/no sex vs −1 hour gel/sex; −24 hour gel/no sex vs −24 hour gel/sex, and −1 hour gel/sex vs 1 dose before and 1 dose after sex). **P < .01; ***P < .001; Wilcoxon rank sum adjusted for multiple comparisons. Abbreviation: BAT, 1 dose before and 1 dose after.
Table 2.
Tenofovir and Tenofovir–Diphosphate Concentrations in Different Compartments
| Sample | Median Concentration (IQR) |
Difference |
|||
|---|---|---|---|---|---|
| Couples | No Sex Visit | Sex Visit | MedPC (IQR) | P Valuea | |
| Cervicovaginal lavage (ng/mL) | |||||
| −1 TFV | 24 | 2.13 × 105 (3.65 × 105) | 4.17 × 104 (1.93 × 105) | 72.2 (37.5) | .0009 |
| −24 TFV | 22 | 1.16 × 104 (2.47 × 104) | 2.56 × 103 (5.79 × 103) | 78.2 (33.0) | <.0001 |
| BAT TFV | 23 | 3.45 × 105 (5.17 × 105) | 80.8 (36.4) | <.0001 | |
| Vaginal tissue (ng/mg) | |||||
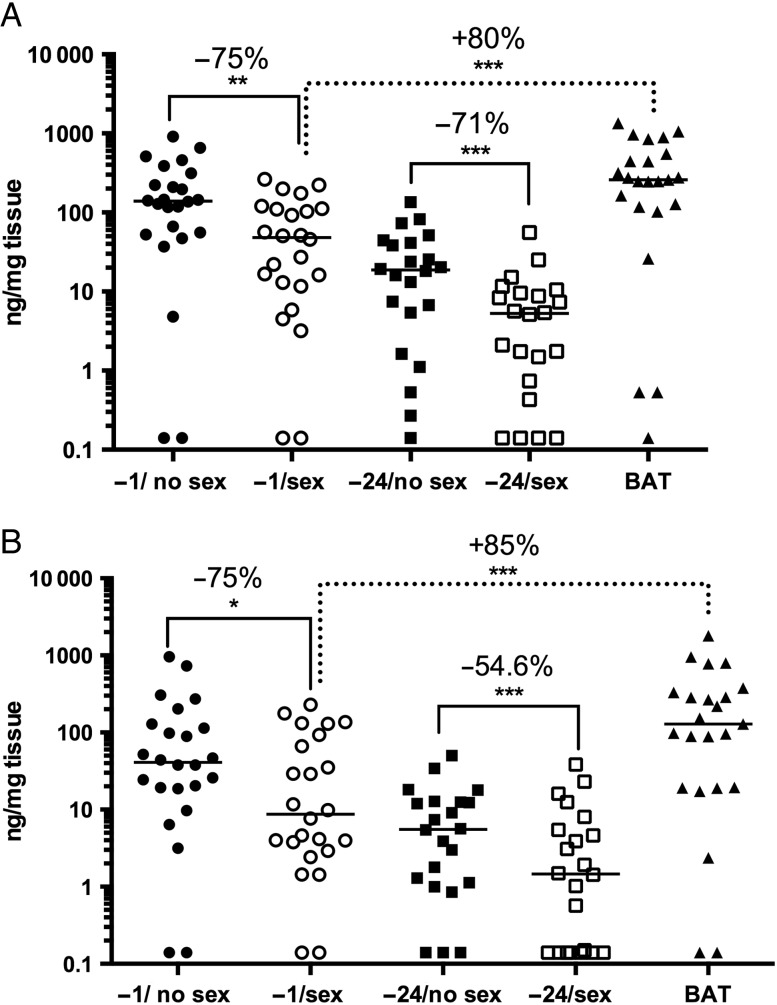
| −1 TFV | 24 | 139.25 (213.18) | 48.12 (98.36) | 75.1 (65.2) | .0014 |
| −24 TFV | 22 | 18.75 (35.88) | 5.28 (8.83) | 71.0 (55.3) | .0003 |
| BAT TFV | 23 | 258.72 (434.44) | 79.9 (43.4) | <.0001 | |
| Cervical tissue (ng/mg) | |||||
| −1 TFV | 24 | 40.97 (102.29) | 8.74 (76.47) | 74.5 (63.4) | .0614 |
| −24 TFV | 22 | 5.56 (11.39) | 1.46 (5.22) | 54.6 (55.2) | .0009 |
| BAT TFV | 23 | 129.15 (311.97) | 85.2 (21.7) | <.0001 | |
| Vaginal tissue (fmol/mg) | |||||
