Abstract
The features of protective murine antibodies to the Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan (GXM) have been rigorously investigated; however, the characteristics of protective human antibodies to GXM have not been defined. We produced monoclonal antibodies (MAbs) from XenoMouse mice (transgenic mice that express human immunoglobulin M [IgM], IgG2, and κ) which were immunized with a C. neoformans serotype D strain 24067 GXM-diphtheria toxoid conjugate. This study reports the specificity and efficacy of three human IgM MAbs, G14, G15, and G19, generated from these mice. Each MAb was specific for GXM, but G14 and G19 had different specificity based on their binding to serotype A strain H99 and SB4 GXMs, to which G15 did not bind. Nucleic acid sequence analysis revealed that G15 uses VH3-64 in the germ line configuration. G14 and G19 use VH6-1, which has somatic mutations. All of the MAbs use Vκ DPK22/A27. Studies of MAb efficacy in BALB/c mice showed that administration of 0.1 mg, but not 1 or 0.01 mg, of G15 prolonged survival against lethal C. neoformans strain 24067 challenge, whereas G14 and G19 were not protective at any dose. This panel of MAbs illustrates that serotype D GXM has epitopes that elicit human antibodies that can be either protective or nonprotective. Our findings suggest that VH gene use may influence GXM specificity and efficacy, and they provide insights into the possible contribution that VH gene use may have in resistance and susceptibility to cryptococcosis.
The specificity, molecular genetic structure, and efficacy of murine antibodies to the Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan (GXM) have been rigorously investigated (10, 11, 37, 38, 40, 43, 47, 51, 54). However, to date, the molecular genetic structures of only two human immunoglobulin M (IgM) monoclonal antibodies (MAbs) to GXM have been reported (49). The efficacy of one of these MAbs in BALB/c mice was established (23). The study of more human antibodies to GXM has been hampered by the lack of defined human MAb reagents and available candidate vaccines to elicit such antibodies in humans. Studies of murine MAbs elicited by an experimental GXM-tetanus toxoid (GXM-TT) vaccine (12, 37) revealed that the vaccine elicited protective, nonprotective, and deleterious antibodies with defined specificities and molecular structures (39, 40, 43, 47). Protective and nonprotective mouse IgM MAbs can be distinguished by their GXM binding characteristics and specificity (35, 43). However, certain protective MAbs display a prozone-like phenomenon, whereby they are nonprotective when administered in large amounts at the same inoculum at which they are protective in smaller amounts (54, 55). Protective and nonprotective mouse MAbs derived from the same VH and Vκ genes manifest distinct VH mutations (37, 42, 43). Based on studies of sera from humans and human immunoglobulin transgenic mice (XenoMouse mice), the VH gene usage of human antibodies to GXM is restricted to VH3 gene elements (22, 23, 34, 49). VH3 is the closest human gene family to the murine clan 3 7183 VH gene family, a clan 3 VH gene family that is used in mouse MAbs to GXM (9, 26). In this study, XenoMouse mice, which are transgenic for human IgM, IgG2 VH, and Vκ loci (36), were used to investigate the specificity and gene use of human antibodies to GXM.
(Parts of this work were presented at the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 14 to 17 September 2003 [R. W. Maitta, Q. Chang, A. Lees, and L. Pirofski, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-374, p. 435, 2003].)
MATERIALS AND METHODS
Animals.
XenoMouse mice, which are transgenic mice expressing human IgM, IgG2, and κ genes (36), were obtained from Abgenix (Fremont, Calif.) and maintained in the barrier facility of the Albert Einstein College of Medicine (AECOM). Six- to 8-week-old female BALB/c, mice used for C. neoformans challenge studies, were obtained from the National Cancer Institute (Bethesda, Md.). The animal research presented in this study complies with all federal, local, and institutional regulations controlling animal use.
Organisms.
C. neoformans serotype D, strain 24067, was obtained from the American Type Culture Collection (Manassas, Va.). C. neoformans serotype A strains H99 and SB4 and strain cap67 (an acapsular C. neoformans strain) were kindly provided by A. Casadevall (AECOM).
Peptide conjugates and adjuvants.
Diphtheria toxoid (DT) was obtained from Sigma (St. Louis, Mo.). GXMs from strains 24067, H99, and SB4 (24067, H99, and SB4 GXMs) were purified as described previously (17). 24067 GXM was conjugated to DT as described previously (21). Briefly, 5 mg of GXM (strain 24067) was activated with 5 mg of CDAP (1-cyano-4-dimethylaminopyridinium tetrafluoroborate) (Sigma) as described previously (21) and conjugated to 4 mg of DT (Sigma). Alhydrogel was obtained from Accurate Chemical and Scientific Corp. (Westbury, N.Y.). The adjuvant CpG (ImmuneEasy), which consists of oligonucleotides that contain unmethylated cytosine-guanine dinucleotide repeats, was obtained from Qiagen (Valencia, Calif.).
Mouse immunizations, bleedings, and generation of MAbs.
XenoMouse G2/κ mice were vaccinated subcutaneously at the base of the tail with a 100-μl injection of 10 μg of GXM-DT per mouse with 50 μl of Alhydrogel and 10 μl of CpG and were revaccinated on days 14 and 28. Mouse blood samples were obtained from the retro-orbital sinus, and sera were separated as previously described to determine levels of antibodies to GXM (see below). The splenocytes of mice with high titers of antibody to GXM were isolated and fused with the mouse myeloma cell line NSO to produce hybridomas as previously described (15, 50). Secreted supernatants from the resulting hybridoma cells were screened by enzyme-linked immunosorbent assay (ELISA) for GXM binding, cloned on soft agar, and tested for production of antibodies to 24067 GXM.
ELISAs for GXM reactivity.
ELISAs to detect MAb reactivity with GXM and the GXM mimotope, P13, selected by the protective human IgM to GXM, 2E9 (64), were performed as previously described (21, 34). Briefly, 96-well polystyrene ELISA plates (Corning Glass Works, Corning, N.Y.) were coated with a 10-μg/ml concentration of GXM from C. neoformans strain 24067 or from serotype A strains SB4 and H99, or with P13-dextran (21), in phosphate-buffered saline (PBS) for 3 h at room temperature (RT), followed by blocking with 0.1% Tween 20 (Fisher Scientific, Pittsburgh, Pa.)-PBS overnight at 4°C. The serum samples were diluted in blocking buffer (0.1% Tween 20-PBS) at 1:50 initially and 1:3 thereafter and, subsequently, the MAb supernatants were added to the plates and incubated for 1 h at 37°C. A myeloma IgM (Calbiochem, San Diego, Calif.) and a MAb to the polysaccharide of type 8 Streptococcus pneumoniae, D11 (65), were used as controls. After blocking, the plates were washed in PBS-0.05% Tween 20 by using a SkanWasher 400 (Molecular Devices, Sunnyvale, Calif.); incubated for 1 h with a 1:1,000 dilution of alkaline phosphatase (AP)-conjugated goat anti-human IgM, IgG, human κ, or mouse λ (Southern Biotechnology, Birmingham, Ala.), each diluted in the blocking buffer; washed; and incubated with p-nitrophenyl phosphate substrate (Sigma) for antibody detection. Absorbances were read at 405 nm with an MRX microplate reader (Dynex Technologies, Chantilly, Va.).
