Abstract
Pneumolysin (PLY) is an important virulence factor of Streptococcus pneumoniae. We examined the ability of three murine monoclonal antibodies (MAbs) to PLY (PLY-4, PLY-5, and PLY-7) to affect the course of pneumococcal pneumonia in mice. The intravenous administration of antibodies PLY-4 and PLY-7 protected the mice from the lethal effect of the purified toxin. Mice treated with PLY-4 before intranasal inoculation of S. pneumoniae type 2 survived longer (median survival time, 100 h) than did untreated animals (median survival time, 60 h) (P < 0.0001). The median survival time for mice treated with a combination of PLY-4 and PLY-7 was 130 h, significantly longer than that for mice given isotype-matched indifferent MAbs (P = 0.0288) or nontreated mice (P = 0.0002). The median survival time for mice treated with a combination of three MAbs was significantly longer (>480 h) than that for mice treated with PLY-5 (48 h; P < 0.0001), PLY-7 (78 h; P = 0.0007), or PLY-4 (100 h; P = 0.0443) alone. Similarly, the survival rate for mice treated with three MAbs (10 of 20 mice) was significantly higher than the survival rate obtained with PLY-5 (1 of 20; P = 0.0033), PLY-4 (2 of 20; P = 0.0138), or PLY-7 (3 of 20; P = 0.0407) alone. These results suggest that anti-PLY MAbs act with a synergistic effect. Furthermore, MAb administration was associated with a significant decrease in bacterial lung colonization and lower frequencies of bacteremia and tissue injury with respect to the results for the control groups.
Streptococcus pneumoniae, a microorganism highly adapted to living in the upper respiratory tract, is nevertheless a human pathogen of major importance, causing high morbidity and mortality worldwide (27). Pneumococci cause serious invasive diseases, mainly pneumonia, meningitis, and bacteremia, in addition to otitis media and acute sinusitis. The 23-valent polysaccharide vaccine fails in groups of immunocompromised persons and children less than 2 years old. The heptavalent conjugate vaccine, which promises to be more effective, does not include some serotypes highly prevalent in Africa, Asia, and Oceania (41). Increasing numbers of antibiotic-resistant strains and serotype shift could lead to the failure of chemotherapy and currently marketed polysaccharide vaccines, respectively. Therefore, efforts have been focused on creating alternative vaccines based on pneumococcal proteins common to all of the serotypes and antigenically more effective (39).
Pneumolysin (PLY) is a 53-kDa toxic protein produced by all of the pneumococcal strains tested so far, independently of their serotype. The ply gene has very limited variability (26). PLY belongs to the family of antigenically related thiol-activated, cholesterol-binding cytolysins secreted by species of five genera of gram-positive bacteria (31). These toxins are able to lyse the plasma membranes of virtually any animal cell. In addition, PLY has additional biological activities implicated in virulence. Direct activation of the classical complement pathway (33) and stimulation of the release of cytokines (19) also contribute to the host inflammatory response.
It has been shown that PLY plays a role in animal models of infection. This toxin has been implicated in mortality from disease and is a protective immunogen in animals (24, 30). Mice immunized with nontoxic versions of PLY were significantly protected from challenge with a range of capsular serotypes (3). Mutations of the ply gene reduced pneumococcal virulence in inoculated mice (7, 8, 23). These observations have led to investigation of the possibility of including this protein in new vaccine formulations.
A panel of monoclonal antibodies (MAbs) to PLY has been raised (15). Several of these antibodies have been demonstrated to have neutralizing effects on the toxin in vitro. PLY-7 and PLY-5 block the binding of PLY to erythrocytes, while PLY-4 inhibits some other stage in the action of this toxin. In this study, we explored the role of these antibodies in neutralizing the in vivo toxic effects of purified PLY and their potential protective effect in pneumococcal pneumonia induced by intranasal challenge of mice with S. pneumoniae type 2.
MATERIALS AND METHODS
Recombinant PLY and antibodies.
The MAbs used in this study were PLY-4, PLY-5, and PLY-7 {mouse anti-PLY immunoglobulin G1 κ chain [IgG1(κ)]}; 1.4G8.66 [mouse anti-pepsinogen C IgG1(κ)] (16) was used as an isotype-matched indifferent MAb. Recombinant PLY and rabbit polyclonal IgG to PLY (anti-PLY IgG) were obtained as already described (13, 14). Purified nonimmune rabbit IgG (NI-IgG) was purchased from Sigma Chemical Co.
