Abstract
Breast cancer survival is reportedly higher in the US than in Europe. The first worldwide study (CONCORD) found wide international differences in age-standardised survival. The aim of this study is to explain these survival differences.
Population-based data on stage at diagnosis, diagnostic procedures, treatment and follow-up were collected for about 20,000 women diagnosed with breast cancer aged 15–99 years during 1996–98 in 7 US states and 12 European countries. Age-standardised net survival and the excess hazard of death up to five years after diagnosis were estimated by jurisdiction (registry, country, European region), age and stage with flexible parametric models.
Breast cancers were generally less advanced in the US than in Europe. Stage also varied less between US states than between European jurisdictions. Early, node-negative tumours were more frequent in the US (39%) than in Europe (32%), while locally advanced tumours were twice as frequent in Europe (8%), and metastatic tumours of similar frequency (5–6%). Net survival in Northern, Western and Southern Europe (82–85%) was similar to that in the US (84%), but lower in Eastern Europe (72%). For the first 3 years after diagnosis the mean excess hazard was higher in Eastern Europe than elsewhere: the difference was most marked for women aged 70–99 years, and mainly confined to women with locally advanced or metastatic tumours.
Differences in breast cancer survival between Europe and the US in the late 1990s were mainly explained by lower survival in Eastern Europe, where low healthcare expenditure may have constrained the quality of treatment.
Keywords: CONCORD, net survival, excess hazard, cancer registries
Introduction
Breast cancer survival has been reported as higher in the US than in Europe1,2. For women diagnosed 1985–89, five-year survival was higher in each of the nine Surveillance, Epidemiology and End Results (SEER) areas than in any of the 22 European countries participating in the EUROCARE-2 study.
The first worldwide analysis of cancer survival (CONCORD3) provided a systematic comparison of survival for adults (15–99 years) diagnosed with a cancer of the breast, colon, rectum or prostate in one of 31 countries during 1990–94 and followed up to 1999. International differences in age-standardised survival were very wide, even after adjustment for differences in mortality from other causes of death. Breast cancer survival in the US and Canada was higher than in other countries, but differences between the US and most European regions were smaller than for women diagnosed during 1985–892. The largest differences were between the US and Eastern Europe.
A population-based comparison of five-year breast cancer survival among women diagnosed in 17 territories in 6 European countries during 1990–92 and in the 9 states and metropolitan areas of the US covered by the SEER programme in 1990 showed that differences were mainly attributable to stage at diagnosis and the diagnostic procedures used to determine the stage4.
Both the assiduity of investigation and the appropriateness of treatment by stage varied widely for women diagnosed in Europe during 1990–92 and 1996–985,6. Primary treatment for breast cancer also varies greatly throughout the US7,8. Following the NIH Consensus Development Conference in 1990, which recommended breast-conserving surgery and radiotherapy instead of mastectomy for women with stage I and II breast cancer, the proportion treated with breast-conserving surgery increased steadily up to 19959, but the percentage who also received radiotherapy and/or axillary lymphadenectomy declined10. Differences in protocol and calendar period make it difficult to draw firm conclusions from these studies about whether the differences in survival between Europe and the US are attributable to differences in stage, or treatment, or both.
The CONCORD protocol incorporated “high-resolution” studies designed to explain the international variations in survival for breast, colorectal and prostate cancer. The analyses involve large random samples of patients, with detailed clinical and pathological data that are not routinely abstracted by population-based cancer registries. The study reported here provides a trans-Atlantic comparison of stage, treatment and survival for women with breast cancer. The aims were to compare the stage distributions in Europe and the US; to determine whether the transatlantic differences in 5-year survival persist and, if so, to assess the extent to which they are attributable to differences in stage. We also set out to compare adherence to “standard care” for breast cancer in relation to age, stage and hormone receptor status, before widespread introduction of clinical guidelines.
Material and methods
Data on stage, diagnostic procedures, treatment and follow-up were collected for a representative sample of about 20,000 women aged 15–99 years who were registered with a diagnosis of breast cancer in the US or one of 12 European countries during 1996–98. A common protocol was used, based on the EUROCARE high-resolution protocols5,6.
The European data were provided by 26 population-based cancer registries in 12 countries, 7 of which with national coverage, denoted by an asterisk (*). For some analyses, the data were grouped into four European regions defined by the United Nations (UN, http://unstats.un.org/unsd/methods/m49/m49regin.htm) - Northern Europe: Denmark*, Finland*, Iceland*, Sweden*; Western Europe: France (Bas-Rhin, Côte d’Or, Doubs, Isère, Tarn) and the Netherlands (Eindhoven, North East Netherlands); Southern Europe: Italy (Firenze, Genova, Modena, Palermo, Ragusa, Varese), Slovenia*, Spain (Basque Country, Castellon, Granada, Navarra); Eastern Europe: Estonia*, Poland (Cracow, Warsaw), Slovakia*. Estonia is classified by the UN as being in Northern Europe, but cancer survival has usually resembled that in Eastern European countries11, and the data from Estonia are included here with Eastern Europe. Data from the US were provided by 7 state-wide registries: California, Colorado, Illinois, Louisiana, New York, Rhode Island and South Carolina. The US registries are part of the National Program of Cancer Registries, based at the Centers for Disease Control and Prevention.
For this study the cancer registries included in the EUROCARE-3 high-resolution study6 made special efforts to update the follow-up to at least five years after diagnosis for all patients. The North East Netherlands registry was not included in EUROCARE-3, but it is unusual in that it routinely collects almost all the data required for high-resolution studies on all registered cancer patients, so it was able to provide such data on virtually all women with breast cancer, not just a sample.
Most European registries provided a random sample of at least 500 women diagnosed during 1996–98, as specified by the protocol. Denmark and Sweden provided a sample of women diagnosed in 1994, and Palermo (Italy) provided data for all women diagnosed in 1999, the first year for which data were available there. The Finnish cases were a population-based sample of women diagnosed in the Tampere hospital region, which is considered representative of the whole of Finland. Despite these slight departures from protocol, these cases were retained to ensure the widest possible geographic coverage. Each of the US registries provided a random sample of at least 500 women diagnosed with breast cancer in 1997.
Anonymised, individual cancer registration records were supplied for 20,150 women diagnosed with a malignant neoplasm of the breast. In situ tumours (1,168, 5.8%) were excluded from the analyses because they were collected systematically in the US, but not in Europe. A further 20 cases (0.1%) were excluded because they did not meet the protocol (2 with benign or uncertain behaviour, 2 with the morphology of leukaemia or lymphoma, and 16 aged less than 15 or more than 100 years). In all, 18,962 women with a primary, invasive, malignant neoplasm of the breast were included in the analysis of stage and treatment. Women whose cancer was only registered from a death certificate (23; 0.1%), or of unknown vital status (18; 0.1%), or for whom the date of last known vital status preceded the date of diagnosis (32; 0.2%), were excluded from the survival analyses, which thus involved 18,889 women.
Information on stage, diagnostic examinations, treatment and follow-up was obtained by direct examination of the clinical record. Where records were incomplete, pathology reports, hospital discharge records and other sources were consulted as necessary.
