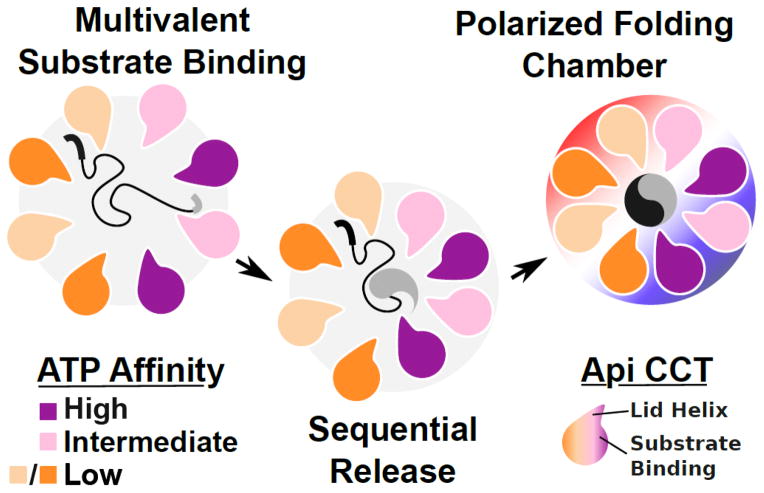
Abstract
Protein folding in the cell requires the assistance of enzymes collectively called chaperones. Among these, the chaperonins are 1 MDa ring-shaped oligomeric complexes that bind unfolded polypeptides and promote their folding within an isolated chamber in an ATP-dependent manner. Group II chaperonins, found in archaea and eukaryotes, contain a built-in lid that opens and closes over the central chamber. In eukaryotes, the chaperonin TRiC/CCT is hetero-oligomeric, consisting of two stacked rings of eight paralogous subunits each. TRiC facilitates folding of approximately 10% of the eukaryotic proteome, including many cytoskeletal components and cell cycle regulators. Folding of many cellular substrates of TRiC cannot be assisted by any other chaperone. A complete structural and mechanistic understanding of this highly conserved and essential chaperonin remains elusive. However, recent work is beginning to shed light on key aspects of chaperonin function, and how their unique properties underlie their contribution to maintaining cellular proteostasis.
Graphical abstract
Chaperonins: The Protein Folding Machines
Among the most striking aspects of protein biology is the manner in which polypeptide chains routinely and rapidly attain an active three dimensional structure with high fidelity. This property, first presented in historic work by Christian Anfinsen [1], implies that both the native conformation and folding trajectory of a protein are encoded in its primary structure. As Levinthal famously argued [2], if the sequence of a peptide did not place some restrictions on the conformational landscape accessible at physiological temperatures, an exhaustive search over all conformational degrees of freedom would take an unreasonably long time. These two observations, namely that small globular proteins attain their native conformations autonomously and that they do so on surprisingly short timescales, serve to frame the biophysical problem of protein folding. The combined weight of many folding studies supports the idea that small globular proteins can fold productively in isolation in a two-state fashion [3, 4]. Nevertheless, the model of two-state folding does not encompass the breadth of the folding problem under physiological conditions. In particular, the cellular environment places folding polypeptides in an environment that disfavors folding and promotes aggregation and misfolding [5]. The vectorial nature of protein synthesis places a topological constraint upon folding, as N-terminal regions of polypeptides are available for folding before the polypeptide is completed [6, 7]. In the cell, proteins also encounter stresses such as temperature, free radicals, and osmolytes which can damage and/or unfold proteins. Unchecked, these perturbations in conjunction with the cytosolic pool of nascent or unfolded polypeptides would lead to protein aggregation en masse in the concentrated cytosol [8]. All of these issues are compounded for the many proteins which cannot fold independently and instead become trapped in intermediate conformations.
To cope with environmental stresses and to facilitate the folding of troublesome or large proteins, cells have evolved a system of molecular chaperones and quality control machinery, often called the “protein homeostasis” or “proteostasis” network. Chaperones are proteins themselves which bind to unfolded or misfolded polypeptides and induce their folding, sequester them, or facilitate their degradation [9]. Members of this cellular proteostasis network constitute the first interacting partners seen by nascent peptides upon departing the ribosome exit tunnel and can be found in both bacteria and eukaryotes [10, 11]. They also commonly represent the final interacting partner of proteins destined for degradation. Among the most important of the molecular chaperones are the chaperonins, large 1 MDa oligomeric complexes comprising two stacked rings, each of which creates a central cavity for polypeptide folding [12, 13]. Chaperonins are ATPases which harness the energy of nucleotide binding and hydrolysis in order encapsulate misfolded proteins in their central cavity such that they may fold in isolation. The chaperonins are present in every kingdom of life and are essential in all sequenced organisms excepting some members of the genus Mycoplasma [14]. The chaperonins are subdivided into two families, termed the group I and group II chaperonins.