Bacteria.
S. pneumoniae D39 type 2 NCTC 7466 was obtained from The Spanish Type Culture Collection (Valencia, Spain). Lyophilized cells were restored in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) and were subcultured twice on blood agar plates (Biomedics, Madrid, Spain). The virulence of the type 2 strain was ensured by intraperitoneal injection of MF-1 mice with 105 CFU and recovery of the strain from the peritoneal cavity during necropsy. This virulent strain was inoculated into 10 ml of BHI broth supplemented with 10% fetal calf serum (Gibco Laboratories, Life Technologies Ltd., Paisley, Scotland) and incubated overnight at 37°C. The culture was diluted to 100 ml and incubated at 37°C until it reached an optical density at 600 nm of 0.15 (108 CFU/ml). This strain was stored as 1-ml aliquots at −70°C in BHI broth. Virulence and CFU were periodically checked. For challenge in mice, frozen suspensions of S. pneumoniae type 2 were thawed, pelleted, and washed three times with 1 ml of Hanks balanced salts solution (HBSS) (Flow Laboratories, Irvine, Scotland) diluted with distilled water. Cells were suspended in 250 μl of HBSS and used immediately.
Hemolysis and in vitro neutralization assays.
Hemolysis and in vitro neutralization assays were performed as already described (15). Briefly, serial dilutions of toxin were incubated with 50 μl of 1.6% sheep erythrocytes in phosphate-buffered saline (PBS) for 30 min at 37°C. The concentration of toxin that lysed 50% of erythrocytes was considered 1 hemolytic unit. For the neutralization test, serial twofold dilutions of MAb were incubated with 2 hemolytic units of PLY for 15 min at 37°C. Fifty microliters of 1.6% sheep erythrocytes in PBS was added, and the plates were incubated at 37°C for 30 min. The minimal concentration of MAb which completely inhibited hemolysis was considered 1 neutralizing unit (NU).
Mice.
MF-1 mice (Oxon, Harland Olac Ltd., Bicester, England) were bred at the University of Oviedo animal house. All animal studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Oviedo. Mice used in this report were 6 to 8 weeks old and weighed 30 ± 3 g (mean and standard deviation).
Mouse toxin lethality model.
The model used for the toxin lethality studies involved injection into the mouse tail vein of various amounts of PLY: 3, 1.5, 0.75, and 0.375 μg diluted in 100 μl of pyrogen-free 10 mM PBS. Groups of 10 mice were injected with each amount of toxin in sterile nonpyrogenic PBS. The same volume of pyrogen-free PBS was injected into control mice. Lethality then was monitored for the subsequent 24 h, and the 50% lethal dose (LD50) was calculated as described by Reed and Muench (34) and Dougherty (17).
Murine infection.
Groups of 20 mice were lightly anesthetized with 3% (vol/vol) halothane over oxygen (3 to 4 liters min−1) by using a methacrylate box connected to Fluovac 240 (Anaesthetizing Systems, Cheshire, England). Mice were intranasally infected with a lethal dose of 5 × 106 CFU of S. pneumoniae D39 type 2 in 50 μl of HBSS applied atraumatically to the tip of the nose and involuntarily inhaled. Deaths were recorded for 20 days.
Passive protection studies.
Groups of 20 mice were injected in the tail vein with 100 μg of PLY-4, PLY-5, PLY-7, or anti-PLY IgG in 200 μl of sterile nonpyrogenic PBS 1 h before purified toxin challenge or intranasal infection with S. pneumoniae D39 type 2. Control mice were injected with 100 μg of indifferent MAb (1.4G8.66), 100 μg of NI-IgG, or 200 μl of PBS. Mice received a second dose of antibodies 36 h after infection, under the same conditions as the first dose. Combinations of 2 MAbs (PLY-4 and PLY-7 at 50 μg each) and 3 MAbs (PLY-4, PLY-7, and PLY-5 at 34 μg each) were administered to mice under the same conditions as single MAbs.
Organ histological analysis and CFU.
