Abstract
Decades of research have established only a few etiological factors for glioma, which is a rare and highly fatal brain cancer. Common methodological challenges among glioma studies include small sample sizes, heterogeneity of tumor subtypes, and retrospective exposure assessment. Here, we briefly describe the Glioma International Case-Control (GICC) Study (recruitment, 2010–2013), a study being conducted by the Genetic Epidemiology of Glioma International Consortium that integrates data from multiple data collection sites, uses a common protocol and questionnaire, and includes biospecimen collection. To our knowledge, the GICC Study is the largest glioma study to date that includes collection of blood samples, which will allow for genetic analysis and interrogation of gene-environment interactions.
Keywords: cancer, case-control studies, glioblastoma, glioma, methodology, study profile
Editor's note: An invited commentary on this article appears on page 92, and the authors’ response appears on page 95.
With an annual incidence rate of 2–3 cases per 100,000 population in the United States, glioma, which comprises approximately 28% of all primary brain tumors, is a rare but highly fatal disease (1–4). Decades of research have established only a few etiological factors (family history, rare genetic cancer predisposition syndromes, ionizing radiation, and 10 independent genetic risk loci) (5–11), partly because glioma is a particularly challenging disease to study. Because it is a rare, highly fatal, and heterogeneous disease (2), it is difficult to accrue enough cases for large-scale epidemiologic studies (6). Due to potential etiological differences by tumor subtype, large sample sizes are needed to stratify results by histology. Additionally, it is not usually feasible to conduct a prospective study, thus necessitating the use of case-control study designs and retrospective exposure assessments. For these reasons, we opted to develop a consortium structure that integrates data from multiple sites, uses a common protocol and questionnaire, and includes biospecimen collection. Studies conducted by such consortia can help overcome some of the above obstacles and may be able to attain sufficient statistical power for identifying novel risk factors for this enigmatic disease.
To examine the genetic factors underlying familial glioma, the Genetic Epidemiology of Glioma International Consortium (GLIOGENE Consortium) was formed in 2006 to recruit families affected by ≥2 cases of glioma from 14 institutions across 5 countries (12). Research carried out by this consortium has yielded a number of high-impact discoveries (13, 14). However, because familial glioma accounts for only about 5% of all gliomas (12), the GLIOGENE investigators recognized the need to study sporadic glioma, which comprises the remaining 95% of gliomas. Thus, capitalizing upon the infrastructure in place from the GLIOGENE familial study, we have launched a large study of glioma that includes biospecimen collection: the Glioma International Case-Control (GICC) Study.
The main goals of the GICC Study are: 1) to identify novel genetic risk variants for glioma, as well as validate variants implicated by previous genome-wide association studies of glioma; and 2) to explore biologically relevant gene-gene and gene-environment interactions in glioma susceptibility. With 4,545 cases and 4,173 controls, the GICC Study confers the opportunity to evaluate both environmental exposures and genetic variation while accounting for tumor subtype. The study's comprehensive questionnaire data will allow for examination of putative risk factors identified from prior literature (i.e., radiation exposure, atopy, childhood infections), which can be reexamined in detail and validated. Additionally, the large study population may enable us to explore gene-environment interactions. Here, we present an overview of the study's structure and methodology, some methodological challenges and solutions, and preliminary demographic data.
THE GICC STUDY: DESIGN AND METHODS
Structure of the GICC Study
There are 14 recruitment sites in the GICC Study: Brigham and Women's Hospital (Boston, Massachusetts), Case Western Reserve University (Cleveland, Ohio), Columbia University (New York, New York), the Danish Cancer Society Research Centre (Copenhagen, Denmark), the Gertner Institute (Tel Hashomer, Israel), Duke University (Durham, North Carolina), the University of Texas MD Anderson Cancer Center (Houston, Texas), Memorial Sloan Kettering Cancer Center (New York, New York), the Mayo Clinic (Rochester, Minnesota), NorthShore HealthSystem (Chicago, Illinois), Umeå University (Umeå, Sweden), the University of California, San Francisco (San Francisco, California), the University of Southern California (Los Angeles, California), and the Institute of Cancer Research (London, United Kingdom).