The group I chaperonins, of which GroE from Escherichia coli is the archetype, are present in the bacterial cytosol as well as the eukaryotic organelles derived from endosymbiosis. Less frequently, group I chaperonins can be found in archaea [15]. The group I chaperonin system consists of two components, a tetradecameric Hsp60 and a heptameric co-chaperone Hsp10. The Hsp60, known as GroEL in E. coli, consists of two 7-fold symmetric rings related by a 2-fold inter-ring symmetry axis. Each GroEL ring harbors a central cavity in which client proteins are encapsulated for folding. The co-chaperone Hsp10, called GroES in E. coli, binds to GroEL in an ATP-dependent manner acting as a ‘lid’ to prevent substrate egress while greatly expanding the size of the folding chamber [16].
By contrast, group II chaperonins are found in archaea and the eukaryotic cytosol. They also consist of two stacked rings, each composed of eight 50–60 KDa subunits, but do not have an obligate co-chaperone in the same manner as the group I chaperonins. Rather, they contain a built-in lid that closes the folding chamber and are thus competent to fold substrates in vitro without the assistance of accessory proteins. This should not be taken to mean that the group IIs function in isolation in the cell. On the contrary, the group II chaperonins appear to be at the heart of a complex network of co-chaperones [17] [18, 19] [20, 21]. Notable examples include the hexameric prefoldin complex which is often thought to bind to and prevent aggregation of unfolded substrates before handing them off to the chaperonin [22, 23] and the phosducin-like proteins which have been shown to enhance TRiC-mediated folding of several substrates [21, 24].
The eukaryotic group II chaperonin, which is known as TRiC/CCT (TRiC hereafter), differs from its simpler archaeal homologues in that it is composed of 8 paralogous subunits. Most notably, TRiC is absolutely required for folding many essential proteins, including cytoskeletal proteins like tubulin and actin as well as cell cycle regulators such as CDC20, CDH1 [25] [26] [27]. It has been estimated that as much as 10% of cytosolic proteins interact with the eukaryotic chaperonin TRiC along their folding trajectory [28].
Architecture of Group II Chaperonins
Like the group I chaperonins, group IIs are composed of two oligomeric rings related by a 2-fold symmetry axis. While group I chaperonins have 7-fold symmetric rings [29, 30], the group IIs have 8-fold and occasionally 9-fold [31–35] symmetry within their rings. Unlike GroEL, most group II chaperonins are heteromeric. The extreme case is the eukaryotic chaperonin, TRiC/CCT in which each ring contains 8 distinct, paralogous subunits occupying fixed positions in the complex [36] [37].
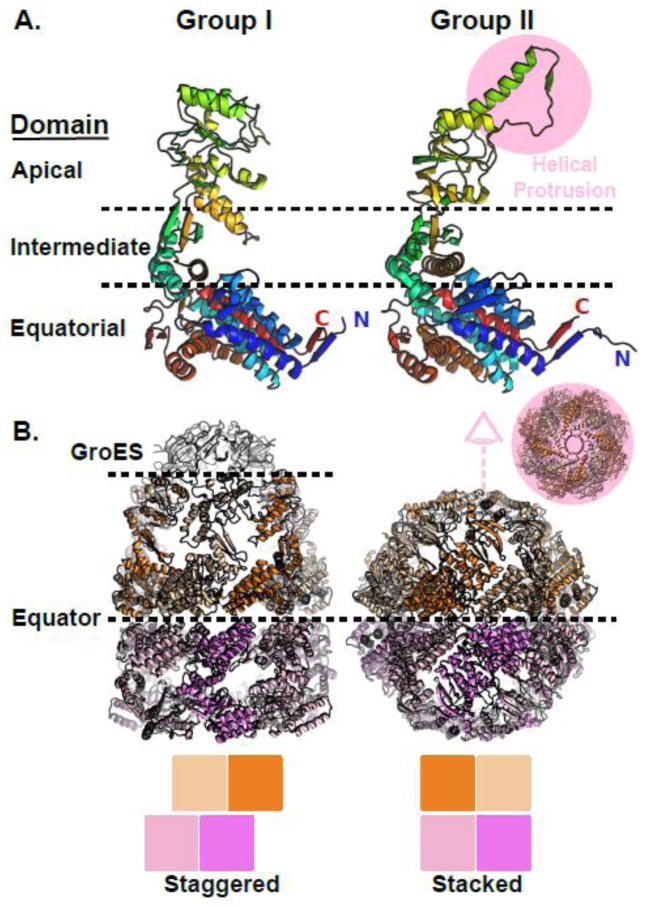
The archetypal group II chaperonin which served as the first structural model for the family is the Thermoplasma acidophilum α/β-thermosome. The first atomic resolution structure of a group II chaperonin was of an isolated apical domain from the thermosome α-subunit [38]. The apical domain, which is the domain that diverges most from the group I in terms of primary sequence, was shown to contain a helical protrusion [38, 39] absent from the structures of E. coli GroEL. A comparison of the domain structures of group I vs group II chaperonins is presented in Figure 1A which highlights the helical protrusion extending from the apical domain of the group II chaperonin MmCpn [40]. The equatorial domain of the chaperonins forms the ring interface and contains most of the residues involved in nucleotide binding. The intermediate domain forms the apical surface of the nucleotide binding pocket and contains the catalytic aspartate which activates water for nucleotide hydrolysis. The structure of the equatorial and intermediate domains is conserved between the group I and group II chaperonins (Figure 1A). When the first structure of the full length thermosome was solved [41] the significance of the apical helix could be appreciated for the first time. The thermosome structure demonstrated that the apical helices form an iris enclosing the folding chamber (Figure 1B inset) thereby allowing the group II chaperonins to function without a co-chaperone lid. The structure also revealed how the subunits of one ring are seated directly in register on a subunit in the second ring, in contrast to the staggered inter-ring registry of the group I chaperonins.
