Abstract
Background:
Drugs administration as a pretreatment regiment before ICSI cycle in PCOs patients could enhance the success rate.
Objectives:
The aim of this study was to compare the effectiveness of metformin with Simvastatin in patients with polycystic ovary syndrome (PCOs) candidates for intra-cytoplasmic sperm injection (ICSI) before starting the cycle.
Patients and Methods:
In this prospective, double blind, randomized clinical trial the efficacy of these drugs was evaluated in 40 women with PCO syndrome (20 patients in each group; A: simvastatin and B: metformin) candidates for ICSI. In the both groups, metformin and simvastatin administrated for eight weeks before starting the ICSI cycle. Endocrine, metabolic and clinical parameters were measured before and after drug therapy; also, the results of ICSI cycle evaluated in the both groups.
Results:
Both drugs improved hirsutism score significantly, but simvastatin better than metformin (Group A, 24.5 ± 3.6 P: 0.0001 VS Group B, 22.9 ± 5.9 P: 0.003). The reduction in body mass index (BMI) was not significant in the groups. Simvastatin reduced some biochemical parameters such as FSH, LH, testosterone, total cholesterol, LDL and increased HDL level significantly, whereas metformin decreased FSH, TG, testosterone and total cholesterol significantly. Overall, respectively 35% and 30% of patients treated with metformin and Simvastatin became pregnant. There was no significant difference between the effects of these two drugs on ICSI cycle results like oocyte in meiosis2 (M2) phase (1.35 ± 1.6 vs. 2 ± 3.87, P value: 0.4) and the number of Grade A, embryo (1.2 ± 1.3 vs. 1.1 ± 1.4, P value: 0.7).
Conclusions:
Simvastatin effectively improved hyperandrogenism signs and symptoms in patients with PCO, but this effect as a pretreatment regiment was not more expressive than metformin in ICSI cycle outcome.
Keywords: Metformin, Simvastatin, Polycystic Ovary Syndrome, Intracytoplasmic Sperm Injection
1. Background
Polycystic ovary syndrome (PCOS) is a heterogeneous and complex endocrine disorder associated with hyperandrogenism and insulin resistance signs and symptoms such as obesity, abnormal glucose tolerance test or type 2 diabetes, abnormal blood lipid profile, high blood pressure and cardiovascular disease (1). This disorder is prevalent and affects 5 - 10% of women of reproductive age (2) and causes infertility in 75% of these women due to chronic anovulation (3).
Drug therapy is one of the most common approaches to control this disease. Many drugs were used as a treatment for PCOs or control its consequences, including metformin (4), Simvastatin (5), Atorvastatin (6), N - acetyl-cysteine (NAC) (7) and Flutamide (8). In general, all of these drugs have positive effects in improving hyperandrogenic symptoms and regulation of menstrual cycles, but simvastatin and metformin have been more commonly used than others for treatment and research purposes. In one study, long-term treatment with simvastatin was more effective than metformin for control of hyperandrogenism sign and increased menstrual regulation in women with PCO (9).
There are several published studies regarding the effectiveness of statins administration in women with PCO (10, 11), but in comparison with metformin there is no published research about drug administration before or during ICSI cycle.
Simvastatin a drug from statins group, inhibits HMG-COA reductase and reduces cholesterol production, increases liver LDL receptor sensitivity and HDL level, meanwhile decreases TG level in blood circulation (12). Moreover, statins prevent theca-interstitial cell proliferation in ovaries and therefore reduce total androgen production (13). Metformin as a Biguanid drug, blocks glucose synthesis in liver and enhances peripheral sensitivity to insulin, which finally decreases androgen level in patients with PCO and enhances the chance of pregnancy (14).
According to latest Cochrane published study about metformin treatment before and during ICSI cycle, live birth or pregnancy rate was not improved (15); the Cochrane database announced that although simvastatin improved lipid profile and hyperandrogenism sign, it had no significant effect on pregnancy chance (16), of course in natural cycle.
2. Objectives
We decided to compare the efficacy of both drugs as a pretreatment regiment before ICSI cycle in patients with PCO.
3. Patients and Methods
In this prospective, double blind, randomized clinical trial, 40 women with PCOS and a history of infertility referring for first ICSI cycle to Imam Khomeini Hospital affiliated with Ahvaz Jundishapur University Of Medical Sciences (AJUMS) were selected based on closed enveloped randomization method from December 2010 to November 2012 to receive simvastatin (group A) or metformin (group B). According to the variance of previous studies, the sample size was calculated as 20 patients for each group.
