Abstract
Haemophilus ducreyi colocalizes with polymorphonuclear leukocytes and macrophages and evades phagocytosis during experimental infection of human volunteers. H. ducreyi contains two genes, lspA1 and lspA2, which encode predicted proteins of 456 and 543 kDa, respectively. Compared to its wild-type parent, an lspA1 lspA2 double mutant does not inhibit phagocytosis by macrophage and myelocytic cell lines in vitro and is attenuated in an experimental rabbit model of chancroid. To test whether expression of LspA1 and LspA2 was necessary for virulence in humans, six volunteers were experimentally infected. Each volunteer was inoculated with three doses (ranging from 85 to 112 CFU) of the parent (35000HP) in one arm and three doses (ranging from 60 to 822 CFU) of the mutant (35000HPΩ12) in the other arm. The papule formation rates were 88% (95% confidence interval [95% CI], 76.8 to 99.9%) at 18 parent sites and 72% (95% CI, 44.4 to 99.9%) at 18 mutant sites (P = 0.19). However, papules were significantly smaller at mutant sites (mean size, 24.8 mm2) than at parent sites (mean size, 39.1 mm2) 24 h after inoculation (P = 0.0002). The pustule formation rates were 44% (95% CI, 5.8 to 77.6%) at parent sites and 0% (95% CI, 0 to 39.4%) at mutant sites (P = 0.009). With the caveat that biosafety regulations preclude testing of a complemented mutant in human subjects, these results indicate that expression of LspA1 and LspA2 facilitates the ability of H. ducreyi to initiate disease and to progress to pustule formation in humans.
Haemophilus ducreyi is a gram-negative, unencapsulated bacterium that causes chancroid, a genital ulcer disease. Although rare in the United States (11), chancroid remains prevalent in some developing countries (33). H. ducreyi is an important pathogen because it facilitates the acquisition and transmission of human immunodeficiency virus type 1 (8, 33).
The H. ducreyi genome contains two extremely large open reading frames (ORFs), lspA1 and lspA2 (large supernatant protein), which encode predicted proteins that have calculated molecular weights of 456 and 543 kDa, respectively, and have 86% identity (40). The protein product of the lspA1 gene is a very large antigen that can be detected in concentrated culture supernatants (40). Although both lspA1 and lspA2 are transcribed in vitro, the LspA2 protein is not reproducibly detected in concentrated culture supernatants from several wild-type H. ducreyi strains (40).
Both LspA1 and LspA2 are detected by their reactivity in Western blot analysis with specific monoclonal antibodies (MAbs) (39). The whole-cell lysate of a double lspA1 lspA2 mutant, 35000HP.12, exhibits weak reactivity with LspA1- and LspA2-reactive MAbs, suggesting that this mutant may express truncated protein products (39). Nevertheless, 35000HP.12 is significantly less virulent than 35000HP in the temperature-dependent rabbit model for chancroid (39). In vitro, 35000HP.12 is readily engulfed by phagocytes (38), whereas strains 35000 and 35000HP resist phagocytosis and inhibit phagocytosis of secondary targets (1, 28, 38, 41).
To study H. ducreyi pathogenesis in humans, we developed an experimental infection model in which strain 35000HP and its derivatives are delivered to the skin of the upper arms of healthy volunteers by puncture wounds made by the tines of an allergy testing device (27, 32). Papules form within 24 h of inoculation and either evolve into pustules in 2 to 5 days or resolve spontaneously. There is a significant effect of dose on pustule formation, and men are twice as likely to form pustules as women (3, 9). In an individual inoculated at multiple sites, a pustule may develop at one site while another site resolves (5, 31). However, outcomes at multiple sites within a subject tend to be similar, suggesting that there is a host effect on susceptibility to disease progression (28). In reinfection experiments, some volunteers are repeatedly prone to pustule formation while others are prone to resolution (4, 28), confirming that different hosts are differentially susceptible to disease progression versus resolution in the model (28).
To test the role of putative virulence determinants in humans, mutant-parent comparison trials have been performed using the model (27). In these trials, volunteers are inoculated with multiple doses of the parent on one arm and a mutant on the other arm. Volunteers serve as their own controls for the gender and host effects. To date, of 13 mutants tested (27, 29), those that lack an intact flp locus, expression of the hemoglobin receptor (HgbA), the peptidoglycan-associated lipoprotein (PAL), or DsrA, an outer membrane protein (OMP) that is the major known determinant of serum resistance, are attenuated in their ability to form pustules (2, 10, 18, 29).
In the human infection model, H. ducreyi colocalizes with polymorphonuclear leukocytes and macrophages (7). However, H. ducreyi remains extracellular and evades phagocytosis in individuals who are prone to pustule formation (6). In view of the facts that expression of either intact LspA1 or intact LspA2 is required for evasion of phagocytosis in vitro, expression of both proteins is necessary for full virulence in animals, and both genes are transcribed during experimental infection in humans (37-39), we speculated that both lspA1 and lspA2 could be required for virulence in humans. For this study, a lspA1 lspA2 double mutant, 35000HPΩ12, which was constructed with Ω cassettes inserted near the very beginning of both the lspA1 and lspA2 ORFs and which lacks reactivity with a MAb that binds to both proteins (39), was characterized and tested for virulence in the human challenge model.