MAb concentration.
ELISA plates were coated with 10 μg of an unlabeled goat anti-human IgM (Southern Biotechnology) per ml in PBS for 3 h at RT and blocked with 1% bovine serum albumin (BSA)-PBS overnight at 4°C. MAbs were purified from hybridoma supernatants with anti-human IgM beads (Sigma). Purified antibodies were diluted 1:100 and then sequentially diluted 1:3 and incubated for 1 h at 37°C. Plates were washed and incubated with a 1:1,000 dilution of AP-conjugated goat anti-human IgM (Southern Biotechnology) in the blocking buffer for 1 h at 37°C. Absorbance was measured as described above. The antibody concentration was determined by extrapolating the values from a standard curve for a control polyclonal IgM (Sigma).
Whole-cell ELISA.
A whole-cell ELISA was developed to examine MAb binding to C. neoformans cells. Whole-cell ELISAs have been used for the detection of MAbs to other fungi (46), and an assay with heat-killed cells has been used for MAbs to Mycobacterium tuberculosis antigens (25). C. neoformans strains 24067, SB4, and H99 were grown in Sabouraud dextrose broth (Becton Dickinson, Sparks, Md.) for 2 days, after which the cells were washed in PBS, counted, and heat killed at 68°C for 2 h. Cell death was verified by incubating heat-killed cells in Sabouraud dextrose broth overnight at 31°C. Heat-killed cells were used so the yeasts would not grow during the course of the assay, which from plating to development takes 15 to 18 h. The cells were diluted in PBS and plated into ELISA plates at a concentration of 107 CFU/ml (50 μl/well; 5 × 105 cells/well), and the plates were incubated overnight at 4°C. Unbound cells were removed, and bound cells were fixed to the plates by incubation with 150 μl of methanol per well for 30 min. The plates were washed and blocked with 1% BSA-PBS for 1 h at 37°C. After washing, MAbs or a control myeloma IgM (Calbiochem) was added to the plates at an initial concentration of 10 μg/ml, diluted 1:3, and incubated for 1 h at 37°C. The reactivities of the MAbs and controls were determined on the same plate for each serotype. After washing, the plates were incubated with a 1:1,000 dilution of AP-conjugated goat anti-human IgM in the blocking buffer for 1 h at 37°C. The plates were developed as described above, and absorbances were read at a wavelength of 405 nm.
C3 deposition ELISA.
ELISA plates were coated with 10 μg of 24067 GXM per ml in PBS and blocked with 1% BSA-PBS, and 25 μl of a 10- or 50-μg/ml concentration of the MAb or the control IgM was added to each well with 25 μl of a human serum complement source (5 or 1%) (Sigma). After incubation for 1 h at 37°C, the plates were washed and incubated with a 1:1,000 dilution of goat anti-human C3 (ICN Biomedicals, Aurora, Ohio) for 1 h at 37°C. After washing, the plates were incubated with AP-labeled rabbit anti-goat immunoglobulin (1:1,000) (Calbiochem) for 1 h at 37°C, and antibody binding was detected as described above.
Inhibition ELISA.
An inhibition ELISA based a method previously used to determine specificity for MAbs to pneumococcal polysaccharide (15) was used to examine the GXM specificities of the MAbs. Briefly, ELISA plates were coated with 10 μg of 24067, SB4, or H99 GXM per ml in PBS for 3 h at RT, washed, and blocked with 1% BSA-PBS overnight at 4°C. Purified MAbs, used at a final concentration of 10 or 1 μg/ml, were mixed with serial 1:3 dilutions of 24067, H99, or SB4 GXM beginning at a concentration of 100 μg/ml and were incubated with the plates for 1 h at 37°C. After washing, the plates were incubated with a 1:1,000 dilution of AP-conjugated goat antibody to human IgM (Southern Biotechnology) for 1 h at 37°C. Plates were washed and antibody binding was detected as described above. The relative apparent affinity constant (aKa) of each MAb for soluble GXM was determined according to the method of Nieto et al. (45). The aKa was defined as the inverse molar concentration of soluble GXM needed to reduce maximal MAb binding to solid-phase GXM by 50%. Although this approach has limitations when applied to antibody-polysaccharide interactions, it is helpful for comparing the relative affinities of antibodies to polysaccharide antigens for which defined epitopes are not available (15, 37).
The MAbs were also used in competition ELISAs with the protective and nonprotective murine IgM MAbs to GXM 12A1 and 13F1, respectively (39, 43) (provided by A. Casadevall, AECOM). For these studies, ELISA plates coated with 10 μg of 24067 GXM per ml were incubated for 1 h at 37°C with 5 or 50 μg of 12A1 or 13F1 per ml, respectively, and serial dilutions of G14, G15, and G19 beginning at an initial concentration of 10 μg/ml. The amounts of the MAbs that were used represented the amounts that led to similar binding intensities on 24067 GXM-coated plates. After washing, the plates were incubated with a 1:1,000 dilution of AP-conjugated goat anti-mouse IgM (Southern Biotechnology) in the blocking buffer (1% BSA-PBS) for 1 h at 37°C, washed, and developed as described above. Another ELISA was performed by incubating the plates with 5 μg of G14, G15, or G19 per ml with serial dilutions of 12A1 and 13F1 beginning at initial concentrations of 10 and 50 μg/ml, respectively, and developing the plates as described above.
Immunofluorescence.