Disease stage was defined according to the 4th edition of the TNM (Tumour, Nodes, Metastasis) manual.12 If pathological data on tumour size and lymph node status (pT and pN) were unavailable, clinical data (cT and cN) were used. Following advice from epidemiologists, pathologists and clinicians, records for which the metastatic status was unknown (MX) were considered as negative (M0), if T and N were known. Patients were grouped into six categories: early, node-negative disease (T1N0M0), larger node-negative (T2-3N0M0), node-positive (T1-3N+M0), locally advanced (T4, any N, M0), metastatic (M1) and unstaged. Within the category of early, node-negative disease, we also assessed the distribution of small tumours by size: less than 5mm (T1a), 5–10mm (T1b) and over 10mm and up to 20mm (T1c). Estrogen-receptor (ER) status was categorized as positive, negative or unknown. Age at diagnosis was categorised into four groups (15–39, 40–49, 50–69, 70–99 years) for survival analysis. Treatment comparisons were made in wider age groups: 15–49 and 50–99 years for chemotherapy and hormone therapy; 15–69 and 70–99 years for breast-conserving surgery plus radiotherapy.
Data on surgical procedures were collected in 7 categories: conservative surgery (including quadrantectomy, tumour excision, lumpectomy), simple mastectomy, any modified radical mastectomy, extended radical (Halsted) mastectomy, surgery (not otherwise specified), unknown if surgery was performed, and no surgery. When a surgical procedure was performed, axillary procedures were collected in 5 categories: for lymph-node sampling, for axillary clearance, unspecified whether for sampling or clearance, not specified if done or not, and not done. Information was also sought on sentinel lymph-node biopsy, with or without lymphadenectomy, but sentinel biopsy was very uncommon during 1996–98. Information on biopsy or needle aspiration of the breast was coded in 5 categories as either done; not done because of refusal or death, or for specified medical contraindications, or for other or unspecified reasons; or unknown if done or not. Chemotherapy, radiotherapy and hormonal therapy were coded as yes, no or unknown.
Primary treatment for early node-negative disease was dichotomised as breast-conserving surgery with radiotherapy (BCS+RT) vs. all other surgical procedures, whether or not followed by radiotherapy. Chemotherapy and endocrine treatment were dichotomised as administered vs. not administered or unknown.
Statistical Analysis
We examined the proportion of women with early, node-negative disease who received breast-conserving surgery plus radiotherapy; the proportion of women with node-positive disease who received chemotherapy; the proportion of women with estrogen-receptor-positive tumours who received tamoxifen, and the proportion of women for whom at least 10 lymph nodes were removed and examined during lymphadenectomy, as recommended in the TNM manual for staging breast cancer from 1992 (4th edition, 2nd revision)12. Cancer registry data sets were excluded if data on stage and/or treatment were missing for 20% or more of patients. Thus Firenze and Ragusa were excluded from the analyses of chemotherapy in node-positive disease, and Firenze, Genova and Ragusa were excluded from the analyses of hormonal treatment in estrogen-receptor-positive disease.
Net survival up to five years after diagnosis was estimated by jurisdiction (registry, country and European region), age and stage using flexible parametric excess hazard models13. Net survival is the survival of cancer patients in the hypothetical situation when the cancer may be assumed to be the only possible cause of death; it may be interpreted as cancer survival after controlling for competing causes of death. Net survival was estimated with a modelling approach14,15, in which the total hazard of death is considered as the sum of the cancer-related mortality hazard (excess hazard), and the hazard of death from other causes (background hazard). The background hazard is derived from life tables of all-cause mortality by sex, single year of age and calendar year in the general population of the country, region or (in the US) state from which the cancer patients are drawn. We constructed period life tables for 1994–2004 with the approaches proposed by Baili et al.16.
Age was included as a continuous variable in all models, in order to avoid the bias in the estimation of net survival that would otherwise arise from differential loss of the oldest patients (informative censoring). Both non-linear and time-dependent (interaction with time since diagnosis) effects of age were initially modelled with cubic splines. The proportionality of the effect of tumour stage on the excess hazard was also assessed. Simpler models, with linear and/or proportional effects, were successively tested and selected using the Akaike Information Criterion for goodness of fit17. We also estimated the instantaneous excess risk (hazard) of death due to breast cancer, after subtracting the hazard from all other causes of death14,15,18,19. We present the mean excess hazard per 1,000 person-years at risk at 1 month, 6 months and 1, 3 and 5 years since diagnosis, both by age and by stage at diagnosis after adjustment for age.
Overall (all-ages) net survival estimates were age-standardised with the international cancer survival standard (ICSS) weights20.
We used a logistic regression model to estimate the odds of women with early node-negative disease receiving breast-conserving surgery and radiotherapy (vs. any other surgical procedure, with or without radiotherapy) in each jurisdiction, after adjustment for age and tumour size.
Survival analyses were performed with stpm218 in Stata version 11 (StataCorp LP, College Station, TX).
Results
We included 18,962 women with invasive primary breast cancer: 15,842 women in 26 jurisdictions in 12 European countries and 3,120 women in 7 US states (Table 1). Microscopic verification was available for 98–99% of the women in each of the US states and 94% in Europe, ranging from 79% in Estonia to 100% in the Basque country (Spain). Data were available on stage for about 90% of cases in both data sets, ranging from 78% (Ragusa, Italy) to 95% or more in 8 of the 26 European registries and from 81% (New York) to 94% (Colorado and S Carolina) in the US.
Table 1.
Stage at diagnosis for women with invasive primary breast cancer, Europe and US: availability (%) of data and distribution.
| EUROPE | Registry | Period of diagnosis | Morphologically verified | Stage data available | Early node-negative (T1 N0 M0)
|
Large node-negative (T2-3 N0 M0)
|
Node-positive (T1-3 N+ M0)
|
Locally advanced (T4 any N M0)
|
Metastases (M1)
|
Unstaged
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | % | % | % | % | % | % | % | |||
| Denmark | National | 1994 | 500 | 99 | 94 | 28 | 11 | 28 | 24 | 3 | 6 |
| Estonia | National | 1997 | 522 | 79 | 94 | 17 | 15 | 35 | 17 | 11 | 6 |
| Finland | National | 1996–1997 | 552 | 95 | 82 | 36 | 9 | 30 | 4 | 2 | 18 |
| France | Bas-Rhin | 1995 | 175 | 95 | 97 | 42 | 15 | 29 | 3 | 7 | 3 |