Groups of six mice were randomly selected 12, 24, 48, and 72 h postinfection and were deeply anesthetized. Blood was obtained from heart puncture and collected in heparinized tubes. After that, mice were euthanatized by cervical dislocation. The lungs were removed, weighed, and homogenized in an Ultra-Turrax T25 (Janke & Kunkel, IKA-Labortechnik, Staufen, Germany). Quantitative culturing on blood agar of blood and lung homogenates was done to determine the number of CFU. At each time point, one animal per group was randomly chosen for histological analysis. Lungs were removed, fixed in 10% buffered formalin, and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin and viewed by light microscopy. Four sections separated by at least 200 μm were studied per animal.
Statistics.
Differences between the median survival times for groups of mice were analyzed by the Mann-Whitney U test. Differences between the overall survival rates for groups of mice were analyzed by the Fisher exact test. Differences between CFU were analyzed by the two-tailed unpaired t test. The limit of statistical significance was a P value of 0.05.
RESULTS
Neutralization of toxemia in mice by anti-PLY MAbs.
The in vitro neutralizing capacity of anti-PLY MAbs was tested; 1 NU of PLY-4, PLY-5, and PLY-7 was estimated to be equivalent to 4, 125, and 68 ng, respectively.
To assess the potential protective efficacy of these MAbs in vivo, we first determined the intravenous LD50 of purified PLY in mice; it was found to be 1.06 μg. Injection of 2 LD50s of the toxin into the tail vein of mice was, as expected, uniformly fatal in 2 h.
In the first in vivo experiment, preincubation of PLY (2 LD50s) with a neutralizing amount of MAb PLY-7 for 30 min at 37°C was highly protective (100% survival). However, all of the mice injected with the same amount of this MAb 1 h prior to toxin administration died a few minutes later. Therefore, twofold increasing amounts of anti-PLY MAbs were sequentially assayed in groups of 10 mice. Mice given indifferent MAb, anti-PLY IgG, and NI-IgG were used as controls. Table 1 shows the doses of antibodies which protected the mice against toxin lethality. Animals injected with PLY-4, PLY-7, and anti-PLY IgG were protected against death, while mice that received indifferent MAb and NI-IgG did not survive. All of the mice given 6.25 μg (1,500 NUs) of PLY-4 survived toxemia, while 50 μg (735 NUs) of PLY-7 was necessary to obtain a similar effect. No degree of protection was observed with PLY-5 at the tested amount (1,600 NUs).
TABLE 1.
Protective doses of anti-PLY antibodies in a mouse toxin lethality model
| Treatmenta | Dose (μg/mouse) | NUs | No. (%) of mice surviving out of 10 testedb |
|---|---|---|---|
| PLY-4 | 6.25 | 1,500 | 10 (100) |
| 3.125 | 750 | 0 (0) | |
| 1.5 | 375 | 0 (0) | |
| PLY-5 | 200 | 1,600 | 0 (0) |
| 125 | 800 | 0 (0) | |
| 75 | 400 | 0 (0) | |
| PLY-7 | 50 | 735 | 10 (100) |
| 25 | 367 | 0 (0) | |
| 12.5 | 183 | 0 (0) | |
| Indifferent MAb | 125 | 0 | 0 (0) |
| Anti-PLY IgG | 3.125 | NDc | 10 (100) |
| NI-IgG | 125 | 0 | 0 (0) |
Mice were injected in the tail vein 1 h before administration of 2 LD50s of PLY. The indifferent MAb was 1.4G8.66.
Survival was monitored for 48 h following challenge.
ND, not determined.
Observation of a synergistic effect of anti-PLY MAbs in protection against pneumococcal pneumonia.
In order to evaluate the potential protection afforded by passive immunization, anti-PLY MAbs were administered singly or in combination. Groups of mice were intranasally challenged with 5 × 106 CFU of S. pneumoniae type 2. Morbidity and mortality were monitored every 12 h. The first signs of disease were observed between 24 and 36 h; mice exhibited an increased rate of breathing, low mobility, fever, and bristly hair. The median survival time was 60 h, and all of the animals died before 6 days postinfection.