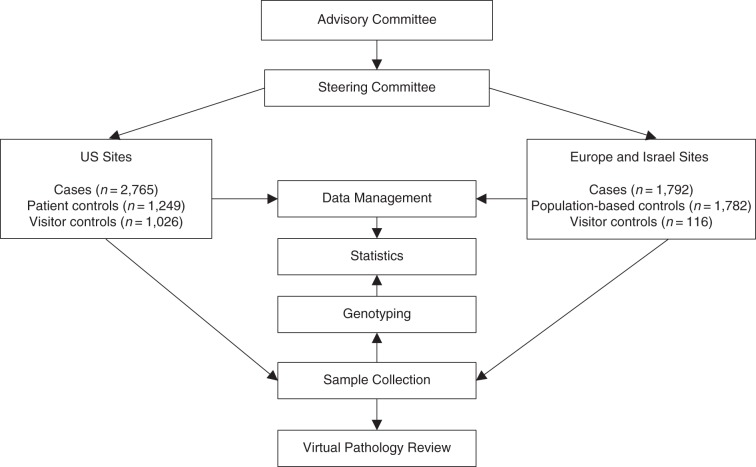
The structure of the GICC Study is presented in Figure 1. A steering committee oversees the administration of the study. Data management and statistical analyses are conducted centrally, with oversight by a topic-specific working group. These working groups were formed for each exposure of interest and are led by members of the GLIOGENE Consortium.
Figure 1.
Infrastructure of the Glioma International Case-Control Study (recruitment, 2010–2013).
A common study protocol was developed and is followed as closely as possible at each study site. However, it was not feasible to conduct the study identically at every site due to site-specific differences in infrastructure, resources, institutional policies, and laws and regulations across 14 institutions and 5 countries. Every site administered the same questionnaire (either the full version or the abbreviated version) and entered the data into a centralized Web-based database.
Ethical considerations
All participating institutions received institutional review board or ethical board approval to conduct the study. All study staff were trained in human subjects research, and informed consent was obtained from all participants.
Study population
Case eligibility and recruitment
Case recruitment began in April 2010 at all sites. Eligibility criteria for cases were as follows:
Diagnosis of histologically confirmed, supratentorial, intracranial glioma (World Health Organization (WHO) grades II–IV): fibrillary astrocytoma (International Classification of Diseases, Ninth Revision, code 9420/3), protoplasmic astrocytoma (code 9410/3), gemistocytic astrocytoma (code 9411/3), oligodendroglioma (code 9450/3), oligoastrocytoma (code 9382/3), anaplastic astrocytoma (code 9401/3), anaplastic oligodendroglioma (code 9451/3), anaplastic oligoastrocytoma (code 9382/3), or glioblastoma (code 9440/3)
Age 18–80 years at diagnosis
Ability to speak the local language
Cases were recruited within 1 year of diagnosis and consented to participation at their clinic visits. Blood/saliva samples and interviews were obtained or scheduled at that time. Interviews were conducted in person (52.5%) or by telephone (21.8%), or the questionnaire was self-administered (mailed to the respondent) (21.3%). At European and Israeli sites, cases were recruited nationwide through the university clinics that had neuro-oncology centers. The consent process for all centers involved written permission to abstract data from medical records and to obtain pathology slides for confirmation of the glioma diagnosis. If the case patient had neurological or other health deficits, we asked an appropriate proxy to assist with questionnaire completion.
Control eligibility and recruitment
Like cases, controls were eligible for the study if they were between 18 and 80 years of age and could speak the local language. Investigators at each site chose which control recruitment method to use based on feasibility and their existing infrastructure.