Figure 1.
Structural comparison of group I and group II chaperonins. A) Domain architecture is conserved between group I and group II chaperonins. Left, chain A from the cis-cavity of a GroE crystal structure. Right, chain A from the MmCpn crystal structure. B) Comparison of the GroEL (left) and MmCpn (right) complex architectures. The GroE and MmCpn crystal structures used were PDBID: 1AON [16] and PDBID: 3RUW [40] respectively.
The Structure of the Eukaryotic Chaperonin TRiC
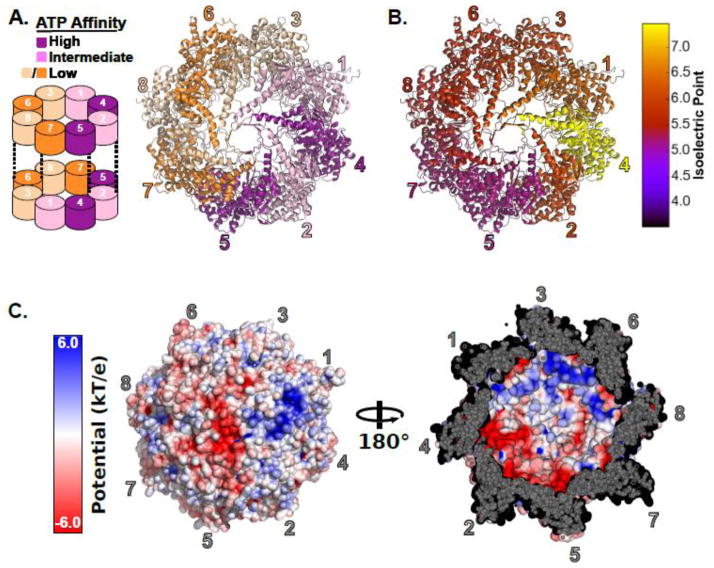
Structures of the TRiC and several of its substrates [42–46] or cofactors [18, 42, 47–49] have been solved by cryoelectron microscopy [46, 50] [51] [52, 53] and small angle x-ray scattering [54]. However, atomic resolution electron density maps have long eluded the field. Owing to the structural similarity of the eight paralogous TRiC subunits, the field has struggled to assign an arrangement to the subunits in the TRiC rings [52, 55, 56]. Only recently, has a hybrid approach utilizing X-ray crystallography and chemical crosslinking mass spectrometry finally yielded the definitive arrangement [57, 58]. The subunit arrangement of TRiC leads to the spatial partitioning of subunits with different chemical properties. Specifically, subunits are segregated by their ATP binding affinities [59] (Figure 2A) and by their net charge (Figure 2B,C). Thus, the hetero-oligomeric nature of TRiC generates functional and chemical asymmetries absent from other chaperonin systems, which likely provide the basis for the unique ability TRiC possesses to fold specific substrates such as actin, which cannot be folded by the GroE chaperonin system[60].
Figure 2.
The subunit arrangement of the hetero-oligomeric eukaryotic chaperonin TRiC. A) Left, a schematic of the subunit arrangement of TRiC showing the inter-ring register. Right, the crystal structure of TRiC showing the subunit arrangement and the partitioning of ATP affinities. Subunits are colored by their ATP affinity. B) Influence of subunit arrangement on charge distribution. Subunits are colored by their isoelectric points. Isoelectric points were estimated for the Saccharomyces cerevisiae CCT subunits using the pepstats program from the EMBOSS suite [112]. C) The surface charge characteristics inside the closed TRiC cavity. Left, a view of the outside of the chaperonin folding chamber colored by surface electrostatic potential. Right, a view of the lid of the folding chamber from the inside illustrating the polarized nature of the TRiC cavity. Surface electrostatics were rendered in PyMOL [113] using APBS [114] Tools [115]. The TRiC structure is PDBID: 4V94 [57] prepared by adding missing heavy atoms in PDBFixer [116].