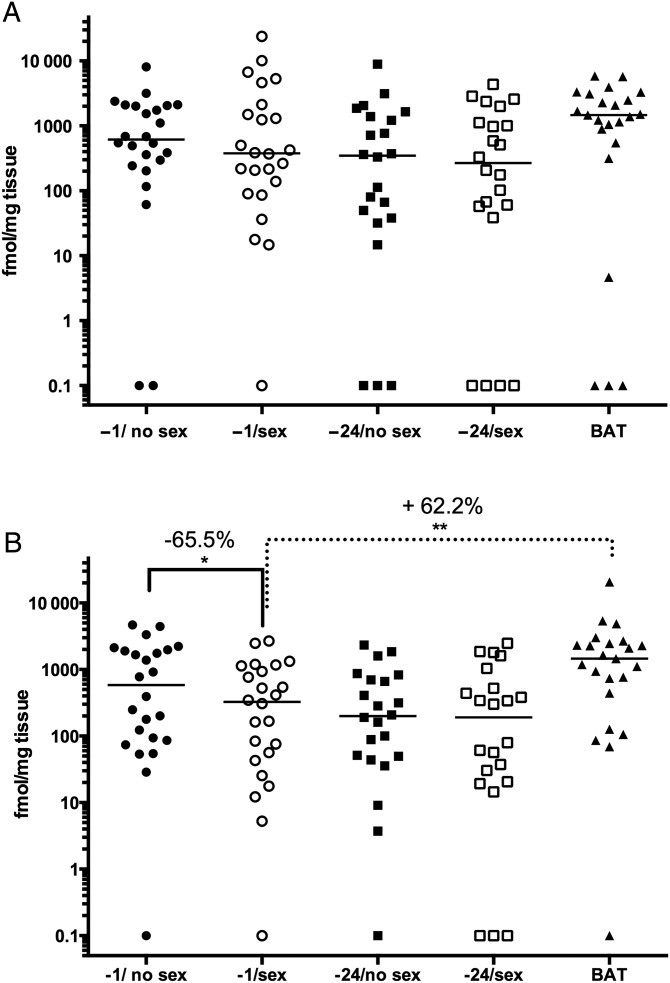
| −1 TFV–DP | 24 | 614.97 (1763.23) | 378.37 (1697.49) | 19.0 (156.1) | .9838 |
| −24 TFV–DP | 22 | 346.75 (1348.75) | 268.38 (1059.81) | 9.7 (117.3) | .9832 |
| BAT TFV–DP | 23 | 1463.83 (2547.93) | 64.7 (160.4) | .9074 | |
| Cervical tissue (fmol/mg) | |||||
| −1 TFV–DP | 24 | 584.12 (1847.18) | 326.90 (982.94) | 65.5 (53.4) | .0226 |
| −24 TFV–DP | 22 | 199.69 (651.15) | 190.34 (501.21) | −22.3 (285.3) | .9457 |
| BAT TFV–DP | 23 | 1458.33 (2024.56) | 62.2 (57.2) | .0032 | |
| Cytobrush (fmol/106 cells) | |||||
| −1TFV–DP | 10 | 5066.67 (13073.53) | 2831.44 (8684.80) | 33.3 (184.3) | 1.0000 |
| −24TFV–DP | 9 | 84.72 (1058.06) | 0.00 (259.39) | 55.5 (51.5) | .3000 |
| BAT TFV–DP | 10 | 2658.82 (5601.30) | −36.4 (817.6) | .9038 | |
| Rectal sponge (ng/mg) | |||||
| −1 TFV | 24 | 0.76 (3.07) | 1.89 (6.40) | 46.5 (269.6) | .9832 |
| −24 TFV | 22 | 0.25 (0.48) | 0.56 (1.58) | −110.9 (1113.1) | .3738 |
| BAT TFV | 23 | 0.99 (4.66) | 56.5 (849.9) | .8014 | |
| Blood plasma (ng/mL) | |||||
| −1 TFV | 24 | 1.11 (1.52) | 2.34 (3.28) | −75.5 (242.3) | .0226 |
| −24 TFV | 22 | 0.16 (0.75) | 0.42 (0.70) | 8.3 (50.2) | .6005 |
| BAT TFV | 23 | 3.81 (10.85) | 64.8 (73.4) | .0045 | |
Data are presented as median (IQR) = IQR (75th percentile minus 25th percentile).
