Abstract
We have previously demonstrated that oral delivery of a disease-promoting particulated antigen of Leishmania amazonensis (LaAg) partially protects mice against cutaneous leishmaniasis. In the present work, we sought to optimize a mucosal vaccine by using the intranasal route for delivery of different antigen preparations, including (i) LaAg, (ii) soluble recombinant p36/LACK leishmanial antigen (LACK), and (iii) plasmid DNA encoding LACK (LACK DNA). BALB/c mice that received two intranasal doses of 10 μg of LaAg and were challenged 1 week postvaccination with L. amazonensis developed delayed but effective control of lesion growth. A diminished parasite burden was accompanied by enhancement of both gamma interferon (IFN-γ) and interleukin-10 levels in the lesion-draining lymph nodes. The vaccine efficacy improved with time. At 4 months postvaccination, when a strong parasite-specific TH1-type response was present in vivo, the infection was controlled for at least 5 months after challenge. In contrast to the particulated LaAg, soluble LACK (10 μg/dose) had no effect. Interestingly, LACK DNA (30 μg/dose), but not empty DNA, promoted rapid and durable protective immunity. Parasite growth was effectively controlled, and at 5 months after challenge LACK-reactive cells in both the mucosal and lesion-draining lymph nodes produced high levels of IFN-γ. These results demonstrate for the first time the feasibility of using the intranasal route for long-lived memory vaccination against cutaneous leishmaniasis with adjuvant-free crude antigens or DNA.
Leishmaniasis is a disease caused by intracellular protozoan parasites belonging to the genus Leishmania, and there is a wide spectrum of clinical manifestations ranging from chronic cutaneous ulcers to fatal visceral infection. Leishmaniasis is broadly distributed in most tropical and Mediterranean countries, and the estimated incidence is 2 million cases/year (38). Despite the recent advances in new antileishmanial compounds (6), the first-line therapy for all forms of the disease is still based on painful multiple injections of pentavalent antimonials which invariably produce serious toxicity. The problem is further aggravated by the surge of antimonial resistance in some areas where the disease is endemic (22).
The development of a vaccine against leishmaniasis is a long-term goal in both human and veterinary medicine. Various subunit and DNA antigens have been identified as potential vaccine candidates (14). However, the development of subunit vaccines for human vaccination against cutaneous leishmaniasis is hampered by the requirement for potentially toxic TH1-inducing adjuvants, which include BCG and interleukin-12 (IL-12). A great number of studies of experimental vaccines against cutaneous leishmaniasis have been based on the murine model of infection with Leishmania major, and these studies have demonstrated the relationship between genetically determined resistance and susceptibility to infection with the expansion of TH1 and TH2 cells, respectively (35). The extreme susceptibility of BALB/c mice to L. major infection has been correlated with early production of IL-4 by LACK (Leishmania homolog of mammalian receptors for activated C kinase)-reactive Vβ4-Vα8+ CD4+ T cells that drive the differentiation of other responding T cells to the TH2 phenotype (20). BALB/c mice made tolerant to LACK by transgenic expression of the antigen in the thymus exhibit both a diminished TH2 response and a healing phenotype, demonstrating the association of LACK reactivity with disease (16). LACK is a well-conserved antigen that is present in many Leishmania species (31), and it may well account at least in part for the increased susceptibility of mice and monkeys vaccinated by intradermal, intramuscular, and subcutaneous routes with whole promastigote antigens of Leishmania amazonensis or L. major in the absence of adjuvants (19, 21, 32).
Mucosal vaccination with immunopathology-related antigens has been used to induce systemic tolerance and protection against TH2-related disorders, such as allergies and some autoimmune diseases (7). In this context, the effect of oral vaccination of susceptible BALB/c mice and the more resistant C57BL/6 mouse strain with a total of 200 μg of L. amazonensis antigen (LaAg) on subsequent parasite challenge was recently evaluated (32). Vaccination rendered both mouse strains more resistant to L. amazonensis and L. major infections. Protectiveness required functional γ/δ T-cell receptor-positive T cells and was accompanied by selective systemic tolerance of TH2-type responses. The naturally particulated form of LaAg may have been critical for protectiveness, as up to 8 mg of soluble LACK administered by mucosal routes (oral and nasal) did not affect the susceptibility of BALB/c mice to L. major (26). In that study, only when LACK was conjugated with the adjuvant cholera toxin beta subunit (CTB) was partial protection achieved. However, no mucosal adjuvant, including the cholera toxin beta subunit, has been approved for use in humans yet (10). Therefore, identification of a mucosal vaccine that is intrinsically immunogenic and effective is highly desirable.
CpG DNA is a potent enhancer of systemic and mucosal immune responses (25), and intranasal DNA vaccines are emerging as a successful noninvasive alternative to DNA injected intramuscularly (2). In leishmaniasis, parenteral injections with LACK-encoding plasmid DNA (LACK DNA) proved to effectively protect BALB/c mice against L. major infection, in contrast to the disease-enhancing effect of adjuvant-free soluble LACK (12, 37). On the other hand, leishmaniasis caused by Leishmania donovani or Leishmania mexicana seems to be more refractory to parenteral LACK DNA (9, 27).