MATERIALS AND METHODS
Bacteria and culture conditions.
35000HP is a human-passaged (HP) variant of 35000 and has been reported previously (5). Construction of 35000HPΩ12 has been reported previously, but details of its construction and characterization were not fully described (39). The recombinant plasmid pDad (39), containing the 5′ half of the lspA1 gene, was digested with BstBI, which cut the lspA1 ORF 103 nucleotides downstream from the translation initiation codon. The Ω-Km2 (Ωkan) cartridge (23) was excised from pUC4-ΩKm2 by digestion with SmaI and ligated into the BstBI site in pDad-5, which had been blunt ended by treatment with the Klenow fragment of DNA polymerase I. This ligation reaction mixture was used to electroporate Escherichia coli DH5α, and recombinant strains were selected on Luria-Bertani plates containing kanamycin (30 μg/ml). One of these recombinant plasmids, designated pDadΩkan, was propagated in E. coli HB101, digested with NdeI, and used to electroporate 35000HP as described elsewhere (20). The H. ducreyi 35000HPΩ1 mutant containing the Ωkan cassette inserted into its lspA1 gene was selected on GC-heme agar (34) containing kanamycin (30 μg/ml). Next, the recombinant plasmid pCW118 (40), containing the 5′ half of the lspA2 gene, was digested with AgeI, which cut the lspA2 ORF 54 nucleotides downstream from its translation initiation codon. The Ωcat cartridge was excised from pHP45Ω-Cm (17) by digestion with SmaI and then ligated into the AgeI site in pCW118, which had been blunt ended by treatment with Pfu polymerase. The ligation reaction mixture was used to electroporate E. coli DH5α, and recombinant strains were selected on Luria-Bertani agar containing chloramphenicol (30 μg/ml). The recombinant plasmid pCW118Ω, after propagation in E. coli HB101, was digested with EcoRI and used to electroporate H. ducreyi 35000HPΩ1; the lspA1 lspA2 double mutant 35000HPΩ12 was selected on chocolate agar containing chloramphenicol (0.6 μg/ml).
The Ωkan and Ωcat cartridges used in the construction of 35000HPΩ12 contain antibiotic resistance cartridges and Ω fragments (17, 23). However, the Ωcat cassette is approximately 3,000 bp and the Ωkan cassette is approximately 2,200 bp, both larger than what one would expect if they contained primarily antibiotic resistance coding sequences. To gain institutional biosafety committee approval for use of 35000HPΩ12, the nucleotide sequence of each cassette was examined. The Ωcat cassette contained a cat ORF of 639 bp and approximately 2,400 bp of flanking sequence. The Ωkan cassette contained a kan ORF of 742 bp and approximately 1,500 bp of flanking sequence. Each cassette contained multiple putative ORFs that could encode short polypeptides but contained no additional antibiotic resistance markers or transposable elements. The mutant was approved for use in human subjects.
All H. ducreyi strains were grown on chocolate agar plates supplemented with 1% IsoVitaleX and incubated at 35°C with 5% CO2, or they were grown in broth consisting of proteose peptone, 50 μg of hemin per ml, 1% IsoVitaleX, and 5% heat-inactivated fetal calf serum, or in supplemented Columbia broth as described elsewhere (38). Where appropriate, the medium was supplemented with kanamycin (30 μg/ml) and chloramphenicol (1.5 μg/ml).
Comparison of the mutant and parent.
Genomic DNAs from strains 35000HP and 35000HPΩ12 were digested with BlpI or EcoRV, and Southern blots were probed with portions of either the lspA1 or lspA2 coding sequence, the Ωkan ORF, or the Ωcat ORF as described previously (39). Lipooligosaccharides (LOS), OMPs, and whole-cell lysates were prepared from 35000HP and 35000HPΩ12 and were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis as described elsewhere (18). In Western blot analyses, whole-cell lysates were probed with MAb 3B9, which binds to PAL; MAb 5A9, which binds to HgbA; MAb 3F12, which binds to a major outer membrane protein (MOMP); MAb 2C7, which binds to MOMP and OmpA2; rabbit polyclonal sera to recombinant DsrA (kindly provided by Christopher Elkins); or rabbit polyclonal sera that bind to Flp1 and Flp2 as described elsewhere (12, 18, 21, 22, 30, 35).
Phagocytosis assays.
The abilities of wild-type and mutant strains of H. ducreyi to inhibit phagocytic activity were measured by using the mouse monocyte-macrophage cell line J774A.1 (TIB-67; American Type Culture Collection, Manassas, Va.) and opsonized fluorescent microspheres as described previously (38). Briefly, bacteria were incubated with the J774A.1 cell monolayers for 1 h at 33°C, and then the microspheres were added. After a 40-min incubation, unbound microspheres were removed by washing. The percentage of phagocytic cells containing fluorescent microspheres was determined by counting at least 100 cells in each sample.