| Côte d’Or | 1996–1997 | 521 | 99 | 96 | 43 | 14 | 31 | 5 | 3 | 4 | |
| Doubs | 1997 | 234 | 97 | 98 | 34 | 21 | 34 | 2 | 7 | 2 | |
| Isère | 1997 | 243 | 95 | 95 | 49 | 12 | 30 | 0 | 4 | 5 | |
| Tarn | 1997 | 231 | 98 | 94 | 36 | 16 | 28 | 6 | 8 | 6 | |
| Iceland | National | 1995–1998 | 458 | 97 | 90 | 36 | 13 | 35 | 1 | 4 | 10 |
| Italy | Firenze | 1997 | 330 | 94 | 82 | 35 | 9 | 25 | 8 | 4 | 18 |
| Genova | 1996 | 523 | 93 | 92 | 34 | 14 | 27 | 10 | 7 | 8 | |
| Modena | 1997 | 478 | 97 | 95 | 48 | 10 | 32 | 3 | 3 | 5 | |
| Palermo | 1999 | 580 | 93 | 89 | 26 | 13 | 35 | 9 | 6 | 11 | |
| Ragusa | 1996–1998 | 392 | 87 | 78 | 20 | 13 | 36 | 3 | 6 | 22 | |
| Varese | 1996–1997 | 1,126 | 97 | 92 | 32 | 12 | 33 | 10 | 5 | 8 | |
| Netherlands | Eindhoven | 1997–1998 | 1,281 | 99 | 97 | 43 | 13 | 31 | 6 | 4 | 3 |
| NE Netherlands | 1997 | 2,237 | 92 | 92 | 32 | 18 | 30 | 6 | 7 | 8 | |
| Poland | Cracow | 1997–1998 | 619 | 84 | 93 | 23 | 13 | 44 | 7 | 5 | 7 |
| Warsaw | 1996 | 597 | 96 | 88 | 23 | 13 | 42 | 6 | 4 | 12 | |
| Slovakia | National | 1996 | 551 | 97 | 98 | 22 | 14 | 36 | 8 | 19 | 2 |
| Slovenia | National | 1997 | 882 | 82 | 91 | 23 | 15 | 32 | 11 | 10 | 9 |
| Spain | Basque Country | 1996 | 541 | 100 | 94 | 28 | 18 | 36 | 8 | 5 | 6 |
| Castellon | 1995–1998 | 769 | 98 | 96 | 33 | 16 | 34 | 6 | 7 | 4 | |
| Granada | 1996–1997 | 500 | 95 | 93 | 16 | 22 | 38 | 11 | 6 | 7 | |
| Navarra | 1996–1997 | 500 | 99 | 93 | 39 | 13 | 31 | 4 | 6 | 7 | |
| Sweden | National | 1994 | 500 | 94 | 90 | 36 | 11 | 25 | 14 | 5 | 10 |
|
|
|||||||||||
| European registries | 15,842 | 94 | 92 | 32 | 14 | 33 | 8 | 6 | 8 | ||
| Northern Europe | 2,010 | 96 | 89 | 34 | 11 | 29 | 11 | 3 | 11 | ||
| Western Europe | 4,922 | 95 | 95 | 37 | 16 | 30 | 5 | 6 | 5 | ||
| Southern Europe | 6,621 | 94 | 91 | 30 | 14 | 33 | 8 | 6 | 9 | ||
| Eastern Europe | 2,289 | 89 | 93 | 21 | 14 | 39 | 9 | 9 | 7 | ||
|
|
|||||||||||
| US | State | ||||||||||
| California | 1997 | 458 | 99 | 92 | 43 | 12 | 28 | 4 | 5 | 8 | |
| Colorado | 1997 | 485 | 99 | 94 | 45 | 14 | 26 | 5 | 4 | 6 | |
| Illinois | 1997 | 467 | 99 | 88 | 37 | 16 | 22 | 6 | 8 | 12 | |
| Louisiana | 1997 | 492 | 99 | 88 | 37 | 17 | 25 | 4 | 6 | 12 | |
| New York | 1997 | 448 | 98 | 81 | 33 | 10 | 29 | 3 | 5 | 19 | |
| Rhode Island | 1997 | 403 | 99 | 87 | 42 | 11 | 27 | 3 | 4 | 13 | |
| South Carolina | 1997 | 367 | 98 | 94 | 38 | 18 | 28 | 5 | 5 | 6 | |
|
|
|||||||||||
| US registries | 3,120 | 99 | 89 | 39 | 14 | 26 | 4 | 5 | 11 | ||
Breast cancers were generally less advanced in the US than in Europe, and the stage distribution varied less between US states than between European jurisdictions. Early node-negative tumours were more frequent in the US (39%, range 33–45%) than in Europe (32%, 16–49%). Large node-negative tumours were of similar frequency (Europe 14%, 9–22%; US 14%, 10–18%), while node-positive tumours were more common in Europe (33%, 25–44%) than the US (26%, 22–29%). Locally advanced tumours were twice as frequent in Europe (8%, 0–24%) as in the US (4%, 3–6%), but the overall frequency of metastatic tumours was similar (5–6%). The proportion of tumours with unspecified stage was slightly higher in the US (11%) than Europe (8%), but up to 18–22% in three European registries (Finland; Italy: Firenze, Ragusa), while only New York (19%) differed much from the US average. Exclusion of these registries did not substantially alter the overall stage distributions in Europe or the US (data not shown).
Lymphadenectomy was reported for 13,687 (86%) women in Europe and 2,531 (81%) in the US, but it was generally more extensive in the US, where 10 or more nodes were examined in 78% (range 76–83%) of procedures, compared with 66% (23–93%) in Europe (Table 2). Among women with early node-negative tumours, the distribution of tumour size was more favourable in the US than in Europe.
Table 2.
No. of lymph nodes examined among women with breast cancer who underwent lymphadenectomy, and distribution (%) of tumour size in women with early, node-negative (T1N0M0) breast cancer treated by surgery: Europe and US
| Lymphadenectomy | 10 or more lymph nodes examined | Women with early node-negative disease treated by surgery1
|
|||||
|---|---|---|---|---|---|---|---|
| Less than 5mm (T1a) | 5 to 10mm (T1b) | Over 10mm up to 20mm (T1c) | |||||
| EUROPE | Registry | No. | % | No. | % | % | % |
| Denmark | National | 445 | 59 | 142 | 7 | 25 | 68 |
| Estonia | National | 383 | 23 | 87 | 0 | 17 | 80 |
| Finland | National | 461 | 33 | 200 | 4 | 34 | 63 |
| France | Bas-Rhin | 168 | 66 | 74 | 5 | 23 | 72 |
| Côte d’Or | 489 | 47 | 226 | 8 | 40 | 52 | |
| Doubs | 220 | 35 | 79 | 6 | 27 | 67 | |
| Isère | 227 | 67 | 118 | 5 | 45 | 50 | |
| Tarn | 208 | 75 | 83 | 5 | 37 | 58 | |
| Iceland | National | 393 | 65 | 165 | 5 | 28 | 64 |
| Italy | Firenze | 256 | 93 | 116 | 4 | 17 | 66 |
| Genova | 454 | 84 | 178 | 9 | 18 | 58 | |
| Modena | 459 | 81 | 226 | 9 | 34 | 56 | |
| Palermo | 568 | 77 | 148 | 9 | 18 | 46 | |
| Ragusa | 299 | 80 | 77 | 6 | 17 | 62 | |
| Varese | 969 | 91 | 359 | 4 | 25 | 70 | |
| Netherlands | Eindhoven | 1,172 | 60 | 542 | 4 | 28 | 65 |
| NE Netherlands | 1,896 | 50 | 715 | 2 | 18 | 48 | |
| Poland | Cracow | 516 | 67 | 144 | 6 | 26 | 68 |
| Warsaw | 487 | 83 | 139 | 6 | 22 | 65 | |
| Slovakia | National | 456 | 27 | 120 | 5 | 26 | 69 |
| Slovenia | National | 688 | 89 | 200 | 4 | 18 | 74 |
| Spain | Basque Country | 483 | 75 | 151 | 5 | 25 | 64 |
| Castellon | 707 | 78 | 256 | 6 | 28 | 63 | |
| Granada | 431 | 86 | 82 | 2 | 22 | 62 | |
| Navarra | 430 | 86 | 193 | 11 | 24 | 65 | |
| Sweden | National | 422 | 49 | 179 | 4 | 36 | 60 |
|
|
|
||||||
| European registries | 13,687 | 66 | 4,999 | 5 | 26 | 61 | |
| US | State | ||||||
| California | 397 | 79 | 198 | 8 | 31 | 59 | |
| Colorado | 399 | 78 | 215 | 12 | 28 | 57 | |
| Illinois | 376 | 78 | 171 | 13 | 32 | 50 | |
| Louisiana | 415 | 75 | 181 | 7 | 32 | 60 | |
| New York | 335 | 83 | 150 | 11 | 34 | 53 | |
| Rhode Island | 313 | 76 | 167 | 11 | 25 | 62 | |
| South Carolina | 296 | 76 | 138 | 9 | 23 | 62 | |
|
|
|
||||||
| US registries | 2,531 | 78 | 1,220 | 10 | 29 | 57 | |
Percentages do not add to 100%: early node-negative tumours with no precise data on tumour size are not shown
More than 90% of women received surgical treatment: 91% in Europe (from 77% in Estonia to 95% or more in 10 of 26 jurisdictions) and 96% in the US (93–97%; Table 3). Among operated women, 35% had early node-negative disease in Europe, compared with 41% in the US. Among women operated for early node-negative disease, breast-conserving surgery plus radiotherapy was received by 55% in Europe and 49% in the US, but the variability was much wider in Europe (9% in Estonia; 78–84% in four of the five French regions) than in the US (34% in S Carolina; 58% in Rhode Island). The proportion of women aged 70–99 years who received breast-conserving surgery and radiotherapy for early node-negative disease varied more between European countries and regions (4–6% in two Polish regions; 84% in Tarn) than between US states (21% in Louisiana; 47–48% in Rhode Island and California).