The noninvasive nasal route of vaccination is more advantageous than the oral route, because less acidic and enzymatic antigen digestion allows a more controlled and reduced vaccine dosage along with consequent reduced costs. In the present work we attempted to optimize mucosal vaccination against cutaneous leishmaniasis by using the intranasal route for evaluation of the effectiveness of various forms of leishmanial antigens.
MATERIALS AND METHODS
Mice.
BALB/c mice were originally purchased from Jackson Laboratory (Bar Harbor, Maine). They were bred and maintained at our facilities by using sterilized bedding, filtered water, and pelleted food. Female animals were used when they were 4 to 6 weeks old. Experimental protocols were approved by the Animal Use Committee of the Institute of Biophysics/Federal University of Rio de Janeiro (Brazil).
Parasites.
L. amazonensis (strain MHOM/BR/75/Josefa) was used at the early stationary growth phase for both vaccine preparation and infection. For the vaccine, wild-type parasites were used. For infection, parasites that were rendered fluorescent by transfection with green fluorescent protein (GFP) (36) were used. Both wild-type and GFP-expressing parasites were routinely isolated from mouse lesions and maintained at 26°C as promastigotes in Dulbecco modified minimum essential medium containing 10% heat-inactivated fetal calf serum and antibiotics (50 U of penicillin per ml and 50 μg of streptomycin per ml). Transfected parasites were periodically cultured with 100 μg of Geneticin per ml for GFP selection.
Vaccines. (i) LaAg.
LaAg was prepared as previously described (32). Briefly, wild-type L. amazonensis promastigotes were washed three times by centrifugation, and the pellet was resuspended at a concentration of 2 × 108 parasites/ml in phosphate-buffered saline (PBS) and subjected to three cycles of freezing and thawing. The resulting particulated cell lysate was termed LaAg. One milliliter of LaAg contained 970 μg of protein, as measured by the Lowry assay. Sample aliquots were kept at −20°C until they were required.
(ii) LACK DNA.
The gene encoding the Leishmania infantum LACK protein was obtained from a genomic library as described previously (11) and was inserted downstream of the cytomegalovirus promoter in the EcoRI/XbaI site of the pCI-neo expression vector (Promega), giving plasmid pCI-neo-LACK (LACK DNA) (34). Empty plasmid pCI-neo was used as a DNA control.
(iii) LACK.
L. infantum p36 recombinant protein was purified from an Escherichia coli BL21 strain transformed with a pRSET expression plasmid as previously described (11).
Nasal immunization.
Mice held upward received by instillation 10 μg of LaAg, 10 μg of LACK, 30 μg of LACK DNA, or 30 μg of control DNA in 20 μl of PBS (10 μl in each nostril) with a fine tip attached to a micropipette. The animals were boosted 7 days later by using the same vaccine dosage. The controls received PBS.
Infection.
Mice were infected in a hind footpad at 1 week or at 17 weeks (4 months) after the second vaccination dose with 105 GFP-labeled promastigotes of L. amazonensis. Lesion sizes were measured with a dial caliper (Mitutoyo, Sao Paolo, Brazil) every 4 to 5 days, and the results were expressed as the difference between the thickness of infected footpads and the thickness of noninfected footpads. For determination of parasite loads in the lesions, infected feet were cut off and individually homogenized in 2 ml of PBS by using a tissue grinder. After removal of tissue debris by gravity sedimentation for 10 min, 200-μl portions of twofold dilutions of the cell suspensions were transferred in triplicate to black microplates, and fluorescence was read with a plate reader fluorometer (Fluoroskan, Ashford, United Kingdom) by using excitation at 435 nm and emission at 538 nm (32, 36).
Cytokines.
The nose-draining cervical lymph nodes and the lesion-draining popliteal lymph nodes were excised, and single-cell suspensions were prepared in Dulbecco modified minimum essential medium containing 10% heat-inactivated fetal calf serum and antibiotics. Cells were plated in triplicate in 24-well culture plates at a concentration of 4 × 106 cells/ml and were stimulated with 2.5 μg of Concanavalin A (ConA) (Sigma Aldrich, St. Louis, Mo.) per ml or with 10 μg of LACK per ml for 48 h at 37°C with 4% CO2 in air. ConA was used for stimulation of cells from LaAg-vaccinated animals since crude antigens of L. amazonensis are anergenic to T cells in vitro (32). The levels of gamma interferon (IFN-γ) and IL-10 were measured in twofold dilutions of the supernatants by an enzyme-linked immunosorbent assay (ELISA). The levels of cytokines were determined with standard curves by using recombinant murine cytokines and paired antibodies according to the instructions of the manufacturer (R&D Systems, Minneapolis, Minn.).
Hypersensitivity reaction.
Four months after boosting, each animal was injected in a hind footpad with 20 μg of LaAg in 20 μl of PBS. Footpad swelling was measured with a dial caliper (Mitutoyo) at various times, and the results were expressed as the difference between the thickness of the footpads inoculated with the antigen and the thickness of the footpads inoculated with 20 μl of PBS.