Seven sites recruited visitors accompanying cancer patients as controls, 4 sites recruited clinic-based controls, and 3 sites used population-based controls (Figure 1). It was not feasible for all sites to recruit controls using identical methods. Ideally, control selection would capture members of the underlying source population for the cases (15, 16). However, there is no ideal method for recruiting such a control population for any case-control study (16), particularly one involving several large referral centers, where the source population includes a mix of both national and international patients and cannot therefore be easily defined. Our goal was to accrue either visitors accompanying non-brain-tumor patients or patients at general medical clinics as controls or, alternatively, population-based controls, to optimize participation at as many sites as possible. Sites with population-based case recruitment accrued population-based controls (Sweden, Denmark, Israel). Most US sites are tertiary-care and/or referral centers and thus used visitor or general clinic-based controls, depending on what was most feasible and/or cost-effective.
There are advantages of using visitor controls. Hospital visitors generally tend to derive from the same hypothetical population as cases, and are likely to be included as cases if they were to develop glioma (17, 18). Published reports also support the use of hospital/clinic visitors for control recruitment in studies with underlying populations that are difficult to define (18–20). Many of the consortium investigators have previously used this method to accrue controls for several ongoing case-control studies.
However, visitors accompanying non-brain-tumor patients may be more likely to report higher prevalences of exposures, such as family history of cancer (18), or to devote more effort to recalling details during questionnaire response than the general population, potentially as a result of being a close contact of someone with cancer. General medical clinic-based controls may be more likely to report comorbid conditions (e.g., allergies/asthma) or medication use (e.g., antihistamine use) than the general population, which could bias a potential association between such factors and glioma towards the null. For these reasons, we will conduct sensitivity analyses by examining each exposure-outcome relationship of interest by control type (discussed below).
Centralized pathology review
Pathology slides from a subset of the cases recruited during the first year of the GICC Study (total n = 588) were subjected to centralized review to ensure that misclassification of tumor type was minimal across sites. Some studies of reviews by independent pathologists have shown large interindividual variability (up to 50% between histopathological entities for both major and minor changes) (21–23).
Our pathology team met in October 2011 to design our pathology review protocol. To ensure a high participation rate, the decision as to whether the original slides from initial diagnosis or freshly cut slides were sent to our team was left to the discretion of the individual study center, according to each country's or hospital's local guidelines. The corresponding pathology reports were also collected. All non–glioblastoma multiforme (GBM) cases (n = 509) and 10% of GBM cases (n = 79) available on that date were selected for review, because GBMs are classified according to established diagnostic criteria (WHO scheme) and have previously been shown to be subject to a very low rate of diagnostic discrepancy (24).
Eight pathologists were randomized into teams of 2, with 1 European pathologist and 1 US pathologist per team. The pathologists were blinded to the primary diagnosis. The teams and the group as a whole employed the WHO general guidelines for typing and grading (25). If the twin team of pathologists did not reach consensus, the case was taken forward to a final panel for a decision by all of the pathologists. A change of diagnosis was defined as a change from low WHO grade to high WHO grade or a complete histological change from oligodendroglioma to astrocytoma or vice versa.
Overall, there was a change of grade from lower to higher in only 4% of glioma cases, and there was a change in both grade and histology in only 2.8% of cases. Given the small proportions of gliomas that were reclassified, we believe that misclassification of tumor type is unlikely to represent a major source of bias in our study.