Human challenge protocol.
Healthy adult male and female volunteers over the age of 18 years were recruited for the study. Volunteers gave informed consent for participation and for human immunodeficiency virus serology, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University-Purdue University of Indianapolis. The experimental challenge protocol, preparation and inoculation of the bacteria, calculation of the estimated delivered dose (EDD), clinical observations, and surface cultures were carried out exactly as described previously (2, 5, 31, 32, 36). When a papule was present, the area of erythema was calculated by measuring the greatest dimension vertically and horizontally in millimeters and then multiplying the two measurements. The areas were measured and recorded by a physician who was blinded to the identity of the inoculum used at each site. Volunteers were observed until they reached a clinical end point, defined as either 14 days after inoculation, development of a pustule that was either painful or greater than 4 mm in diameter, or resolution of infection at all sites. Once a clinical end point was achieved, the code was broken, and sites with active disease, if present, were biopsied with punch forceps. The volunteers were then treated with two doses of oral ciprofloxacin as described previously (5, 32).
Statistical analysis.
Comparisons of papule and pustule formation rates for the two strains were performed using a logistic regression model with generalized estimating equations (GEE) to account for the correlation among sites within the same individual, as described previously (29). The GEE sandwich estimate for the standard errors was used to calculate 95% confidence intervals (95% CI) for these rates except when the rate was zero, and the estimate could not be calculated. For those cases (pustule formation rates for the 35000HPΩ12 strain), the exact binomial confidence intervals were calculated based on the number of volunteers rather than the number of sites.
Phenotypes of recovered bacteria.
To confirm that the inocula were correct and that no phenotypic changes occurred during infection, individual colonies from the inocula, surface cultures, and biopsy specimens were picked, suspended in freezing medium, and frozen in 96-well plates. The colonies were scored for susceptibility to chloramphenicol and kanamycin on chloramphenicol- and kanamycin-containing chocolate agar plates. If available, sufficient colonies (n ≥ 30) from an individual specimen were scored so that there was a 95% probability that ≤11% of the colonies would have the incorrect phenotype (2).
RESULTS
Characterization of 35000HPΩ12.
To confirm that 35000HPΩ12 contained only single copies of the appropriate antibiotic resistance cartridges in lspA1 and lspA2, genomic DNAs isolated from 35000HP and 35000HPΩ12 were subjected to Southern blot analysis using probes for lspA1, lspA2, Ωkan, and Ωcat. Southern blotting demonstrated that a single copy of the Ωkan cassette was present in the lspA1 gene and a single copy of the Ωcat cassette was present in the lspA2 gene (data not shown).
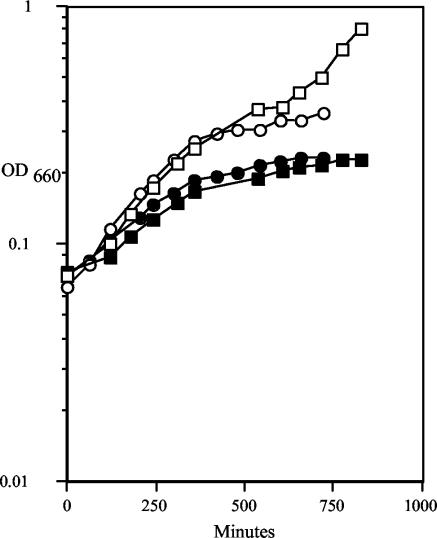
In supplemented Columbia broth, 35000HP and 35000HPΩ12 had similar growth rates and grew to similar extents (data not shown). In the proteose peptone broth approved for preparation of the human challenge inocula, the 35000HPΩ12 mutant had a slower generation time (5 h) than 35000HP (2.5 h) (Fig. 1). The final density of the 35000HPΩ12 culture was also consistently lower than that of the 35000HP culture, as was reported for the previous double mutant, 35000HP.12 (39). Like 35000HP.12, 35000HPΩ12 autoagglutinated more readily than wild-type 35000HP (data not shown).
FIG. 1.
Growth rates of 35000HP (open symbols) and 35000HPΩ12 (solid symbols) measured over time. The graph shows results from two representative experiments, represented by squares and circles, respectively.