Statistical analysis.
The statistical significance of differences between vaccinated and control groups of mice was determined by Student's t test.
RESULTS
Intranasal vaccination with LaAg confers protection against L. amazonensis infection.
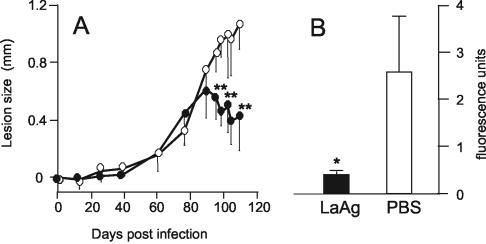
To evaluate whether the nasal mucosa could be used for controlled administration of vaccine doses lower than those previously shown to be required for oral immunization (≥100 μg) (32), BALB/c mice were doubly immunized with 10 μg of LaAg prior to subcutaneous infection with GFP-expressing L. amazonensis. Figure 1A shows that following a lag period of 80 days, the vaccinated animals were able to significantly control lesion growth (P ≤ 0.02), unlike the nonvaccinated animals, in which the lesions continued to grow steadily. The protection conferred by nasally administered LaAg was confirmed by a significantly lower (P ≤ 0.05) parasite burden, as determined by decreased fluorescence in the infected feet (Fig. 1B). This finding shows that the protection against homologous infection after mucosal immunization with LaAg is not restricted to the gastrointestinal system but extends to the nasal mucosa when a lower dosage is used.
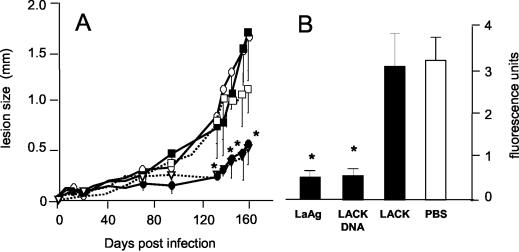
FIG. 1.
Protective effect of nasal immunization with LaAg against subsequent infection with L. amazonensis. BALB/c mice were vaccinated intranasally with 10 μg of LaAg and received a booster vaccination 7 days later (• and solid bar). The controls received PBS alone (○ and open bar). One week after the booster vaccination each of the animals was infected with 105 fluorescent L. amazonensis cells in the footpad. (A) Lesion sizes on different days. (B) On day 110 of infection, the parasite loads in individual feet were expressed in fluorescence units. The values are means and standard deviations (n = 5). One asterisk, P ≤ 0.05; two asterisks, P ≤ 0.02.
Intranasally administered LaAg induces mixed cytokine responses during infection.
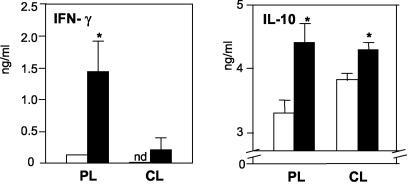
To more specifically determine the modulation of the immune responses during control of leishmanial infection in vaccinated animals, cytokine responses were measured in the peripheral popliteal (lesion-draining) lymph nodes and in the cervical (nose-draining) lymph nodes. As shown in Fig. 2, nonvaccinated animals produced little or no IFN-γ in the late stages of infection, but they produced relatively high levels of IL-10 in both sites, which is compatible with a progressive disease. Nasal vaccination resulted in a 7.4-fold increase in the production of IFN-γ in the peripheral lymph nodes, whereas production of IL-10 was increased 1.4-fold compared to production in nonvaccinated mice, which was indicative of a preferential increase in the IFN-γ response during protection. The IFN-γ and IL-10 levels measured just prior to infection (day 0) in the peripheral lymph nodes were enhanced 2.5- and 1.5-fold (P ≤ 0.05 for both), whereas on day 30 they were enhanced 2-fold (P ≤ 0.01) and 5-fold (P ≤ 0.05), respectively (data not shown). The IL-4 level measured on day 30 showed eightfold enhancement compared to the level in nonvaccinated mice (P ≤ 0.01) (data not shown). Together, these findings indicate that short-term vaccination did not prevent the development of TH2 responses in the early weeks of infection, which may have been critical to the lack of protection in the earlier stages of infection, but the increased IFN-γ response that followed may have contributed to the effective control of lesion growth at the later stages. The absolute levels of IL-10 and IL-4 were generally higher than the IFN-γ levels, but it is worth emphasizing that the measurements were obtained by using a quantitative method, not a qualitative method, which detected relative variations for a given cytokine but not variations for different cytokines.
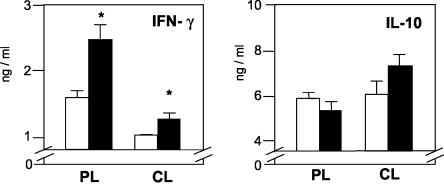
FIG. 2.
Cytokine production by mice infected 1 week after LaAg vaccination. Mice (n = 5) were vaccinated intranasally with LaAg (solid bars) or with PBS alone (open bars) prior to infection, as described in the legend to Fig 1. On day 110 of infection, the levels of IFN-γ and IL-10 produced by ConA (2.5 μg/ml)-stimulated peripheral lymph node cells (PL) and cervical lymph node cells (CL) were measured in the culture supernatants by ELISA. The values are means and standard deviations for triplicate samples. An asterisk indicates that the P value was ≤0.05 for a comparison with the PBS controls. nd, not detectable.