C. neoformans strains 24067, SB4, H99, and cap67 were grown for 48 h at 31°C in Sabouraud dextrose broth. Strain 24067 has a medium capsule size in vitro, and the capsule is not induced in size by growth in Sabouraud dextrose broth, although it can manifest some size variation (61, 62). As measured by India Ink staining with light microscopy as described previously (62), the sizes of the encapsulated cells were 6.3, 4.6, and 6.3 μm for 24067, SB4, and H99 cells, respectively. The cells were washed three times with PBS and centrifuged at 2.5 × g (3,000 rpm) for 15 min. Cells were resuspended in PBS, counted, resuspended at a concentration of 2 × 106 to 4 × 106 cells/100 μl, and incubated for 1 h at 37°C with10 μg of each MAb or the control IgM (Calbiochem) per ml. After incubation, the cells were washed three times with PBS, resuspended in a 1:100 dilution of goat anti-human IgM-tetramethyl rhodamine isothiocyanate (Southern Biotechnology) in 1% BSA-PBS, and incubated for 1 h at 37°C in the dark. Negative control cells were stained with the fluorochrome-conjugated antibody only. Cells were washed three times with PBS and a 1:100 solution of Calcofluor white (Fluostain; Sigma), incubated for 5 min at RT, extensively washed, and resuspended in 100 μl of 0.1 M N-propyl gallate (Sigma) antiquenching solution. For viewing, 3 to 10 μl of the cell suspension was placed on a slide and visualized with an Axioskop 2 mot plus (Zeiss, Thornwood, N.Y.).
Nucleic acid sequence analysis.
Antibody VH (heavy-chain) and VL (light-chain) cDNAs were generated from the hybridomas by reverse transcription of RNA and amplified with a set of sense variable-region primers and the antisense constant-region primers. VH cDNAs were amplified with a mixture of all VH primers which cover all of the VH genes in the XenoMouse mice, as follows. The VH sense primers were VH3-07, CACCATGGARTTGGGGCTGAGCTGG; VH3-09, CACCATGGAGTTKGGACTGAGCTGG; VH3-11, CACCATGGAGTTTGGGCTKAGCTGG; VH3-21, CACCATGGAACTGGGGCTCCGCTGG; VH3-48, CACCATGGAGTTGGGGCTGTGCTGG; VH3-53, CACCATGGAGTTTTGGCTGAGCTGG; VH3-64, CACCATGACGGAGTTTGGGCTGAGC; and VH6, CACCATGTCTGTCTCCTTCCTCATCTT. The antisense VH primer was IgM ASO, GTGCTGCTGATGTCAGAGTTG. The oligonucleotides generated were sequenced by using the ABI-PRISM Big Dye terminator cycle sequencing ready reaction kit (Perkin-Elmer, Torrance, Calif.). Determination of V, D, and J gene usage was performed by using in silico methods.
VL cDNA was amplified as described previously (42). The primers used for the light chain were as follows: VL(κ) sense, 5′GAA(CT)ATC(T)GAGCTCACC(GT)CAGTCTCCA-3′; VL(κ) antisense, 5′CCTGTTGAAGCTCTTTGTGAC-3′. The light-chain primers were synthesized at the DNA synthesis facility of the Cancer Center of AECOM. PCR products were gel purified, and direct sequencing of the Vκ gene was performed. Variable-region sequences were compared to the database of human immunoglobulin sequences by using DNA-plot (V Base Index; MRC Centre for Protein Engineering, University of Cambridge, Cambridge, United Kingdom) (http://www.mrc-cpe.cam.ac.uk/vbase-ok.php?menu= 901) and BLAST search (National Center for Biotechnology Information, Bethesda, Md.) (http://www.ncbi.nlm.nih.gov/BLAST).
Mouse protection experiments.
C. neoformans strain 24067 cells were grown for 48 h in Sabouraud dextrose agar at 31°C with shaking, washed, and counted prior to use. An intraperitoneal (i.p.) infection model in which MAb was administered 1 h prior to infection of BALB/c mice was used. The i.p. infection model was based on the serum potency model that was used to establish the efficacy of therapeutic antisera for administration to patients in the serum therapy era (8) and has been used to study the efficacies of a human IgM to GXM (23) and of antibodies to other pathogens (15, 50, 56, 65). A similar model has been used by different groups to establish the efficacies of mouse MAbs (38, 40, 41), including a MAb that was used in a phase I clinical trial (10). BALB/c mice were used because the efficacy of another human IgM MAb was also evaluated with this strain (23). G14, G15, G19, a control (myeloma) IgM (Calbiochem), or PBS was administered i.p. to BALB/c mice at 1, 0.1, 0.05, 0.005, and 0.0005 mg, and the mice were challenged i.p. 1 h later with 5 × 106 CFU of C. neoformans/mouse in a volume of 100 μl of PBS. Mice were observed daily, or twice daily when they manifested evidence of clinical illness, and survival was recorded daily.
Statistical analysis.
Animal survival was analyzed by using the Kaplan-Meier log rank test (Prism version 3.0.2; Graphpad Software, Inc., San Diego, Calif.) A P value of <0.05 was considered significant.
RESULTS
MAbs and MAb gene use.
Human IgM/κ MAbs reactive with GXM from strain 24067 were recovered. Three MAbs that demonstrated the strongest GXM binding upon initial screening, i.e., G14, G15, and G19, are described here. The molecular genetic elements that compose each of the MAbs and their complementarity determining region 3 (CDR3) sequences are shown in Table 1, and their CDR1 and CDR2 sequences are shown in Table 2. G15 uses VH3-64 and Vκ A27, and G14 and G19 use VH6-1 and Vκ A27. G14 and G19 differ in CDR2, where G14 has an A-to-T substitution in codon 55 that changes the residue from Y to F and G19 has substitutions in codon 58 that change the first and last bases from A and G to T and C, respectively, changing the residue from K to Y (compared to the germ line). G14 also has a silent mutation in codon 101. G14 and G19 share substitutions in codon 33, where a G-to-A change changes the germ line S to N. G15 uses germ line segments. Each MAb uses the same light-chain gene element DPK22/A27 (Table 1). The G15 A27 gene is germ line. The G14 A27 gene has a C-to-T change in codon 30 that changes the germ line S to I, and G19 has substitutions in CDR1 codons 25, 29, 30, and 31, changing them from A, V, S, and S to T, I, T, and N, respectively. Based on the putative germ line genes of the MAbs, the replacement-to-silent (R/S) mutation ratios were as follows: for the G14 VH, 5:1 (five replacements [one CDR1, one CDR2, and three CDR3); for the G19 VH, 6:0 (one CDR1, two CDR2, and three CDR3); for the G14 Vκ, 2:0 (one CDR1 and one CDR3); and for the G19 Vκ, 5:0 (four CDR1 and one CDR3). The R/S ratios in the CDRs indicate that the G14 and G19 somatic mutations were likely to have been selected by antigen (28). Comparison of the CDR1 and CDR2 sequences of the MAbs to previously reported human and murine IgMs to GXM are shown in Table 2.
TABLE 1.