Data and specimens
The GICC risk factor questionnaire included questions on demographic characteristics, exposure to ionizing radiation, medical and medication history, and occupational exposure history. The family history section of the questionnaire documents the numbers of and dates of birth and death for all first- and second-degree relatives and all cancer diagnoses in the family. The questionnaire has a fixed script, including transition statements where necessary. The consortium utilized previously validated scales and questions (i.e., the Charlson Comorbidity Index (26) and tobacco use questions from the WHO's Global Adult Tobacco Survey (http://www.who.int/tobacco/surveillance/gats/en/)) for as many sections of the questionnaire as possible. Two versions of the questionnaire were utilized: a full version and an abbreviated version. The abbreviated version excluded only detailed questions on brain tumor symptoms, seizures, medical history, immunosuppressant use, dental x-rays, and physical activity. The study sites that administered the abbreviated version (due to time constraints) were: Duke University; Memorial Sloan Kettering Cancer Center; the University of California, San Francisco; the Institute of Cancer Research; and the University of Southern California.
Data collection procedures were similar at each site. All study coordinators attended an initial 2-day central training session to ensure site-to-site homogeneity in protocols and data collection procedures. The study manager conducted site visits to ensure that appropriate procedures were being followed. Staff from all recruitment centers participated in monthly conference calls that focused on data collection, eligibility, and study procedures.
Each participant was asked to submit to venipuncture (30 mL of blood) or, if unable/unwilling to do so, to provide a saliva sample. Each site stored an aliquot of whole blood (or saliva) from each participant and extracted DNA from the remaining blood/saliva using standard methods. The US sites then submitted 10 μg of DNA per participant to the Mayo Clinic's Biospecimen Accessioning and Processing Core for central long-term storage.
Data reliability
One study site, the Mayo Clinic, utilized clinic-based controls from the Mayo Clinic Biobank, which collects medical records and questionnaire data. Controls for this site were selected from the 50,000 available biobank participants, frequency-matched to cases, and recontacted for participation in the GICC Study. Approximately 60% of controls consented to GICC participation about a year after being entered into the biobank. Because these individuals (n = 453) completed both questionnaires in a relatively short period of time, we were able to assess concordance between certain variables that were ascertained through similar questions, and we calculated the percentage of concordance between the responses. Only 1 of the 51 variables examined had less than 90% concordance (see Web Table 1, available at http://aje.oxfordjournals.org/).
Genotyping
Illumina's Infinium OncoArray-500K BeadChip array (Illumina Inc., San Diego, California) was used for genotyping. We customized the array to include an additional 37,000 bead types. Customized content included genes previously implicated in glioma etiology. We plan to provide additional details related to the GICC genetic data in a future publication.
Plan of analysis
Statistical analysis
Due to both innate heterogeneity (i.e., geographic/cultural) and differences in study conduct/methodology between sites, a multipronged analytical approach is planned for each exposure of interest. All estimates will be calculated and presented for all gliomas, as well as separately for GBMs and non-GBMs. First, we will compare exposure proportions/means between cases and controls across the 14 study sites. Then site-specific crude and adjusted odds ratios will be calculated for each exposure-outcome relationship, using logistic regression. All 14 sets of site-specific odds ratios, their corresponding 95% Wald confidence intervals, and P values will be presented in each manuscript to show the potential variability in results across sites. These odds ratios and 95% confidence intervals will be compared with each other; specifically, if 1 (or 2) of the site-specific odds ratios are in opposite directions and have nonoverlapping confidence intervals, we will conduct a series of sensitivity analyses to help explain why this odds ratio is outside of the observed range. These sensitivity analyses will vary based on the exposure of interest (behavioral, occupational, etc.).
We will then use meta-analysis methods to quantify potential site-specific heterogeneity. We will combine site-specific estimates through a random-effects model to consider variability within and between studies (27). Final combined meta-analysis odds ratios, calculated by both a 1-stage and a 2-stage meta-analysis approach, will be provided. The 1-stage approach synthesizes the individual-level data from all sites simultaneously, while also accounting for clustering of subjects within sites. The 1-stage approach consists of a multilevel logistic regression model with random effects. We will use the adaptive Gauss-Hermite quadrature approximation method (28). In the 2-stage approach, we will use a maximum likelihood estimate to summarize the exposure-outcome association for each study site in the first stage and maximum likelihood estimation/restricted maximum likelihood to combine those aggregate data across study sites in the second stage. To formally measure statistical heterogeneity, we will calculate Cochran's Q statistic and the I2 statistic to assess the inconsistency of results (29).