Abbreviations: BAT, 1 dose before and 1 dose after; IQR, interquartile range; MedPC, median percentage change comparing paired no sex to sex visits or, for BAT dosing, comparing BAT to −1 hour dosing with sex (a positive [negative] percentage change reflects a decrease [increase] in concentration); TFV, tenofovir; TFV–DP, tenofovir–diphosphate.
a P value adjusted by the false discovery rate method to account for the multiple comparisons among biological assessments (8) and paired visits (4). BAT compared with −1 hour dosing without coitus factored into P value adjustment, but data are not presented for this pair-wise comparison.
Vaginal and cervical tissue concentrations fell by a median of 75.1% (65.2%; P = .001; Figure 2A) and 74.5% (63.4%; P = .061; Figure 2B), respectively, when gel was applied at −1 hour and by 71.0% (55.3%; P = .0003) and 54.6% (55.2%; P = .0009), respectively, at −24 hours compared with matched no sex visits (Table 2). Adding a post-coital dose significantly increased vaginal and cervical tissue TFV levels compared with the −1 hour gel/sex visit (P < .0001; Table 2).
Figure 2.
Tenofovir (TFV) drug levels are reduced in vaginal and cervical tissue following unprotected sex. The concentrations of TFV in vaginal (A) and cervical (B) tissue at each visit were measured (ng/mg tissue). Biopsies with drug levels below the lower limits of quantification (LLOQ) were set at the LLOQ. The line indicates the median, and the percentage change in drug levels in paired samples is shown (−1 hour gel/no sex vs −1 hour gel/ sex; −24 hour gel/no sex vs −24 hour gel/sex; and −1 hour gel/sex vs 1 dose before and 1 dose after sex). *P < .05; **P < .01; ***P < .001; Wilcoxon rank sum adjusted for multiple comparisons. Abbreviation: BAT, 1 dose before and 1 dose after.
Plasma TFV concentrations were low following a single dose of gel, increased following sex (Table 2; P = .023), and increased further with BAT dosing (P = .0045). Plasma concentrations with −24 hour dosing were lower and unaffected by sex. Low TFV concentrations in rectal sponge samples were also unaffected by sex (Table 2).
TFV–DP Concentrations Are Lower in Cervical, but not Vaginal, Tissue Following Coitus
Cervical tissue concentrations of TFV–DP decreased by 65.5% (53.4%) when gel was applied 1 hour before sex compared with no sex (P = .023) and increased 62.2% (57.2%; P = .003) following an additional post-coital dose (Figure 3A). Despite similar trends, there were no significant differences in TFV–DP in vaginal tissue or cytobrush samples (Figure 3B, Table 2). There were also no differences in biopsy or cytobrush TFV–DP concentrations in the paired −24 hour samples.
Figure 3.
Tenofovir–diphosphate (TFV–DP) levels are reduced in cervical tissue following unprotected sex. The concentrations of the active metabolite, TFV–DP, were measured in cervical (A) and vaginal (B) tissue at each visit (fmol/mg tissue). Biopsies with drug levels below the lower limits of quantification are shown (−1 hour gel/no sex vs −1 hour gel/ sex; −1 hour gel/sex vs 1 dose before and 1 dose after sex). *P < .05; **P < .01; Wilcoxon rank sum adjusted for multiple comparisons. Abbreviation: BAT, 1 dose before and 1 dose after.