Molecular genetic derivation and CDR3 sequences of MAbs produced from GXM-DT-vaccinated XenoMouse mice
| Gene use | MAb | Isotype | VHDHJH or VLJL | CDR3 | GenBank accession no. |
|---|---|---|---|---|---|
| VH | G15 | IgM | VH3-64/D3-9/JH4b | DHTIFGLVPPLDY | AY452136 |
| G14 | IgM | VH6-1/D3-10/JH5b | EGTMIRGIINWFDS | AY452137 | |
| G19 | IgM | VH6-1/D3-10/JH5b | EGTMIRGIINWFDS | AY452138 | |
| VL | G15 | κ | A27/JK1 | QQYGSSRT | AY538254 |
| G14 | κ | A27/JK1 | QQYGNSRT | AY538256 | |
| G19 | κ | A27/JK1 | QQYGNSRT | AY538255 |
TABLE 2.
CDR1 and CDR2 sequences of XenoMouse mouse human, human, and murine-derived MAbs to GXM
| MAb-VH | Origin, immunogen | Sequencea
|
Efficacy | Reference | |
|---|---|---|---|---|---|
| CDR1 (positions 31 to 35) | CDR2 | ||||
| 2E9-VH3 | Human, GXM-TT | NYWMT | NIKQDGGERYYVGSVTG | Protective | 49 |
| 3B6-VH3 | Human, GXM-TT | NYAMG | GISGSGGTSHFADSVKG | Unknown | 49 |
| G15-VH3 | XenoMouse mouse, GXM-DT | SYAMH | AISSNGGsTYYADSVKG | Protective | This report |
| 12A1-7183 | Mouse, GXM-TT | NYFMS | MININGNNTYYPDTVKG | Protective | 40, 43 |
| 13F1-7183 | Mouse, GXM-TT | SYyMS | AINSNGGsTYYPDTVKG | Nonprotective | 40, 43 |
| G14-VH6 | XenoMouse mouse, GXM-DT | SNNAAWN | RTYFRSKWYNDYAVSVKS | Nonprotective | This report |
| G19-VH6 | XenoMouse mouse, GXM-DT | SNNAAWN | RTYYRSYWYNDYAVSVKS | Nonprotective | This report |
| Human VH3/mouse, GXM binding | N/SY--- | -I---G----YYb---V-G | |||
Boldface underlining, residues shared between mouse and XenoMouse mouse MAbs; boldface italics, residues generated by somatic mutation (36) (this report); italics, residues similar between MAbs but in different position; lowercase, residues associated with diminished GXM binding (36).
Not in 3B6 because of somatic mutations.
GXM reactivity.
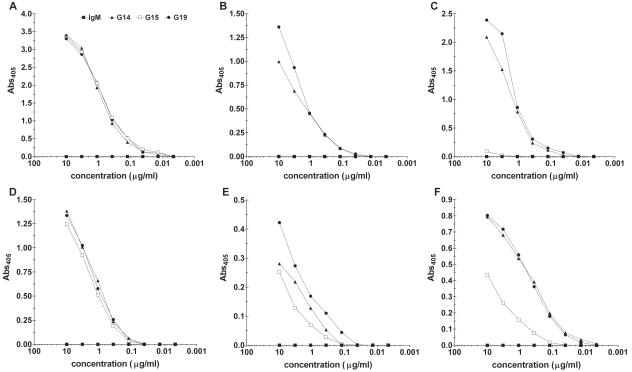
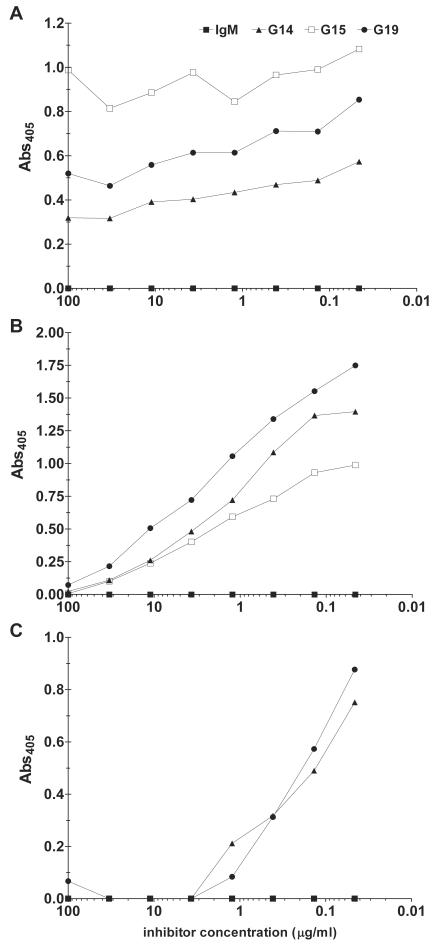
The MAbs had similar reactivities with 24067 GXM and 24067 cells (Fig. 1A and D). G14 and G19 bound to the serotype A strains in both the GXM and whole-cell ELISAs (Fig. 1B, C, E, and F). G15 did not bind to serotype A GXMs by GXM ELISA (Fig. 1B and C) but did bind in the whole-cell assay (Fig. 1E and F). Soluble SB4 GXM did not inhibit G14 or G19 binding to 24067 GXM (Fig. 2A). The calculated aKa values for the MAbs, defined as the inverses of the soluble 24067 GXM concentrations at 50% maximal binding to solid-phase 24067, were 1.3 × 103, 2 ×103, and 2.1 ×103 M−1, respectively (Fig. 2B). The calculated aKa values for G14 and G19 inhibition by soluble SB4 GXM binding to H99 GXM were 2 ×104 and 1 × 104 M−1, respectively (Fig. 2C). Each MAb mediated complement deposition on solid-phase GXM by ELISA. G14, G15, and G19 deposited C3 on solid-phase GXM with absorbances of 0.97, 0.64, and 0.96, respectively, while the control MAbs (an IgA [NAD] and IgM [D11] MAb to S. pneumoniae serotype 8 [9]) had absorbances of 0.07 and 0.1, respectively, for the IgM and the IgA. In competition ELISAs, the human MAbs did not inhibit the binding of the murine MAbs 12A1 and 13F1 to 24067 GXM and vice versa (data not shown). None of the MAbs bound the acapsular strain cap67 or the GXM mimotope P13 (data not shown).
FIG. 1.
Binding of MAbs G14, G15, and G19 to serotype A and D GXMs. (A, B, and C) Binding of MAbs to purified GXM from strains 24067, SB4, and H99, respectively. (D, E, and F) Binding of MAbs to heat-killed whole cells from strains 24067, SB4, and H99, respectively. Binding is represented by the absorbance at 405 nm (Abs405) as shown on the y axis for the amount of the indicated MAb on the x axis.
FIG. 2.