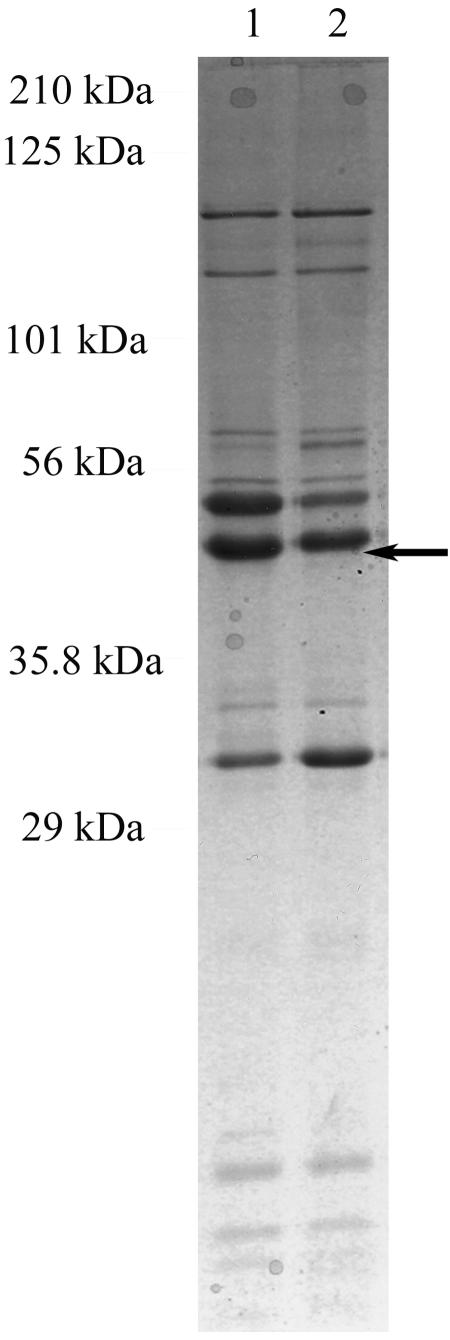
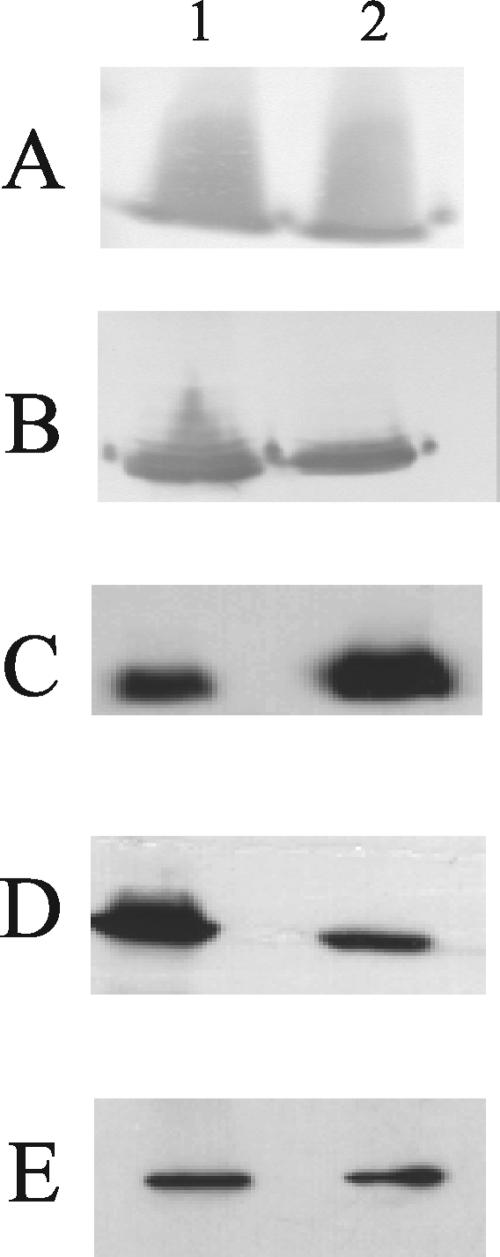
OMP and LOS prepared from 35000HPΩ12 and 35000HP were analyzed by SDS-PAGE. The LOS profiles demonstrated no differences between the mutant and the parent (data not shown). The OMP pattern of 35000HPΩ12 was similar to that of 35000HP, except for decreased expression of a band with an apparent molecular weight of 40 kDa (Fig. 2). The 35000HPΩ12 mutant also expressed a reduced amount of MOMP, as revealed by Western blot analysis with MAbs 3F12 and 2C7 (Fig. 3). Both of these changes had been observed previously for the original 35000HP.12 mutant (39). Western blots of whole-cell lysates were probed with antibodies that bind proteins required for virulence in the human challenge model, including PAL, DsrA, and HgbA (2, 10, 18). The amount of each protein expressed by the mutant was similar to that for the parent (Fig. 3 and data not shown). In addition, the mutant showed slightly increased expression of Flp1 and Flp2 (Fig. 3). Thus, these lspA1 and lspA2 mutations not only abolish expression of LspA1 and LspA2 but also result in detectable perturbations in OMP profiles, as has been described for 35000HP.12 (39).
FIG. 2.
SDS-15% PAGE and Coomassie blue staining of OMPs prepared from 35000HP (lane 1) and 35000HPΩ12 (lane 2). Arrow points to the 40-kDa protein.
FIG. 3.