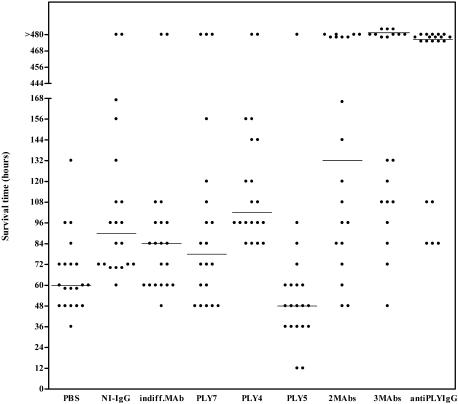
Anti-PLY MAbs (PLY-4, PLY-5, and PLY-7) and indifferent MAb were assayed individually in four groups of 20 mice. Mice treated with PLY-4 lived significantly longer than either PBS-treated mice (P < 0.0001) or indifferent MAb-treated mice (P = 0.0048) (Fig. 1). However, neither PLY-5 nor PLY-7 alone conferred significant protection against infection. Three groups of 20 mice were treated with a combination of antibodies. PLY-4 and PLY-7 were mixed prior to injection into mice. A significant increase in the median survival time was obtained for mice treated with the MAb mixture compared with both indifferent MAb-treated mice (P = 0.028) and PBS-treated mice (P = 0.0002). However, this treatment did not provide better protection than PLY-4 alone (Table 2). Mice that received a combination of three MAbs survived significantly longer than those that received PLY-4, indifferent MAb, or PBS. However, there was no significant difference from the results obtained for mice that received PLY-4 and PLY-7 in combination. Similarly, mice given anti-PLY IgG survived longer than mice given PLY-4, NI-IgG, or PBS. The median survival time for these mice was not significantly different from that for mice given either two MAbs or three MAbs.
FIG. 1.
Antibody-mediated protection of mice by intravenous administration of anti-PLY antibodies in pneumococcal infection. Groups of 20 mice were intranasally infected with 2 LD50s of S. pneumoniae type 2 and treated with antibodies. Each dot represents one mouse. Horizontal lines denote median survival times. indiff., indifferent.
TABLE 2.
| Immunization group |
P value for the following groupc:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PBS | NI-IgG | Indifferent MAb | PLY-7 | PLY-4 | PLY-5 | 2 MAbs | 3 MAbs | Anti-PLY IgG | |
| PBS | 0.0003 | 0.0198 | NS | <0.0001 | NS | 0.0002 | <0.0001 | <0.0001 | |
| NI-IgG | NS | ND | ND | ND | ND | ND | ND | 0.0001 | |
| Indifferent MAb | NS | ND | NS | 0.0048 | NS | 0.0288 | 0.0010 | ND | |
| PLY-7 | NS | ND | NS | ND | ND | ND | 0.0007 | ND | |
| PLY-4 | NS | ND | NS | ND | ND | NS | 0.0443 | 0.0032 | |
| PLY-5 | NS | ND | NS | ND | ND | ND | <0.0001 | ND | |
| 2 MAbs | 0.0033 | ND | NS | NS | NS | 0.0197 | NS | NS | |
| 3 MAbs | 0.0004 | ND | 0.0138 | 0.0407 | 0.0138 | 0.0033 | NS | NS | |
| Anti-PLY IgG | <0.0001 | <0.0001 | 0.0003 | 0.0003 | <0.0001 | <0.0001 | NS | NS | |
Differences in median survival times between groups of mice protected with the indicated antibody were analyzed by using the Mann-Whitney U test.
Differences in survival rates between groups of mice were analyzed by using the Fisher exact test.
2 MAbs: PLY-4 and PLY-7; 3 MAbs: PLY-4, PLY-7, and PLY-5. NS, not significant; ND, not determined.
The highest protection rate was observed with anti-PLY IgG (15 of 20 mice survived), but this rate was not significantly different from that observed after treatment with a combination of either two or three MAbs. The survival rates for mice receiving single MAbs were not significantly different from that of PBS-treated mice (Table 2). Although the overall survival rate for mice that received two MAbs (8 of 20) was numerically higher than that for mice that received PLY-4 (2 of 20) or PLY-7 (3 of 20), the difference did not reach statistical significance. The survival rate for mice that received three MAbs (10 of 20) was significantly higher than that for mice that received single MAbs but was not significantly higher than that for mice that received a combination of two MAbs (8 of 20).
Protection of mice against bacteremia and lung tissue injury by passive immunization with anti-PLY MAbs.