Sex and Gel Affected Antiviral Activity
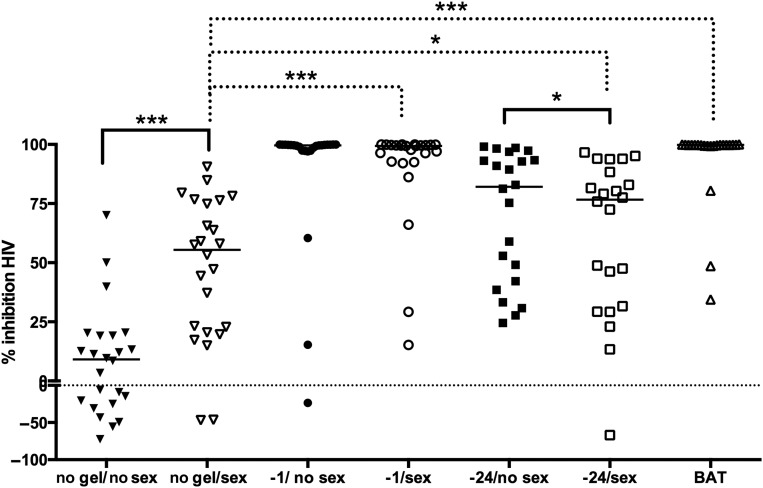
The CVL baseline (no gel/no sex) anti-HIV activity was highly variable, with some CVL inhibiting and others enhancing HIV infection in vitro. Anti-HIV activity increased significantly (P < .001) following sex from a baseline of 9.09% (41.84%) to 55.46% (53.92%) and further increased to 99.4% (7.16%) if gel was applied 1 hour prior to sex (P < .001 compared with no gel/sex). However, the anti-HIV activity increased more modestly to 76.68% (56.69%) if gel was applied 14 hours prior to sex (P < .05). Anti-HIV activity was highest in CVL obtained after BAT dosing (99.81% [0.41%]; P < .001; Figure 4). There was no significant difference in anti-HIV activity when the paired −1 hour visits were compared. However, there was a small but significant decrease in anti-HIV activity when the paired −24 hour visits were compared (82.07% [51.10%] no sex vs 76.68% [56.69%] postcoital; P = .04). The anti-HIV activity correlated significantly with TFV concentrations (Spearman correlation coefficient [SCC], 0.91; P < .001; Supplementary Figure 1A).
Figure 4.
Inhibitory activity of cervicovaginal lavage (CVL) against human immunodeficiency virus (HIV) infection in vitro. The percentage inhibition of HIV infection by CVL at each visit was determined. The lines indicate the median for the group, and values below zero indicate enhancement. The anti-HIV activity is compared between the paired no gel baseline visits (no gel/no sex vs no gel/sex) and between post-coital gel applications and no gel/sex visits. *P < .05; ***P < .001; Wilcoxon rank sum adjusted for multiple comparisons. Abbreviation: BAT, 1 dose before and 1 dose after.
The baseline anti-HSV activity of CVL was also variable (but, in contrast to HIV, there was minimal enhancing activity) and decreased following sex from a median (IQR) of 58.0% (49.0%) to 24.0% (25.5%; P < .0001). There was a significant increase when gel was applied −1 hour, −24 hours, or BAT when compared with the no gel/sex visit (57.0% [49.0%], 53.5% [60.0%], and 67.0% [50.0%], respectively) but not when compared with the no gel/no sex visit (Figure 5). There were no significant differences between the paired gel with or without sex visits. Moreover, the anti-HSV activity correlated only modestly with the TFV drug concentrations (SCC = 0.36; P = .002; Supplementary Figure 1B). One participant was HSV-2 seropositive at enrollment and exclusion of this participant had no impact on the findings.
Figure 5.
Inhibitory activity of cervicovaginal lavage (CVL) against herpes simplex virus type 2 (HSV-2) infection in vitro. The percentage inhibition of HSV-2 infection by CVL at each visit was determined. The lines indicate the median for the group. The anti-HSV activity is compared between the paired no gel baseline visits (no gel/no sex vs no gel/sex) and between post-coital gel applications and no gel/sex visits. **P < .01; ***P < .001; Wilcoxon rank sum adjusted for multiple comparisons. Abbreviation: BAT, 1 dose before and 1 dose after.
No Impact of Sex/Gel on Mucosal Immune Cells
There were no significant differences in number of viable cells recovered from cervical cytobrushes (median [IQR] 2.01 × 105 [4.35 × 105]). Similarly, there were no significant differences in the percentage of T cells (CD3+), total, CCR5+, CD25+ or CD69+ CD4 + T cells, total, CCR5+, CD25+ or CD69+ CD8+ T cells, monocytes (CD14+), neutrophils (CD 177+), or dendritic cells (CD209+) when different visits were compared (Supplementary Table 3).