Western blot-based detection of selected protein antigens expressed by wild-type and mutant H. ducreyi strains. Whole-cell lysates of 35000HP (lanes 1) and 35000HPΩ12 (lanes 2) were probed with the following primary antibodies: the PAL-specific MAb 3B9 (A), the MOMP- and OmpA2-reactive MAb 2C7 (B), a polyclonal antiserum against Flp1 and Flp2 (C), the MOMP-specific MAb 3F12 (D), and the HgbA-specific MAb 5A9 (E).
Phagocytosis assays.
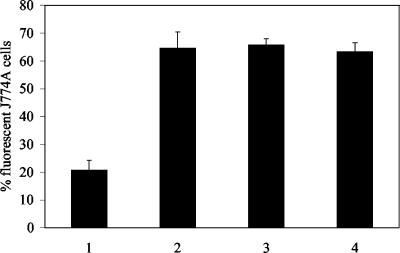
The H. ducreyi wild-type strain 35000HP, the original lspA1 lspA2 mutant 35000HP.12 (39), and 35000HPΩ12 were tested for their abilities to inhibit phagocytosis of opsonized fluorescent microspheres by murine J774A.1 cells, a macrophage-like cell line. This was done to confirm that the new 35000HPΩ12 mutant also lacked the ability to inhibit phagocytic activity. As expected, in contrast to the wild-type parent strain (Fig. 4, lane 1), both the original lspA1 lspA2 mutant 35000HP.12 (Fig. 4, lane 2) and the 35000HPΩ12 mutant (Fig. 4, lane 3) were unable to inhibit phagocytosis of these microspheres.
FIG. 4.
Inhibition of the phagocytic activity of J774A.1 cells by wild-type and mutant strains of H. ducreyi. Phagocytosis of opsonized fluorescent microspheres by J774A.1 cells was determined after incubation of these cells with the H. ducreyi strains as described in Materials and Methods. The percentage of J774A.1 cells containing fluorescent microspheres is shown. Each bar represents the mean and standard error from four independent experiments. Bars: 1, wild-type 35000HP; 2, the lspA1 lspA2 double mutant 35000HP.12; 3, the lspA1 lspA2 double mutant 35000HPΩ12; 4, medium control.
Human inoculation experiments.
Six healthy adults (five females, one male; age range, 18 to 56 years; mean age ± standard deviation, 30 ± 14 years) volunteered for the study. Three individuals (volunteers 236, 237, and 238) were challenged in the first iteration, and three (volunteers 239, 240, and 241) were challenged in the second iteration.
An escalating dose-response study was used to compare the virulence of the mutant and the parent. We initially inoculated three volunteers on both arms. One arm was inoculated at three sites with 35000HP, with an EDD of 85 CFU. The other arm was inoculated at three sites with 35000HPΩ12, with EDDs of 60, 120, and 240. Papules developed at seven of nine sites inoculated with the parent strain and four of nine sites inoculated with the mutant (Table 1). All mutant papules resolved (Table 1). Pustules developed at five of nine sites inoculated with the parent strain and at none of the nine sites inoculated with the mutant (Table 1).
TABLE 1.
Response to inoculation of live H. ducreyi strainsa
| Volunteer no. | Genderb | Days observed | Isolate | No. of initial papules | Final outcome of initial papule
|
|
|---|---|---|---|---|---|---|
| No. of pustules | No. resolved | |||||
| 236 | F | 8 | 35000HP | 1 | 1 | |
| 35000HPΩ12 | 0 | |||||
| 237 | M | 5 | 35000HP | 3 | 2 | 1 |
| 35000HPΩ12 | 1 | 1 | ||||
| 238 | F | 6 | 35000HP | 3 | 3 | |
| 35000HPΩ12 | 3 | 3 | ||||
| 239 | F | 8 | 35000HP | 3 | 3 | |
| 35000HPΩ12 | 3 | 3 | ||||
| 240 | F | 6 | 35000HP | 3 | 3 | |
| 35000HPΩ12 | 3 | 3 | ||||
| 241 | F | 6 | 35000HP | 3 | 3 | |
| 35000HPΩ12 | 3 | 3 | ||||
Volunteers 236, 237, and 238 were inoculated in the first iteration, and volunteers 239, 240, and 241 were inoculated in the second iteration. Each volunteer was inoculated at three sites with 35000HP and at three sites with 35000HPΩ12.
M, male; F, female.
Since the ability of the mutant to cause pustules was impaired, we infected three more volunteers and increased the dose of the mutant. For this group of volunteers, three sites were inoculated with EDDs of 206, 411, and 822 CFU of 35000HPΩ12. Three sites were inoculated with 112 CFU of the parent strain. All parent sites (nine of nine) and all mutant sites (nine of nine) developed papules (Table 1). No mutant sites developed pustules, while three of nine parent sites developed pustules (Table 1). Thus, the ability of 35000HPΩ12 to form pustules was impaired, even at doses 10-fold greater than that of the parent.