Forest plots will be provided as a simple representation of the site-specific and overall summary (meta-analysis) estimates and of the variation between study results (30). Additionally, for each association, we will examine the differences in effect estimates between sites with different types of controls (visitor, clinic, or population-based) to ensure that no patterns by control type are present. In addition, when we are examining exposures for which geographic differences may be meaningful (e.g., allergies/asthma), our analyses will be stratified and results presented accordingly.
Other methodological and analytical challenges and solutions
Two key methodological challenges are related to differences in questionnaire administration methods and the accuracy of proxy-reported information.
Questionnaire administration methods differed by site (Web Table 2). To assess whether reporting bias may exist by questionnaire administration method, we will calculate site-specific odds ratios for each exposure and will assess potential heterogeneity between sites by primary method of questionnaire administration (in-person interview, telephone interview, mixed methods (by phone and in person), or self-administration; see example in Web Table 3). Differences in questionnaire administration are less likely to be causing a major bias in the results if clear patterns do not emerge in the odds ratios by category.
As an additional sensitivity analysis, we will investigate the association between questionnaire administration method and the probability of reporting a positive response for each exposure of interest, using logistic regression (see example in Web Table 4). This analysis will allow us to determine whether the questionnaire administration method is resulting in misclassification of the factor of interest and whether that misclassification is nondifferential or differential by case-control status. If we find differential misclassification by questionnaire administration method, we will consider weighting our future analyses accordingly. Unfortunately, we cannot simply adjust for questionnaire administration method in our final models, because of the distribution of administration methods within sites and by case-control status (Web Table 2).
Because glioma may affect the patient's cognitive functioning (or patients may die after giving consent), proxy responses for cases are sometimes necessary. The impact of proxy responses may differ by exposure of interest, partly because some exposures may be less likely to be accurately reported by a proxy than others (e.g., severe allergies in adulthood vs. ages at common childhood illnesses). The proportion of proxy-only respondents in our study is relatively low (7.8% of respondents overall; Table 1), and most proxy respondents are cohabitants of the case, including spouses (approximately 50%). Cases who used proxy respondents at recruitment were more likely to be older and male and more likely to have a high-grade tumor than cases who self-reported (data not shown). As a result, we will compare odds ratios for each exposure including and excluding proxy responses. If the results are similar, proxy responses will be included in the final analyses of that exposure. If they differ, both estimates will be reported. We cannot adjust for proxy response in our regression models, since proxies were not used for controls.
Table 1.
Characteristics of the Study Population, Glioma International Case-Control Study, 2010–2013
| Characteristic | Cases |
Controls |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total | 4,545 | 100 | 4,173 | 100 |
| Sex | ||||
| Male | 2,679 | 58.9 | 2,350 | 56.3 |
| Female | 1,866 | 41.1 | 1,823 | 43.7 |