DISCUSSION
Most phase 1 PrEP studies are conducted in sexually abstinent women. However, sex can modify drug concentrations through dilution, redistribution, leakage, and/or protein binding. Seminal proteins may directly impact drug activity as illustrated with several polyanionic drugs [10, 11, 20–22]. While in vitro studies showed no adverse effects of semen on TFV activity [12, 17, 22], we hypothesized that application of TFV gel shortly before sex could adversely impact drug concentrations and that if less drug reached target cells there could be a decrease in antiviral effects (PD). Findings from this study support these hypotheses. Lower drug concentrations were recovered from CVL and vaginal and cervical tissue when gel was applied 1 hour or 24 hours before sex compared with paired samples obtained without sex. Addition of a post-coital dose resulted in concentrations at least as high as without sex. Interestingly, plasma TFV concentrations increased slightly following sex (−1 hour dosing), which could reflect redistribution of secretions and/or increased blood flow.
A decrease in the active metabolite, TFV–DP, was also observed in the −1 hour paired cervical tissue samples (trend in vaginal tissue) but not in the −24 hour paired sample. The latter presumably reflects the rapid intracellular phosphorylation and subsequent prolonged half-life of intracellular TFV–DP that maintains concentrations in the face of coital influences [14]. Consistent with this explanation, TFV–DP is formed very rapidly after topical dosing, unlike oral dosing [16].
Determining the clinical implications of the observed statistically significant decreases in drug levels requires further study as there are no validated biomarkers predictive of PrEP efficacy. While direct challenge of tissue with HIV (or HSV) ex vivo has the potential to serve as a biomarker, the variability in tissue susceptibility to HIV infection and need for multiple biopsies limit the usefulness of this approach [23–25]. An alternative is to use CVL as the drug source and to test antiviral activity in cell culture. This approach is most directly applicable for drugs that act extracellularly, such as binding and fusion inhibitors. For drugs such as TFV, which must be converted into TFV–DP intracellularly, it may provide a less direct measure of drug activity. In the current study, anti-HIV activity correlated strongly with TFV concentrations and was lowest −24 hours post-sex and highest with BAT. These findings suggest that single or infrequent applications temporally separated from potential HIV exposure may not be protective. Notably, there was minimal increase in anti-HSV activity of CVL over the background endogenous levels following gel application, which is consistent with the higher concentrations of TFV needed to inhibit HSV plaque formation. For example, the concentration of TFV required to inhibit 50% of HSV-2 plaque formation in a similar assay (50% inhibition/inhibitory concentration [IC50]) is 593 µg/mL, whereas the IC50 for HIV in the TZM-bl assay ranges from 1 to 10 µg/mL [13, 26].
We detected no differences in the number or phenotype of immune cells between visits, suggesting that neither TFV nor sex causes rapid (within 2 hours) recruitment of immune cells into the cervix. These findings differ from those from a prior study (n = 10) that compared immune cells by immunohistochemistry in cervical biopsies obtained at baseline and approximately 12 hours after vaginal intercourse. That study showed an increase in macrophages, dendritic cells, and CD8 T cells following sex [27]. The differences may reflect the timing of sampling relative to sex (2 vs 12 hours), the site of sampling (cytobrush vs ectocervical biopsy), and the technique (flow vs immunohistochemistry). More research is needed to understand whether sex modifies susceptibility to HIV infection through alterations of target cells and/or the mucosal immune environment.
While difficulties with adherence may preclude further development of vaginal gels for HIV prevention, our finding that sex affects TFV PK has implications for other delivery systems. Similar studies with sustained drug delivery systems such as intravaginal rings are needed. Coitus may have little impact on rings designed to deliver drugs with intracellularly active metabolites (such as TFV disoproxil fumarate [28, 29] or TFV [7, 30]) even if removed shortly before sex. In contrast, sex may adversely impact rings that deliver drugs such as dapivirine that bind to seminal proteins [22] or drugs that diffuse rapidly along intracellular–extracellular concentration gradients [31]. Thus, these data highlight the importance of conducting post-coital PK studies to inform the selection of drugs or drug combinations and to optimize dosing recommendations.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We acknowledge the significant contribution to this research from the research participants and the clinical site support staff. We also thank Sarah Yandura for performing the anti-human immunodeficiency virus assays, Ashley Huber for performing the anti-herpes simplex virus assays, and Kathryn Duffill and Rhonda Brand for technical assistance with flow studies. From Statistical Center for HIV/AIDS Research & Prevention, we thank Corey Miller for project management, Debbie Lands for data management, Jill Zeller for clinical support, and Barbra Richardson, Karen Liu, Holly Gundacker, Janne Abullarade, Della Wilson, and Rick Westcott for statistical and programming support. We thank CONRAD for providing the tenofovir gel and Gilead for providing tenofovir drug substance.