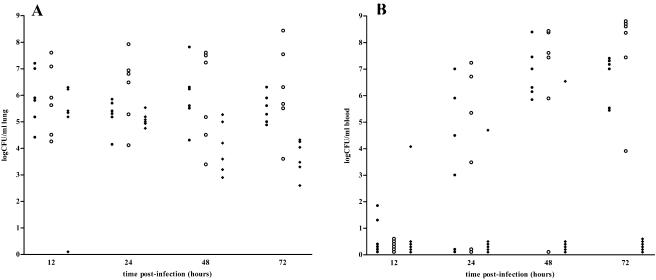
The effects on the number of bacteria in blood and lungs were examined in mice treated with a combination of three MAbs (PLY-4, PLY-5, and PLY-7), indifferent MAb, and PBS. Because the majority of the control mice succumbed to infection by day 3, collection of samples was carried out before 72 h postinfection. Determinations of CFU in lungs of mice intranasally challenged with S. pneumoniae type 2 are shown in Fig. 2A. All of the mice exhibited intrapulmonary colonization with pneumococci throughout the period of experimentation. After 48 h, a significantly lower pneumococcal count was observed in lung homogenates from anti-PLY MAb-treated mice than in those from either PBS-treated mice (P = 0.0106) or indifferent MAb-treated mice (P = 0.0480). After 72 h postinfection, mice that had been treated with anti-PLY MAbs showed lower CFU than either PBS-treated mice (P = 0.0004) or indifferent MAb-treated mice (P = 0.0070). In both indifferent MAb- and PBS-treated mice, after 12 h postinfection, increasing numbers of bacteria were detected in blood samples, and all mice became bacteremic at 48 h postinfection (Fig. 2B). Lower frequencies of bacteremia were seen in MAb-treated mice than in PBS-treated mice at 48 h postinfection (16.6 versus 100%; P = 0.0152) or in both groups of control mice at 72 h postinfection (0 versus 100%; P = 0.0022).
FIG. 2.
(A) Passive immunization with anti-PLY MAbs significantly decreased pneumococcal colonization of lungs with respect to that in PBS-treated and indifferent MAb-treated groups (P = 0.0106 and 0.0480, respectively) at 48 and 72 h postinfection (P = 0.0004 and 0.0070). (B) Anti-PLY MAb treatment decreased the number of bacteremic mice during pneumococcal pneumonia with respect to that in the PBS-treated group at 48 h (P = 0.0152) and with respect to that in both control groups at 72 h (P = 0.0022) postinfection. Symbols: •, PBS-treated mice; ○, mice treated with indifferent MAb; ⧫, mice treated with PLY-4, PLY-5, and PLY-7.
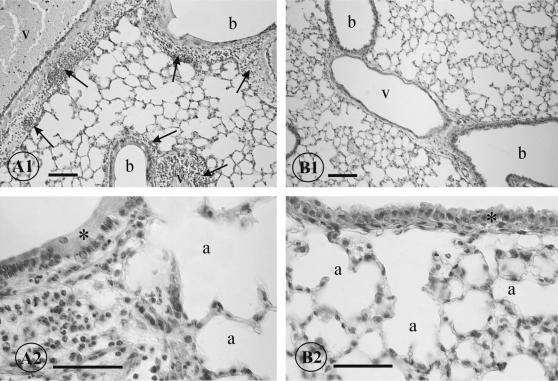
When lung tissues from PBS-treated mice were examined by light microscopy (Fig. 3, A1 and A2), severe inflammation was observed in areas surrounding vessels, bronchi, and bronchioles starting at 24 h postinfection. Numerous polymorphonuclear leukocytes and occasional hemorrhages were detected in these areas. Some intra-alveolar leukocytes were also seen. The inflammatory process increased during the following days. In contrast, most of the lung tissues from mice treated with anti-PLY MAbs appeared histologically normal (Fig. 3, B1 and B2), and only a few inflammatory cells could be detected in the alveoli and perivascular areas.
FIG. 3.
(A1 and A2) Histological appearance of lungs of mice infected with S. pneumoniae type 2. Perivascular and peribronchiolar inflammation (arrows) can be seen at 48 h postinfection (A1). Numerous leukocytes can be seen in this peribronchiolar area. The bronchiolar epithelium (asterisk) is altered, and the alveoli show slight distension (A2). (B1 and B2) Lung sections of mice treated with anti-PLY MAbs at 48 h postinfection. Vessels, bronchioles, and alveoli appear structurally normal, with no signs of acute inflammation (B1). The bronchiolar epithelium (asterisk) and the alveoli do not show any signs of pathological alterations (B2). a, alveolus; b, bronchiole; v, vessel. Calibration bars: A1 and B1, 100 μm; A2 and B2, 50 μm.