Inhibition of G14, G15, and G19 binding by soluble serotype A and D GXMs. Binding by ELISA is shown. (A) Inhibition of the binding of the MAbs to 24067 by soluble SB4; (B) inhibition of the binding of the MAbs to 24067 by soluble 24067; (C) inhibition of the binding of the MAbs to H99 by soluble SB4. Binding is represented by the absorbance at 405 nm (Abs405) as shown on the y axis for the concentration of the indicated soluble GXM on the x axis. The curve representing G15 in panel A is not seen because it is the same as that of the control IgM.
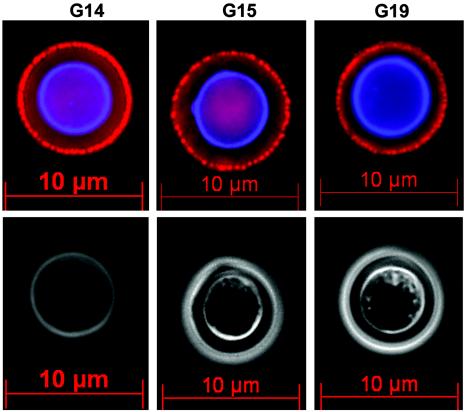
Immunofluorescence.
Each of the MAbs produced a similarly stippled binding pattern that was concentrated at the outermost aspect of the polysaccharide capsule on serotype D (24067) (Fig. 3). Similar binding was observed with serotype A strains (SB4 and H99) (data not shown). The binding of each of the MAbs was limited to the C. neoformans capsule, as there was no binding to acapsular strain cap67 (not shown). Based on the conditions used, the results of the immunofluorescence studies indicate that the MAbs bind capsular polysaccharide but not noncapsular determinants.
FIG. 3.
Immunofluorescent staining of C. neoformans serotype D (strain 24067) with GXM MAbs G14, G15, and G19. Staining was performed as detailed in the text with anti-human IgM rhodamine to detect MAb binding and Calcofluor white to detect the cell wall. The top panels show the immunofluorescent image, and the bottom panels show the phase image of the corresponding MAb shown in the upper panels. Magnification, ×100.
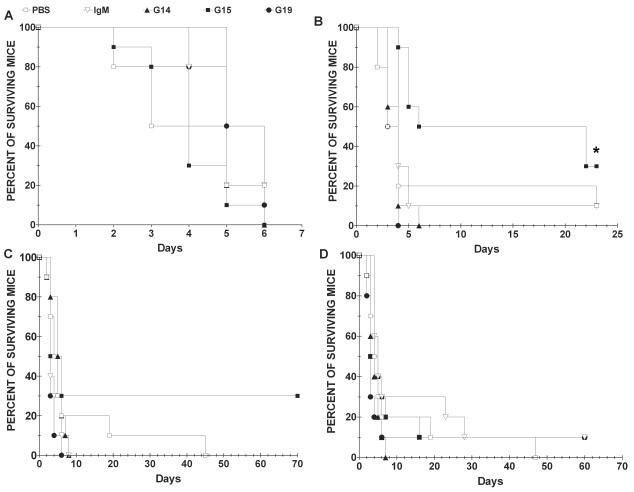
Protection studies.
The administration of 100 μg of G15 prolonged the survival of C. neoformans-infected mice compared to those that received PBS, control IgM, G14, and G19 (P < 0.04 [Kaplan-Meier log rank test]) (Fig. 4). Other amounts of G15 did not prolong survival, although there was a trend towards prolonged survival with the 50-μg dose (Fig. 4C). These data are most consistent with a prozone-like phenomenon (47, 48). G14 and G19 did not prolong survival at any dose. G15 produced statistically significant prolongation of survival when given in a 100-μg dose in two independent experiments. The degree of protection that we observed is in the same range as for certain mouse MAbs that were protective when larger amounts of MAb were given before an inoculum of the same C. neoformans strain, which was less lethal (38), and is greater than that for a mouse-human chimeric MAb that was given in a 1-mg dose (63).
FIG. 4.
Survival of BALB/c mice treated with G14, G15, or G19 after challenge with C. neoformans strain 24067. MAb treatment and C. neoformans challenge were performed i.p. (A) Survival of mice given 1 mg of the MAbs per mouse; (B) survival of mice given 100 μg of the MAbs per mouse; (C) survival of mice given 50 μg of the MAbs per mouse; (D) survival of mice given 5 μg of the MAbs per mouse. In each panel, the y axis represents the percentage of surviving mice, and the x axis depicts the number of days after i.p. infection (n = 10 mice/group; *, P < 0.04 [Kaplan-Meier log rank survival]).
DISCUSSION
The MAbs to GXM described here reveal that GXM-DT immunization of human immunoglobulin transgenic mice elicited human IgMs to GXM with different VH gene usages, specificities, and efficacies against C. neoformans in mice. Based on the inhibition ELISAs with 24067 GXM, the human MAbs have different specificity than the murine MAbs 12A1 and 13F1 (a protective MAb and a nonprotective MAb, respectively). Since the murine MAbs were elicited by GXM-TT, the specificity differences between the human and murine IgM MAbs could reflect differences in the response to 24067 GXM in GXM-DT versus serotype A 371 in GXM-TT (39). Carrier-specific differences in the fine specificity of antibodies elicited by capsular polysaccharide-protein conjugate vaccines have been described previously (31). Since genetic factors have been shown to influence the immune response to C. neoformans (20), the genetic backgrounds of the mouse strains used for immunization or mouse versus human antibody repertoire differences could also have contributed to specificity differences. Studies with defined GXM epitopes are required to fully explain specificity differences between the antibodies. Nonetheless, our data provide further evidence and extend to human GXM-specific antibodies the observation that polysaccharide conjugate vaccines elicit protective and nonprotective antibodies with different specificities and efficacies (15, 39, 43, 50).
Our data show that G15, G14, and G19 binding to 24067 GXM and cells was similar. However, G15 has different GXM specificity, since it did not bind serotype A GXMs and had lesser reactivity with H99 cells than G14 and G19. The G15 GXM epitope is either not present or poorly accessible on soluble serotype A GXMs but is present on serotype A cells. The diminished serotype A reactivity of G15 could reflect an affinity difference, but precise affinity determinations require defined epitopes, which are not available. Nonetheless, our data suggest that G15 is 24067 GXM specific, whereas G14 and G19 have broader GXM reactivity. In addition, since G15 does not react with the GXM mimotope selected by the protective human IgM to GXM, 2E9 (64), the epitope that it recognizes defines a second GXM epitope that can elicit a protective human antibody.
The specificity difference between G15 and G14-G19 is paralleled by different VH gene use. G15 uses a VH3 gene, whereas G14 and G19 use VH6-1. Maitta et al. found that the ability of human immunoglobulin transgenic mice to produce antibodies to GXM (24067) was associated with VH3 expression (34), and the two previously reported human IgM MAbs to GXM use VH3 (49), as do naturally occurring antibodies to serotypes A and D in human sera (2, 22, 23, 49). The reagents used for previous serological analyses could not identify VH6 expression. Therefore, it is not known whether human sera also contain VH6-expressing antibodies to GXM, and additional reagents are needed to establish the full range of VH expression by naturally occurring human antibodies to GXM.
