Abstract
We aimed to model the incidence of infection with Mycobacterium tuberculosis among adults using data on infection incidence in children, disease prevalence in adults, and social contact patterns. We conducted a cross-sectional face-to-face survey of adults in 2011, enumerating “close” (shared conversation) and “casual” (shared indoor space) social contacts in 16 Zambian communities and 8 South African communities. We modeled the incidence of M. tuberculosis infection in all age groups using these contact patterns, as well as the observed incidence of M. tuberculosis infection in children and the prevalence of tuberculosis disease in adults. A total of 3,528 adults participated in the study. The reported rates of close and casual contact were 4.9 per adult per day (95% confidence interval: 4.6, 5.2) and 10.4 per adult per day (95% confidence interval: 9.3, 11.6), respectively. Rates of close contact were higher for adults in larger households and rural areas. There was preferential mixing of close contacts within age groups and within sexes. The estimated incidence of M. tuberculosis infection in adults was 1.5–6 times higher (2.5%–10% per year) than that in children. More than 50% of infections in men, women, and children were estimated to be due to contact with adult men. We conclude that estimates of infection incidence based on surveys in children might underestimate incidence in adults. Most infections may be due to contact with adult men. Treatment and control of tuberculosis in men is critical to protecting men, women, and children from tuberculosis.
Keywords: disease burden, infection incidence, social contact pattern, tuberculosis
Tuberculosis remains a major global public health problem. Approximately 1 in 3 people globally might be infected with Mycobacterium tuberculosis and at risk of progressing to tuberculosis disease (1). In 2014, there were approximately 9.6 million new tuberculosis disease cases and 1.5 million deaths (2).
Despite the huge global burden of tuberculosis disease, there are important gaps in the understanding of patterns of M. tuberculosis transmission. The primary mechanism of M. tuberculosis infection is inhaling droplets containing bacilli aerosolized by an infectious tuberculosis case (1), but how M. tuberculosis infection incidence varies by age, particularly among adults, and the characteristics of the source cases are poorly known.
The incidence of M. tuberculosis infection by age can be estimated using direct methods that rely on the follow-up of initially uninfected individuals over time or indirect methods that typically rely on statistical analysis of data on the prevalence of M. tuberculosis infection (3). Direct estimates from Canada, South India, the United States, and Malawi suggest that the incidence rate of M. tuberculosis infection is higher in adults (4–8), but this has been attributed in part to the instability of the tuberculin skin test (TST) (4). Historic decisions on the upper age limits in TST prevalence surveys have meant that estimates using indirect methods in persons older than 20 years of age are rare (9, 10). The limited data that do exist from TST prevalence surveys suggest that infection incidence increased with increasing age into adulthood in Uganda and Saskatchewan but decreased in South Africa (11–13). As such, data on the incidence of M. tuberculosis infection among adults are limited and equivocal.
There is also substantial uncertainty about where M. tuberculosis transmission takes place. Sustained household exposure has traditionally been the assumed route of transmission. More recently, molecular and other data have suggested a substantial role for transmission outside the household (14–19) and led to investigations in indoor settings in the community, where people congregate (20, 21). Mathematical models of the transmission of acute respiratory infections are sensitive to assumptions about contact patterns between different age groups (22–24). This has led to diary and interview-based attempts to empirically measure and analyze social mixing patterns in Europe (25–32), Asia (33), and more recently, South Africa (34).
Understanding infection incidence by age and the characteristics of source cases is critical to informing control programs. In the absence of reliable estimates of M. tuberculosis infection incidence among adults, we performed a survey of social contact patterns and modeled the age- and sex-specific M. tuberculosis infection incidence and the sexes of source cases. We combined social contact data, data on the incidence M. tuberculosis infection among schoolchildren, and data on the prevalence of adult tuberculosis from 24 communities in Zambia and South Africa (35–37).
METHODS
Ethics statement
Ethics approval was obtained from University of Stellenbosch (N04/10/173) Health Research Ethics Committee, the University of Zambia Biomedical Research Ethics Committee (007-10-04), and the London School of Hygiene and Tropical Medicine Ethics Committee (A211 3008).
Social contact survey
Adults (≥18 years of age) enrolled in the Zambia-South Africa TB and AIDS Reduction (ZAMSTAR) Study (35) final tuberculosis prevalence survey that was carried out in 2010 in 16 communities in Zambia and 8 communities in the Western Cape, South Africa, were randomly selected for face-to-face interviews. Interviews took place during daylight hours in February and March 2011 in Zambia and in May and July 2011 in South Africa. Four ZAMSTAR standard enumeration areas (SEAs) in each community were randomly selected proportional to size, and within each SEA, 10 individuals were randomly selected from 4 age and sex strata: men 18–29 years of age, men ≥30 years of age, women 18–29 years of age, and women ≥30 years of age (160 per community). Individuals were not eligible if they had not spent the previous night in the SEA. If an individual was ineligible, did not consent, or was not found after 2 visits, another individual was randomly selected from the same stratum in that SEA.
Interviews were carried out in participants' homes using a standardized questionnaire. The questionnaire was piloted in Zambia in early 2011 based on insights from participatory research carried out in both countries in 2005 (21). Interviewees were asked about their age, sex, number of cohabitees (hereafter referred to as household size), and their recent contact history. Interviewees were asked to report contacts that occurred in the 24 hours preceding the midnight before the interview. Two types of contact were measured: “close” and “casual” contacts. A close contact was defined as contact with someone with whom the interviewee had a face-to-face conversation that was longer than a greeting and within an arm's reach. Information was gathered on the age and sex of each person contacted, the place and duration of the contact (see below), and the frequency of contact with this person. Casual contacts were defined as contacts with people who were inside buildings other than the interviewee's home that the interviewee had visited. Interviewees were asked to report close contacts with individuals of all ages, as well as the number of casual contacts with individuals 5–12 years of age and 13 years of age or older, over the previous 24 hours. The study questionnaire is included in Web Appendix 1 (available at http://aje.oxfordjournals.org/).
Location and duration of contacts
The durations and locations of close and casual contacts were recorded. Casual contact locations were chosen following the social science surveys in both countries in 2005–2006 and in Zambia in 2011 (21, 38) (see Web Appendix 2 for detail of locations).
Data analysis
Data were double-entered into an SQL server database and analyzed using Microsoft Excel 2011 (39), Stata (40), and R (41). The contact rate for a given type of contact was defined as the mean number of individuals contacted by each adult per day. Unadjusted and adjusted mean contact rates were calculated to identify differences in contact rates between communities as a whole, accounting for the sample design. Adjusted mean contact rates were calculated by weighting data on individuals for community population size and the age and sex proportions in the SEA (see Web Appendix 2 for weighting methodology). The number of casual contacts was based on the category midpoint (excepting the “>20” category, for which 21 contacts was assumed) and modeled with a zero-inflated negative binomial distribution (42). Contact rates were examined by interviewee age category, sex, household size, day of the week, urban or rural community, and setting. Analyses were repeated for close and casual contacts and contacts with children (≤12 years of age).
The duration of casual contact for each interviewee was defined as the sum of the reported time spent in each location multiplied by the number of casual contacts reported there. Duration category midpoints were used.