| Race/ethnicity | ||||
| Missing data | 17 | 0.4 | 4 | 0.1 |
| White | 4,174 | 91.8 | 3,692 | 88.5 |
| Black | 71 | 1.6 | 140 | 3.4 |
| Asian | 84 | 1.9 | 87 | 2.1 |
| Hispanic | 161 | 3.5 | 224 | 5.4 |
| Other | 38 | 0.8 | 26 | 0.6 |
| Age at diagnosis/ enrollment, yearsa | ||||
| 18–29 | 307 | 6.8 | 294 | 7.1 |
| 30–39 | 526 | 11.6 | 474 | 11.4 |
| 40–49 | 816 | 18.0 | 680 | 16.3 |
| 50–59 | 1,157 | 25.5 | 1,079 | 25.9 |
| 60–69 | 1,238 | 27.2 | 1,098 | 26.3 |
| 70–80 | 501 | 11.0 | 548 | 13.1 |
| Education | ||||
| Missing data | 477 | 10.5 | 120 | 2.9 |
| High school or less | 1,125 | 24.8 | 912 | 21.9 |
| Some college | 1,105 | 24.3 | 1,292 | 31.0 |
| Bachelor's degree | 1,026 | 22.6 | 958 | 23.0 |
| Advanced degree | 812 | 17.9 | 891 | 21.4 |
| Glioma grade | ||||
| Not applicable | 0 | 0 | 4,173 | 100 |
| Grade II | 870 | 19.1 | 0 | 0 |
| Grade III | 819 | 18.0 | 0 | 0 |
| Grade IV | 2,784 | 61.3 | 0 | 0 |
| Unclassified | 72 | 1.6 | 0 | 0 |
| Marital status | 475 | 10.5 | 117 | 2.8 |
| Missing data | ||||
| Married/living with partner | 3,161 | 69.6 | 3,017 | 72.3 |
| Divorced/separated | 324 | 7.1 | 325 | 7.8 |
| Widowed | 131 | 2.9 | 131 | 3.1 |
| Never married | 454 | 10.0 | 583 | 14.0 |
| Interview type | ||||
| Missing data | 19 | 0.4 | 0 | 0 |
| Self-reported | 3,993 | 87.9 | 4,173 | 100 |
| Equally self- and proxy-reported | 163 | 3.6 | 0 | 0 |
| Proxy-reported | 370 | 8.1 | 0 | 0 |
| Questionnaire administration | ||||
| Missing data | 155 | 3.4 | 79 | 1.9 |
| By telephone | 1,194 | 26.3 | 646 | 15.5 |
| In person | 2,182 | 48.0 | 2,520 | 60.4 |
| Both by phone and in person | 98 | 2.2 | 42 | 1.0 |
| Self-administered | 916 | 20.2 | 886 | 21.2 |
a The mean age was 53.4 (standard deviation, 13.8) years in cases and 53.8 (standard deviation, 14.2) years in controls; the median age was 55 (range, 18–80) years in cases and 56 (range, 18–80) years in controls.
PRELIMINARY RESULTS: DEMOGRAPHIC PROFILE OF THE GICC POPULATION
Demographic characteristics of the GICC study population are summarized in Table 1 (data are shown by site in Web Table 2). The median ages at diagnosis/enrollment for cases and controls were 55 (standard deviation, 13.8) years and 56 (standard deviation, 14.2) years, respectively. Approximately 59% of cases were male, as compared with 56% of controls, and the majority of the study population was non-Hispanic white (91.8% of cases; 88.5% of controls). Among cases, 61.3% had high-grade (WHO grade IV) gliomas. This demographic profile is consistent with previous studies of adult glioma (31).
SUMMARY
Limitations
As a study conducted by an international multi-institution consortium, the GICC Study is subject to some limitations. Site-to-site heterogeneity in control selection and other differences in study conduct can influence our findings, but we have carefully planned our analyses to consider these differences as much as possible. Additional geographic and cultural heterogeneity across international sites may convolute some associations, necessitating that both site-specific and overall meta-regression results be reported for each exposure. Nevertheless, such challenges are unavoidable in large consortium studies, partly due to differences in infrastructure and resources, institutional policies, and national regulations.
Planned analyses
The initial set of GICC analyses will focus on validating previously implicated epidemiologic risk factors for glioma (viral exposures, atopy, antihistamine use, nonsteroidal antiinflammatory drug use, family history, radiation exposure) in our large study population. Future analyses will include a genome-wide association study and will explore the roles of gene-environment and gene-gene interactions in glioma etiology.