The cumulative results for the two iterations showed that papules developed at 88% (95% CI, 76.8 to 99.9%) of sites inoculated with 35000HP and at 72% (95% CI, 44.4 to 99.9%) of sites inoculated with 35000HPΩ12. During the trial, we noted that papules on one arm appeared smaller than papules on the other arm. We calculated the surface area of papule erythema at each site 24 h after inoculation, after the code was broken. The surface areas of mutant papules (mean size, 24.8 mm2) were significantly smaller than those of the parent papules (mean size, 39.1 mm2) at 24 h (P = 0.0002). Thus, the ability of the mutant to initiate infection seemed impaired.
Overall, pustules formed at 8 of 18 (44%; 95% CI, 5.8 to 77.6%) sites inoculated with 35000HP compared to 0 of 18 (0%; 95% CI, 0 to 39.4%) sites inoculated with 35000HPΩ12 (P = 0.009). Thus, the ability of the mutant to form pustules was also impaired compared to that of the parent.
Recovery of bacteria and confirmation of phenotypes.
For the two parent and two mutant broth cultures used to prepare the inocula, all 94 parent colonies and all 93 mutant colonies tested were phenotypically correct (the mutant was chloramphenicol resistant [Cmr] and kanamycin resistant [Kanr]; the parent was Cms and Kans). Of the 18 sites that were inoculated with the parent, 8 (44%) yielded at least one positive surface culture, while 0 of 18 mutant sites yielded a positive surface culture. No mutant sites were biopsied. Eight parent pustules were biopsied. Five were processed for other purposes and were not cultured. Of three parent biopsy specimens that were cultured, two yielded H. ducreyi. All colonies obtained from surface cultures (n = 245) and biopsy specimens (n = 39) from parent sites were phenotypically correct (Cms and Kans). Thus, all colonies tested from the inocula, surface cultures, and biopsy specimens had the expected phenotype.
DISCUSSION
35000HPΩ12 was similar to 35000HP.12 in terms of its phenotype, including detectable perturbations in its OMP profile, tendency to autoagglutinate, and the inability to inhibit phagocytosis in vitro. Although loss of expression of LspA1 and LspA2 has pleiotropic effects, we thought comparing the virulence of 35000HP with that of 35000HPΩ12 in humans was justified, given that evasion of phagocytosis is a major feature of the pathogenesis of experimental H. ducreyi 35000HP infection in humans (6, 27). 35000HPΩ12 formed papules at a rate similar to that of 35000HP, but mutant papules were significantly smaller. H. ducreyi was recovered intermittently from surface cultures of parent-inoculated sites and was not recovered from mutant-inoculated sites. The pustule formation rates were also significantly different. These data indicate that 35000HPΩ12 was unable to survive host defenses as well as 35000HP.
35000HP resists uptake by phagocytic cell lines, polymorphonuclear leukocytes, and macrophages in vitro (1, 38, 41), but 35000HPΩ12 is unable to inhibit phagocytic activity (Fig. 4). In the human challenge model, 35000HP remains extracellular and evades phagocytosis in individuals who form pustules (6). We cannot visualize 35000HP in lesions until the organism has achieved a density of approximately 500 CFU (6). With the parent, this likely occurs 48 h after inoculation (6). Our protocol permits biopsy of sites with active disease only at the clinical end point. All sites inoculated with 35000HPΩ12 resolved clinically. Although we did not biopsy any mutant-inoculated sites and examine the relationship between the mutant and host cells, it seems likely that 35000HPΩ12 was unable to resist uptake and killing in vivo.
35000HPΩ12 grew at a rate similar to that of 35000HP in supplemented Columbia broth but grew at half the rate of the parent in the proteose peptone medium approved for the human challenge studies. Whether 35000HPΩ12 and 35000HP had different growth rates in vivo is not known, and we cannot exclude a decreased growth rate as a mechanism of attenuation. The inocula were prepared from mid-log-phase cultures, and the estimated CFU injected at mutant sites was as much as eightfold higher than that at parent sites. Given the propensity of 35000HPΩ12 to autoagglutinate, the delivered dose may have actually been higher than what we estimated by colony counts, yet 35000HPΩ12 formed no pustules.
Given the pleiotropic effects of elimination of LspA1 and LspA2 expression, we investigated whether other virulence determinants of the organism were affected in the mutant. In previous studies, mutations in the hgbA, pal, dsrA, and tadA genes impaired the ability of H. ducreyi to form pustules in humans (2, 10, 18, 29). 35000HPΩ12 and 35000HP expressed similar amounts of the proteins encoded by these genes. 35000HPΩ12 did express increased amounts of Flp1 and Flp2, but these are unlikely to affect virulence negatively, since the tadA mutant, which still expresses Flp1 and Flp2, the first two gene products of the flp operon that contains tadA, was less virulent in humans than its parent, 35000HP (29). Western blotting also showed decreased expression of MOMP and parental levels of OmpA2, both of which are OmpA homologues (21). A MOMP mutant, which expressed parental levels of OmpA2, was fully virulent in the human infection model (36), and it is unlikely that a change in the expression of MOMP affected the virulence of 35000HPΩ12. National and local biosafety committees have precluded our testing of a mutant complemented in trans in human subjects because they do not want normal skin flora to acquire a plasmid encoding a virulence determinant. We did not test a repaired mutant, because a challenge with a repaired mutant would not address the possibility that the mutant was impaired due to polar effects of the Ωkan or Ωcat insertion in lspA1 and lspA2 on downstream genes or that another mutation had occurred during the repair. With the caveat that we did not test a complemented or repaired mutant, this is the fifth demonstration that a putative virulence factor of H. ducreyi facilitates pustule formation in humans.