VH6-1 is the only member of VH6, the most 3′ family in the human immunoglobulin VH locus and a major component of the naturally occurring B-cell repertoire (18, 58). Somatically unmutated and mutated IgM VH6-1 gene products have previously been reported to bind lipid A (53) and Bordetella pertussis lipopolysaccharide (4), respectively. Unlike G15, which is germ line, G14 and G19 have somatic mutations in their CDR1 and CDR2 sequences. Somatic mutations among VH6-expressing B-cell transcripts have been attributed to antigen-driven mechanisms, which can occur in IgM and be CD40 ligand independent (28, 57). Hence, the expressed VH6 repertoire can be shaped by antigen selection in a T-cell-independent manner. VH6 expression is most prevalent in fetal life and early childhood, after which a mature repertoire that is dominated by VH3 emerges (6). Although VH3 is more prevalent in the resting B-cell repertoire, VH3 and VH6 expression is nearly equal in the naturally occurring B-cell repertoire (18). With this observation, our findings suggest that VH6-expressing antibodies may contribute to the reactivity of human sera with GXM but that such antibodies may not recognize epitopes that enhance resistance to cryptococcosis. Studies of additional human MAbs and B cells could validate this concept and establish or refute the existence of a naturally occurring GXM-reactive B-cell repertoire.
The specificity differences of murine IgM MAbs to GXM that resulted from somatic hypermutation of a canonical V-D-J gene element were found to translate into efficacy differences against C. neoformans (39, 43). In contrast, we found that the XenoMouse mouse-derived human IgM MAbs were either germ line or had only a few somatic mutations. G15 uses germ line VH and VL gene segments, providing proof of principle that combinatorial diversity, an antigen-independent mechanism, is sufficient to generate human antibodies to certain GXM determinants that are protective. Reports that VH gene use in antibodies to capsular polysaccharides is restricted (11, 30, 48, 59) and that CDR structure enables germ line antibodies to bind microbial carbohydrate epitopes (44) support the concept that clans of structurally related immunoglobulins mediate binding to certain antigens (29). The functional efficacy of germ line IgM has also been established for experimental influenza and pneumococcal infections (15, 27, 50). With our finding that G15 is protective, these observations underscore the increasingly recognized importance of B cells and T-cell-independent immunity in pathogen resistance (reviewed in reference 13).
The VH CDR1 and CDR2 of protective human and murine IgMs to GXM share common residues (Table 2). Nakouzi and Casadevall found that the CDR2s of the murine MAbs had predominantly germ line residues at positions 54/G, 58/Y, 59/Y, and 63/V (42). These residues are also found in the protective human MAbs. Interestingly, 4 of 22 germ line VH3 sequences have 32/Y-52a/I-56/G-60/Y-61/Y-65/V-65b/G, which we found in both the murine and human VH3 MAbs, including V3-07, which is used by 2E9; V3-23, which is used by 3B6; V3-64, which is used by G15; and V3-43 (V base index). In 2E9, the previously reported protective VH3 MAb, the germ line 56/A was changed by somatic mutation to G (49), the germ line residue in the G15 gene. At the beginning of CDR3, G15 has a 95/R-96/D motif, whereas G14 and G19 have an RE motif. An R in position 95 (which is part of the VH) was found to be essential for GXM binding, but an RE motif retained GXM binding (42). Otherwise, the CDR3 sequences of the mouse, human, and XenoMouse mouse-derived MAbs to GXM are each unique. Hence, the VL and VH gene family used and the mutations in CDR1 and CDR2 may be more important determinants of GXM binding and specificity than CDR3 diversity. This is consistent with a report that CDR3 diversity was sufficient to confer binding to proteins and haptens but insufficient for carbohydrate binding (60). Further studies with defined epitopes are required to precisely identify the structural correlates of MAb GXM binding.
All three MAbs use the same Vκ A27 gene, which has been previously reported to occur in human antibodies to capsular polysaccharides (3, 32, 33). As we found for the VH, the G14 and G19 A27 genes had a few somatic mutations, whereas G15 did not. Variable VL use with the same VH has been shown to confer reactivity with different viruses (52). The use of the same Vκ and Vλ genes has previously been described for murine and human MAbs to GXM, respectively (37, 49). Therefore, although its presence is not predictive of or sufficient for antibody efficacy, the A27 gene product may contribute to GXM specificity for human IgMs. Taken together, the VH and VL use of the MAbs reveals that human antibodies with different GXM specificities and efficacies can be generated by differential VH gene use.
G15 was protective when administered to mice in a 100-μg dose but not when administered in higher or lower doses. This is consistent with the prozone-like phenomenon described by Taborda and Casadevall for mouse MAbs (54). Along the same lines, XenoMouse mice that generated a robust human IgM anamnestic response to GXM after priming with a GXM mimotope were not protected from cryptococcal challenge (34). Since sera from human immunodeficiency virus (HIV)-infected individuals have been found to have higher GXM antibody levels than sera from non-HIV-infected individuals (19, 22), these observations lead us to wonder if a prozone-like phenomenon could contribute to the pathogenesis of HIV-associated cryptococcosis. To date, we have found two VH3 IgMs to be protective against C. neoformans and two VH6-1 IgMs to be nonprotective. The study of more antibodies is needed to establish the contribution, if any, of VH gene usage to antibody specificity and/or efficacy. However, in light of VH3 dysregulation (1, 5, 7, 14, 22) and specificity differences among antibodies to GXM in HIV-infected individuals (22, 64), our data support the hypothesis that antibody repertoire defects could contribute to the apparent lack of serum antibody efficacy in certain HIV-infected individuals (48). Since C. neoformans infection is nearly universal (2, 16, 24, 26) and CD4 T-cell deficiency is insufficient to predict susceptibility to HIV-associated cryptococcosis, additional factors must influence susceptibility and resistance to cryptococcosis. The findings presented here lead us to hypothesize that certain antibodies in the naturally occurring repertoire are GXM reactive and protective and as such could contribute to natural resistance to cryptococcosis, whereas the apparent failure of antibodies from HIV-infected individuals to mediate resistance could be a function of their VH gene use and/or GXM specificity.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI035370, R01AI045459, and R01AI044374 (to L.P.) and by Individual National Research Service Award F31AI010129 (to R.W.M.).
We thank Arturo Casadevall for providing reagents for this study.