DISCUSSION
In this study, we attempted to explore the potential capacity of MAbs to inhibit the progression of pneumococcal infection in a mouse intranasal challenge model. The first experimental approach was to demonstrate that anti-PLY MAbs maintained toxin-neutralizing capacity in vivo in a mouse toxemia assay. We showed that PLY-4 and PLY-7 neutralized the lethal effect of purified toxin, while PLY-5 had no apparent effect. The reason for the different behaviors of the anti-PLY MAbs when given in doses corresponding to the same NUs is unclear. Several studies with site-specific mutants demonstrated that both cytotoxicity and complement activation caused by PLY contribute to pathogenesis (4). The mutation Asp385→Asn, located in domain 4, abolished complement-activating activity without changing hemolytic activity. This residue does not belong to the amino acid stretches recognized by anti-PLY MAbs (15, 20, 38). Nevertheless, the PLY-5 and PLY-7 epitopes also belong to domain 4. We cannot exclude the possibility that the binding of some of the MAbs to PLY interferes with classical complement pathway activation. Furthermore, it has been demonstrated that PLY has additional toxic properties which are not eliminated by the neutralization of cytolytic activity (7). Because anti-PLY MAbs are isotype matched, our observations suggest that activities involved in toxic shock are neutralized by PLY-4 and PLY-7 but not by PLY-5.
The second experimental approach was to examine the neutralizing potential of MAbs in mice intranasally challenged with S. pneumoniae (36). PLY-4 significantly prolonged the median survival time of animals with respect to control animals. A combination of two or three MAbs increased the median survival time and the number of mice that survived the period of experimentation with respect to both PBS-treated and indifferent MAb-treated mice. These results were significantly better than those obtained for groups of mice treated with single MAbs, suggesting that PLY-4, PLY-5, and PLY-7 synergistically neutralize the toxin effect. These data could be related to the previously described positive modulation induced by PLY-7 on the binding of PLY-5 (15). Both MAbs recognize epitopes that are localized in domain 4, at the carboxyl terminus of PLY, and that are involved in cellular membrane binding. PLY-5 recognizes the undecapeptide motif, a conserved hydrophobic Trp-rich motif shared by all thiol-activated cytolysins (20). The PLY-7 epitope would conform to one of the loops at the bottom of domain 4, on the side opposite that of the PLY-5 epitope (38). It is possible that PLY-7 alters the conformation of the native molecule in such a way as to assist in the binding of PLY-5 (15). Surprisingly, the most effective MAb, PLY-4, recognizes a molecular stretch in domain 1 which does not interfere with cell binding but which could be involved in the oligomerization process.
A higher survival rate was observed in the anti-PLY IgG-treated group, as might be expected if some property other than lytic activity but also involved in the host inflammatory response had been neutralized (19, 33). Studies of passive protection in mice with polyclonal antibodies against PLY were recently described (12, 28, 29); various survival rates were observed and could be attributed to different pneumococcal serotypes, mouse strains, and routes of inoculation used (1, 11). It is conceivable that the administration of a cocktail of MAbs targeted against different regions of PLY could be as effective as anti-PLY polyclonal antibodies, and an advantage of using the former is the possibility of genetic manipulation.
Intranasal inoculation of pneumococci mimics the natural route of infection, and the invasion of lung tissue and the breakdown of mucosal and capillary barriers can be studied. Lungs of mice infected with S. pneumoniae type 2 showed an acute inflammation process, and bacteremia started 24 h after challenge. These observations are essentially similar to those previously described (6, 22, 23). Pneumococcal tissue colonization was observed over the period of experimentation in the lungs of mice treated with anti-PLY-MAbs. In contrast, histological examination showed low levels of inflammatory responses in most of the lungs. It has been demonstrated that PLY causes damage to the alveolar lining (18) and is the main trigger of inflammation (22). The administration of antibodies to PLY would neutralize the toxic and inflammatory activities of this bacterial component. Therefore, it would be interesting to determine the potential capacity of such MAbs for modulating inflammatory sequelae of other pneumococcal infections, such as meningitis and otitis media (10).
An interesting observation was the significant decrease in lung bacterial counts in anti-PLY MAb-treated mice relative to control mice after 48 h postinfection. Since anti-PLY antibodies would not be expected to promote opsophagocytic clearance, this finding could be explained by the capacity for an inhibitory effect of PLY on polymorphonuclear phagocytic function (32).