Nucleotide Driven Conformational Cycle of the Group II Chaperonins
Productive folding of proteins by the group II chaperonins is an ATP-dependent process [36, 61]. The use of archaeal model systems for structural and biochemical work has greatly benefited the group II chaperonin field and contributed to our understanding of the nucleotide cycle of group II chaperonins [62]. Recently, the structures of the apo, nucleotide bound, and closed states of archaeal group II chaperonin have been solved at atomic or near atomic resolution leading to an improved understanding of the domain motions which occur upon nucleotide binding and hydrolysis [31, 63–65]. Furthermore, the first atomic resolution structure of a monomeric archaeal chaperonin was solved in the unliganded state [66] revealing a more spacious nucleotide binding face than what is observed in the closed state structures. The more accessible pocket bears a striking resemblance to that of apo GroEL.
The formation of the closed chaperonin complex begins with the binding of ATP. The lidless variant of the homo-oligomeric archaeal group II chaperonin from Methanococcus maripaludis (MmCpn hereafter)[67] was observed under different nucleotide conditions by cryo-EM. When compared to the apo-state conformation, the apical domains of ATP-bound subunits are rotated approximately 45° [65]. This observation has been corroborated by directly monitoring movements resulting from ATP binding of the thermosome by diffracted X-ray tracking. The phase associated with ATP binding was observed to finish within 1s after the freeing of caged nucleotide [68]. Substrate binding sites on the apical domain are still accessible in this transient state, though not to the extent of the fully open complex.
Transit of the complex along the reaction cycle requires that the state of the bound nucleotide be monitored and communicated across the domains of a single subunit and to neighbors within the complex. This role is fulfilled by a conserved lysine in the intermediate domain of the group II chaperonins [40]. The orientation of Lys-161 is reliant upon the phosphate state of bound nucleotide, moving to interact with either the γ-or ∝-phosphates of ATP and ADP, respectively. This results in significant rearrangement of the loop comprised of residues 160–169, termed the Nucleotide Sensing Loop (NSL), altering intra-and inter-subunit points of communication. Homologous residues in the CCT subunits can be seen to adopt similar conformations when crystallized with ADP-BeF3 [57]. Interestingly, one notable difference between the archaeal and eukaryotic NSLs is the expected flexibility of the each sequence. In the archeal chaperonin MmCpn, the NSL is relatively unencumbered, with glycines flanking Lys-161. By comparison, the CCT sequences contain more sterically restrictive β-branched amino acids. Like other archaeal chaperonins, MmCpn has a much faster nucleotide hydrolysis rate than TRiC [67]. However, the “TRiC-like” mutant G160S of MmCpn was shown to have a drastically slower rate of ATP hydrolysis, roughly equivalent to the steady state hydrolysis of TRiC[40]. The identity of this loop may represent the simplest determinant for controlling the overall speed of the nucleotide hydrolysis cycle, hence acting as a “timer” that determines the residence time of the polypeptide within the closed folding chamber. However, detailed analyses of the discrete steps of nucleotide hydrolysis need to be carried out for mutants of both archaeal and eukaryotic complexes in order to define the particular transitions that are being delayed[69]. Advances in expression and purification of recombinantly sourced TRiC are beginning to make necessary experiments such as these a posibility[70].
Hydrolysis is required to bring the lid helices into close proximity, forming the iris and parallel β-barrel responsible for capping the folding chamber[71]. Opening of the lid occurs in conjunction with releasing ADP from the active site. This slow reopening and ADP release represent the rate limiting steps of the reaction cycle[69]. In MmCpn, this reopening has been shown to be strongly dependent upon the nature of a specific loop within the apical domain[72]. The Release Loop for Substrate, RLS, is a five residue stretch located opposite the substrate binding site and mutation to alanines causes the complex to stall in a doubly closed configuration (Figure 3 & Figure 4). The complex can exist in a symmetrically closed conformation even during ATP cycling conditions, raising questions about the mechanisms that lead to reopening of the lid, and their coordination across the rings. For GroE, cycling is suggested to occur through a two-stroke mechanism [67, 73]. Failure of a chaperonin to release GroES has been observed in the single ring mutant of GoEL SR1. As a result, GroES stays permanently associated with the single-ring chaperonin [74], this observation was viewed as evidence that proper cycling relies on the interaction between the two chambers. This mechanism is less clear in the case of the group II chaperonins, as the allosteric communication that occurs between the rings is less well understood.