Intranasally administered LaAg induces a long-lasting immunological memory.
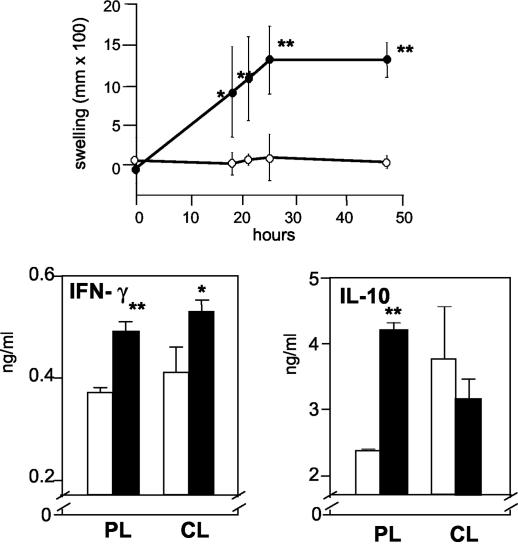
Surrogate markers of protective immunity in murine cutaneous leishmaniasis include TH1 responses, such as IFN-γ production and delayed-type hypersensitivity (DTH) responses in vivo (8, 29). These recall responses were evaluated 4 months after vaccination with LaAg. Figure 3 shows that vaccinated animals, but not nonvaccinated animals, displayed a strong DTH response after antigen challenge. The swelling kinetics showed that there was a peak response at 24 h, which was maintained for at least 48 h. A significant (P ≤ 0.05) increase in the production of IFN-γ in both popliteal and cervical lymph nodes was seen in vaccinated animals (Fig. 3, lower left panel), which was compatible with the in vivo DTH response, even though the IL-10 level was also augmented in the popliteal lymph nodes (Fig. 3, lower right panel). Together, these findings indicate that nasal vaccination with adjuvant-free LaAg induced a long-lasting mixed immune response in peripheral lymphoid tissues, which resulted in an inflammatory TH1-type immunity.
FIG. 3.
Hypersensitivity response and cytokine production 4 months after nasal vaccination. Mice (n = 9) received LaAg (• and solid bars) or PBS alone (○ and open bars), as described in the legend to Fig 1. (A) Four months after the booster vaccination, some of the animals (n = 5) were challenged in the footpads with 20 μg of LaAg, and the local swelling response was scored at different times. (B) The levels of IFN-γ and IL-10 produced by popliteal lymph node cells (PL) and cervical lymph node cells (CL) of unchallenged animals (n = 4) were measured in the culture supernatants of ConA-stimulated cells by ELISA. The values are means and standard deviations. One asterisk, P ≤ 0.05 for a comparison with the corresponding PBS controls; two asterisks, P ≤ 0.02 for a comparison with the corresponding PBS controls.
Intranasally administered LaAg confers long-lasting protective immunity.
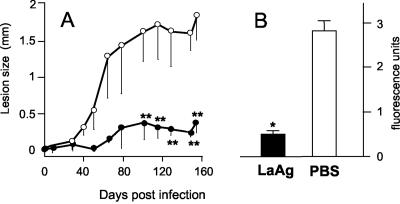
To evaluate whether the long-lasting immunogenicity of nasally administered LaAg remained protective, animals were challenged 4 months after vaccination, and the course of infection was then monitored for a further 5 months (160 days). As shown in Fig. 4A, vaccinated animals managed to control lesion growth throughout the infection. Protection was confirmed by a significantly (P ≤ 0.02) lower parasite burden compared with that of nonvaccinated animals (Fig. 4B). It is worth noting that the efficacy of vaccination increased with time. The delayed control seen in animals challenged only 1 week after vaccination (Fig. 1A) was suppressed 4 months later (Fig. 4A), when the animals were able to control the infection since the early days.
FIG. 4.
Long-lasting protective effect of nasal LaAg vaccination. Mice were immunized intranasally twice with LaAg (• and solid bar) or with PBS alone (○ and open bar) as described in the legend to Fig. 1. Animals were infected 4 months later with 105 fluorescent L. amazonensis cells in a footpad. (A) Lesion growth on different days. (B) On day 160 of infection, the parasite loads in the infected feet were expressed in fluorescence units. The values are means and standard deviations (n = 5). One asterisk, P ≤ 0.01; two asterisks, P ≤ 0.05.
The cytokine levels measured after 5 months of infection were increased (Fig. 5) compared with the levels measured just prior to infection (Fig. 3), probably due to active stimulation by live parasitism. Interestingly, the levels of IFN-γ but not the levels of IL-10 were further increased in the lesion-draining popliteal lymph nodes of vaccinated animals, suggesting that the IFN-γ skewed response was related to infection control. Although the effect was not as pronounced, the levels of IFN-γ were still elevated in the cervical lymph nodes (P ≤ 0.05), indicating that after such a long time after vaccination the mucosa-associated sites were still sensitized. Together, these findings demonstrate that the long-term memory produced by nasal vaccination with LaAg prompted effective control of infection.