Table 3.
Breast-conserving surgery plus radiotherapy (BCS+RT) in early node-negative breast cancer (T1N0M0), by age: Europe and US
| EUROPE | Registry | All women
|
Surgically treated
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Any stage
|
Early node-negative
|
||||||||||||||
| No. | % | All ages (15–99 years)
|
15–69 years
|
70–99 years
|
||||||||||||
| No. | % | BCS + RT
|
No. | % | BCS + RT
|
No. | % | BCS + RT
|
||||||||
| No. | % | No. | % | No. | % | |||||||||||
| Denmark | National | 500 | 487 | 97 | 142 | 29 | 38 | 27 | 122 | 86 | 35 | 29 | 20 | 14 | 3 | 15 |
| Estonia | National | 522 | 404 | 77 | 87 | 22 | 8 | 9 | 73 | 84 | 7 | 10 | 14 | 16 | 1 | 7 |
| Finland | National | 552 | 520 | 94 | 200 | 38 | 120 | 60 | 163 | 82 | 111 | 68 | 37 | 19 | 9 | 24 |
| France | Bas-Rhin | 175 | 165 | 94 | 74 | 45 | 47 | 64 | 61 | 82 | 40 | 66 | 13 | 18 | 7 | 54 |
| Côte d’Or | 521 | 501 | 96 | 226 | 45 | 189 | 84 | 193 | 85 | 164 | 85 | 33 | 15 | 25 | 76 | |
| Doubs | 234 | 225 | 96 | 79 | 35 | 62 | 78 | 63 | 80 | 53 | 84 | 16 | 20 | 9 | 56 | |
| Isère | 243 | 233 | 96 | 118 | 51 | 97 | 82 | 101 | 86 | 84 | 83 | 17 | 14 | 13 | 76 | |
| Tarn | 231 | 220 | 95 | 83 | 38 | 70 | 84 | 58 | 70 | 49 | 84 | 25 | 30 | 21 | 84 | |
| Iceland | National | 458 | 438 | 96 | 165 | 38 | 74 | 45 | 139 | 84 | 72 | 52 | 26 | 16 | 2 | 8 |
| Italy | Firenze | 330 | 299 | 91 | 116 | 39 | 86 | 74 | 85 | 73 | 70 | 82 | 31 | 27 | 16 | 52 |
| Genova | 523 | 487 | 93 | 178 | 37 | 123 | 69 | 145 | 81 | 107 | 74 | 33 | 19 | 16 | 48 | |
| Modena | 478 | 465 | 97 | 226 | 49 | 124 | 55 | 177 | 78 | 114 | 64 | 49 | 22 | 10 | 20 | |
| Palermo | 580 | 555 | 96 | 148 | 27 | 104 | 70 | 126 | 85 | 91 | 72 | 22 | 15 | 13 | 59 | |
| Ragusa | 392 | 337 | 86 | 77 | 23 | 41 | 53 | 62 | 81 | 37 | 60 | 15 | 19 | 4 | 27 | |
| Varese | 1,126 | 1,053 | 94 | 359 | 34 | 158 | 44 | 285 | 79 | 138 | 48 | 74 | 21 | 20 | 27 | |
| Netherlands | Eindhoven | 1,281 | 1,219 | 95 | 542 | 44 | 359 | 66 | 428 | 79 | 300 | 70 | 114 | 21 | 59 | 52 |
| NE Netherlands | 2,237 | 1,989 | 89 | 715 | 36 | 366 | 51 | 580 | 81 | 330 | 57 | 135 | 19 | 36 | 27 | |
| Poland | Cracow | 619 | 518 | 84 | 144 | 28 | 16 | 11 | 119 | 83 | 15 | 13 | 25 | 17 | 1 | 4 |
| Warsaw | 597 | 532 | 89 | 139 | 26 | 28 | 20 | 121 | 87 | 27 | 22 | 18 | 13 | 1 | 6 | |
| Slovakia | National | 551 | 485 | 88 | 120 | 25 | 42 | 35 | 99 | 83 | 38 | 38 | 21 | 18 | 4 | 19 |
| Slovenia | National | 882 | 718 | 81 | 200 | 28 | 121 | 61 | 169 | 85 | 117 | 69 | 31 | 16 | 4 | 13 |
| Spain | Basque Country | 541 | 506 | 94 | 151 | 30 | 103 | 68 | 131 | 87 | 91 | 69 | 20 | 13 | 12 | 60 |
| Castellon | 769 | 730 | 95 | 256 | 35 | 98 | 38 | 220 | 86 | 93 | 42 | 36 | 14 | 5 | 14 | |
| Granada | 500 | 458 | 92 | 82 | 18 | 37 | 45 | 72 | 88 | 36 | 50 | 10 | 12 | 1 | 10 | |
| Navarra | 500 | 459 | 92 | 193 | 42 | 149 | 77 | 173 | 90 | 140 | 81 | 20 | 10 | 9 | 45 | |
| Sweden | National | 500 | 471 | 94 | 179 | 38 | 82 | 46 | 138 | 77 | 72 | 52 | 41 | 23 | 10 | 24 |
|
|
|
|
|
|
||||||||||||
| European registries | 15,842 | 14,474 | 91 | 4,999 | 35 | 2,742 | 55 | 4,103 | 82 | 2,431 | 59 | 896 | 22 | 311 | 35 | |
| US | State | |||||||||||||||
| California | 458 | 440 | 96 | 198 | 45 | 106 | 54 | 138 | 70 | 77 | 56 | 60 | 30 | 29 | 48 | |
| Colorado | 485 | 470 | 97 | 215 | 46 | 114 | 53 | 163 | 76 | 97 | 60 | 52 | 24 | 17 | 33 | |
| Illinois | 467 | 436 | 93 | 171 | 39 | 94 | 55 | 117 | 68 | 73 | 62 | 54 | 32 | 21 | 39 | |
| Louisiana | 492 | 475 | 97 | 181 | 38 | 67 | 37 | 119 | 66 | 54 | 45 | 62 | 34 | 13 | 21 | |
| New York | 448 | 430 | 96 | 150 | 35 | 77 | 51 | 95 | 63 | 55 | 58 | 55 | 37 | 22 | 40 | |
| Rhode Island | 403 | 380 | 94 | 167 | 44 | 97 | 58 | 109 | 65 | 70 | 64 | 58 | 35 | 27 | 47 | |
| South Carolina | 367 | 351 | 96 | 138 | 39 | 47 | 34 | 96 | 70 | 35 | 36 | 42 | 30 | 12 | 29 | |
|
|
|
|
|
|
||||||||||||
| US registries | 3,120 | 2,982 | 96 | 1,220 | 41 | 602 | 49 | 837 | 69 | 461 | 55 | 383 | 31 | 141 | 37 | |
For early node-negative disease, and relative to Southern Europe (1,848 women, reference category), the odds of receiving both breast-conserving surgery and radiotherapy (vs. any other surgical procedure, with or without radiotherapy), adjusted for age and tumour size, were lower in the US (OR=0.80; 95%CI 0.69–0.94) and Northern Europe (OR=0.60; 0.50–0.72); much lower in Eastern Europe (OR=0.16; 0.12–0.20), and higher in Western Europe (OR=1.57; 1.36–1.81) (Table 4). The odds of receiving this treatment were significantly lower for women aged 70–99 years than for those aged 60–69 years (OR=0.48; 0.41–0.56), after adjustment for region and tumour size. Women with tumours of 5–10mm (T1b) received this treatment more than women with larger tumours (up to 20mm, T1c) (OR=1.31; 1.16–1.48).