Estimated incidence of M. tuberculosis infection
We estimated the age- and sex-specific incidence rate of M. tuberculosis infection (λi), where the index i = 1, …, 5 represents female participants 0–4, 5–12, 13–25, 26–45, and ≥46 years of age, respectively, and i = 6, …, 10 represents male participants of the corresponding ages. These age groups were chosen to approximately match the ages for which we have data on the incidence of M. tuberculosis infection in children (≈5–12 years of age) and the groups we interviewed in our contact study. We use index α to denote the groups interviewed in our contact study, where α = 1,2,3 for women 18–25, 26–45, and ≥46 years old, respectively, and α = 4,5,6 for men 18–25, 26–45, and ≥ 46 years old, respectively. We let βαi be the rate at which individuals in a group α come into effective contact with individuals in group i and pα be the per capita prevalence of culture-positive individuals in group α. Assuming mass-action mixing within groups, we arrive at equation 1 below. In equation 2, we further assume that the effective contact rate is proportional (with coefficient β0) to the corresponding close contact rates measured in our survey (c). In equation 3, we further assume that the total numbers of contacts per unit time between 2 groups (i and α) are symmetrical, so that Nαcαi = Niciα, where Ni and Nα are the number of individuals in groups i and α, respectively. For example, the total number of contacts per unit time reported by men 18–25 years of age with women 26–45 years of age is equal to the total number of contacts per unit time reported by women 26–45 years of age with men 18–25 years of age. The cαi were estimated directly from our contact data.
| (1) |
| (2) |
| (3) |
In these communities, the prevalence of culture-positive tuberculosis disease in adults (>18 years of age) was measured in the 2011 ZAMSTAR final prevalence survey (35) (giving pα), and the annual risk of M. tuberculosis infection in children 5–12 years old was estimated from a TST survey in 2005 to be 4.2% per year in Western Cape and 1.2% per year in Zambia (37). We used this incidence rate of M. tuberculosis infection that was empirically measured in children (37) to set the constant of proportionality (β0) in the model and thus to predict infection rates in older age groups. The relative infectiousness of smear-positive versus smear-negative culture tuberculosis disease cases was ignored because a similar proportion of culture positive cases were also smear-negative by country, and this overall proportion would be absorbed into β0 on scaling to the incidence of M. tuberculosis infection in children. We used estimates of the annual risk of M. tuberculosis infection based on the mixture method (37), which might be more robust to differences in the prevalence of nontuberculous mycobacteria between countries than are estimates based on other methods. Confidence intervals were computed from 104 bootstraps assuming the numbers of contacts were Poisson-distributed and the demographic proportions were Dirichlet-distributed. The demographic populations in equation 3 were from the household enumeration part of our study and are given in the Web Table 1. Sensitivity of results to mixing is investigated in Web Appendix 3.
RESULTS
Participants
Of the 5,875 eligible individuals sought, 14% could not be located on 2 attempts, 23% had moved, 1% had died, 2% refused to participate, and 60% consented; therefore, 3,528 interviewees participated in the survey (Table 1). This represented 3% (3,528 of 123,790) of adults enumerated in the ZAMSTAR final prevalence survey. A total of 1,831 (52%) were female and 2,256 (64%) were in Zambia; 1,176 (33%), 1,489 (42%), and 863 (25%) were 18–25 years old, 26–45 years old, and ≥46 years old, respectively. Interviewees in Zambia reported larger households than did those in South Africa (mean number of people per household = 4.6, 95% confidence interval (CI): 4.5, 4.7, and 3.6, 95% CI: 3.5, 3.7, respectively), and fewer South African households than Zambian households included a 5–12-year-old child (40% versus 60%; P < 0.001).
Table 1.
Characteristics of Social Contact Survey Interviewees in 16 Communities in Zambia and 8 Communities in Western Cape, South Africa, 2011
| Interviewee Characteristic | Zambia (n = 2,256) |
Western Cape, South Africa (n = 1,272) |
Total (n = 3,528) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age of interviewee, years | ||||||
| 18–25 | 779 | 34.5 | 397 | 31.2 | 1,176 | 33.3 |
| 26–45 | 911 | 40.4 | 578 | 45.4 | 1,489 | 42.2 |
| ≥46 | 566 | 25.1 | 297 | 23.4 | 863 | 24.5 |
| Sex of interviewee | ||||||
| Female | 1,193 | 52.9 | 638 | 50.2 | 1,831 | 51.9 |
| Male | 1,063 | 47.1 | 633 | 49.8 | 1,696 | 48.1 |
| Missing | 1 | 0.1 | 1 | 0.0 | ||
| Years lived in household | ||||||
| <6 months | 73 | 3.2 | 12 | 0.9 | 85 | 2.4 |
| 6–11 months | 109 | 4.8 | 8 | 0.6 | 117 | 3.3 |
| 1–4 years | 613 | 27.2 | 225 | 17.7 | 838 | 23.8 |
| 5–9 years | 395 | 17.5 | 303 | 23.8 | 698 | 19.8 |
| ≥10 years | 1,022 | 45.3 | 709 | 55.7 | 1,731 | 49.1 |
| Missing | 44 | 2.0 | 15 | 1.2 | 59 | 1.7 |
| Years lived in community | ||||||
| <6 months | 14 | 0.6 | 3 | 0.2 | 17 | 0.5 |
| 6–11 months | 59 | 2.6 | 5 | 0.4 | 64 | 1.8 |
| 1–4 years | 419 | 18.6 | 159 | 12.5 | 578 | 16.4 |
| 5–9 years | 366 | 16.2 | 273 | 21.5 | 639 | 18.1 |
| ≥10 years | 1,343 | 59.5 | 819 | 64.4 | 2,162 | 61.3 |
| Missing | 55 | 2.4 | 13 | 1.0 | 68 | 1.9 |
| No. of people in householda,b | 4.6 | 4.5, 4.7 | 3.6 | 3.5, 3.7 | 4.3 | 4.2, 4.4 |
| No. of children in household | ||||||
| 0 | 860 | 38.1 | 763 | 60.0 | 1,623 | 46.0 |
| 1 | 545 | 24.2 | 355 | 27.9 | 900 | 25.5 |
| 2 | 423 | 18.8 | 119 | 9.4 | 542 | 15.4 |
| 3 | 222 | 9.8 | 27 | 2.1 | 249 | 7.1 |
| 4 | 70 | 3.1 | 3 | 0.2 | 73 | 2.1 |
| ≥5 | 39 | 1.7 | 5 | 0.4 | 44 | 1.3 |
| Missing | 97 | 4.3 | 0 | 0.0 | 97 | 2.8 |
| No. of children 5–12 years old in householda,c | 1.2 | 1.1, 1.2 | 0.6 | 0.5, 0.6 | 1.0 | 0.9, 1.0 |
| No. of hours spent inside the previous day (including sleeping)a,d | 14.0 | 13.8, 14.3 | 17.8 | 17.5, 18.1 | 15.4 | 15.2, 15.6 |
Abbreviation: CI, confidence interval.
a Values are expressed as means and 95% confidence intervals.
b Data were missing for 134 interviewees.
c Data were missing for 97 interviewees.
d Data were missing for 255 interviewees.