Conclusions
To our knowledge, the GICC Study is the largest case-control study of glioma to date that includes both epidemiologic data and biospecimens, allowing for the evaluation of genetic variants and environmental factors and the exploration of gene-environment interactions. The study includes detailed data on key suspected risk factors for glioma, permitting us to examine these factors in an international context. Many previous glioma studies have been hampered by problems related to small sample sizes, lack of generalizability, and issues regarding exposure assessment. We hope that our consortium study can help overcome some of these challenges.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, Texas (E. Susan Amirian, Georgina N. Armstrong, Renke Zhou, Ching C. Lau, Michael E. Scheurer, Melissa L. Bondy); Department of Epidemiology and Public Health, School of Medicine, Yale University, New Haven, Connecticut (Elizabeth B. Claus); Department of Neurosurgery, Brigham and Women's Hospital, Boston, Massachusetts (Elizabeth B. Claus); Case Comprehensive Cancer Center, School of Medicine, Case Western Reserve University, Cleveland, Ohio (Jill S. Barnholtz-Sloan); Department of Epidemiology and Biostatistics, School of Public Health, Georgia State University, Atlanta, Georgia (Dora Il'yasova); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, North Carolina (Dora Il'yasova, Joellen Schildkraut); Department of Surgery, Duke University Medical Center, Durham, North Carolina (Francis Ali-Osman); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer, Israel (Siegal Sadetzki); Department of Epidemiology and Preventive Medicine, School of Public Health, Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel (Siegal Sadetzki); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (Christoffer Johansen); Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (Christoffer Johansen, Helle Broholm); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, United Kingdom (Richard S. Houlston, Caterina Giannini); Department of Laboratory Medicine and Pathology, Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Robert B. Jenkins); Department of Neurology, Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, Minnesota (Daniel Lachance); Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, New York (Sara H. Olson, Jonine L. Bernstein); Department of Neurology, NorthShore University HealthSystem, Evanston, Illinois (Ryan T. Merrell); Department of Neurological Surgery, School of Medicine, University of California, San Francisco, San Francisco, California (Margaret R. Wrensch); Department of Public Health Services, School of Public Health, University of Alberta, Edmonton, Alberta, Canada (Faith G. Davis); Departments of Neurology, Neurosurgery, and Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California (Rose Lai); Department of Biostatistics, University of Texas MD Anderson Cancer Center, Houston, Texas (Sanjay Shete); Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire (Christopher I. Amos); Department of Laboratory Medicine and Pathobiology, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada (Kenneth Aldape); Department of Immunology, Genetics, and Pathology, Faculty of Medicine, Uppsala University, Uppsala, Sweden (Irina Alafuzoff); Department of Medical Biosciences, Faculty of Medicine, Umeå University, Umeå, Sweden (Thomas Brännström); Department of Pathology, Cambridge Cancer Centre, University of Cambridge, Cambridge, United Kingdom (Peter Collins); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Marc Rosenblum); Department of Pathology, School of Medicine, University of California, San Francisco, San Francisco, California (Tarik Tihan); and Department of Radiation Sciences, Faculty of Medicine, Umeå University, Umeå, Sweden (Beatrice S. Melin).
This work was supported by grants from the National Institutes of Health (grants R01CA139020, R01CA52689, P50097257, and P30CA125123). Additional support was provided by the McNair Medical Institute at Baylor College of Medicine (Houston, Texas) and the Population Sciences Biorepository at Baylor College of Medicine.
The Glioma International Case-Control Study is being conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each study subject or from his/her guardian. Approval from local institutional review boards was received at each institution participating in the Genetic Epidemiology of Glioma International Consortium.
Conflict of interest: none declared.