Table 4.
Odds ratio (OR) for women with early node-negative disease (T1N1M0) being treated with breast-conserving surgery and radiotherapy (vs. any other surgical procedure, with or without radiotherapy) in each jurisdiction, adjusted for age and tumour size
| No.1 | OR | 95% CI
|
||
|---|---|---|---|---|
| Jurisdiction | ||||
| Northern Europe | 681 | 0.60 | 0.50 | 0.72 |
| Western Europe | 1,595 | 1.57 | 1.36 | 1.81 |
| Southern Europe | 1,848 | 1 | ||
| Eastern Europe | 477 | 0.16 | 0.12 | 0.20 |
| US | 1,185 | 0.80 | 0.69 | 0.94 |
| Age (years) | ||||
| 15–39 | 244 | 1.33 | 0.99 | 1.78 |
| 40–49 | 1,039 | 1.44 | 1.21 | 1.70 |
| 50–59 | 1,558 | 1.38 | 1.19 | 1.60 |
| 60–69 | 1,614 | 1 | ||
| 70–99 | 1,331 | 0.48 | 0.41 | 0.56 |
| Tumour size | ||||
| Less than 5mm (T1a) | 380 | 0.94 | 0.75 | 1.17 |
| 5–10mm (T1b) | 1,650 | 1.31 | 1.16 | 1.48 |
| >10mm, up to 20mm (T1c) | 3,756 | 1 | ||
|
|
||||
Number of women with early node-negative disease who were operated, with information on tumour size available
Among women with node-positive tumours, 58% received chemotherapy in the 26 European jurisdictions, compared with 69% in the 7 US states (Table 5). Among women aged less than 50 at diagnosis, the overall proportion was similar in Europe and the US (90%), but the range was wider in Europe (54–100%) than the US (84–94%). Among older women, the proportion who received chemotherapy was higher, and varied less, in the US (60%, range 53–67%) than in Europe (46%, range 14–75%).
Table 5.
Chemotherapy in node-positive disease and endocrine treatment in estrogen-receptor-positive disease, by age: registry and country
| Women with node-positive tumours
|
Women with estrogen-receptor-positive tumours
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–49 years
|
50–99 years
|
No. | Endocrine treatment |
15–49 years
|
50–99 years
|
||||||||||||||||||
| EUROPE | Registry | No. | Chemo- therapy |
Total
|
Chemo- therapy |
Total
|
Chemo- therapy |
Total
|
Endocrine treatment |
Total
|
Endocrine treatment |
||||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| Denmark | National | 204 | 43 | 21 | 35 | 17 | 19 | 54 | 169 | 83 | 24 | 14 | 340 | 115 | 34 | 52 | 15 | 4 | 8 | 288 | 0 | 111 | 39 |
| Estonia | National | 300 | 236 | 79 | 81 | 27 | 76 | 94 | 219 | 73 | 160 | 73 | 110 | 94 | 85 | 34 | 31 | 24 | 71 | 76 | 69 | 70 | 92 |
| Finland | National | 189 | 71 | 38 | 52 | 28 | 41 | 79 | 137 | 72 | 30 | 22 | 376 | 125 | 33 | 89 | 24 | 21 | 24 | 287 | 76 | 104 | 36 |
| France | Bas-Rhin | 63 | 48 | 76 | 17 | 27 | 17 | 100 | 46 | 73 | 31 | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Côte d’Or | 194 | 110 | 57 | 67 | 35 | 58 | 87 | 127 | 65 | 52 | 41 | 396 | 220 | 56 | 106 | 27 | 33 | 31 | 290 | 73 | 187 | 64 | |
| Doubs | 94 | 59 | 63 | 25 | 27 | 21 | 84 | 69 | 73 | 38 | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Isère | 78 | 50 | 64 | 20 | 26 | 19 | 95 | 58 | 74 | 31 | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Tarn | 86 | 52 | 60 | 23 | 27 | 21 | 91 | 63 | 73 | 31 | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Iceland | National | 178 | 97 | 54 | 59 | 33 | 53 | 90 | 119 | 67 | 44 | 37 | 303 | 162 | 53 | 77 | 25 | 32 | 42 | 226 | 75 | 130 | 58 |
| Italy | Genova1 | 193 | 139 | 72 | 46 | 24 | 42 | 91 | 147 | 76 | 97 | 66 | |||||||||||
| Modena | 169 | 129 | 76 | 45 | 27 | 40 | 89 | 124 | 73 | 89 | 72 | 320 | 221 | 69 | 75 | 23 | 59 | 79 | 245 | 77 | 162 | 66 | |
| Palermo | 252 | 198 | 79 | 77 | 31 | 75 | 97 | 175 | 69 | 123 | 70 | 209 | 148 | 71 | 70 | 33 | 53 | 76 | 139 | 67 | 95 | 68 | |
| Varese | 455 | 250 | 55 | 131 | 29 | 90 | 69 | 324 | 71 | 160 | 49 | 685 | 246 | 36 | 157 | 23 | 42 | 27 | 528 | 77 | 204 | 39 | |
| Netherlands | Eindhoven | 477 | 181 | 38 | 144 | 30 | 133 | 92 | 333 | 70 | 48 | 14 | 552 | 189 | 34 | 149 | 27 | 18 | 12 | 403 | 73 | 171 | 42 |
| NE Netherlands | 823 | 323 | 39 | 236 | 29 | 220 | 93 | 587 | 71 | 103 | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Poland | Cracow | 336 | 184 | 55 | 84 | 25 | 74 | 88 | 252 | 75 | 110 | 44 | 104 | 66 | 63 | 22 | 21 | 13 | 59 | 82 | 79 | 53 | 65 |
| Warsaw | 290 | 157 | 54 | 96 | 33 | 80 | 83 | 194 | 67 | 77 | 40 | 89 | 61 | 69 | 26 | 29 | 10 | 38 | 63 | 71 | 51 | 81 | |
| Slovakia | National | 288 | 219 | 76 | 99 | 34 | 91 | 92 | 189 | 66 | 128 | 68 | 93 | 49 | 53 | 34 | 37 | 12 | 35 | 59 | 63 | 37 | 63 |
| Slovenia | National | 402 | 282 | 70 | 108 | 27 | 106 | 98 | 294 | 73 | 176 | 60 | 385 | 227 | 59 | 88 | 23 | 24 | 27 | 297 | 77 | 203 | 68 |
| Spain | Basque Country | 228 | 190 | 83 | 87 | 38 | 84 | 97 | 141 | 62 | 106 | 75 | 260 | 213 | 82 | 76 | 29 | 49 | 64 | 184 | 71 | 164 | 89 |
| Castellon | 327 | 240 | 73 | 85 | 26 | 82 | 96 | 242 | 74 | 158 | 65 | 355 | 290 | 82 | 86 | 24 | 70 | 81 | 269 | 76 | 220 | 82 | |