Close contacts
The 3,528 interviewees reported 17,451 close contacts with persons of all other ages in the preceding 24 hours (Table 2). Fifteen percent (2,695 contacts) were with 0–12-year-old children. The adjusted rate of close contact was 4.9 (95% CI: 4.6, 5.2) contacts per adult per day; the rate for close contact with 0–12-year-old children was 0.8 (95% CI: 0.7, 0.9).
Table 2.
Close Contact Rate by Interviewee Characteristic in 16 Communities in Zambia and 8 Communities in Western Cape, South Africa, 2011
| Interviewee Characteristic | Contacts With Persons of All Ages |
Contacts With Children 0–12 Years Old Only |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Contacts | No. of Interviewees | Unadjusted Contact Rate per Adult per Daya |
Adjusted Contact Rate per Adult per Daya |
No of Contacts | No. of Intervieweesb | Unadjusted Contact Rate per Adult per Dayc |
Adjusted Contact Rate per Adult per Dayc |
|||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||||
| All interviewees | 17,451 | 3,528 | 4.9 | 4.8, 5.0 | 4.9 | 4.6, 5.2 | 2,695 | 3,426 | 0.8 | 0.7, 0.8 | 0.8 | 0.7, 0.9 |
| Age of interviewee, years | ||||||||||||
| 18–25 | 6,014 | 1,176 | 5.1 | 4.9, 5.3 | 5.0 | 4.5, 5.4 | 787 | 1,146 | 0.7 | 0.6, 0.7 | 0.7 | 0.6, 0.9 |
| 26–45 | 7,399 | 1,489 | 5.0 | 4.8, 5.1 | 4.9 | 4.5, 5.2 | 1,339 | 1,447 | 0.9 | 0.9, 1.0 | 0.9 | 0.8, 1.1 |
| ≥46 | 4,038 | 863 | 4.7 | 4.5, 4.9 | 4.7 | 4.4, 5.1 | 569 | 833 | 0.7 | 0.6, 0.8 | 0.7 | 0.6, 0.8 |
| Sex of interviewee | ||||||||||||
| Female | 9,101 | 1,831 | 5.0 | 4.8, 5.1 | 4.9 | 4.5, 5.3 | 1,711 | 1,767 | 1.0 | 0.9, 1.0 | 0.9 | 0.8, 1.1 |
| Male | 8,346 | 1,696 | 4.9 | 4.8, 5.1 | 4.8 | 4.4, 5.2 | 982 | 1,658 | 0.6 | 0.5, 0.6 | 0.6 | 0.5, 0.7 |
| Missing | 4 | 1 | 4.0 | N/A | N/A | N/A | 2 | 1 | 2.0 | N/A | N/A | N/A |
| Household size | ||||||||||||
| 1 | 1,043 | 319 | 3.3 | 3.0, 3.5 | 3.2 | 2.8, 3.5 | 40 | 310 | 0.1 | 0.1, 0.2 | 0.1 | 0.1, 0.2 |
| 2 | 2,036 | 526 | 3.9 | 3.7, 4.0 | 3.9 | 3.6, 4.2 | 221 | 516 | 0.4 | 0.4, 0.5 | 0.4 | 0.3, 0.5 |
| 3 | 2,715 | 627 | 4.3 | 4.1, 4.5 | 4.3 | 3.9, 4.6 | 413 | 614 | 0.7 | 0.6, 0.7 | 0.7 | 0.6, 0.8 |
| 4 | 2,984 | 583 | 5.1 | 4.9, 5.3 | 5.0 | 4.6, 5.4 | 454 | 570 | 0.8 | 0.7, 0.9 | 0.8 | 0.6, 0.9 |
| 5 | 2,515 | 447 | 5.6 | 5.3, 5.9 | 5.8 | 5.3, 6.3 | 487 | 433 | 1.1 | 1.0, 1.3 | 1.2 | 1.0, 1.5 |
| 6 | 1,844 | 323 | 5.7 | 5.4, 6.1 | 5.8 | 5.2, 6.3 | 401 | 313 | 1.3 | 1.1, 1.4 | 1.3 | 1.1, 1.5 |
| 7 | 1,363 | 216 | 6.3 | 5.9, 6.8 | 6.4 | 5.7, 7.0 | 233 | 205 | 1.1 | 1.0, 1.3 | 1.3 | 0.9, 1.6 |
| 8 | 1,132 | 171 | 6.6 | 6.1, 7.2 | 6.6 | 5.8, 7.3 | 202 | 168 | 1.2 | 1.0, 1.4 | 1.2 | 0.9, 1.6 |
| 9 | 1,409 | 182 | 7.7 | 7.0, 8.5 | 7.8 | 6.9, 8.6 | 232 | 177 | 1.3 | 1.0, 1.6 | 1.4 | 1.0, 1.8 |
| Missing | 410 | 134 | 3.1 | 2.6, 3.5 | 2.7 | 2.2, 3.3 | 12 | 120 | 0.1 | 0.0, 0.2 | 0.1 | 0.0, 0.1 |
| Day of the week | ||||||||||||
| Sunday | 3,129 | 637 | 4.9 | 4.7, 5.2 | 4.8 | 4.3, 5.3 | 493 | 622 | 0.8 | 0.7, 0.9 | 0.8 | 0.7, 1.0 |
| Monday | 3,438 | 693 | 5.0 | 4.7, 5.2 | 4.7 | 4.1, 5.2 | 546 | 676 | 0.8 | 0.7, 0.9 | 0.8 | 0.6, 1.0 |
| Tuesday | 3,409 | 684 | 5.0 | 4.8, 5.2 | 5.1 | 4.7, 5.4 | 574 | 657 | 0.9 | 0.8, 1.0 | 0.9 | 0.8, 1.0 |
| Wednesday | 3,317 | 644 | 5.2 | 4.9, 5.4 | 5.2 | 4.7, 5.7 | 488 | 630 | 0.8 | 0.7, 0.9 | 0.8 | 0.7, 1.0 |
| Thursday | 3,192 | 665 | 4.8 | 4.6, 5.0 | 4.7 | 4.1, 5.3 | 494 | 652 | 0.8 | 0.7, 0.9 | 0.8 | 0.6, 1.0 |
| Friday | 451 | 103 | 4.4 | 3.8, 5.0 | 4.9 | 3.1, 6.7 | 37 | 94 | 0.4 | 0.2, 0.6 | 0.5 | 0.1, 0.9 |
| Saturday | 515 | 102 | 5.0 | 4.4, 5.7 | 5.1 | 4.0, 6.2 | 63 | 95 | 0.7 | 0.5, 0.9 | 0.8 | 0.2, 1.4 |
| Urban or rural community | ||||||||||||
| Urban | 15,740 | 3,232 | 4.9 | 4.8, 5.0 | 4.8 | 4.5, 5.2 | 2,352 | 3,132 | 0.8 | 0.7, 0.8 | 0.8 | 0.7, 0.9 |
| Rural | 1,711 | 296 | 5.8 | 5.4, 6.2 | 5.6 | 5.3, 6.0 | 343 | 294 | 1.2 | 1.0, 1.3 | 1.0 | 0.7, 1.4 |
| Setting | ||||||||||||
| Zambia | 10,787 | 2,256 | 4.8 | 4.6, 4.9 | 4.6 | 4.2, 5.1 | 1,447 | 2,159 | 0.7 | 0.6, 0.7 | 0.7 | 0.5, 0.8 |
| Western Cape, South Africa | 6,664 | 1,272 | 5.2 | 5.1, 5.4 | 5.2 | 4.8, 5.7 | 1,248 | 1,267 | 1.0 | 0.9, 1.0 | 1.0 | 0.9, 1.2 |
Abbreviations: CI, confidence interval; N/A, not applicable.
a Excluding 1 interviewee (with 4 close contacts) for whom a sampling weight could not be calculated because information on sex was not available. Therefore, values are based on data from 3,527 interviewees who reported 17,347 close contacts.
b Excluding 102 interviewees for whom information about close contacts with children 0–12 years of age was not available.
c Excluding 1 interviewee (with 2 close contacts with children 0–12 years of age) for whom a sampling weight could not be calculated because information on sex was unavailable.