In accordance with the guidelines of the Declaration of Helsinki, after approval of university Ethical committee (ETH-3251), the study was submitted on Iranian health ministry website for clinical trials (www.IRRCT.IR). (Registration number: IRCT 201202138994N).
An informed constant was obtained from patients before initiation of study. PCOS considered as presence of two of the following criteria including menstrual irregularities (oligomenorrhea or amenorrhea), clinical and laboratory findings of hyperandrogenism and polycystic ovaries on ultrasound evaluation. Besides, inclusion criteria were aged 20-35 years, a history of infertility for at least two years (either primary or secondary), normal TSH and prolactin and candidates for the first cycle of ICSI. Patients with other causes of infertility, including tubal factors, endometriosis and male factor infertility were excluded. Patients who used metformin, lipid control drugs, clomiphene, letrozole or doing IUI (Intrauterine insemination) in the past 3-months before study or having medical problems such as kidney or liver diseases, diabetes or a history of elevated blood lipids were excluded.
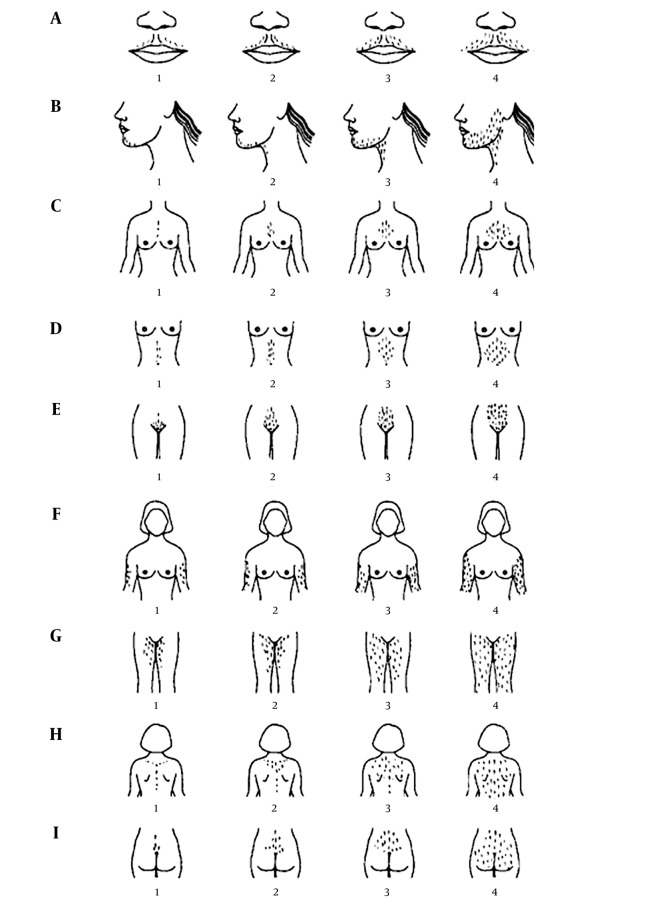
Demographic data obtained from both groups. Body mass index (BMI) and the score of hirsutism were assessed according to the Ferriman–Gallwey scoring system (Figure 1). In this system, hair growth in a male pattern on a woman shown in four different degrees of severity in 11 different body parts, namely the upper lip, chin, chest, upper back, lower back, upper abdomen, lower abdomen, arm, forearm, thigh and lower leg (17). On the third day of menstruation, all patients were evaluated for total testosterone, LH, FSH, TG, total Cholesterol ,LDL, HDL, FBS, GTT with 75 grams glucose (blood sugar measured one and two hours after drinking it), Prolactin and TSH.
Figure 1. The Ferriman–Gallwey Scoring System.
After getting the first blood sample for biochemical evaluation, in group A, patients received simvastatin 20 mg daily (Osvah Pharmaceutical Company, Iran) plus low dose oral contraceptive pill (OCP) daily for eight weeks. In group B, patients received metformin 500 mg TDS (Osvah Pharmaceutical Company, Iran) plus low dose OCP daily for eight weeks. Then, all patients were again evaluated for previous biochemical parameters (except prolactin and TSH) and BMI measurement and hirsutism scoring. Patients were enrolled in the ICSI protocol according to the long protocol; from the day of twenty-one of the second cycle, subcutaneous superfact (0.5 mg daily, Triptrolein, Hoechst, Frankfort, Germany) injection was started. After menstruation on the third day of cycle, both drugs (simvastatin and metformin) were discontinued and after vaginal sonography control and reducing superfact to 0.25 mg daily, induction of ovulation with folliotropin alfa (Gonal-f , Merck, Serono. Germany) started at dosage of 150 IU per day. Ovarian response was examined by transvaginal sonography six days later and Gonal-f dosage adjusted according to the patient response; when at least three follicles with a size of 18mm detected, human chorionic gonadotropin (HCG) 10000 units (Profasi. Serono. Switzerland) was injected intramuscularly and ovarian puncture was performed 36 - 40 hours later.