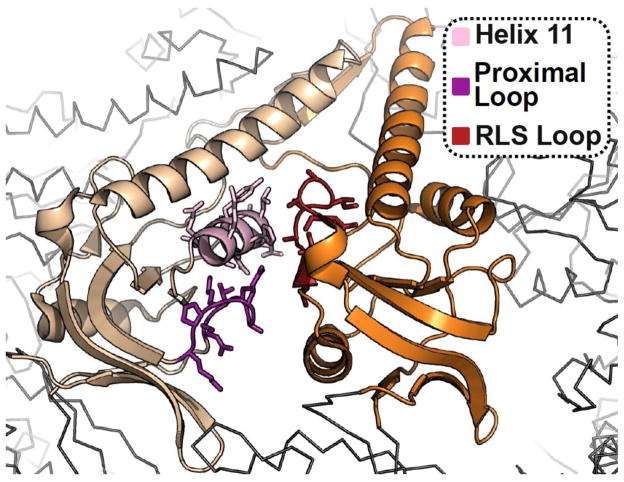
Figure 3.
Structural basis for substrate release during lid closure. The apical domains of the group II chaperonin MmCpn in the closed state, highlighting the substrate binding surface at the interface between two adjacent apical subunits, from PDBID: 3RUW [40]. The substrate binding surface of the left subunit comprising Helix 11 and the Proximal Loop is indicated as well as the RLS Loop of the right hand subunit which is responsible for evicting bound substrate during closure.
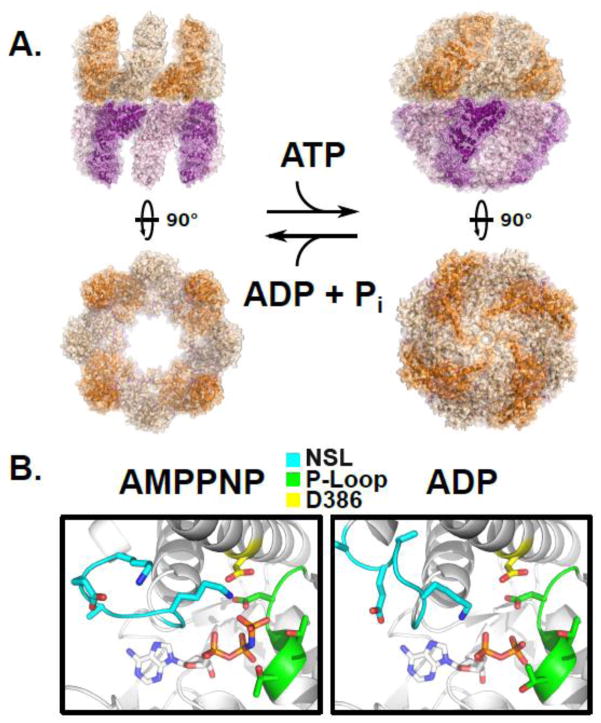
Figure 4.
The ATP driven conformational cycle of the group II chaperonin. A) Left, the open apo state of a group II chaperonin MmCpn determined by cryoelectron microscopy, PDBID: 3IYF. Right, the cryoelectron microscopy structure of wild type MmCpn in the ATP-induced closed state, PDBID: 3LOS [64]. B) The position of the nucleotide sensing loop (NSL) is dependent upon the presence of the γ-phosphate. Left, the crystal structure of the archaeal chaperonin MmCpn in a pre-hydrolysis state, complexed with the non-hydrolyzable ATP analog AMPPNP, PDBID: 3RUV. Right, the crystal structure of the same MmCpn complexed with ADP, PDBID: 3RUW [40]. The nucleotide sensing loop, P-loop, and catalytic aspartate, D386 are indicated in cyan, green, and yellow respectively. The conformation of the NSL is altered by the scission of the gamma phosphate between the left and right panels.
The stoichiometry of ATP utilization by the subunits of the group II chaperonin rings is very different than the 1:1 ratio observed in GroEL[16, 75]. It has been shown that the heteromeric α/β-thermosome behaves quite differently than the homomeric all α-complex[76]. TRiC provides the extreme example of differentiation of subunits leading to novel allosteric mechanisms. There, specialization has led to a mechanism wherein only four out of the eight CCT subunits appreciably bind ATP[59]. Studies performed in S. cerevisiae demonstrated the in vivo significance of this observation. Mutations that removed the ability to bind or hydrolyze ATP had severe phenotypes in the four “high affinity” TRiC/CCT subunits, but showed no effect on growth or viability when made in the four low affinity subunits[59]. The role that this decentralization of nucleotide usage plays in the context of productive folding remains an area of ongoing research.