Adjusted rates of close contact with all ages showed little difference by interviewee age, sex, or day of the week (Table 2). There was strong evidence of higher contact rates in communities with larger households (P for trend < 0.001) and in rural communities. Adjusted rates of close contact with children 0–12 years old were higher for participants 26–45 years old, women, residents of larger households, and those in South Africa (Table 2).
Casual contacts
Data on casual contacts were available for 93% (3,277) of interviewees, who reported 38,128 contacts with persons 5 years of age or older (Table 3). Of these, 34% (12,779) were with children who were 5–12 years of age. The overall adjusted casual contact rate was 10.4 (95% CI: 9.3, 11.6) per adult per day and for contact with children 5–12 years old was 3.5 (95% CI: 3.1, 4.0).
Table 3.
Casual Contact Rate by Interviewee Characteristics in 16 Communities in Zambia and 8 Communities in Western Cape, South Africa, 2011
| Interviewee Characteristic | Contacts With Children ≥5 Years of Age |
Contacts With Children 5–12 Years of Age Only |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Contacts | No. of Intervieweesa | Unadjusted Contact Rate per Adult per Dayb |
Adjusted Contact Rate per Adult per Dayb |
No. of Contacts | No. of Intervieweesc | Unadjusted Contact Rate per Adult per Dayd |
Adjusted Contact Rate per Adult per Dayd |
|||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||||
| All interviewees | 38,128 | 3,277 | 11.6 | 11.1, 12.2 | 10.4 | 9.3, 11.6 | 12,779 | 3,309 | 3.9 | 3.6, 4.1 | 3.5 | 3.1, 4.0 |
| Age of interviewee, years | ||||||||||||
| 18–25 | 13,542 | 1,081 | 12.5 | 11.5, 13.6 | 10.8 | 9.2, 12.3 | 4,297 | 1,095 | 3.9 | 3.5, 4.3 | 3.4 | 2.8, 4.1 |
| 26–45 | 16,921 | 1,394 | 12.1 | 11.3, 13.0 | 10.8 | 9.3, 12.4 | 5,866 | 1,404 | 4.2 | 3.8, 4.6 | 3.8 | 3.2, 4.4 |
| ≥46 | 7,665 | 802 | 9.6 | 8.5, 10.6 | 8.8 | 7.1, 10.4 | 2,616 | 810 | 3.2 | 2.8, 3.7 | 3.0 | 2.4, 3.5 |
| Sex of interviewee | ||||||||||||
| Female | 17,573 | 1,722 | 10.2 | 9.4, 11.0 | 9.5 | 8.2, 10.7 | 6,507 | 1,733 | 3.8 | 3.4, 4.1 | 3.5 | 3.0, 4.0 |
| Male | 20,555 | 1,554 | 13.2 | 12.4, 14.0 | 12.1 | 10.3, 13.9 | 6,272 | 1,575 | 4.0 | 3.7, 4.3 | 3.6 | 3.0, 4.1 |
| Missing | 0 | 1 | 0.0 | N/A | N/A | N/A | 0 | 1 | 0.0 | N/A | N/A | N/A |
| Household size | ||||||||||||
| 1 | 3,676 | 305 | 12.1 | 10.4, 13.7 | 11.6 | 9.5, 13.8 | 1,112 | 306 | 3.6 | 3.0, 4.3 | 3.6 | 2.8, 4.4 |
| 2 | 5,675 | 490 | 11.6 | 10.1, 13.0 | 10.2 | 8.1, 12.2 | 1,878 | 494 | 3.8 | 3.2, 4.4 | 3.4 | 2.6, 4.2 |
| 3 | 6,235 | 574 | 10.9 | 9.6, 12.2 | 8.7 | 7.3, 10.2 | 2,138 | 582 | 3.7 | 3.1, 4.2 | 3.0 | 2.3, 3.6 |
| 4 | 6,369 | 550 | 11.6 | 10.2, 12.9 | 11.0 | 9.2, 12.8 | 2,203 | 555 | 4.0 | 3.4, 4.5 | 3.8 | 3.1, 4.5 |
| 5 | 4,883 | 410 | 11.9 | 10.4, 13.5 | 11.6 | 9.4, 13.7 | 1,600 | 414 | 3.9 | 3.2, 4.5 | 3.9 | 3.0, 4.7 |
| 6 | 2,983 | 309 | 9.7 | 8.0, 11.3 | 9.4 | 6.6, 12.2 | 998 | 311 | 3.2 | 2.5, 3.9 | 3.1 | 1.9, 4.2 |
| 7 | 2,612 | 198 | 13.2 | 10.5, 15.9 | 11.4 | 7.9, 14.9 | 850 | 201 | 4.2 | 3.2, 5.3 | 3.7 | 2.3, 5.1 |
| 8 | 1,809 | 150 | 12.1 | 9.1, 15.0 | 9.0 | 6.3, 11.8 | 675 | 154 | 4.4 | 3.2, 5.6 | 3.4 | 2.0, 4.8 |
| 9 | 2,500 | 164 | 15.2 | 12.2, 18.3 | 13.7 | 9.7, 17.7 | 863 | 164 | 5.3 | 4.0, 6.6 | 4.9 | 3.4, 6.3 |
| Missing | 1,386 | 127 | 10.9 | 8.3, 13.6 | 9.9 | 7.3, 12.5 | 462 | 128 | 3.6 | 2.5, 4.7 | 2.9 | 2.0, 3.9 |
| Day of the week | ||||||||||||
| Sunday | 8,419 | 600 | 14.0 | 12.5, 15.5 | 11.9 | 9.7, 14.0 | 3,309 | 605 | 5.5 | 4.8, 6.2 | 4.7 | 3.6, 5.7 |
| Monday | 6,543 | 646 | 10.1 | 9.0, 11.3 | 9.4 | 7.3, 11.4 | 2,088 | 655 | 3.2 | 2.7, 3.6 | 2.9 | 2.2, 3.5 |
| Tuesday | 6,621 | 630 | 10.5 | 9.3, 11.7 | 9.5 | 7.7, 11.4 | 2,147 | 635 | 3.4 | 2.9, 3.9 | 3.2 | 2.5, 3.8 |
| Wednesday | 7,196 | 590 | 12.2 | 10.8, 13.6 | 10.6 | 8.8, 12.5 | 2,273 | 597 | 3.8 | 3.3, 4.3 | 3.3 | 2.6, 3.9 |
| Thursday | 6,667 | 619 | 10.8 | 9.6, 11.9 | 10.4 | 8.7, 12.1 | 2,112 | 623 | 3.4 | 2.9, 3.9 | 3.4 | 2.8, 4.1 |
| Friday | 1,260 | 96 | 13.1 | 9.4, 16.8 | 10.1 | 7.3, 12.8 | 387 | 97 | 4.0 | 2.5, 5.5 | 3.4 | 2.1, 4.7 |
| Saturday | 1,422 | 96 | 14.8 | 11.2, 18.4 | 13.2 | 7.3, 19.1 | 463 | 97 | 4.8 | 3.4, 6.1 | 4.2 | 2.0, 6.5 |
| Urban or rural community | ||||||||||||
| Urban | 35,426 | 3,011 | 11.8 | 11.2, 12.4 | 10.5 | 9.3, 11.8 | 11,843 | 3,040 | 3.9 | 3.7, 4.1 | 3.5 | 3.1, 4.0 |
| Rural | 2,702 | 266 | 10.2 | 8.5, 11.9 | 8.7 | 6.5, 10.9 | 936 | 269 | 3.5 | 2.8, 4.2 | 3.0 | 2.1, 3.8 |
| Setting | ||||||||||||
| Zambia | 26,331 | 2,032 | 13.0 | 12.2, 13.7 | 11.6 | 10.1, 13.1 | 9,057 | 2,057 | 4.4 | 4.1, 4.7 | 4.0 | 3.4, 4.6 |
| Western Cape, South Africa | 11,797 | 1,245 | 9.5 | 8.7, 10.2 | 8.9 | 7.2, 10.5 | 3,722 | 1,252 | 3.0 | 2.7, 3.3 | 2.9 | 2.3, 3.4 |
Abbreviations: CI, confidence interval; N/A, not applicable.