After ovarian puncture, the number of MII oocytes and after sperm injection, on the day 3, the number of embryos of grade A was recorded. The incidence of ovarian hyperstimulation syndrome (OHSS) was reported in the both groups. Seventy-two hours after ovarian puncture, transfer of the fetus was performed. Then, support of luteal phase was started with vaginal suppository of micronized progesterone 400 mg twice daily. The pregnancy outcome with β-HCG blood test was investigated two weeks after embryo transfer. The first ultrasound was performed five weeks after embryo transfer in cases of pregnancy positive test. Cases of abortion, ectopic pregnancy (EP), ongoing pregnancy (passing 14 weeks of gestational age) and negative pregnancy were detected.
Statistical analysis was performed using SPSS 17.0 for Windowse. All data had normal variation. Student’s t-test for unpaired data and sample t-test for paired information were used. Also Chi-Square and Fisher’s tests were used to assess frequency distribution and clinical outcome. Regression and ANOVA tests were used for comparison between the groups. For GTT result interpretation, online software Area under the curve (AUC) calculator was used.
P value < 0.05 was considered as significant and the power of study was 80%.
4. Results
Demographic information in the both groups described in Table 1. In each group, 75% of patients had primary infertility and 25% secondary infertility.
Table 1. Demographic Dataa.
| Variable | Group A, Simvastatin | Group B, Metformin | P Valueb |
|---|---|---|---|
| Age, y | 27.3 ± 3.2 | 27 ± 4.7 | 0.8 |
| BMI, Kg/m 2 | 31.5 ± 5.3 | 32.2 ± 4 | 0.6 |
| Duration of infertility, y | 6.3 ± 4.6 | 5.7 ± 3.1 | 0.6 |
aData are presented as mean ± SD.
bP value < 0.05 significant.
75% of patients in group A, were obese (BMI > 30 kg/m2) and in group B, 65%.
After eight weeks treatment in both groups, reduction of mean hirsutism score was significant, although it was more meaningful in simvastatin group (Table 2); this reduction in mean BMI level was not significant (Table 3).
Table 2. Differences in Mean ± SD Hirsutism Score Before and After the Treatmenta.
| Before Treatment | After Treatment | P Valueb | |
|---|---|---|---|
| Group A | 0.0001 | ||
| Simvastatin | 27.6 ± 4.3 | 24.5 ± 3.6 | |
| Group B | |||
| Metformin | 23.8 ± 5.6 | 22.9 ± 5.4 | 0.003 |
aData are presented as mean ± SD.
bP value < 0.05 significant.
Table 3. Differences in BMI Level (Mean ± SD) Before and After the Treatmenta.
| Before treatment | After Treatment | P Valueb | |
|---|---|---|---|
| Group A | 0.65 | ||
| Simvastatin | 31.5 ± 5.3 | 31.7 ± 5.2 | |
| Group B | |||
| Metformin | 32.2 ± 4 | 32.3 ± 4 | 0.60 |
aData are presented as mean ± SD.
bP value < 0.05 significant.
Biochemical parameters before the treatment in the both groups were not different (Table 4). After eight weeks in group A receiving simvastatin, testosterone, FSH, LH, Cholesterol (Chol) and LDL significantly decreased; meanwhile HDL level elevated. In group B, after metformin administration the level of FSH, Testosterone, TG and Chol reduced meaningfully (Table 5).
Table 4. Biochemical Parameters (Mean ± SD) in the Both Groups Before Treatmenta.
| Variable | Group A | Group B | P Value |
|---|---|---|---|
| Simvastatin | Metformin | ||
| FSH, IU/liter | 7.86 ± 5.33 | 5.80 ± 2.33 | 0.123 |
| LH, IU/liter | 7.07 ± 5.81 | 6.36 ± 3.26 | 0.639 |
| Testosterone, ng/mL | 0.582 ± 0.547 | 612 ± 418 | 0.849 |
| Triglycerides, mg/dL | 145 ± 92.8 | 149 ± 57.3 | 0.856 |
| Cholesterol, mg/dL | 176 ± 43.3 | 179 ± 41.9 | 0.83 |
| LDL, mg/dL | 105.4 ± 33.7 | 95.8 ± 27.2 | 0.335 |
| HDL, mg/dL | 43.3 ± 10.3 | 43.2 ± 7.51 | 0.983 |
| FBS, mg/dL | 94.6 ± 18.8 | 95.15 ± 15.8 | 0.925 |
aData are presented as mean ± SD.