FIG. 5.
Cytokine production by mice infected 4 months after vaccination. Mice (n = 5) were immunized intranasally with LaAg (solid bars) or received PBS alone (open bars) and were infected as described in the legend to Fig 4. On day 160 of infection, the levels of IFN-γ and IL-10 produced by popliteal lymph node cells (PL) and cervical lymph node cells (CL) were measured in the culture supernatants of ConA-stimulated cells by ELISA. The values are means and standard deviations for triplicate samples. An asterisk indicates that the P value is ≤0.05.
Intranasally administered LACK DNA is also effective in conferring protection against infection.
To verify the efficacy of a soluble defined leishmanial antigen, we used recombinant LACK (rLACK) to vaccinate BALB/c mice by the nasal route using the same protocol that was used for LaAg, in which animals were challenged 7 days after the booster dose. We found that administration of 10 μg of rLACK/dose had no effect (Fig. 6A) compared with administration of LaAg. The possibility of a suboptimal dosage was discarded since we used the same dose that we used for LaAg, in which LACK was present in a fraction. We then evaluated whether plasmid DNA expressing LACK could be effective. LACK DNA (30 μg/dose) significantly delayed lesion growth (Fig. 6A) and prevented parasite growth (Fig. 6B) for at least 5 months. This effect was not due to an adjuvant effect of CpG sequences in the bacterial DNA, as empty control DNA did not affect lesion growth. Interestingly, the delayed onset of infection was accompanied by increased LACK recall production of IFN-γ not only in the peripheral lymph nodes but also in the cervical lymph nodes (Fig. 7). The same cell populations restimulated in vitro with ConA showed a similar pattern of responses (data not shown). These results indicate that the DNA induced long-term sensitization in the mucosa-draining lymph nodes.
FIG. 6.
Effect of nasally administered rLACK and LACK DNA on subsequent infection with L. amazonensis. Mice were vaccinated intranasally with 10 μg of rLACK (▪ and solid bar), 30 μg of LACK DNA (• and solid bar), 10 mg of LaAg (▿ and solid bar), or 30 μg of DNA (□) and received a booster vaccination 7 days later. The controls received PBS alone (○ and open bar). One week after the booster vaccination animals were infected with 105 fluorescent L. amazonensis cells in a footpad. (A) Lesion sizes on different days. (B) On day 160 of infection, the parasite loads in the infected feet were expressed in fluorescence units. The values are means and standard deviations (n =5). An asterisk indicates that the P value is ≤0.05.
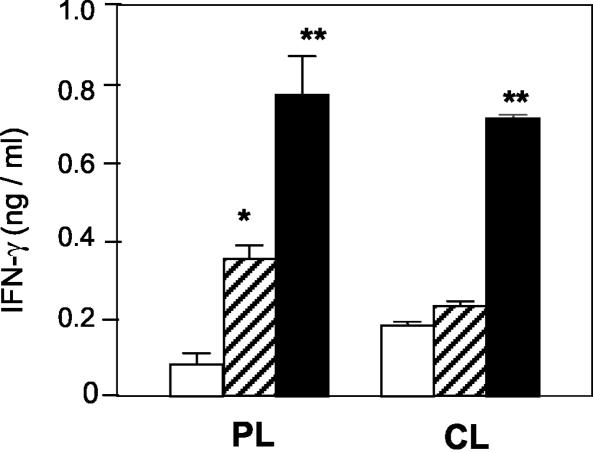
FIG. 7.
IFN-γ production by mice infected 1 week after LACK vaccination. Mice (n = 5) were vaccinated intranasally with rLACK (striped bars), LACK DNA (solid bars), or PBS alone (open bars) and infected as described in the legend to Fig 6. On day 160 of infection, the levels of IFN-γ produced by rLACK (10 μg/ml)-stimulated popliteal lymph node cells (PL) and cervical lymph node cells (CL) in the culture supernatants were measured by ELISA. The values are means and standard deviations for triplicate samples. One asterisk, P ≤ 0.05 for a comparison with PBS controls; two asterisks, P ≤ 0.01 for a comparison with PBS controls.
DISCUSSION
Previous studies showed that injecting killed promastigotes of L. major or L. amazonensis into the skin rendered mice and rhesus monkeys more susceptible to cutaneous leishmaniasis (19, 21). Interestingly, an opposing protective effect can be produced if killed promastigotes of L. amazonensis (LaAg) are administered orally to mice. There is an association between increased resistance in animals orally vaccinated with LaAg and increased production of IFN-γ in the lesion-draining lymph nodes, and there is concomitant suppression of parasite-specific TH2-type responses in vivo (32).