| Granada | 237 | 178 | 75 | 72 | 30 | 71 | 99 | 165 | 70 | 107 | 65 | 157 | 127 | 81 | 45 | 29 | 29 | 64 | 112 | 71 | 98 | 88 | |
| Navarra | 169 | 138 | 82 | 67 | 40 | 67 | 100 | 102 | 60 | 71 | 70 | 317 | 252 | 79 | 86 | 27 | 72 | 84 | 231 | 73 | 180 | 78 | |
| Sweden | National | 159 | 45 | 28 | 34 | 21 | 27 | 79 | 125 | 79 | 18 | 14 | 248 | 123 | 50 | 41 | 17 | 7 | 17 | 207 | 83 | 116 | 56 |
|
|
|||||||||||||||||||||||
| European registries | 6,191 | 3,619 | 58 | 1,790 | 29 | 1,607 | 90 | 4,401 | 71 | 2,012 | 46 | 5,299 | 2,928 | 55 | 1,313 | 25 | 572 | 44 | 3,986 | 75 | 2,356 | 59 | |
| US | State | ||||||||||||||||||||||
| California | 154 | 107 | 69 | 55 | 36 | 46 | 84 | 99 | 64 | 61 | 62 | 287 | 157 | 55 | 68 | 24 | 31 | 46 | 219 | 76 | 126 | 58 | |
| Colorado | 155 | 113 | 73 | 51 | 33 | 48 | 94 | 104 | 67 | 65 | 63 | 290 | 192 | 66 | 73 | 25 | 47 | 64 | 217 | 75 | 145 | 67 | |
| Illinois | 133 | 87 | 65 | 43 | 32 | 39 | 91 | 90 | 68 | 48 | 53 | 233 | 129 | 55 | 45 | 19 | 23 | 51 | 188 | 81 | 106 | 56 | |
| Louisiana | 155 | 102 | 66 | 38 | 25 | 32 | 84 | 117 | 75 | 70 | 60 | 260 | 116 | 45 | 40 | 15 | 18 | 45 | 220 | 85 | 98 | 45 | |
| New York | 155 | 106 | 68 | 47 | 30 | 42 | 89 | 108 | 70 | 64 | 59 | 228 | 147 | 64 | 42 | 18 | 20 | 48 | 186 | 82 | 127 | 68 | |
| Rhode Island | 128 | 84 | 66 | 34 | 27 | 32 | 94 | 94 | 73 | 52 | 55 | 301 | 245 | 81 | 68 | 23 | 52 | 76 | 233 | 77 | 193 | 83 | |
| South Carolina | 121 | 89 | 74 | 35 | 29 | 31 | 89 | 86 | 71 | 58 | 67 | 186 | 121 | 65 | 39 | 21 | 26 | 67 | 147 | 79 | 95 | 65 | |
|
|
|||||||||||||||||||||||
| US registries | 1,001 | 688 | 69 | 303 | 30 | 270 | 89 | 698 | 70 | 418 | 60 | 1,785 | 1,107 | 62 | 375 | 21 | 217 | 58 | 1,410 | 79 | 890 | 63 | |
n/a - not available
Registries were excluded if data on treatment were unavailable for more than 20% of women: Genova (hormonal treatment), Firenze and Ragusa (both treatments)
Overall, endocrine treatment in ER-positive tumours was slightly higher in the US (62%) than in Europe (55%). The proportion was similar in women aged 50 and over (63% in the US; 59% in Europe), but younger women received tamoxifen more often in the US (58% vs. 44%).
Overall, age-standardised net survival at five years was 81% in Europe and 84% in the US (Figure 1). Survival in Northern, Western and Southern Europe (81–84%) was similar to that in the US (84%), but it was lower in Eastern Europe (69%). Survival varied more widely between European jurisdictions (88% in Iceland to 62% in Estonia) than between US states (from 91% in Colorado to 76% in South Carolina).
Figure 1.
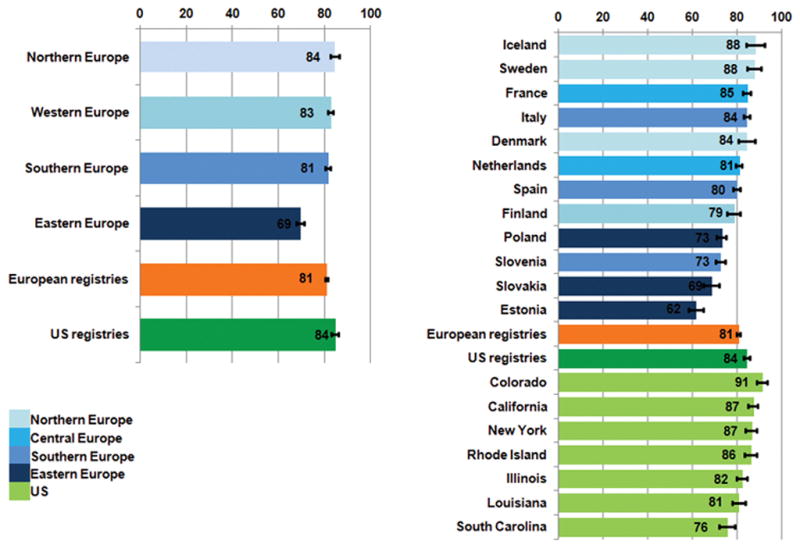
Five-year age-standardised net survival (%), women diagnosed with primary invasive breast cancer in Europe and the US in the late 1990s: country and region
Five-year age-standardised net survival was broadly similar in all European regions and the US for early, node-negative tumours (96–98%) and for large, node-negative tumours (85–90%) (Figure 2, available in web-appendix). The geographic range in survival was much wider for locally advanced disease, from 37% in Eastern Europe to 77% in Northern Europe, and 44% in the US. As with overall survival, stage-specific five-year survival was similar in Northern, Western and Southern Europe and the US. In Eastern Europe, survival for node-positive, locally advanced and metastatic tumours was lower than in other European regions or the US.
The mean excess hazard was higher in Eastern Europe than in other jurisdictions at 1 month, 6 months and 1, 3 and 5 years after diagnosis, both for all ages and in each of 5 age categories (Figure 3, available in web-appendix). The difference was most marked for women aged 70–99 years. No striking differences were found between Northern, Western, Southern Europe and the US. The high excess hazard of death in Eastern Europe was mainly confined to women with locally advanced or metastatic tumours (Figure 4, available in web-appendix).
Discussion
Transatlantic differences in cancer survival have raised questions about early diagnosis and the adequacy of investigation and treatment. To our knowledge, this is the first population-based high-resolution study to use clinical data that were collected by trained abstractors from the primary medical records under a common protocol, subjected to standard quality control procedures and analysed centrally with the same statistical methods. We compared survival using clinical data on stage, diagnostic procedures and treatment. The survival differences appear likely to be related to differences in diagnosis and patterns of care shortly after diagnosis. The women were diagnosed more than 10 years ago, but most diagnostic and therapeutic approaches used at that time remain in widespread use: understanding their role in international differences in survival remains relevant.