Editor: T. R. Kozel
REFERENCES
- 1.Abadi, J., J. Friedman, R. Jefferis, R. A. Mageed, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J. Infect. Dis. 178:707-716. [DOI] [PubMed] [Google Scholar]
- 2.Abadi, J., and L. Pirofski. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without HIV infection. J. Infect. Dis. 180:915-919. [DOI] [PubMed] [Google Scholar]
- 3.Adderson, E. E., P. G. Shackelford, R. A. Insel, A. Quinn, P. M. Wilson, and W. L. Carroll. 1992. Immunoglobulin light chain variable region gene sequences for human antibodies to Haemophilus influenzae type b capsular polysaccharide are dominated by a limited number of V kappa and V lambda segments and VJ combinations. J. Clin. Investig. 89:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andris, J. S., B. R. Brodeur, and J. D. Capra. 1993. Molecular characterization of human antibodies to bacterial antigens: utilization of the less frequently expressed VH2 and VH6 heavy chain variable region gene families. Mol. Immunol. 30:1601-1616. [DOI] [PubMed] [Google Scholar]
- 5.Berberian, L., J. Shukla, R. Jefferis, and J. Braun. 1994. Effects of HIV infection on VH3 (D12 idiotope) B cells in vivo. J. Acquir. Immune Defic. Syndr. 7:641-646. [PubMed] [Google Scholar]
- 6.Berman, J. E., K. G. Nickerson, R. R. Pollock, J. E. Barth, R. K. Schuurman, D. M. Knowles, L. Chess, and F. W. Alt. 1991. VH gene usage in humans: biased usage of the VH6 gene in immature B lymphoid cells. Eur. J. Immunol. 21:1311-1314. [DOI] [PubMed] [Google Scholar]
- 7.Bessudo, A., L. Rassenti, D. Havlir, D. Richman, E. Feigal, and T. J. Kipps. 1998. Aberrant and unstable expression of immunoglobulin genes in persons infected with human immunodeficiency virus. Blood 92:1317-1323. [PubMed] [Google Scholar]
- 8.Buchwald, U. K., and L. Pirofski. 2003. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 9:945-968. [DOI] [PubMed] [Google Scholar]
- 9.Burns, T., Z. Zhong, M. Steinitz, and L. Pirofski. 2003. Modulation of polymorphonuclear cell IL-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide. Infect. Immun. 71:6775-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall, A., W. Cleare, M. Feldmesser, R. Glatman-Freedman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to C. neoformans polysaccharide which is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall, A., M. DeShaw, M. Fan, F. Dromer, T. R. Kozel, and L. Pirofski. 1994. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infect. Immun. 62:3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall, A., J. Mukherjee, S. J. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 165:1086-1093. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall, A., and L. Pirofski. 2003. Antibody mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 24:474-478. [DOI] [PubMed] [Google Scholar]
- 14.Chang, Q., P. Alpert, J. Abadi, and L. Pirofski. 2000. A pneumococcal capsular polysaccharide vaccine induces a repertoire shift with increased VH3 expression in peripheral B cells from HIV-uninfected, but not HIV-infected individuals. J. Infect. Dis. 181:1313-1321. [DOI] [PubMed] [Google Scholar]
- 15.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, L. C., D. L. Goldman, T. L. Doering, L. Pirofski, and A. Casadevall. 1999. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect. Immun. 67:2218-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherniak, R., L. C. Morris, B. C. Anderson, and S. A. Meyer. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 59:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidkova, G., S. Pettersson, D. Holmberg, and I. Lundkvist. 1997. Selective usage of VH genes in adult human B lymphocyte repertoires. Scand. J. Immunol. 45:62-73. [DOI] [PubMed] [Google Scholar]
- 19.DeShaw, M., and L. Pirofski. 1995. Antibodies to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan are ubiquitous in the serum of HIV+ and HIV− individuals. Clin. Exp. Immunol. 99:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dromer, F., P. Yeni, and J. Charreire. 1988. Genetic control of the humoral response to cryptococcal capsular polysaccharide in mice. Immunogenetics 28:417-424. [DOI] [PubMed] [Google Scholar]
- 21.Fleuridor, R., A. Lees, and L. Pirofski. 2001. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J. Immunol. 166:1087-1096. [DOI] [PubMed] [Google Scholar]
- 22.Fleuridor, R., R. H. Lyles, and L. Pirofski. 1999. Quantitative and qualitative differences in the serum antibody profiles of HIV-infected persons with and without Cryptococcus neoformans meningitis. J. Infect. Dis. 180:1526-1536. [DOI] [PubMed] [Google Scholar]
- 23.Fleuridor, R., Z. Zhong, and L. Pirofski. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178:1213-1216. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Hermoso, D., G. Janbon, and F. Dromer. 1999. Epidemiologic evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glatman-Freedman, A., J. M. Martin, P. F. Riska, B. R. Bloom, and A. Casadevall. 1996. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J. Clin. Microbiol. 34:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman, D. L., H. Khine, J. Abadi, D. L. Lindenberg, L. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:e66. [DOI] [PubMed] [Google Scholar]
- 27.Harada, Y., M. Muramatsu, T. Shibata, T. Honjo, and K. Kuroda. 2003. Unmutated immunoglobulin M can protect mice from death by influenza virus infection. J. Exp. Med. 197:1779-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Insel, R. A., W. S. Varade, and E. Marin. 1994. Human splenic IgM immunoglobulin transcripts are mutated at high frequency. Mol. Immunol. 31:383-392. [DOI] [PubMed] [Google Scholar]
- 29.Kirkham, P. M., R. F. Mortari, J. A. Newton, and H. W. Schroeder. 1992. Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 11:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lara-Ochoa, F., J. C. Almagro, E. Vargas-Madrazo, and M. Conrad. 1996. Antibody-antigen recognition: a canonical structure paradigm. J. Mol. Evol. 43:678-684. [DOI] [PubMed] [Google Scholar]
- 31.Lucas, A. H., and D. M. Granoff. 1995. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type b polysaccharide-protein conjugates. J. Immunol. 154:4195-4202. [PubMed] [Google Scholar]
- 32.Lucas, A. H., D. M. Granoff, R. E. Mandrell, C. C. Connolly, A. S. Shah, and D. C. Powers. 1997. Oligoclonality of serum immunoglobulin G antibody responses to Streptococcus pneumoniae capsular polysaccharide serotypes 6B, 14, and 23F. Infect. Immun. 65:5103-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas, A. H., J. W. Larrick, and D. C. Reason. 1994. Variable region sequences of a protective human monoclonal antibody specific for the Haemophilus influenzae type b capsular polysaccharide. Infect. Immun. 62:3873-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maitta, R. W., K. Datta, A. Lees, S. S. Belouski, and L. A. Pirofski. 2004. Immunogenicity and efficacy of Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan peptide mimotope-protein conjugates in human immunoglobulin transgenic mice. Infect. Immun. 72:196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean, G. R., M. Torres, N. Elguezabal, A. Nakouzi, and A. Casadevall. 2002. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 169:1379-1386. [DOI] [PubMed] [Google Scholar]