Mucosal membranes of the various tissues are linked together in a common mucosal immune system network. This is due to the expression of specific lymphocyte homing receptors, such as α4β7, that recognize the mucosal addressin cell adhesion molecule MadCAM-1 (13). Thus, in the present work we attempted to optimize mucosal immunization against cutaneous leishmaniasis by using the nasal route of vaccination for smaller vaccine doses and testing different leishmanial antigen types. Here we show for the first time that intranasal immunization with an adjuvant-free antigen could protect mice against cutaneous leishmaniasis. Passive inhalation of aerosol LaAg was also effective in delaying the onset of infection and reducing the parasite burden, but an amount of LaAg that was 30 times larger than the amount used for nasal instillation was necessary (unpublished observations). When we used a protocol similar to the protocol previously used for oral vaccination, in which animals were challenged 1 week postvaccination (32), intranasal vaccination with a 10-fold-lower LaAg dose also promoted increased resistance in BALB/c mice against L. amazonensis infection (Fig. 1). Interestingly, unlike orally vaccinated BALB/c mice, which responded more rapidly although at a lower intensity (32), nasally vaccinated mice were able to effectively control an infection only late in the infection, as shown by the significantly reduced parasite burden measured on day 110 of infection (Fig. 1B). It is possible that the late control of infection after short-term vaccination was due to the time lag required for an effective IFN-γ response. A very slow IFN-γ response has also been observed in humans, and some individuals may take up to 6 months following intramuscular vaccination with L. amazonensis antigens to optimally respond with IFN-γ production (33). The time lag requirement for an effective immune response may be further substantiated by the observation that the vaccinating effect is better 4 months after immunization (Fig. 4) than 1 week after immunization (Fig. 1). The long-term effectiveness of intranasal vaccination against intracellular pathogens has a corollary in tuberculosis, in which intranasal vaccination with BCG was recently shown to induce IFN-γ production in the lungs and in the spleen (5). In that study, similar to the results observed with Leishmania, intranasal vaccination was more effective at 6 months than at 3 months postvaccination for preventing Mycobacterium tuberculosis growth at those sites.
We showed here that intranasal vaccination with LaAg promoted a balanced TH1-TH2 response in the periphery, as demonstrated by upregulation of both IFN-γ and IL-10 production in the popliteal lymph nodes at either 1 week or 4 months postvaccination. In spite of this, subsequent infection with L. amazonensis resulted in controlled parasite growth. The following mechanisms were proposed to explain the effective protection achieved in the presence of upregulated IL-10: (i) the eventual predominant TH1 response (IFN-γ) in later stages of infection was sufficient for protection; (ii) upregulated TH2 cytokines (IL-10 and IL-4) are not sufficient to downregulate the TH1 responses during L. amazonensis infection of vaccinated mice (this possibility is supported by the finding that susceptibility of mice to L. amazonensis is not controlled by TH2 responses to the same extent as L. major infection [15]); and (iii) immunomodulatory factors other than the classical TH1 and TH2 cytokines may be favorably modulated by nasal vaccination (this possibility is supported by the finding that upon vaccination mice were capable of mounting a stronger DTH response to parasite antigens in vivo despite the higher level of IL-10 production in vitro [Fig. 3]; in this sense, tumor growth factor β is a good candidate as this cytokine is upregulated in aggravated murine cutaneous leishmaniasis [3]).
The protection achieved by intranasal immunization was accompanied by long-lasting immunological memory and adaptive immunity, as if LaAg possessed some kind of self-adjuvanticity. Intestinal or nasal antigen uptake may result in peripheral tolerance, and this was the starting point of the present work with disease-promoting parasite antigens. However, systemic immunity may also be achieved after antigen administration through the mucosa (1), and the balance between tolerance and immunity is a function of the nature of the antigen, the antigen dosage, the antigen form (soluble or particulated), the site of antigen administration, and the association with adjuvants (23). Thus, it seems that although both the nasal and oral mucosa may be sites for effective vaccination with LaAg, the antigen may be differentially presented at the two sites, as indicated by the partial tolerance and the active systemic immunity, respectively.
The advantage of a crude vaccine, such as LaAg, for mucosal vaccination is that the vaccine is already considered safe for parenteral use in humans (24). However, a defined vaccine may be more appropriate for standardization reasons. We found that soluble rLACK, at doses similar to those used for LaAg, had no effect against infection with L. amazonensis in short-term immunized animals (Fig. 6), which is consistent with the finding of McSorley et al. showing that 10 μg to 1 mg of nasally administered rLACK failed to protect BALB/c mice against infection with L. major (26). However, the possibility that the effectiveness of rLACK becomes apparent in long-term immunized mice should not be discarded. The slight increase in IFN-γ production after rLACK immunization was insufficient to induce protection and was not due to upregulated TH2 cytokines, as both the IL-10 and IL-4 levels were comparable with those of PBS controls (data not shown). It is possible that the soluble nature and the low glycosylation level of the recombinant protein impaired its uptake by M cells in the nasal mucosal epithelium (18), thus explaining the absence of activity. Use of LACK entrapped in biodegradable microspheres would better clarify this issue. On the other hand, LACK DNA effectively delayed the onset of infection and induced strong IFN-γ production in both peripheral and nose-associated cervical lymph nodes (Fig. 6 and 7). Kinetic studies with intranasally administered β-galactosidase-encoding plasmid DNA revealed an absorption rate of 14% and active expression of mRNA in mucosa-associated and peripheral lymph nodes 24 h later (30), but the exact mechanism by which plasmid DNA vaccines enter the nasal mucosa and tissue cells remains unclear (2). Susceptibility of BALB/c mice to L. major infection has been correlated with early production of IL-4 by LACK-reactive CD4+ T cells that drive the TH2 responses (20). It is worth noting that the presence of LACK-reactive memory T cells in animals not previously exposed to the parasites has been explained by their cross-reactivity with antigens from microbes of the normal intestinal flora (17). In the study of Julia et al., antigen-presenting cells from mesenteric lymph nodes of naive BALB/c mice induced the proliferation of LACK-specific T cells in vitro. Thus, it is possible that LACK-specific mucosal system-associated cells respond differently than peripheral T cells to challenge by mucosally administered LACK antigen as present in LaAg or as DNA transcripts, promoting the development of systemic protective immune responses.