Overall, five-year net survival was not very different in Europe (81%) and the US (84%). Differences were mainly confined to the three Eastern European countries, Estonia, Poland and Slovakia, where average five-year survival was 69%. Estonia and Slovakia are both covered by national cancer registries, and the women from those countries were thus nationally representative. Survival varied more widely between the 26 European jurisdictions than between the 7 US states.
The differences in survival between Europe and the US in the late 1990s are smaller than for women diagnosed at the beginning of the decade3,4. In the previous high-resolution study4, the US data were taken from the SEER public-use data set21 and harmonised to the extent possible with the data collected under the EUROCARE-2 high-resolution protocol. By contrast, the data for this study were collected directly from the clinical records using a standard protocol; European coverage rose from 17 to 26 registries (11 contributed to both studies), and US coverage changed from the 5 metropolitan areas and 4 states covered by the SEER program to 7 state-wide registries in the National Program of Cancer Registries (NPCR). Survival in the 1990s was lower in the NPCR territories than the SEER areas3,22. Finally, in the previous high-resolution study, differences in background mortality in the US were controlled with a single national life table for 1990, weighted for the proportion of Blacks, Whites and other races, whereas we were able to use state-specific life tables for each calendar year 1994–2004.
The modelling approach used to estimate net survival is a strength of this study, but it does not explain the smaller transatlantic differences than those obtained with relative survival in the previous study. We found similar patterns with all the other widely used methods for survival estimation (data not shown).
The European differences in survival were generally similar to those reported for the same countries among women diagnosed 1995–9911. Survival was higher than expected in Denmark (84%): the data in this study are from eastern Denmark, greater Copenhagen and Copenhagen (Zeeland), where most of the population has undergone mammographic screening since 199123. In these areas, survival after mammographic diagnosis is higher than in Denmark as a whole, regardless of whether it was a screening mammography. Survival in Slovenia was lower than in other Southern European countries, and more similar to that in Eastern Europe. Variation in survival between the 7 US states was less marked than in Europe, mostly in the range 81–87%, but ranging from 91% in Colorado to 76% in South Carolina, where Blacks represent approximately 30% of the population (http://www.ipspr.sc.edu/publication/Older%20SC.pdf).
The availability of information about race in this data set would have strengthened the international survival comparisons, but information about race is not available in many European countries. Race in the US and geographical area in Europe are often considered as a proxy for socio-economic status. In future studies, it would be preferable to use life tables that are specific for race and/or socio-economic status.
Stage-specific net survival was similar in most European jurisdictions and US states. In Eastern Europe, survival from node-positive, large and metastatic tumours (N+; T4; M1) was lower than in other European regions or the US, and the proportion of metastatic tumours was also high, mainly in Estonia and Slovakia.
The mean excess hazard of death by time since diagnosis was similar in Europe and the US for women with early node-negative disease, large node-negative disease or node-positive disease, and up to five years after diagnosis. The hazard was somewhat higher in Eastern Europe for locally advanced disease, and much higher for metastatic disease, especially in the first three years after diagnosis. Adjustment for the number of examined lymph nodes, necessarily restricted to women who underwent lymphadenectomy (86%), did not modify this pattern, either overall, or within each category of stage. In other words, the geographic pattern in the mean excess hazard of death was not affected by the number of nodes examined during lymphadenectomy (data not shown). This suggests that, in contrast with the findings from the study of women diagnosed in 19904, stage migration does not affect the comparison of stage-specific survival between European regions and the US. This could be because the recording of stage has become more homogeneous, or because the quality and completeness of diagnostic investigation is less variable now than previously.
The mean excess hazard of death for women with late-stage disease was very high in Eastern Europe. This suggests that fewer effective treatment options were available for these women, although higher levels of co-morbidity may have restricted therapeutic options. Hormonal treatment and adjuvant chemotherapy were used more extensively in Slovakia, Estonia and Poland than in other European countries, and not just for node-positive and estrogen-receptor positive disease. Mastectomy was often used instead of breast-conserving surgery and radiotherapy, in part because radiotherapy facilities were not always available. Total national expenditure on health was low, and this is also likely to have affected the quality of treatment6.
Data on stage were remarkably complete, because they were collected directly from clinical records. Complete data on stage and lymph nodes were unavailable for all but 5–11% of women in the 5 broad European regions, although for up to 20% in 3 of the 26 European registries. However, exclusion of women with unknown stage or lymph node status did not change the geographic pattern of the excess hazard of death within any of the categories for which stage was known. More complex analyses after imputation of missing values are unlikely to change this picture.
Pattern of care studies and survival have been conducted separately in Europe5,6 and the US24. Here, we could make a direct comparison between Europe and the US with data on stage at diagnosis and treatment collected and coded with the same rules.
Overall, women received breast-conserving surgery and radiotherapy for early node-negative breast cancer somewhat more often in Europe (55%) than the US (49%), but the distribution by age was similar. The lower proportion in the US is mainly determined by Louisiana (37%) and South Carolina (34%) and may be explained by the attitude of some US clinicians during the late 1990s, when radiotherapy may have been considered unnecessary after breast-conserving surgery10. Another explanation may be the paucity of radiotherapy centres and/or the distance of the nearest radiotherapy facility25 in these two states.
After adjusting for age and tumour size within the category of early node-negative disease, the odds of being treated with breast-conserving surgery and radiotherapy were almost 60% higher in Western Europe than Southern Europe (reference), 20–40% lower in the US and Northern Europe, and more than 80% lower in Eastern Europe.
In Denmark, the low level of breast-conserving surgery and radiotherapy was probably related to the fact that most breast cancers were treated in local or regional hospitals (not specialist centres), rather than any lack of radiotherapy facilities, although the Danish national cancer plan of 2000 recognised the need to modernize and expand radiotherapy services. Most women receiving breast-conserving surgery also received radiotherapy, but breast-conserving surgery was hardly ever done in areas where breast cancer screening was not performed26.
About 90% of women aged less than 50 years with node-positive disease received chemotherapy in both Europe and the US, in accordance with contemporary clinical protocols27.
The proportion of women aged 50–99 years with positive lymph nodes who received chemotherapy was notably higher in the US (60% vs. 46%). The proportion was similar in all 7 states, but slightly lower in Illinois and Rhode Island. The finding of more active treatment for older women in the US echoes the finding for women diagnosed in 1990, and may indicate the importance of health insurance programs such as MEDICARE. The US National Institutes of Health had also recommended chemotherapy for node-positive breast cancer in 198528.
In the late 1990s, tamoxifen was recommended for estrogen-receptor positive tumours on both sides of the Atlantic29,30, especially for women aged over 50 years. In the US, the proportion of women aged less than 50 years with ER+ tumours treated with hormonal therapy was 58%, higher than in Europe (44%).
The low proportion of women with early stage disease who receive breast-conserving surgery is correlated with total national expenditure on health6. The wider use of chemotherapy and hormonal treatment may reflect the fact that costs are lower than for surgery and radiotherapy. Taken with the findings of this study, this suggests that low healthcare expenditure in Eastern European countries may have had an important effect on the quality of breast cancer treatment, and on survival.
Differences in breast cancer survival between Europe and the US in the late 1990s were mainly explained by lower survival in Eastern Europe, where low healthcare expenditure may have constrained the quality of breast cancer treatment. Similarly wide variation has also been reported within the US, where non-Hispanic Black women were less likely to receive guideline-concordant treatment than non-Hispanic White women8.