Table 5. Differences in Biochemical Parameters in the Both Groups Before and After the Treatmenta.
| Group A, Simvastatin | P Value | Group B, Metformin | P Value | |
|---|---|---|---|---|
| FSH, IU/L | 0.007b | 0.004b | ||
| Before | 7.8 ± 5.3 | 5.8 ± 2.3 | ||
| After | 6.4 ±3.8 | 4.1 ± 1.5 | ||
| LH, IU/L | 0.01b | 0.1 | ||
| Before | 7 ± 5.8 | 6.3 ± 3.2 | ||
| After | 5.4 ± 3.8 | 5.3 ± 3.6 | ||
| Testosterone, ng/mL | 0.013b | 0.001b | ||
| Before | 0.58 ± 0.5 | 0.61 ± 0.41 | ||
| After | 0.32 ± 0.1 | 0.42 ± 0.22 | ||
| TG, mg/dL | 0.259 | 0.005b | ||
| Before | 145 ± 92.8 | 149 ± 57.3 | ||
| After | 116 ± 52.1 | 119 ± 52.4 | ||
| Total Cholesterol, mg/dL | 0.001b | 0.044b | ||
| Before | 176 ± 43.3 | 179 ± 41.9 | ||
| After | 144 ± 36.9 | 163 ± 30.9 | ||
| LDL, mg/dL | 0.004b | 0.269 | ||
| Before | 105 ± 33.7 | 95 ± 27.2 | ||
| After | 87 ± 21.5 | 91 ± 23.1 | ||
| HDL, mg/dL | 0.001b | 0.249 | ||
| Before | 43 ± 10.3 | 43 ± 7.5 | ||
| After | 53 ± 12.8 | 45 ± 8.1 | ||
| FBS, mg/dL | 0.738 | 0.156 | ||
| Before | 94 ± 18.8 | 95 ± 15.8 | ||
| After | 93 ± 14.6 | 89 ± 9.4 |
aData are presented as mean ± SD.
bP value < 0.05 significant.
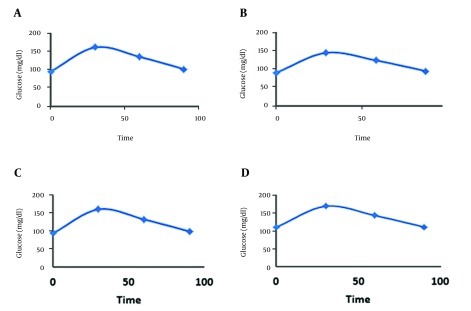
The mean values of three blood sugar measurements (FBS/1 hour/2 hours) before starting the treatment were not different between the two groups (group A: 12208 ± 1658.03 mg/dL vs. group B, 12302 ± 1789.87 mg/dL) (P value 0.8). After the treatment, ROC showed improvement area under curve (AUC) in glucose tolerance test in the both groups; although there were no significant differences between the two groups (P value 0.11) (Figure 2).
Figure 2. Glucose Tolerance Graphs.
A, Before the treatment in Metformin; and C, Simvastatin; B, after the treatment in Metformin; and D, Simvastatin.
After ovarian puncture, the number of oocytes in phase2 meiosis (M2) in simvastatin treated group was 1.35 ± 1.6, and in metformin treated group 2 ± 3.87 (P value 0.48). On the third day after ovarian puncture, the number of grade A embryos in group A was 1.2 ± 1.3 versus 1.1 ± 1.4 in group B (P value 0.79), which was not significantly different.
In the both groups, we had no case of ovarian hyperstimulation syndrome (OHSS).
The pregnancy rate in group A was six cases (30%) ongoing and seven cases in group B (35%). One abortion in each group happened (P value 0.78)
5. Discussion
To the best of our knowledge, this is the first published study comparing simvastatin and metformin as a pretreatment regiment for ICSI cycle in PCOs women and there is one study regarding the effectiveness of simvastatin before IVF (18).