Previous studies have demonstrated the efficacy of parenteral DNA vaccines against murine leishmaniasis (4, 12, 28, 34, 39), but this is the first study to report the efficacy of a mucosal DNA vaccine for this disease. Altogether, the work presented here demonstrates the feasibility of using the nasal mucosa for effective delivery of both crude antigens and DNA against cutaneous leishmaniasis. The demonstration of long-lasting and strong effects of nasally administered LaAg and LACK DNA may open new frontiers for noninvasive human vaccination against leishmaniasis.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. V.L. thanks the Spanish Ministry of Science and Technology for grant BIO 2000-0149-P4-04.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bakke, H., K. Lie, I. L. Haugen, G. E. Korsvold, E. A. Høiby, L. M. Næss, J. Holst, I. S. Aaberge, F. Oftung, and B. Haneberg. 2001. Meningococcal outer membrane vesicle vaccine given intranasally can induce immunological memory and booster responses without evidence of tolerance. Infect. Immun. 69:5010-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, A. G. C., C. Barnfield, R. Brew, and L. S. Klavinskis. 2000. Recent developments in mucosal delivery of pDNA vaccines. Curr. Opin. Mol. Ther. 2:87-93. [PubMed] [Google Scholar]
- 3.Belkaid, Y. 2003. The role of CD4+ CD25+ regulatory T cells in Leishmania infection. Expert Opin. Biol. Ther. 3:875-885. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, M., H. Diao, J. X. Ji, and L. Soong. 2003. DNA immunization with the gene encoding P4 nuclease of Leishmania amazonensis protects mice against cutaneous leishmaniasis. Infect. Immun. 71:6270-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, L. H., J. Wang, A. Zganiacz, and Z. Xing. 2004. Single intranasal mucosal Mycobactetium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect. Immun. 72:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 7.Czerkinsky, C., F. Anjuere, J. R. McGhee, A. George-Chandy, J. Holmgren, M. Kieny, K. Fujiyashi, J. F. Mestecky, V. Pierrefite-Carle, C. Rask, and J. Sun. 1999. Mucosal immunity and tolerance: relevance to vaccine development. Immunol. Rev. 170:197-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhaliwal, J. S., and F. Y. Liew. 1987. Induction of delayed-type hypersensitivity to Leishmania major and the concomitant acceleration of disease development in progressive murine cutaneous leishmaniasis. Infect. Immun. 55:645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumonteil, E., R. S. M. Jesus, E. O. Javier, and G. M. M. del Rosario. 2003. DNA vaccines induce partial protection against Leishmania mexicana. Vaccine 21:2161-2168. [DOI] [PubMed] [Google Scholar]
- 10.Fujihashi, K., T. Koga, F. W. van Ginkel, Y. Hagiwara, and J. R. McGhee. 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20:2431-2438. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Aseguinolaza, G., S. Taladriz, A. Marquet, and V. Larraga. 1999. Molecular cloning, cell localization and binding affinity to DNA replication proteins of the p36/LACK protective antigen from Leishmania infantum. Eur. J. Biochem. 259:909-916. [DOI] [PubMed] [Google Scholar]
- 12.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamann, A., D. P. Andrew, D. Jablonskiwestrich, B. Holzmann, and E. C. Butcher. 1994. Role of alpha (4)-integrins in lymphocyte homing to mucosal tissues in-vivo. J. Immunol. 152:3282-3293. [PubMed] [Google Scholar]
- 14.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, D. E., M. R. Ackermann, U. Wille, C. A. Hunter, and P. Scott. 2002. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect. Immun. 70:2151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julia, V., M. Rassoulzadegan, and N. Glaichenhaus. 1996. Resistance to Leishmania major induced by tolerance to a single antigen. Science 274:421-423. [DOI] [PubMed] [Google Scholar]
- 17.Julia, V., S. S. McSorley, L. Malherbe, J. P. Breittmayer, F. Girard-Pipau, A. Beck, and N. Glaichenhaus. 2000. Priming by microbial antigens from the intestinal flora determines the ability of CD4+ T cells to rapidly secrete IL-4 in BALB/c mice infected with Leishmania major. J. Immunol. 165:5637-5645. [DOI] [PubMed] [Google Scholar]