a Excluding 251 interviewees for whom the number of casual contacts was not available.
b Excluding 1 interviewee (with 0 casual contacts) for whom a sampling weight could not be calculated because information on sex not available. Therefore, values are based on 3,276 interviewees who reported 38,128 casual contacts.
c Excluding 219 interviewees for whom number of casual contacts with children 0–12 years of age was not available.
d Excluding 1 interviewee (with 0 casual contacts 0–12 years of age) for whom a sampling weight could not be calculated because information on sex was not available.
Adjusted rates of casual contact with adults and persons of all ages did not show strong evidence of an association with interviewee characteristics (Table 3). The mean community rate of casual contact with children (5–12 years of age) was higher in Zambia than in South Africa (in Zambia, rate = 4.0, 95% CI: 3.4, 4.6; in South Africa, rate = 2.9, 95% CI: 2.3, 3.4) and higher on Sundays than on most other days (Table 3).
Location and duration of contacts
Most close contacts were reported to have occurred at home (in Zambia, 51%, 95% CI: 47, 55; in South Africa, 73%, 95% CI: 70, 78) (Web Figure 1A). Close contacts were also reported to have occurred in other homes, outside, in work buildings, and in schools (12%, 9%, 5%, and 3%, respectively). Excluding participants' own homes, a higher proportion of close contact time was spent in work buildings than was suggested by the proportion of close contacts in work buildings (25% of contact duration but 15% of contacts) because of longer duration contacts at work (Web Figure 1B).
Of reported casual contacts (Web Figure 1C), 19% occurred in church, 16% in shops, 15% in the interviewee's work building, and 12% in other homes (combined percentages for both settings not shown). Comparing the proportions of casual contacts and casual contact duration by location shows shorter contact episodes in shops, churches, and bars than at work or school (Web Figure 1D).
Mixing by age and sex
The age distribution of close contacts reported by interviewees indicated strong mixing within age groups (Web Table 2 and Figure 1). There was also strong evidence of within-sex preferential mixing: 63% (5,614 of 8,928) of female interviewees' close contacts and 61% (4,935 of 8,083) of male interviewees' close contacts were reported to be of the same sex as the interviewee (P < 0.001). Web Table 2 shows the percentage of close contacts of interviewees who were 18–25, 26–45, and ≥46 years old with male and female contacts by age.
Figure 1.
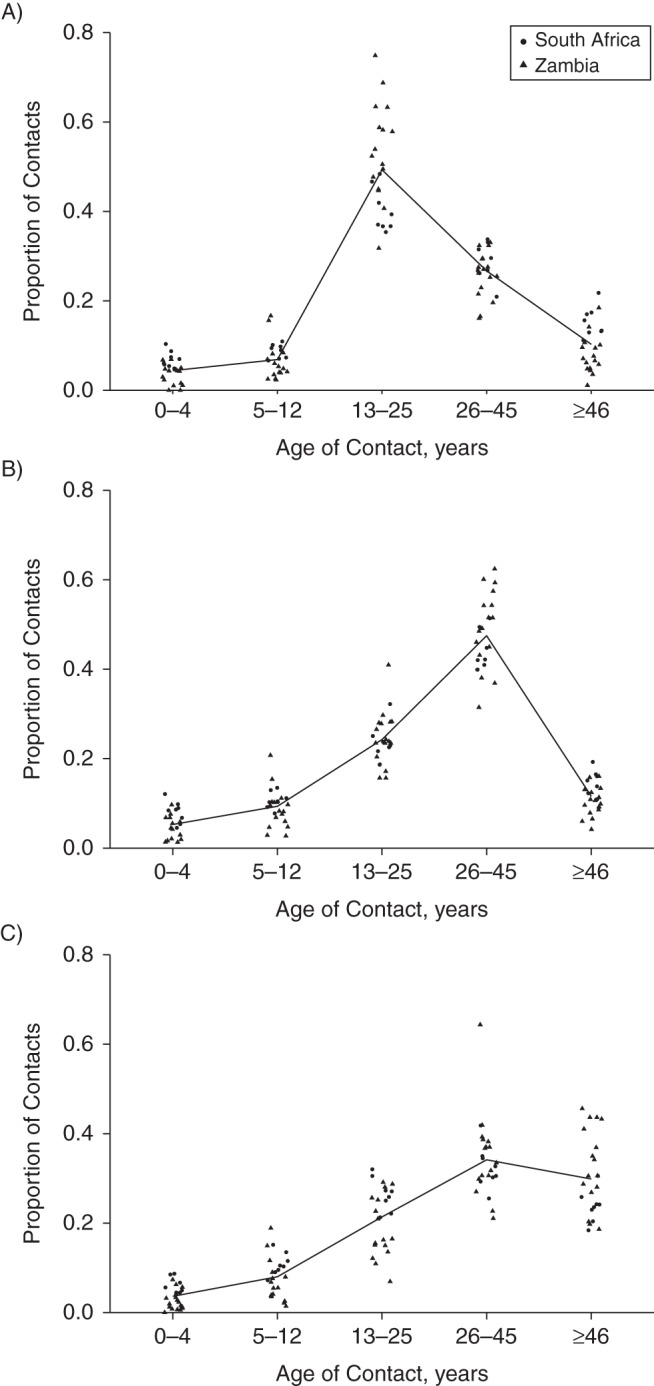
Proportion of close contacts, by contact age and setting, for interviewees aged 18–25 years (A), 26–45 years (B), and ≥46 years (C), Zambia and the Western Cape, South Africa, 2011. Each point is the mean for a community; lines join overall means.