Women with PCOs have enlarged ovaries with an increased number of small follicles with more theca cells, which are androgen-producing cells. It is also clear that theca cells of women with PCOS produce higher amount of androgens compared with the theca cells of normal women. Increased expression of genes involved in androgen production, such as CYP11A and CYP17 has been observed in these cells (9). Metformin-induced reduction in testosterone is not only because of decreasing size of the ovarian theca cells, but also due to the direct action of metformin. Studies have shown that metformin inhibits the production of testosterone and androstenedione in the cultured theca cells. Metformin also decreased expression of steroidogenic acute regulatory (StAR) protein and 17α-hydroxylase/17, 20-desmolase (CYP17) (19). Studies have shown that statins reduce cell proliferation, increase apoptosis and inhibit producing testosterone in humans and rat theca cells (10, 13).
The positive effects of Simvastatin in reducing hirsutism and testosterone have been confirmed by other researchers (5); this reduction in testosterone level, but not the hirsutism score after metformin administration has been reported, but in our study BMI decreased after six months therapy (20).
In another study, after six months of treatment, Simvastatin improved hyperandrogenism parameters like total testosterone, hirsutism score, total cholesterol, LDL and BMI better than metformin (9). Similarly in contrast to our study, both drugs decreased BMI.
Our study indicated that Simvastatin is more effective to improve lipid profile than metformin. Lipid profile analysis showed that Simvastatin significantly reduced LDL and total cholesterol, and increased HDL. This effect reflects direct competitive prevention of the controlling step of cholesterol production by inhibiting 3-hydroxy3methylglutaryl coenzyme A reductase (21). However, metformin significantly decreased triglyceride and LDL by its anti-lipolytic effects, reducing circulating free fatty acid concentrations and therefor the level of triglycerides (22, 23), although this reduction in LDL level in our study was not significant.
In the present study, LH and FSH hormones were significantly lower in Simvastatin group after treatment, but in the metformin group only FSH concentrations decreased. In women with PCO, reduction in FSH level reflects decreased GnRH frequency secretion due to reduction of hypothalamic dopamine and opioid production and from negative feedback of chronically elevated estrone concentration (increased peripheral aromatization of elevated androstenedione) and decreased progesterone level (chronic anovulation) (24). In contrast to FSH, abnormal LH production is due to increased pulse amplitude and frequency secondary to altered pulsatile GnRH secretion; this elevated LH led to ovarian hyperandrogenism (25).
Metformin directly affected gonadotropin-secreting cells and reduced LH production and elevated FSH level by regulating FSH gene expression (26). Simvastatin could reduce LH level, but had no effect on FSH (11); this impressive effect for normalized gonadotropins level improved in other study (27). Simvastatin reduced LH level by decreasing ovarian testosterone production secondary to inhibition of theca-interstitial proliferation. In our study, reduction in LH level was seen in the both groups, although it was more significant in group A; we could not find any explanation about reduced level of FSH in the both groups.
In conclusion, according to only published study about simvastatin administration before IVF cycle (18) and in agreement with Cochrane review about co-administration of metformin and ICSI cycle (15), the outcome of ICSI cycle does not progress significantly, but biochemical parameters, signs and symptom in women with PCO improved. These drugs as a pretreatment protocol before ICSI cycle could alter the results of cycle if used in more patients, or in longer or higher doses. All of these comments could be researched in further investigations.
Acknowledgments
This article was based on a residential thesis and received a financial grant from Ahvaz Jundishapur university of medical science (AJUMS) (ETH-3251), also, the study was submitted on Iranian health ministry website for clinical trials (www.IRRCT.IR). (Registration number: IRCT 201202138994N).
Footnotes
Authors’ Contribution:Elham Pourmatroud: original idea of study and administrator and wrote the manuscript. Razieh Mohammadjafari and Mandana Roozitalab coworkers for collecting data, analysis and writing the manuscript.
Funding/Support:This article was based on a residential thesis and received a financial grant from Ahvaz Jundishapur university of medical science (AJUMS) (ETH-3251).