- 36.Mendez, M. J., L. L. Green, J. R. Corvalan, X. C. Jia, C. E. Maynard-Currie, X. D. Yang, M. L. Gallo, D. M. Louie, D. V. Lee, K. L. Erickson, J. Luna, C. M. Roy, Abderrahim, H., F. Kirschenbaum, M. Noguchi, D. H. Smith, A. Fukushima, J. F. Hales, Finer, M. H., C. G. Davis, K. M. Zsebo, and A. Jakobovits. 1997. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat. Genet. 15:146-156. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee, J., A. Casadevall, and M. D. Scharff. 1993. Molecular characterization of the antibody responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J. Exp. Med. 177:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee, J., T. R. Kozel, and A. Casadevall. 1998. Monoclonal antibodies reveal additional epitopes of serotype D Cryptococcus neoformans capsular glucuronoxylomannan that elicit protective antibodies. J. Immunol. 161:3557-3567. [PubMed] [Google Scholar]
- 39.Mukherjee, J., G. Nussbaum, M. D. Scharff, and A. Casadevall. 1995. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J. Exp. Med. 181:405-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1995. Variable efficacy of passive antibody administration against diverse Cryptococcus neoformans strains. Infect. Immun. 63:3353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakouzi, A., and A. Casadevall. 2003. The function of conserved amino acids in or near the complementarity determining regions for related antibodies to Cryptococcus neoformans glucuronoxylomannan. Mol. Immunol. 40:351-361. [DOI] [PubMed] [Google Scholar]
- 43.Nakouzi, A., P. Valadon, J. D. Nosanchuk, N. Green, and A. Casadevall. 2001. Molecular basis for immunoglobulin M specificity to epitopes in Cryptococcus neoformans polysaccharide that elicit protective and nonprotective antibodies. Infect. Immun. 69:3398-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen, H. P., N. O. Seto, C. R. MacKenzie, L. Brade, P. Kosma, H. Brade, and S. V. Evans. 2003. Germline antibody recognition of distinct carbohydrate epitopes. Nat. Struct. Biol. 10:1019-1025. [DOI] [PubMed] [Google Scholar]
- 45.Nieto, A., A. Gaya, M. Jansa, C. Moreno, and J. Vives. 1984. Direct measurement of antibody affinity distribution by hapten-inhibition enzyme immunoassay. Mol. Immunol. 21:537-543. [DOI] [PubMed] [Google Scholar]
- 46.Nosanchuk, J. D., J. N. Steenbergen, L. Shi, G. S. Deepe, Jr., and A. Casadevall. 2003. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Investig. 112:1164-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nussbaum, G., W. Cleare, A. Casadevall, M. D. Scharff, and P. Valadon. 1997. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J. Exp. Med. 185:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirofski, L. 2001. Polysaccharides, mimotopes and vaccines for encapsulated pathogens. Trends Microbiol. 9:445-452. [DOI] [PubMed] [Google Scholar]
- 49.Pirofski, L., R. Lui, M. DeShaw, A. B. Kressel, and Z. Zhong. 1995. Analysis of human monoclonal antibodies elicited by vaccination with a Cryptococcus neoformans glucuronoxylomannan capsular polysaccharide vaccine. Infect. Immun. 63:3005-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell, N., J. R. Corvalan, M. L. Gallo, C. G. Davis, and L. Pirofski. 2000. Production of protective human antipneumococcal antibodies by transgenic mice with human immunoglobulin loci. Infect. Immun. 68:1820-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanford, J. E., D. M. Lupan, A. M. Schlageter, and T. R. Kozel. 1990. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect. Immun. 58:1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senn, B. M., C. Lopez-Macias, U. Kalinke, A. Lamarre, A. Isibasi, R. M. Zinkernagel, and H. Hengartner. 2003. Combinatorial immunoglobulin light chain variability creates sufficient B cell diversity to mount protective antibody responses against pathogen infections. Eur. J. Immunol. 33:950-961. [DOI] [PubMed] [Google Scholar]
- 53.Settmacher, U., S. Jahn, P. Siegel, R. von Baehr, and A. Hansen. 1993. An anti-lipid A antibody obtained from the human fetal repertoire is encoded by VH6-V lambda 1 genes. Mol. Immunol. 30:953-954. [DOI] [PubMed] [Google Scholar]
- 54.Taborda, C., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 55.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 56.Teitelbaum, R., R. Glatman-Freedman, B. Chen, J. B. Robbins, E. Unanue, A. Casadevall, and B. R. Bloom. 1998. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 95:15688-15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varade, W. S., and R. A. Insel. 1993. Isolation of germinal centerlike events from human spleen RNA. Somatic hypermutation of a clonally related VH6DJH rearrangement expressed with IgM, IgG, and IgA. J. Clin. Investig. 91:1838-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varade, W. S., E. Marin, A. M. Kittelberger, and R. A. Insel. 1993. Use of the most JH-proximal human Ig H chain V region gene, VH6, in the expressed immune repertoire. J. Immunol. 150:4985-4995. [PubMed] [Google Scholar]
- 59.Vargas-Madrazo, E., F. Lara-Ochoa, and J. C. Almagro. 1995. Canonical structure repertoire of the antigen-binding site of immunoglobulins suggests strong geometrical restrictions associated to the mechanism of immune recognition. J. Mol. Biol. 254:497-504. [DOI] [PubMed] [Google Scholar]
- 60.Xu, J. L., and M. M. Davis. 2000. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 13:37-45. [DOI] [PubMed] [Google Scholar]
- 61.Zaragoza, O., B. C. Fries, and A. Casadevall. 2003. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO2. Infect. Immun. 71:6155-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaragoza, O., C. P. Taborda, and A. Casadevall. 2003. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur. J. Immunol. 33:1957-1967. [DOI] [PubMed] [Google Scholar]
- 63.Zebedee, S. L., R. K. Koduri, J. Mukherjee, S. Mukherjee, S. Lee, D. F. Sauer, M. D. Scharff, and A. Casadevall. 1994. Mouse-human immunoglobulin G1 chimeric antibodies with activities against Cryptococcus neoformans. Antimicrob. Agents Chemother. 38:1507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, H., Z. Zhong, and L. Pirofski. 1997. Peptide epitopes recognized by a human anti-cryptococcal glucuronoxylomannan antibody. Infect. Immun. 65:1158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]