The unusual stoichiometry of ATP utilization by the subunits raises the question of how many nucleotides the complex requires for cycling. Single molecule experiments have been performed where fluorescently labeled ATP bound to individual complexes were counted by recording discrete photobleaching steps [77]. The observed distributions centered on 8 bound nucleotides under both cycling and non-cycling conditions, meaning that half of the total nucleotide binding sites are occupied, consistent with the finding that only four subunits per ring bind ATP. Under these ATP concentrations, biochemical experiments demonstrated that both rings contained a closed lid. The doubly-closed conformation has also been observed under cycling conditions by SAXS [71] and in cases with stalled complexes by cryoelectron microscopy [64, 72]. A GroEL-type inter-ring allosteric model, which predicts an asymmetrically closed conformation, does not explain these results[78–80] Given that under nearly identical conditions certain subunits never bind ATP[59], it is likely that the ATP binding subunits of each ring are occupied simultaneously. The hydrolysis state of these bound nucleotides is unknown. It may be the case that the negative cooperativity observed in ATP hydrolysis[67] is a result of a distinct mode of allosteric regulation that remains to be examined.
Substrate properties and interaction
Knowledge of how TRiC recognizes and folds proteins has increased as the list of known substrates of the eukaryotic complex continues to grow. TRiC interacts with approximately 10% of the proteome and its function is absolutely essential for viability [28]. TRiC disfunction is associated with a growing number of diseases. Spontaneous and inherited mutations in subunits CCT5 and CCT4 of TRiC are linked to sensory neuropathy [81, 82]. Tumor-associated mutations are found in the TRiC-binding sites of the Von Hippel-Lindau protein that lead to misfolding and loss of function [83–85]. Cancer-linked proteins p53 and STAT3 are known to be TRiC substrates [86, 87]. TRiC has been shown to mediate the folding of a number of beta-propeller rich proteins, including telomerase cofactor TCAB1 [88], the cell cycle regulators CDC20, CDH1 as well as Gβ subunits of signaling complexes containing WD40 domains [20, 21, 26, 89–92]. TRiC also participates in suppressing aggregation and toxicity of Huntingtin in Huntington’s Disease [93–96] and has recently been linked to susceptibility to Alzheimers’ Disease [97]. Interestingly, several viral proteins have evolved to require TRiC for proper folding and processing [98–101]. As a result, downregulation of TRiC impairs replication of several important human pathogens, including HCV and HIV [98, 101]. TRiC interacts with the HIV proteins Gag, Vif and p6, and is required for HIV replication [102, 103]. The expanding list of processes that TRiC has been shown to be involved in highlights the global importance the group II chaperonin plays in maintenance of the proteome and proper cellular physiology.
A robust definition of the specific sequence or structural properties that define chaperonin dependence remain underdetermined. This difficulty is compounded by the fact that each subunit in the chaperonin recognizes diverse sequence determinants. While some motifs are frequently found in proteins that interact with TRiC, such as the WD40 β-propellers [89, 90], they cannot be said to be clear identifiers. Consistent with the notion that there is no one feature that dictates chaperonin requirement, studies carried out in in vitro translation systems predict that approximately 10% of all cytosolic proteins transit through the TRiC complex [28]. This is in strong agreement with pulse-chase efforts that sought to quantitate the portion of the proteome that required the chaperonin for folding[104]. The proteins identified by Yam and colleagues lack clear sequence or fold conservation, however they do have properties consistent with a greater potential for aggregation. Substrate proteins are often larger, have extended hydrophobic stretches, or are involved in multi-protein complexes [28]. The ability to assist such a breadth of proteins with diverse folds and sequence properties raises the potential for multiple mechanisms through which the complex can promote folding.
Recent in vitro work has illuminated the molecular determinants of substrate interaction with the apical domains of TRiC/CCT subunits. Using known subunit-substrate pairs, Joachimiak and colleagues have demonstrated that substrate motifs are recognized by a cleft formed between Helix 11 (H11) and a Proximal Loop (PL) in the CCT apical domains (Figure 3). While chemically distinct, this region bears structural semblance to the substrate binding site of GroEL [107]. They further demonstrate that the amino acid composition of the Helix 11/Proximal Loop region in each subunit dictates the sequences that can be bound, whereby each apical domain presents a unique arrangement of charged and polar residues around a hydrophobic core. Kinetics of substrate binding for a panel of mutant apical CCT3 domains revealed that the kon and koff of binding were controlled by the flexible PL and H11, respectively. Interestingly, the PL of CCT3 is comprised of a string of positively charged residues while H11 varies between polar and non-polar along the helix. In this model, the flanking hydrophilic residues define the nature of the substrate that a given apical domain recognizes, promoting association to a particular sequence. The hydrophobic patch then serves as an anchoring point for the substrate [108].