Anti-PLY MAb treatment was associated with a smaller number of mice with bacteremia relative to the results for control mice. Because the survival rate in this group was 50% and all mice were nonbacteremic at 72 h postinfection, we cannot rule out the possibility that some nonbacteremic mice could have succumbed to the infection. Previous passive immunization experiments with antibodies to PLY showed effectiveness in avoiding bacteremia (28). Bacteremia is considered a negative prognostic factor in life-threatening situations. Because anti-PLY MAbs restrain lung colonization and bacteremia, the administration of derivatives adapted for human use could have therapeutic utility, particularly in immunocompromised patients.
Passive protection with antibodies against several pneumococcal antigens has been described. Anticapsular antibodies protect mice against bacteremia and clear pneumococci from the lungs due to their opsonophagocytic capacity, but they are serotype dependent (21, 37). Increased protection against lethal pneumococcal infection was observed after passive transfer of sera from mice that were immunized with pneumococcal surface protein A (PspA) (5, 9, 25, 29). Polyclonal serum raised against a mixture of choline-binding proteins protected mice against challenge with the strain from which these proteins were derived. CbpA seems to be the main protective antigen (35). Antisera raised against other pneumococcal surface proteins, such as Sp36, Sp46 (40), and PhtA (2), significantly protected mice from challenge with some pneumococcal strains. Sera against cellular membrane components PiuA and PiaA significantly delayed mortality (12).
Despite the previous body of evidence supporting the role of PLY in pneumococcal pathogenesis, to our knowledge, this is the first description of passive protection afforded by MAbs to this toxin. Future studies should include an evaluation of the protection afforded by a combination of MAbs targeted against determined regions of PLY when given after an infection is established.
Acknowledgments
This work was supported by CICYT BIO92-0755 and FICYT PB-MED01-08. M.D.C.-C. was supported financially by Foundation Universidad de Oviedo.
We thank T. Hernández for invaluable assistance with histological studies and A. F. Caldevilla and S. Melon for statistical analysis.
Editor: J. N. Weiser
REFERENCES
- 1.Aaberge, I. S., J. Eng, G. Lermark, and M. Lovik. 1995. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of experimental bacterial inoculum. Microb. Pathog. 18:141-152. [DOI] [PubMed] [Google Scholar]
- 2.Adamou, J. E., J. H. Heinrichs, A. L. Erwin, W. Walsh, T. Gayle, M. Dormitzer, R. Dagan, Y. A. Brewah, P. Barren, R. Lathigra, S. Langermann, S. Koenig, and S. Johnson. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander, J. E., R. A. Lock, C. C. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24:167-174. [DOI] [PubMed] [Google Scholar]
- 5.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergeron, Y., N. Ouellet, A. M. Deslauriers, M. Simard, M. Oliver, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosarge, J. R., J. M. Watt, D. O. McDaniel, E. Swiatlo, and L. S. McDaniel. 2001. Genetic immunization with the region encoding the α-helical domain of PspA elicits protective immunity against Streptococcus pneumoniae. Infect. Immun. 69:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, J. S., A. D. Ogunniyi, M. C. Woodrow, D. W. Holden, and J. C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cima-Cabal, M. D., F. Vázquez, J. R. de los Toyos, and F. J. Méndez. 1999. Rapid and reliable identification of Streptococcus pneumoniae isolates by pneumolysin-mediated agglutination. J. Clin. Microbiol. 37:1964-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cima-Cabal, M. D. 1999. PhD thesis. University of Oviedo, Oviedo, Spain.
- 15.de los Toyos, J. R., F. J. Méndez, J. F. Aparicio, F. Vázquez, M. M. García-Suárez, A. Fleites, C. Hardisson, P. J. Morgan, P. W. Andrew, and T. J. Mitchell. 1996. Functional analysis of pneumolysin by use of monoclonal antibodies. Infect. Immun. 64:480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamandis, E. P., S. Nadkarni, B. Bhaumik, A. Abdelrahman, D. N. Melegos, G. Borchert, M. H. Black, M. Alonso, A. Salas, J. R. de los Toyos, A. Sampedro, and C. Lopez-Otin. 1997. Immunofluorometric assay of pepsinogen C and preliminary clinical applications. Clin. Chem. 43:1365-1371. [PubMed] [Google Scholar]
- 17.Dougherty, R. H. 1974. Animal virus titration techniques, p. 169-223. In R. J. C. Harris (ed.), Techniques in experimental virology. Academic Press, Inc., New York, N.Y.