The mechanism by which expression of LspA1 and LspA2 confers resistance to phagocytosis is unclear. However, several proteins that share homology with LspA1 and LspA2 are important virulence determinants for other species. A central region of both the LspA1 and LspA2 proteins shares >70% identity with the Haemophilus somnus P76 protein (15). P76 is linked to the ability of H. somnus to resist the complement-mediated bactericidal activity of bovine serum (13, 14). The same region of both LspA1 and LspA2 shares 36% identity with the YopT cytotoxin of Yersinia enterocolitica (26, 42). Interestingly, YopT, a cysteine protease (25), is associated with resistance to phagocytosis (26, 42). The H. ducreyi LspA1 and LspA2 proteins are 43% similar over their N-terminal halves to the Bordetella pertussis filamentous hemagglutinin (FHA), an important adherence factor (16, 24). LspA1 and LspA2 are 45% similar to the Pasteurella multocida proteins PfhB1 and PfhB2, which are essential for virulence in the septicemic mouse model (19).
In summary, these data show that expression of LspA1 and LspA2 is required for full virulence in humans. The mechanism by which the LspA1 and LspA2 proteins exert their inhibitory effect on phagocytosis is not yet clear. Further studies to determine the roles of LspA1 and LspA2 in phagocytosis and virulence expression will be required.
Acknowledgments
This work was supported by grants AI31494 and AI27863 (to S.M.S.), and by grant AI32011 (to E.J.H.), from the National Institute of Allergy and Infectious Diseases (NIAID). The human challenge trials were also supported by grant MO1RR00750 to the GCRC at Indiana University.
We thank Kevin S. McIver for providing pUC4-ΩKm2, Martha Greenwald and Beth Zwickl for enrolling the volunteers, and the volunteers who participated in the trial.
Editor: D. L. Burns
REFERENCES
- 1.Ahmed, H. J., C. Johansson, L. A. Svensson, K. Ahlman, M. Verdrengh, and T. Lagergard. 2002. In vitro and in vivo interactions of Haemophilus ducreyi with host phagocytes. Infect. Immun. 70:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tawfiq, J. A., J. Harezlak, B. P. Katz, and S. M. Spinola. 2000. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex. Transm. Dis. 27:111-114. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq, J. A., K. L. Palmer, C.-Y. Chen, J. C. Haley, B. P. Katz, A. F. Hood, and S. M. Spinola. 1999. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J. Infect. Dis. 179:1283-1287. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer, M. E., and S. M. Spinola. 2000. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 68:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bong, C. T. H., M. E. Bauer, and S. M. Spinola. 2002. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect. 4:1141-1148. [DOI] [PubMed] [Google Scholar]
- 9.Bong, C. T. H., J. Harezlak, B. P. Katz, and S. M. Spinola. 2002. Men are more susceptible to pustule formation than women in the experimental model of Haemophilus ducreyi infection. Sex. Transm. Dis. 29:114-118. [DOI] [PubMed] [Google Scholar]
- 10.Bong, C. T. H., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. A DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2002. Summary of notifiable diseases, United States, 2000. Morb. Mortal. Wkly. Rep. 49:1-100. [PubMed] [Google Scholar]
- 12.Cole, L. E., T. H. Kawula, K. L. Toffer, and C. Elkins. 2002. The Haemophilus ducreyi serum resistance antigen DsrA confers attachment to human keratinocytes. Infect. Immun. 70:6158-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole, S. P., D. G. Guiney, and L. B. Corbeil. 1992. Two linked genes for outer membrane proteins are absent in four non-disease strains of Haemophilus somnus. Mol. Microbiol. 6:1895-1902. [DOI] [PubMed] [Google Scholar]
- 14.Cole, S. P., D. G. Guiney, and L. B. Corbeil. 1993. Molecular analysis of a gene encoding a serum-resistance-associated 76 kDa surface antigen of Haemophilus somnus. J. Gen. Microbiol. 139:2135-2143. [DOI] [PubMed] [Google Scholar]
- 15.Corbeil, L. B., F. D. Bastida-Corcuera, and T. J. Beveridge. 1997. Haemophilus somnus immunoglobulin binding proteins and surface fibrils. Infect. Immun. 65:4250-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domenighini, M., D. A. Relman, C. Capiau, S. Falkow, A. Prugnola, V. Scarlato, and R. Rappuoli. 1990. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol. Microbiol. 4:787-800. [DOI] [PubMed] [Google Scholar]
- 17.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 18.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller, T. E., M. J. Kennedy, and D. E. Lowery. 2000. Identification of Pasteurella multocida virulence genes in a septicemic mouse model using signature-tagged mutagenesis. Microb. Pathog. 29:25-38. [DOI] [PubMed] [Google Scholar]