Estimated incidence of M. tuberculosis infection
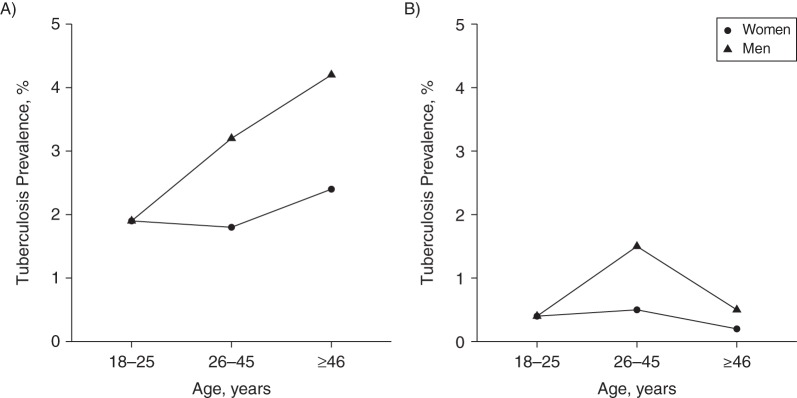
The prevalence of culture-positive tuberculosis disease was higher in males than in females in both settings (for females vs. males: in Zambia, 0.4% vs. 0.9%; in South Africa, 2.0% vs 3.0%). The prevalence of culture-positive tuberculosis disease by age, sex, and setting is shown in Figure 2.
Figure 2.
Prevalence of culture positive tuberculosis disease among adults in the Western Cape, South Africa (A) and Zambia (B) from the 2011 Zambia-South Africa TB and AIDS Reduction Study final prevalence survey (35), shown by age, sex and setting. This is used as a model input for estimating Mycobacterium tuberculosis infection rates in adults.
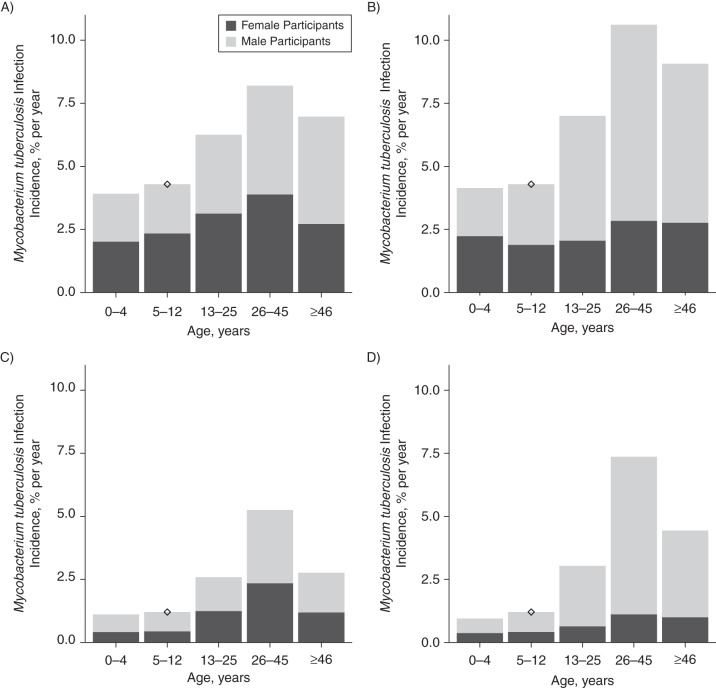
The infection incidence was estimated to be 6%–8% and 7%–10% per year for adult (≥13 years of age) females and males, respectively, in South Africa and 2.5%–5% and 3%–7% per year, respectively, in Zambia (Figure 3). The incidence was 1.5–6 times higher than what was empirically measured in children. This ratio increased with the age assortativity in mixing (Web Figure 2). The estimated overall percentage of infections due to contact with adult men was 57.3% (95% CI: 56.3, 58.2) in Western Cape and 65.7% (64.4, 66.8) in Zambia (Web Table 3); it was 50% or higher in all age groups, in both sexes, and in both settings except in 0–12-year-old girls and 0–4-year-old boys in Western Cape.
Figure 3.
Estimated incidence of Mycobacterium tuberculosis infection in women (A and C) and men (B and D) by age and sex of infectious person, Western Cape, South Africa (A and B) and Zambia (C and D). Diamonds show the M. tuberculosis infection incidence estimated directly from tuberculin skin test sensitivity data among schoolchildren. Data from Shanaube et al. (37).
DISCUSSION
This work represents the first multicountry quantitative study of social mixing patterns in sub-Saharan Africa, and it was based on a large sample drawn from multiple communities in each country. Our results suggest that rates of close contact with persons of all ages were higher for adults in larger households and in rural areas, whereas overall casual contact rates did not vary by interviewee characteristics. Rates of close contact with children were higher among interviewees who were 26–45 years old, women, adults in larger households, and from South African communities, whereas rates of casual contact with children were higher in interviewees from Zambian communities. There was strong evidence of preferential mixing for close contacts within age groups and within sexes. Our results suggest that the estimated incidence of M. tuberculosis infection in adults might be 1.5–6 times higher than what was empirically measured in children because of higher rates of contact between adults and cases with infectious tuberculosis disease, usually other adults. Our observation of within-sex mixing may amplify exposure among men, who have a higher prevalence of the disease. Our results suggest that more than 50% of all infections might be due to contact with adult men.
Our finding that the incidence of M. tuberculosis infection might be higher in adults than in children is consistent with previous direct and indirect estimates from Canada, South India, the United States, Malawi, Uganda, and Saskatchewan (4–8, 12, 13) but not a recent indirect estimate from South Africa (11). Our ratio of adult-to-child M. tuberculosis infection incidence of 1.5–6 compared with a median ratio of 1.5 (interquartile range, 1.1–7) in those other studies for which comparison was possible. Therefore, it may be that infection rates are often higher in adults than in children, despite concerns about TST instability (4). Our finding that most infections in these communities were due to contact with men was largely because tuberculosis prevalence was higher in male interviewees, and it might be generalizable to other populations because the prevalence tuberculosis disease tends to be higher in men (43). The high proportion of M. tuberculosis infection incidence due to contact with men was particularly surprising in young children in Zambia because contact rates with women were higher, which suggests that even in this age group, the higher prevalence in males tended to outweigh the higher contact rates between young children and women. The higher estimated proportion of tuberculosis infections due to men in Zambia is due to the higher relative concentration of tuberculosis disease in men in Zambia.
A potential limitation of the present study was that reported contact rates were lower than rates found in other studies (30, 33, 34). Poor recall and interview fatigue might have led to smaller numbers of contacts being recorded in our study. However, only the relative contact rates, not the absolute contact rates, were used to determine our results regarding M. tuberculosis infection incidence, and similar patterns of more intensive mixing within age groups have been observed in other contact studies (30, 34), which supports our conclusions.