The need for population-based data on stage and treatment is recognised by clinicians and epidemiologists. High-resolution studies still seem to be the only valid way to collect this information. More funding should be directed to help cancer registries obtain timely high-resolution data for all registered patients.
Supplementary Material
Acknowledgments
Some of the data for this study were collected with the support of the Compagnia di San Paolo, Turin, Italy. Support was also obtained from the Health Department of the Navarra Government, Spain (research grant 79/2000). Alleanza Contro il Cancro, the Italian Cancer Network (http://www.alleanzacontroilcancro.it) supported a CONCORD Working Group meeting in London, 29–30 September 2010. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Footnotes
Novelty and impact of the work: Most of the diagnostic and therapeutic modalities used for breast cancer more than 10 years ago remain in widespread use today. Understanding the extent to which access to those modalities can explain international differences in cancer survival therefore remains highly relevant. This is the largest population-based high-resolution study, with a common protocol, standard quality-control procedures and central analyses. The modelling approach to estimate net survival is a methodological strength.
References
- 1.Cutler SJ, editor. International symposium on end results of cancer therapy. Bethesda MD: National Cancer Institute; 1964. NCI Monograph 15. [PubMed] [Google Scholar]
- 2.Gatta G, Capocaccia R, Coleman MP, Ries LAG, Hakulinen T, Micheli A, Sant M, Verdecchia A, Berrino F. Toward a comparison of survival in American and European cancer patients. Cancer. 2000;89:893–900. [PubMed] [Google Scholar]
- 3.Coleman MP, Quaresma M, Berrino F, Lutz J-M, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T, Micheli A, Sant M, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–56. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 4.Sant M, Allemani C, Berrino F, Coleman MP, Aareleid T, Chaplain G, Coebergh JWW, Colonna M, Crosignani P, Danzon A, Federico M, Gafà L, et al. Breast carcinoma survival in Europe and the USA: a population-based study. Cancer. 2004;100:715–22. doi: 10.1002/cncr.20038. [DOI] [PubMed] [Google Scholar]
- 5.Sant M EUROCARE Working Group. Differences in stage and therapy for breast cancer across Europe. Int J Cancer. 2001;93:894–901. doi: 10.1002/ijc.1408. [DOI] [PubMed] [Google Scholar]
- 6.Allemani C, Storm H, Voogd AC, Holli K, Izarzugaza I, Torrella-Ramos A, Bielska-Lasota M, Aareleid T, Ardanaz E, Colonna M, Crocetti E, Danzon A, et al. Variation in ‘standard care’ for breast cancer across Europe: a EUROCARE-3 high resolution study. Eur J Cancer. 2010;46:1528–36. doi: 10.1016/j.ejca.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Sariego J. Regional variation in breast cancer treatment throughout the United States. Am J Surg. 2008;196:572–4. doi: 10.1016/j.amjsurg.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Richardson LC, Kahn AR, Fulton JP, Cress RD, Shen T, Wolf HJ, Bolick-Aldrich S, Chen VW. Survival difference between non-Hispanic black and non-Hispanic white women with localised breast cancer: the impact of guideline-concordant therapy. J Natl Med Assoc. 2008;100:490–8. doi: 10.1016/s0027-9684(15)31295-5. [DOI] [PubMed] [Google Scholar]
- 9.Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the United States following the 1990 National Institutes of Health consensus development conference on the treatment of patients with early-stage invasive breast carcinoma. Cancer. 1999;86:628–37. [PubMed] [Google Scholar]
- 10.Nattinger AB, Hoffmann RG, Kneusel RT, Schapira MM. Relation between the appropriateness of primary therapy for early-stage breast carcinoma and increased use of breast-conserving surgery. Lancet. 2000;356:1148–53. doi: 10.1016/S0140-6736(00)02757-4. [DOI] [PubMed] [Google Scholar]
- 11.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999: results and commentary. Eur J Cancer. 2009;45(Suppl 6):931–91. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Spiessl B, Beahrs OH, Hermanek P, Hutter RVP, Scheibe O, Sobin LH, Wagner KF, editors. TNM Atlas: illustrated guide to the TNM/pTNM classification of malignant tumours. Berlin: Springer Verlag; 1992. [Google Scholar]
- 13.Nelson CP, Lambert PC, Squire IB, Jones DR. Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med. 2007;26:5486–98. doi: 10.1002/sim.3064. [DOI] [PubMed] [Google Scholar]
- 14.Estève J, Benhamou E, Raymond L. Statistical methods in cancer research, volume IV. Descriptive epidemiology. Lyon: International Agency for Research on Cancer; 1994. IARC Scientific Publications No. 128. [PubMed] [Google Scholar]
- 15.Pohar Perme M, Stare J, Estève J. On estimation in relative survival [epub ahead of print] Biometrics. 2011 doi: 10.1111/j.1541-0420.2011.01640.x. doi:10,1111/j.1541–0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 16.Baili P, Micheli A, De Angelis R, Weir HK, Francisci S, Santaquilani M, Hakulinen T, Quaresma M, Coleman MP CONCORD Working Group. Life-tables for world-wide comparison of relative survival for cancer (CONCORD study) Tumori. 2008;94:658–68. doi: 10.1177/030089160809400503. [DOI] [PubMed] [Google Scholar]
- 17.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- 18.Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–90. [Google Scholar]
- 19.Danieli C, Remontet L, Bossard N, et al. Estimating net survival: the importance of allowing for informative censoring. Stat Med. 2011 doi: 10.1002/sim.4464. in press. [DOI] [PubMed] [Google Scholar]
- 20.Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–16. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. SEER Stat - cancer incidence public use database 1973–95. Bethesda, MD: National Cancer Institute; 1998. version 1.1. [Google Scholar]
- 22.Merrill RM, Dearden KA. How representative are the surveillance, epidemiology, and end results (SEER) Program cancer data of the United States? Cancer Causes Control. 2004;15:1027–34. doi: 10.1007/s10552-004-1324-5. [DOI] [PubMed] [Google Scholar]
- 23.Christensen LH, Engholm G, Cortes R, Ceberg J, Tange U, Andersson M, Bladström A, Mouridsen HT, Möller T, Storm H. Reduced mortality for women with mammography-detected breast cancer in east Denmark and south Sweden. Eur J Cancer. 2006;42:2773–80. doi: 10.1016/j.ejca.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Alley LG, Chen VW, Wike JM, Schymura MJ, Rycroft R, Shen T, Bolick-Aldrich S, Roshala W, Fulton JP. CDC and NPCR’s breast, colon, and prostate cancer data quality and patterns of care study: overview and methodology. J Registry Manag. 2007;34:148–57. [Google Scholar]
- 25.Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA. Relationship of distance from radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–6. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 26.Tange UB, Jensen MB, Vejborg IM, Rank FE, Blichert-Toft M, Mouridsen HT, Lynge E. Clinical impact of introduction of mammography screening in a non-screening country with special reference to the Copenhagen service mammography screening programme. Scand J Surg. 2002;91:293–303. doi: 10.1177/145749690209100314. [DOI] [PubMed] [Google Scholar]
- 27.Anonymous. Update of the NCCN guidelines for treatment of breast cancer. Oncology. 1997;11:199–220. [PubMed] [Google Scholar]
- 28.Anonymous. Consensus conference: adjuvant chemotherapy for breast cancer. J Amer Med Assoc. 1985;254:3461–3. [PubMed] [Google Scholar]
- 29.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic or immune therapy. Lancet. 1992;339:1–15. 71–85. [PubMed] [Google Scholar]
- 30.Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