Disclaimer. The content does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This study was supported by the Microbicide Trials Network, which is is funded by the National Institute of Allergy and Infectious Diseases (NIAID) (UM1AI068633, UM1AI068615, UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the US NIH. The anti-HSV studies were supported by NIAID U19AI03461 (B. C. H.).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thigpen MC, Kebaabetswe PM, Paxton LA et al. . Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P et al. . Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P et al. . Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA et al. . Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Ramjee G, Richardson BA et al. . Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rees H, Delan-Moretlwe S, Baron D et al. . FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women. In: CROI 2015 Vol. Abstract number 26LB Seattle, WA, 2015. [Google Scholar]
- 8.Dai JY, Hendrix CW, Richardson BA et al. . Pharmacological measures of adherence and risk of HIV acquisition in the VOICE study. J Infect Dis 2015; doi:10.1093/infdis/jiv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashuba ADM, Gengiah TN, Werner L et al. . Genital tenofovir concentrations correlate with protection against HIV infection in the CAPRISA 004 trial; importance of adherence for microbicide effectiveness. JAIDS 2015; 69:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller MJ, Mesquita PM, Torres NM et al. . Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS One 2010; 5:e8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel S, Hazrati E, Cheshenko N et al. . Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis 2007; 196:1394–402. [DOI] [PubMed] [Google Scholar]
- 12.Keller MJ, Madan RP, Torres NM et al. . A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 2011; 6:e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dezzutti CS, Shetler C, Mahalingam A et al. . Safety and efficacy of tenofovir/IQP-0528 combination gels—a dual compartment microbicide for HIV-1 prevention. Antiviral Res 2012; 96:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louissaint NA, Cao YJ, Skipper PL et al. . Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013; 29:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer KH, Maslankowski LA, Gai F et al. . Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS 2006; 20:543–51. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix CW, Chen BA, Guddera V et al. . MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 2013; 8:e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold BC, Dezzutti CS, Richardson BA et al. . Antiviral activity of genital tract secretions after oral or topical tenofovir pre-exposure prophylaxis for HIV-1. J Acquir Immune Defic Syndr 2014; 66:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP et al. . In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One 2010; 5:e9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller MJ, Zerhouni-Layachi B, Cheshenko N et al. . PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis 2006; 193:27–35. [DOI] [PubMed] [Google Scholar]
- 20.Neurath AR, Strick N, Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect Dis 2006; 6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segarra TJ, Fakioglu E, Cheshenko N et al. . Bridging the gap between preclinical and clinical microbicide trials: blind evaluation of candidate gels in murine models of efficacy and safety. PLoS One 2011; 6:e27675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesquita PM, Srinivasan P, Johnson TJ et al. . Novel preclinical models of topical PrEP pharmacodynamics provide rationale for combination of drugs with complementary properties. Retrovirology 2013; 10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen BA, Panther L, Marzinke MA et al. . Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr 2015; 70:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicol MR, Emerson CW, Prince HM et al. . Models for predicting effective HIV chemoprevention in women. J Acquir Immune Defic Syndr 2015; 68:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dezzutti CS, Uranker K, Bunge KE, Richardson-Harman N, Macio I, Hillier SL. HIV-1 infection of female genital tract tissue for use in prevention studies. J Acquir Immune Defic Syndr 2013; 63:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesquita PM, Rastogi R, Segarra TJ et al. . Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother 2012; 67:1730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 2012; 188:2445–54. [DOI] [PubMed] [Google Scholar]
- 28.Smith JM, Rastogi R, Teller RS et al. . Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A 2013; 110:16145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JM, Srinivasan P, Teller RS et al. . Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate-treated macaques from multiple SHIV exposures. J Acquir Immune Defic Syndr 2015; 68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss JA, Malone AM, Smith TJ et al. . Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother 2012; 56:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugano K, Kansy M, Artursson P et al. . Coexistence of passive and carrier-mediated processes in drug transport. Nat Rev Drug Discov 2010; 9:597–614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.