Our choice to base the calculation of the incidence of M. tuberculosis infection on close contact rates reflects the general uncertainty over what constitutes an effective contact for M. tuberculosis transmission and the lower quality of our data on casual contacts. Our analysis suggests that most close contact time was spent within people's homes. Historical studies in developed settings found a higher prevalence of TST positivity among contacts who were members of the case's household (44), and being the spouse of a tuberculosis case was a strong risk factor for M. tuberculosis infection in contemporary Malawi (45). However, whether a contact is sufficient for transmission is likely to depend on the duration of contact, volume of contacts, ventilation in the room, and host and pathogen characteristics (1). Further analysis of data on contact patterns combined with measures of tuberculosis disease burden and infection may help identify key locales and activities associated with M. tuberculosis transmission and clarify which contacts are more likely to facilitate transmission (46). Our main conclusions depend on mixing within age groups of effective contacts (Web Appendix 3); this is supported by an analysis of DNA fingerprinting data from the Netherlands in which the authors concluded that tuberculosis cases preferentially transmitted infection to people close to their own ages (47).
Another limitation was that data on the prevalence of tuberculosis disease were only available on individuals older than 18 years of age. Some tuberculosis disease cases will have been due to infection from persons younger than 18 years. However, the risk of M. tuberculosis infection developing into the infectious forms of tuberculosis disease is much lower in persons younger than 15 years of age than in those who are older (48). Because the proportion of the population 15–17 years of age is very small, transmission from this group is unlikely to affect our main conclusions.
It is possible that individuals with active tuberculosis might have different contact patterns than the rest of the population. We were not able to investigate this directly because sputum was not taken at interview, and the number of interviewees known to have been prevalent cases in the preceding prevalence survey was too small for meaningful comparison. However, the fact the typical duration of active tuberculosis disease exceeds the typical reported duration of symptoms at diagnosis and the substantial fraction of asymptomatic tuberculosis found in prevalence surveys (49) suggest that active tuberculosis may not strongly influence behavior during much of its infectious period. It remains an interesting possibility that individuals with tuberculosis have systematically different contact patterns that put them at greater risk of tuberculosis exposure and infection in the first place. Such heterogeneities might have important implications for transmission, and longitudinal or case-control studies could inform this issue.
It is uncertain how our results may generalize to settings with a different prevalence of human immunodeficiency virus (HIV). Although an HIV-infected individual may be more susceptible to infection/progression, this increased susceptibility applies equally to exposures from different subgroups and should therefore not affect the proportion of infection due to each. However, our results on proportions of infections due to each group may be sensitive to HIV-infected individuals having different contact patterns. In settings with a lower HIV prevalence, one might expect a larger contribution of men to the proportion of M. tuberculosis infections because HIV prevalence is typically higher among women than among men in settings with a high HIV prevalence.
We followed Sutherland's definition of the incidence of M. tuberculosis infection as the annual rate of infection with tubercle bacilli among individuals who had never previously been infected. (3). However, rates of M. tuberculosis infection among never-infected adults might be lower than we estimated. Individuals who remain uninfected at older ages may differ biologically or behaviorally from already-infected individuals; for example, they might benefit from higher innate immunological protection and/or less frequent contact with tuberculosis cases due to preferential mixing by characteristics other than age and sex (e.g., by socioeconomic status). Generalizing our results to other adults, M. tuberculosis reinfection rates (infection rates in already-infected adults) may be lower or higher than our estimated infection rates for never-infected adults, for example, because of protection conferred by existing latent infection (which would tend to decrease infection incidence) or more frequent contact with tuberculosis cases (which would tend to increase infection incidence).
Our results suggest that mixing within age groups implies that estimates of M. tuberculosis infection incidence based on surveys in children might underestimate infection incidence in adults, and most infections may be due to contact with adult men. For hyperendemic communities in South Africa, which may have an incidence of M. tuberculosis infection in children as high as 4% per year (11, 37, 50), our findings imply that M. tuberculosis infection incidence rates in never-infected adults may be as high as 10% per year, rates which have rarely been seen outside institutional settings. Care and control of tuberculosis in males is critical to protecting men, women, and children from tuberculosis.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: TB Modelling Group, Department of Infectious Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom (Peter J. Dodd, Clare Looker, Ian D. Plumb, Emilia Vynnycky, Richard G. White); Health Economics and Decision Science, School of Health and Related Research, University of Sheffield, Sheffield, United Kingdom (Peter J. Dodd); Global Health and Development Department, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, London, United Kingdom (Virginia Bond); Zambia-South Africa TB and AIDS Reduction Project, School of Medicine, University of Zambia, Lusaka, Zambia (Virginia Bond, Ab Schaap, Kwame Shanaube, Monde Muyoyeta, Helen Ayles); Statistics Modelling and Economics Department, Public Health England, London, United Kingdom (Emilia Vynnycky); Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom (Peter Godfrey-Faussett, Elizabeth L. Corbett, Helen Ayles); TB and HIV Theme, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi (Elizabeth L. Corbett); and Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Stellenbosch University, Cape Town, South Africa (Nulda Beyers).
R.G.W., P.J.D., C.L., and I.D.P. were supported by the Consortium to Respond Effectively to the AIDS TB Epidemic funded by the Bill and Melinda Gates Foundation (grant 19790.01). R.G.W. was also supported by the Medical Research Council (UK) (grant G0802414), and E.L.C was supported by The Wellcome Trust (grant GR095878).
We thank the Zambia AIDS Related Tuberculosis study team and Dr. Rory Dunbar, Dr. Ken Eames, Professor John Edmunds, Dr. Amelia Crampin, and Dr. Immo Kleinschmidt, all of whom contributed advice in the design and analysis of the social contact survey. We also thank the Ministry of Health, District Health Management teams and the communities at institutions where the studies were undertaken for their help and advice.
The funders had no involvement in the design, collection, analysis or interpretation of the data, in writing the report or in the decision to submit.
Conflict of interest: none declared.