The Huntingtin protein contains a polyglutamine tract (polyQ), that when expanded beyond 35 consecutive glutamines promotes neuronal toxicity and aggregation, leading to Huntington’s Disease[93–96]. TRiC can modulate the aggregation of Huntingtin through interactions with specific subunits [93, 105]. TRiC action is not due to direct binding of the expanded polyQ stretch. Rather, an amphipathic helix at the N-terminus of Htt-exon1 was found to be responsible for binding solely to the apical domain of CCT 1. Disruption of the hydrophobic face of this helix was shown to fully eliminate the interaction with both TRiC and the soluble apical domain of CCT 1 [106]. This clearly demonstrates what has come to be suspected about the nature of substrate binding within the group II chaperonins; the distinct features of the paralogous CCT subunits have evolved to recognize divergent sequence motifs.
While diversification at each subunit appears to have evolved to recognize a somewhat narrowly defined stretch of amino acids, the combinatorial nature of binding would afford the complex a large sequence space through which to interact with substrates. This suggests a model where subunit-specific contacts between TRiC and specific regions interspersed in the primary sequence of the unfolded polypeptides allow the hetero-oligomeric complex to bind stably to substrates through polyvalent interactions. For each subunit, the interaction relies on a recognition code integrating polar and hydrophobic contributions. As a result of the fixed arrangement of subunits in TRiC, the distribution of binding sites in the substrate will stipulate the global topology of the bound polypeptide, which in turn may direct substrate folding along a productive pathway. Indeed, instances of specific expansion within actin have been reported based on FRET based measurements[109, 110]. Such a mode of interaction demonstrates the capability the complex has to direct a folding trajectory.
One further challenge that the chaperonin must overcome is the fact that it has only a limited volume in which to protect proteins from the crowded cytoplasm. It has been estimated that the complex can encapsulate proteins up to ~60 kDa in size, given the observed chamber volume [41]. While many substrates of the chaperonin are smaller than 60 kDa [28, 104], it has been shown that TRiC interacts with proteins that are larger than 70 kDa. The potential exists for proteins to be enclosed co-translationally, or for specific domains to be sequestered within the chamber while the remainder of the protein sticks out into the cytosol. The latter has been demonstrated to occur with TRiC in in vitro translation systems using several fusions between actin and fluorescent proteins as well a natural substrate, hSnu114, which exceeds the chamber size [111]. There, encapsulation was monitored by the pattern of proteolytic fragments produced under different nucleotide conditions. In the case of actin constructs that were too large to fit within the chamber, fragments corresponding to the chaperonin independent fluorescent proteins could be detected upon protease treatment of closed complexes. This was the case for all constructs observed when actin was terminal to the fused proteins, suggesting that the remainder of the polypeptide was capable of being threaded out of the complex. However, no protection was observed when actin was flanked by the fluorescent proteins. Taken together, this suggests that despite the walls of the chaperonin having space between subunits [63], threading of protein out of the chamber likely only occurs through the lid iris.
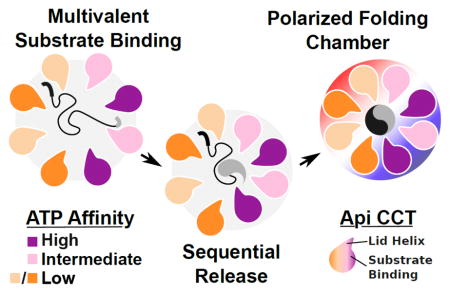
Combining this new appreciation for differential substrate recognition [108], the demonstration of substrate folding on a per-domain basis [111], and the knowledge that there is an asymmetric utilization of nucleotide among the CCT subunits [59] presents an exciting picture of how a substrate may be released into the central chamber and folded upon ATP hydrolysis (Figure 5). With the apical domains possessing specificity for a particular sequence, each substrate would be bound in an orientation defined by the presence and arrangement of different recognition motifs within its sequence. These would then be released in a defined, stepwise, progression as the complex closes upon ATP hydrolysis. A sequential release of substrate into the chamber could therefore be employed to control the folding trajectory of proteins with complex topologies. That these events occur, let alone serve as integral components to the chaperone mechanism, remains purely speculative. Demonstrating such time-resolved substrate release events or capturing the intermediates structurally represent some of the greatest opportunities for furthering the understanding of the group II chaperonin mechanism.
Figure 5.
A schematic view of factors promoting substrate folding by TRiC and other group II chaperonins. The substrate folding process involves multivalent binding of distinct substrate epitopes by the different TRiC apical domains. Release of substrate may proceed in a sequential fashion owing to the asymmetric usage of ATP by the eukaryotic group II chaperonin and the nonconcerted nature of lid closure. Once encapsulated, substrates experience a polarized charge environment with one lobe of the complex demonstrating a positive electrostatic potential while the other is negative.