- 18.Feldman, C., R. Anderson, R. Cockeran, T. Mitchell, P. Cole, and R. Wilson. 2002. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir. Med. 98:580-585. [DOI] [PubMed] [Google Scholar]
- 19.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimules production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs, T., M. D. Cima-Cabal, A. Darji, F. J. Méndez, F. Vázquez, A. A. C. Jacobs, Y. Shimada, Y. Ohno-Iwashita, S. Weiss, and J. R. de los Toyos. 1999. The conserved undecapeptide shared by thiol-activated cytolysins is involved in membrane binding. FEBS Lett. 459:463-466. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, S. E., L. Rubin, S. Romero-Steiner, J. K. Dykes, L. B. Pais, A. Rizvi, E. Ades, and G. M. Carlone. 1999. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia of Streptococcus pneumoniae. J. Infect. Dis. 180:133-140. [DOI] [PubMed] [Google Scholar]
- 22.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadioglu, A., S. Taylor, F. Iannelli, G. Pozzi, T. J. Mitchell, and P. W. Andrew. 2002. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect. Immun. 70:2886-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lock, R. A., D. Hansman, and J. C. Paton. 1992. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection Streptococcus pneumoniae. Microb. Pathog. 12:137-143. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel, L. S., G. Scott, J. F. Kearney, and D. E. Briles. 1984. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J. Exp. Med. 160:386-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, T. J., F. Méndez, J. C. Paton, P. W. Andrew, and G. J. Boulnois. 1990. Comparison of pneumolysin genes and proteins from Streptococcus pneumoniae types 1 and 2. Nucleic Acids Res. 18:4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musher, D. M. 1999. Streptococcus pneumoniae, p. 2129-2156. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed., vol. 2. Churchill Livingstone, New York, N.Y. [Google Scholar]
- 28.Musher, D. M., H. M. Phan, and R. Baughn. 2001. Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J. Infect. Dis. 183:827-830. [DOI] [PubMed] [Google Scholar]
- 29.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogunniyi, A. D., M. C. Woodrow, J. T. Poolman, and J. C. Paton. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer, M. 2001. The family of thiol-activated, cholesterol-binding cytolysins. Toxicon 39:1681-1689. [DOI] [PubMed] [Google Scholar]
- 32.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43:1085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 35.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 36.Saeland, E., G. Vidarsson, and I. Jonsdottir. 2000. Pneumococcal pneumonia and bacteremia model in mice for the analysis of protective antibodies. Microb. Pathog. 29:81-91. [DOI] [PubMed] [Google Scholar]
- 37.Saeland, E., H. Jakobsen, G. Ingolfsdottir, S. T. Sigurdardottir, and I. Jonsdottir. 2001. Serum samples from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by Streptococcus pneumoniae serotypes 6A and 6B. J. Infect. Dis. 183:253-260. [DOI] [PubMed] [Google Scholar]
- 38.Suárez-Álvarez, B., M. M. García-Suárez, F. J. Méndez, and J. R. de los Toyos. 2003. Characterisation of mouse monoclonal antibodies for pneumolysin: fine epitope mapping and V gene usage. Immunol. Lett. 88:227-239. [DOI] [PubMed] [Google Scholar]
- 39.Swiatlo, E., and D. Ware. 2003. Novel vaccine strategies with protein antigens of Streptococcus pneumoniae. FEMS Immunol. Med. Microbiol. 38:1-7. [DOI] [PubMed] [Google Scholar]
- 40.Wizemann, T., J. H. Heinrichs, J. E. Adamou, A. L. Erwin, C. Kunsch, G. H. Choi, S. C. Barash, C. A. Rosen, H. R. Masure, E. Tuomanen, A. Gayle, Y. A. Brewah, W. Walsh, P. Barren, R. Lathigra, M. Hanson, S. Langermann, S. Johnson, and S. Koenig. 2001. Use of a whole-genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect. Immun. 69:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wuorimaa, T., and H. Käyhty. 2002. Current state of pneumococcal vaccines. Scand. J. Immunol. 56:111-129. [DOI] [PubMed] [Google Scholar]