REFERENCES
- 1.Rieder HL. Epidemiologic Basis of Tuberculosis Control. Paris, France: International Union Against Tuberculosis and Lung Disease (IUATLD); 1999. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2015. Geneva, Switzerland: World Health Organization; 2015. http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1 Accessed May 25, 2015. [Google Scholar]
- 3.Sutherland I. Recent studies in the epidemiology of tuberculosis, based on the risk of being infected with tubercle bacilli. Adv Tuberc Res. 1976;19:1–63. [PubMed] [Google Scholar]
- 4.Fine PE, Bruce J, Ponnighaus JM et al. . Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;311:962–975. [PubMed] [Google Scholar]
- 5.Grzybowski S, Allen EA. The challenge of tuberculosis in decline. A study based on the epidemiology of tuberculosis in Ontario, Canada. Am Rev Respir Dis. 1964;90:707–720. [DOI] [PubMed] [Google Scholar]
- 6.Narain R, Nair SS, Chandrasekhar P et al. . Problems connected with estimating the incidence of tuberculosis infection. Bull World Health Organ. 1966;344:605–622. [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson NJ, Glassroth JL, Snider DE Jr et al. . The booster phenomenon in serial tuberculin testing. Am Rev Respir Dis. 1979;1194:587–597. [DOI] [PubMed] [Google Scholar]
- 8.Tuberculosis Prevention Trial Madras. Trial of BCG vaccines in South India for tuberculosis prevention. Indian J Med Res. 1980;72(1-74). [PubMed] [Google Scholar]
- 9.Cauthen G, Pio A, ten Dam H. Annual Risk of Tuberculous Infection Unpublished Document WHO/TB/88.154. Geneva, Switzerland: World Health Organization; 1988. [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland I, Fayers PM. The association of the risk of tuberculous infection with age. Bull Int Union Tuberc. 1975;501:70–81. [PubMed] [Google Scholar]
- 11.Wood R, Liang H, Wu H et al. . Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;144:406–412. [PMC free article] [PubMed] [Google Scholar]
- 12.Stott H, Patel A, Sutherland I et al. . The risk of tuberculous infection in Uganda, deprived from the findings of national tuberculin surveys 1958 and 1970. Tubercle. 1973;541:1–22. [DOI] [PubMed] [Google Scholar]
- 13.Fayers PM, Barnett GD. The risk of tuberculous infection in Saskatchewan. Bull Int Union Tuberc. 1975;501:62–69. [PubMed] [Google Scholar]
- 14.Crampin AC, Glynn JR, Traore H et al. . Tuberculosis transmission attributable to close contacts and HIV status, Malawi. Emerg Infect Dis. 2006;125:729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buu TN, van Soolingen D, Huyen MN et al. . Tuberculosis acquired outside of households, rural Vietnam. Emerg Infect Dis. 2010;169:1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson D, Pillay M, Crump J et al. . Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in rural Africa. Trop Med Int Health. 1997;28:747–753. [DOI] [PubMed] [Google Scholar]
- 17.Crampin AC, Floyd S, Ngwira BM et al. . Assessment and evaluation of contact as a risk factor for tuberculosis in rural Africa. Int J Tuberc Lung Dis. 2008;126:612–618. [PMC free article] [PubMed] [Google Scholar]
- 18.Schaaf HS, Michaelis IA, Richardson M et al. . Adult-to-child transmission of tuberculosis: household or community contact? Int J Tuberc Lung Dis. 2003;75:426–431. [PubMed] [Google Scholar]
- 19.Madico G, Gilman RH, Checkley W et al. . Community infection ratio as an indicator for tuberculosis control. Lancet. 1995;3458947:416–419. [DOI] [PubMed] [Google Scholar]
- 20.Classen CN, Warren R, Richardson M et al. . Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax. 1999;542:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray EJ, Marais BJ, Mans G et al. . A multidisciplinary method to map potential tuberculosis transmission ‘hot spots’ in high-burden communities. Int J Tuberc Lung Dis. 2009;136:767–774. [PubMed] [Google Scholar]
- 22.Edmunds WJ, Gay NJ, Kretzschmar M et al. . The pre-vaccination epidemiology of measles, mumps and rubella in Europe: implications for modelling studies. Epidemiol Infect. 2000;1253:635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallinga J, Lévy-Bruhl D, Gay NJ et al. . Estimation of measles reproduction ratios and prospects for elimination of measles by vaccination in some Western European countries. Epidemiol Infect. 2001;1272:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trotter CL, Gay NJ, Edmunds WJ. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;1621:89–100. [DOI] [PubMed] [Google Scholar]
- 25.Beutels P, Shkedy Z, Aerts M et al. . Social mixing patterns for transmission models of close contact infections: exploring self-evaluation and diary-based data collection through a web-based interface. Epidemiol Infect. 2006;1346:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmunds WJ, O'Callaghan CJ, Nokes DJ. Who mixes with whom? A method to determine the contact patterns of adults that may lead to the spread of airborne infections. Proc Biol Sci. 1997;2641384:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hens N, Ayele GM, Goeyvaerts N et al. . Estimating the impact of school closure on social mixing behaviour and the transmission of close contact infections in eight European countries. BMC Infect Dis. 2009;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kretzschmar M, Mikolajczyk RT. Contact profiles in eight European countries and implications for modelling the spread of airborne infectious diseases. PLoS One. 2009;46:e5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaw JM, Forbes K, Nathan PM et al. . Comparison of three methods for ascertainment of contact information relevant to respiratory pathogen transmission in encounter networks. BMC Infect Dis. 2010;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mossong J, Hens N, Jit M et al. . Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;53:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read JM, Eames KT, Edmunds WJ. Dynamic social networks and the implications for the spread of infectious disease. J R Soc Interface. 2008;526:1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol. 2006;16410:936–944. [DOI] [PubMed] [Google Scholar]
- 33.Horby P, Pham QT, Hens N et al. . Social contact patterns in Vietnam and implications for the control of infectious diseases. PLoS One. 2011;62:e16965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnstone-Robertson SP, Mark D, Morrow C et al. . Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol. 2011;17411:1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayles H, Muyoyeta M, Du Toit E et al. . Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;3829899:1183–1194. [DOI] [PubMed] [Google Scholar]
- 36.Ayles HM, Sismanidis C, Beyers N et al. . ZAMSTAR, The Zambia South Africa TB and HIV Reduction study: design of a 2 × 2 factorial community randomized trial. Trials. 2008;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanaube K, Sismanidis C, Ayles H et al. . Annual risk of tuberculous infection using different methods in communities with a high prevalence of TB and HIV in Zambia and South Africa. PLoS One. 2009;411:e7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bond G, Chilikwela L, Muyoyeta M et al. . Using mixed survey methods to investigate contact patterns of risk of TB infection in Zambia (PC-1260-28). Presented at 42nd Union World Conference on Lung Health, Lille, France, October 26–30, 2011. [Google Scholar]
- 39.Microsoft Excel for Mac 2011. Microsoft Office. Redmond, WA: Microsoft Corporation; 2010. [Google Scholar]
- 40.Stata Corporation. Stata Statistical Software, Release 12. College Station, TX: Stata Corporation; 2011. [Google Scholar]
- 41.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 42.Yau KKW, Wang K, Lee AH. Zero-inflated negative binomial mixed regression modeling of over-dispersed count data with extra zeros. Biom J. 2003;454:437–452. [Google Scholar]
- 43.Borgdorff MW, Nagelkerke NJ, Dye C et al. . Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis. 2000;42:123–132. [PubMed] [Google Scholar]
- 44.van Geuns HA, Meijer J, Styblo K. Results of contact examination in Rotterdam, 1967–1969. Bull Int Union Tuberc. 1975;501:107–121. [PubMed] [Google Scholar]
- 45.Crampin A, Kasimba S, Mwaungulu NJ et al. . Married to M. tuberculosis: risk of infection and disease in spouses of smear-positive tuberculosis patients. Trop Med Int Health. 2011;167:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews JR, Morrow C, Wood R. Modeling the role of public transportation in sustaining tuberculosis transmission in South Africa. Am J Epidemiol. 2013;1776:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borgdorff MW, Nagelkerke NJ, van Soolingen D et al. . Transmission of tuberculosis between people of different ages in The Netherlands: an analysis using DNA fingerprinting. Int J Tuberc Lung Dis. 1999;33:202–206. [PubMed] [Google Scholar]
- 48.Marais BJ, Gie RP, Schaaf HS et al. . The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;84:392–402. [PubMed] [Google Scholar]
- 49.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;3789785:57–72. [DOI] [PubMed] [Google Scholar]
- 50.Middelkoop K, Bekker LG, Myer L et al. . Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis. 2008;473:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.