- 18.Kaiserlian, D., and N. Etchart. 1999. Entry sites for oral vaccines and drugs: a role for M cells, enterocytes and dendritic cells? Semin. Immunol. 11:217-224. [DOI] [PubMed] [Google Scholar]
- 19.Kenney, R. T., D. L. Sacks, J. P. Sypek, L. Vilela, A. A. Gam, and K. Evans-Davis. 1999. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J. Immunol. 163:4481-4488. [PubMed] [Google Scholar]
- 20.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 21.Liew, F. Y., C. Hale, and J. G. Howard. 1985. Prophylactic immunization against experimental leishmaniasis. IV. Subcutaneous immunization prevents the induction of protective immunity against fatal Leishmania major infection. J. Immunol. 135:2095-2101. [PubMed] [Google Scholar]
- 22.Lira, R., S. Sundar, A. Makharia, R. Kenney, A. Gam, E. Saraiva, and D. Sacks. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 180:564-567. [DOI] [PubMed] [Google Scholar]
- 23.Makala, L. H. C., Y. Nishikawa, N. Suzuki, and H. Nagasawa. 2004. Immunology—antigen-presenting cells in the gut. J. Biomed. Sci. 11:130-141. [DOI] [PubMed] [Google Scholar]
- 24.Marzochi, K. B. F., M. C. A. Marzochi, A. F. Silva, N. Grativol, R. Duarte, E. M. Conort, and F. Modabber. 1998. Phase 1 study of an inactivated vaccine against American tegumentary leishmaniasis in normal volunteers in Brazil. Mem. Inst. Oswaldo Cruz 93:205-212. [DOI] [PubMed] [Google Scholar]
- 25.McCluskie, M. J., and H. L. Davis. 1998. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161:4463-4466. [PubMed] [Google Scholar]
- 26.McSorley, S. J., C. Rask, R. Pichot, V. Julia, C. Czerkinsky, and N. Glaichenhaus. 1998. Selective tolerization of Th1-like cells after nasal administration of a cholera toxoid-LACK conjugate. Eur. J. Immunol. 28:424-432. [DOI] [PubMed] [Google Scholar]
- 27.Melby, P. C., J. Yang, W. Zhao, L. E. Perez, and J. Cheng. 2001. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 69:4719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendez, A., Y. Belkaid, R. A. Seder, and D. Sacks. 2002. Optimisation of DNA vaccination against cutaneous leishmaniasis. Vaccine 20:3702-3708. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 30.Oh, Y. K., J. P. Kim, T. S. Hwang, J. J. Ko, J. M. Kim, J. S. Yang, and C. K. Kim. 2001. Nasal absorption and biodistribution of plasmid DNA: an alternative route of DNA vaccine delivery. Vaccine 19:4519-4525. [DOI] [PubMed] [Google Scholar]
- 31.Okuno, T., M. Takeuchi, Y. Matsumoto, H. Otsuka, and Y. Matsumoto. 2002. Pretreatment of Leishmania homologue of receptors for activated C kinase (LACK) promotes disease progression caused by Leishmania amazonensis. Exp. Anim. 51:335-341. [DOI] [PubMed] [Google Scholar]
- 32.Pinto, E. F., M. M. Cortezia, and B. Rossi-Bergmann. 2003. Interferon-gamma-inducing oral vaccination with Leishmania amazonensis antigens protects BALB/c and C57BL/6 mice against cutaneous leishmaniasis. Vaccine 21:3534-3541. [DOI] [PubMed] [Google Scholar]
- 33.Pompeu, M. M. L., C. Brodskyn, M. J. Teixeira, J. Clarencio, J. Van Weyenberg, I. C. B. Coelho, S. A. Cardoso, A. Barral, and M. Barral-Netto. 2001. Differences in gamma interferon production in vitro predict the pace of the in vivo response to Leishmania amazonensis in healthy volunteers. Infect. Immun. 69:7453-7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramiro M. J., J. J. Zarate, T. Hanke, D. Rodriguez, J. R. Rodriguez, M. Esteban, J. Lucientes, J. A. Castillo, and V. Larraga. 2003. Protection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACK. Vaccine 21:2474-2484. [DOI] [PubMed] [Google Scholar]
- 35.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 36.Rossi-Bergmann B., A. Lenglet, C. R. Bezerra-Santos, D. Costa-Pinto, and Y. M. Traub-Czeko. 1999. Use of fluorescent Leishmania for faster quantitation of parasite growth in vitro and in vivo. Mem. Inst. Oswaldo Cruz 94(Suppl. II):74. [Google Scholar]
- 37.Shah, J. A., P. A. Darrah, D. R. Ambrozak, T. N. Turon, S. Mendez, J. Kirman, C. Y. Wu, N. Glaichenhaus, and R. A. Seder. 2003. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J. Exp. Med. 198:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization/TDR.1999. Tropical diseases research—leishmaniasis. Fourteen Programme Report 22. World Health Organization, Geneva, Switzerland.
- 39.Xu, D., and F. Y. Liew. 1994. Genetic vaccination against leishmaniasis. Vaccine 12:1534-1536. [DOI] [PubMed] [Google Scholar]