REFERENCES
- 1.Adamson C, Kanu OO, Mehta AI et al. . Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;188:1061–1083. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;1091:93–108. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin H, Bray F. GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide in 2008. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 4.Ostrom QT, Gittleman H, Liao P et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajaraman P, Melin BS, Wang Z et al. . Genome-wide association study of glioma and meta-analysis. Hum Genet. 2012;13112:1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrom QT, Gittleman H, Stetson L et al. . Epidemiology of gliomas. Cancer Treat Res. 2015;163:1–14. [DOI] [PubMed] [Google Scholar]
- 7.Shete S, Hosking FJ, Robertson LB et al. . Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;418:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrensch M, Jenkins RB, Chang JS et al. . Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;418:905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Chen Y, Zhao Y et al. . Association of sequence variants on chromosomes 20, 11, and 5 (20q13.33, 11q23.3, and 5p15.33) with glioma susceptibility in a Chinese population. Am J Epidemiol. 2011;1738:915–922. [DOI] [PubMed] [Google Scholar]
- 10.Ostrom QT, Bauchet L, Davis FG et al. . The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;167:896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan KM, Thompson RC, Nabors LB et al. . Cancer susceptibility variants and the risk of adult glioma in a US case-control study. J Neurooncol. 2011;1042:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malmer B, Adatto P, Armstrong G et al. . GLIOGENE—an international consortium to understand familial glioma. Cancer Epidemiol Biomarkers Prev. 2007;169:1730–1734. [DOI] [PubMed] [Google Scholar]
- 13.Jalali A, Amirian ES, Bainbridge MN et al. . Targeted sequencing in chromosome 17q linkage region identifies familial glioma candidates in the Gliogene Consortium. Sci Rep. 2015;5:8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bainbridge MN, Armstrong GN, Gramatges MM et al. . Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst. 2015;1071:dju384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 16.Wacholder S, Silverman DT, McLaughlin JK et al. . Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol. 1992;1359:1029–1041. [DOI] [PubMed] [Google Scholar]
- 17.Breslow NE, Day NE. Statistical methods in cancer research. Volume I—the analysis of case-control studies. IARC Sci Publ. 1980;32:5–338. [PubMed] [Google Scholar]
- 18.Mendonça GA, Eluf-Neto J. Hospital visitors as controls in case-control studies. Rev Saude Publica. 2001;355:436–442. [DOI] [PubMed] [Google Scholar]
- 19.Armenian HK, Lakkis NG, Sibai AM et al. . Hospital visitors as controls. Am J Epidemiol. 1988;1272:404–406. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Zhang M, Holman D. Population versus hospital controls for case-control studies on cancers in Chinese hospitals. BMC Med Res Methodol. 2011;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta T, Nair V, Epari S et al. . Concordance between local, institutional, and central pathology review in glioblastoma: implications for research and practice: a pilot study. Neurol India. 2012;601:61–65. [DOI] [PubMed] [Google Scholar]
- 22.Gilles FH, Tavaré CJ, Becker LE et al. . Pathologist interobserver variability of histologic features in childhood brain tumors: results from the CCG-945 study. Pediatr Dev Pathol. 2008;112:108–117. [DOI] [PubMed] [Google Scholar]
- 23.Bruner JM, Inouye L, Fuller GN et al. . Diagnostic discrepancies and their clinical impact in a neuropathology referral practice. Cancer. 1997;794:796–803. [DOI] [PubMed] [Google Scholar]
- 24.Scott CB, Nelson JS, Farnan NC et al. . Central pathology review in clinical trials for patients with malignant glioma. A report of Radiation Therapy Oncology Group 83-02. Cancer. 1995;762:307–313. [DOI] [PubMed] [Google Scholar]
- 25.Louis DN, Ohgaki H, Wiestler OD et al. , eds. WHO Classification of Tumours of the Central Nervous System. 4th ed Lyon, France: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;405:373–383. [DOI] [PubMed] [Google Scholar]
- 27.Hedges L, Vevea J. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;34:486–504. [Google Scholar]
- 28.Debray TP, Moons KG, Abo-Zaid GM et al. . Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS One. 2013;84:e60650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;2111:1539–1558. [DOI] [PubMed] [Google Scholar]
- 30.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;3227300:1479–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010;393:675–694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.