- 20.Hansen, E. J., J. L. Latimer, S. E. Thomas, M. Helminen, W. L. Albritton, and J. D. Radolf. 1992. Use of electroporation to construct isogenic mutants of Haemophilus ducreyi. J. Bacteriol. 174:5442-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klesney-Tait, J., T. J. Hiltke, I. Maciver, S. M. Spinola, J. D. Radolf, and E. J. Hansen. 1997. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J. Bacteriol. 179:1764-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nika, J. R., J. L. Latimer, C. K. Ward, R. J. Blick, N. J. Wagner, L. D. Cope, G. G. Mahairas, R. S. Munson, Jr., and E. J. Hansen. 2002. Haemophilus ducreyi requires the flp gene cluster for microcolony formation in vitro. Infect. Immun. 70:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Casal, J., M. G. Caparon, and J. R. Scott. 1991. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J. Bacteriol. 173:2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucelotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao, F., P. M. Merritt, Z. Bao, W. Innes, and J. E. Dixon. 2002. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109:575-588. [DOI] [PubMed] [Google Scholar]
- 26.Sorg, I., U. M. Goehring, K. Aktories, and G. Schmidt. 2001. Recombinant Yersinia YopT leads to uncoupling of RhoA-effector interaction. Infect. Immun. 69:7535-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spinola, S. M., M. E. Bauer, and R. S. Munson, Jr. 2002. Immunopathogenesis of Haemophilus ducreyi infection (chancroid). Infect. Immun. 70:1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinola, S. M., C. T. H. Bong, A. L. Faber, K. R. Fortney, S. L. Bennett, C. A. Townsend, B. E. Zwickl, S. D. Billings, T. L. Humphreys, M. E. Bauer, and B. P. Katz. 2003. Differences in host susceptibility to disease progression in the human challenge model of Haemophilus ducreyi infection. Infect. Immun. 71:6658-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinola, S. M., K. R. Fortney, B. P. Katz, J. L. Latimer, J. R. Mock, M. Vakevainen, and E. J. Hansen. 2003. Haemophilus ducreyi requires an intact flp gene cluster for virulence in humans. Infect. Immun. 71:7178-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinola, S. M., G. E. Griffiths, K. L. Shanks, and M. S. Blake. 1993. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect. Immun. 61:1346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinola, S. M., A. Orazi, J. N. Arno, K. Fortney, P. Kotylo, C.-Y. Chen, A. A. Campagnari, and A. F. Hood. 1996. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J. Infect. Dis. 173:394-402. [DOI] [PubMed] [Google Scholar]
- 32.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 169:1146-1150. [DOI] [PubMed] [Google Scholar]
- 33.Steen, R. 2001. On eradicating chancroid. Bull. W. H. O. 79:818-826. [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens, M., L. Cope, J. Radolf, and E. Hansen. 1995. A system for generalized mutagenesis of Haemophilus ducreyi. Infect. Immun. 63:2976-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens, M. K., S. Porcella, J. Klesney-Tait, S. R. Lumbley, S. E. Thomas, M. V. Norgard, J. D. Radolf, and E. J. Hansen. 1996. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect. Immun. 64:1724-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Throm, R. E., J. A. Al-Tawfiq, K. R. Fortney, B. P. Katz, A. F. Hood, E. J. Hansen, and S. M. Spinola. 2000. Evaluation of an isogenic major outer membrane protein-deficient mutant in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:2602-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Throm, R. E., and S. M. Spinola. 2001. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect. Immun. 69:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vakevainen, M., S. Greenberg, and E. J. Hansen. 2003. Inhibition of phagocytosis by Haemophilus ducreyi requires expression of the LspA1 and LspA2 proteins. Infect. Immun. 71:5994-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward, C. K., J. L. Latimer, J. Nika, M. Vakevainen, J. R. Mock, K. Deng, R. J. Blick, and E. J. Hansen. 2003. Mutations in the lspA1 and lspA2 genes of Haemophilus ducreyi affect the virulence of this pathogen in an animal model system. Infect. Immun. 71:2478-2486. (Author's correction, 72:1221, 2004.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, C. K., S. R. Lumbley, J. L. Latimer, L. D. Cope, and E. J. Hansen. 1998. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J. Bacteriol. 180:6013-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood, G. E., S. M. Dutro, and P. A. Totten. 2001. Haemophilus ducreyi inhibits phagocytosis by U-937 cells, a human macrophage-like cell line. Infect. Immun. 69:4726-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]