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90(8):4650–8. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 3.Gorry A, White DM, Franks S. Infertility in polycystic ovary syndrome: focus on low-dose gonadotropin treatment. Endocrine. 2006;30(1):27–33. doi: 10.1385/ENDO:30:1:27. [DOI] [PubMed] [Google Scholar]
- 4.Heutling D, Schulz H, Nickel I, Kleinstein J, Kaltwasser P, Westphal S, et al. Asymmetrical dimethylarginine, inflammatory and metabolic parameters in women with polycystic ovary syndrome before and after metformin treatment. J Clin Endocrinol Metab. 2008;93(1):82–90. doi: 10.1210/jc.2007-0842. [DOI] [PubMed] [Google Scholar]
- 5.Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;922(2):456–61. doi: 10.1210/jc.2006-1988. [DOI] [PubMed] [Google Scholar]
- 6.Kaya C, Pabuccu R, Cengiz SD, Dunder I. Comparison of the effects of atorvastatin and simvastatin in women with polycystic ovary syndrome: A prospective, randomized study. Exp Clin Endocrinol Diabetes. 2010;118(3):161–6. doi: 10.1055/s-0029-1220770. [DOI] [PubMed] [Google Scholar]
- 7.Oner G, Muderris II. Clinical, endocrine and metabolic effects of metformin vs N-acetyl-cysteine in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):127–31. doi: 10.1016/j.ejogrb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2004;60(2):241–9. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 9.Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Effects of simvastatin and metformin on polycystic ovary syndrome after six months of treatment. J Clin Endocrinol Metab. 2011;96(11):3493–501. doi: 10.1210/jc.2011-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rzepczynska IJ, Piotrowski PC, Wong DH, Cress AB, Villanueva J, Duleba AJ. Role of isoprenylation in simvastatin-induced inhibition of ovarian theca-interstitial growth in the rat. Biol Reprod. 2009;81(5):850–5. doi: 10.1095/biolreprod.109.078667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: Lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87(4):1451–8. doi: 10.1210/jcem.87.4.8412. [DOI] [PubMed] [Google Scholar]
- 12.Izquierdo D, Foyouzi N, Kwintkiewicz J, Duleba AJ. Mevastatin inhibits ovarian theca-interstitial cell proliferation and steroidogenesis. Fertil Steril. 2004;82 Suppl 3:1193–7. doi: 10.1016/j.fertnstert.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Kazerooni T, Ghaffarpasand F, Kazerooni Y, Kazerooni M, Setoodeh S. Short-term metformin treatment for clomiphene citrate-resistant women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2009;107(1):50–3. doi: 10.1016/j.ijgo.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Tso LO, Costello MF, Albuquerque LE, Andriolo RB, Freitas V. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2009;(2):CD006105. doi: 10.1002/14651858.CD006105.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Raval AD, Hunter T, Stuckey B, Hart RJ. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565. doi: 10.1002/14651858.CD008565.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 17.Rashidi B, Abediasl J, Tehraninejad E, Rahmanpour H, Sills ES. Simvastatin effects on androgens, inflammatory mediators, and endogenous pituitary gonadotropins among patients with PCOS undergoing IVF: Results from a prospective, randomized, placebo-controlled clinical trial. J Investig Med. 2011;59(6):912–6. doi: 10.231/JIM.0b013e31821bfd9c. [DOI] [PubMed] [Google Scholar]
- 18.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76(3):517–24. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 19.Kriplani A, Agarwal N. Effects of metformin on clinical and biochemical parameters in polycystic ovary syndrome. J Reprod Med. 2004;49(5):361–7. [PubMed] [Google Scholar]
- 20.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 21.Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199(6):596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev. 2003;(3):CD003053. doi: 10.1002/14651858.CD003053. [DOI] [PubMed] [Google Scholar]
- 23.Barnes RB, Lobo RA. Central opioid activity in polycystic ovary syndrome with and without dopaminergic modulation. J Clin Endocrinol Metab. 1985;61(4):779–82. doi: 10.1210/jcem-61-4-779. [DOI] [PubMed] [Google Scholar]
- 24.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–56. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 25.Oride A, Kanasaki H, Purwana IN, Miyazaki K. Effects of metformin administration on plasma gonadotropin levels in women with infertility, with an in vitro study of the direct effects on the pituitary gonadotrophs. Pituitary. 2010;13(3):236–41. doi: 10.1007/s11102-010-0223-x. [DOI] [PubMed] [Google Scholar]
- 26.Duleba AJ, Banaszewska B, Spaczynski RZ, Pawelczyk L. Simvastatin improves biochemical parameters in women with polycystic ovary syndrome: Results of a prospective, randomized trial. Fertil Steril. 2006;85(4):996–1001. doi: 10.1016/j.fertnstert.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Comparison of simvastatin and metformin in treatment of polycystic ovary syndrome: prospective randomized trial. J Clin Endocrinol Metab. 2009;94(12):4938–45. doi: 10.1210/jc.2009-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]