Highlights.
Chaperones are enzymes that assist protein folding in the cell and maintain cellular proteostasis
The eukaryotic chaperonin TRiC/CCT consists of two stacked rings of eight paralogous subunits each
TRiC promote ATP-dependent folding of polypeptides (10% of the eukaryotic proteome)
A structural and mechanistic understanding of this essential chaperonins starts to emerge
Unusual design principles of this class of chaperone that underlie its unique role are revealed
Acknowledgments
we thank members of the Frydman lab for stimulating discussions. Work in the Frydman lab on chaperonins is supported by grant GM074074 NIH and DE-SC0008504 from the Department of Energy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anfinsen CB, Haber E, Sela M, White FH., Jr The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proceedings of the National Academy of Sciences of the United States of America. 1961;47:1309–14. doi: 10.1073/pnas.47.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levinthal C. How To Fold Graciously. University of Illinois Bulletin. 1969;67:22–4. [Google Scholar]
- 3.Baldwin RL. The search for folding intermediates and the mechanism of protein folding. Annual review of biophysics. 2008;37:1–21. doi: 10.1146/annurev.biophys.37.032807.125948. [DOI] [PubMed] [Google Scholar]
- 4.Sosnick TR, Barrick D. The folding of single domain proteins--have we reached a consensus? Current opinion in structural biology. 2011;21:12–24. doi: 10.1016/j.sbi.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Berg B, Ellis RJ, Dobson CM. Effects of macromolecular crowding on protein folding and aggregation. The EMBO journal. 1999;18:6927–33. doi: 10.1093/emboj/18.24.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annual review of biochemistry. 2001;70:603–47. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 7.Braselmann E, Chaney JL, Clark PL. Folding the proteome. Trends in biochemical sciences. 2013;38:337–44. doi: 10.1016/j.tibs.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annual review of biochemistry. 2013;82:323–55. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 9.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annual review of biochemistry. 2013;82:323–55. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 10.Albanese V, Yam AY, Baughman J, Parnot C, Frydman J. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell. 2006;124:75–88. doi: 10.1016/j.cell.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Maier T, Ferbitz L, Deuerling E, Ban N. A cradle for new proteins: trigger factor at the ribosome. Current opinion in structural biology. 2005;15:204–12. doi: 10.1016/j.sbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: physiology and mechanism. Annual review of cell and developmental biology. 2007;23:115–45. doi: 10.1146/annurev.cellbio.23.090506.123555. [DOI] [PubMed] [Google Scholar]
- 13.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–8. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 14.Lund PA. Multiple chaperonins in bacteria--why so many? FEMS microbiology reviews. 2009;33:785–800. doi: 10.1111/j.1574-6976.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Hirtreiter AM, Calloni G, Forner F, Scheibe B, Puype M, Vandekerckhove J, et al. Differential substrate specificity of group I and group II chaperonins in the archaeon Methanosarcina mazei. Molecular microbiology. 2009;74:1152–68. doi: 10.1111/j.1365-2958.2009.06924.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Horwich AL, Sigler PB. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature. 1997;388:741–50. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- 17.Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111–7. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- 18.Stirling PC, Cuellar J, Alfaro GA, El Khadali F, Beh CT, Valpuesta JM, et al. PhLP3 modulates CCT-mediated actin and tubulin folding via ternary complexes with substrates. The Journal of biological chemistry. 2006;281:7012–21. doi: 10.1074/jbc.M513235200. [DOI] [PubMed] [Google Scholar]
- 19.Siegert R, Leroux MR, Scheufler C, Hartl FU, Moarefi I. Structure of the molecular chaperone prefoldin: unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell. 2000;103:621–32. doi: 10.1016/s0092-8674(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 20.Plimpton RL, Cuellar J, Lai CW, Aoba T, Makaju A, Franklin S, et al. Structures of the Gbeta-CCT and PhLP1-Gbeta-CCT complexes reveal a mechanism for G-protein beta-subunit folding and Gbetagamma dimer assembly. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2413–8. doi: 10.1073/pnas.1419595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin JN, Thulin CD, Hart SJ, Resing KA, Ahn NG, Willardson BM. Regulatory interaction of phosducin-like protein with the cytosolic chaperonin complex. Proc Natl Acad Sci U S A. 2002;99:7962–7. doi: 10.1073/pnas.112075699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegers K, Waldmann T, Leroux MR, Grein K, Shevchenko A, Schiebel E, et al. Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. The EMBO journal. 1999;18:75–84. doi: 10.1093/emboj/18.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroux MR, Fandrich M, Klunker D, Siegers K, Lupas AN, Brown JR, et al. MtGimC, a novel archaeal chaperone related to the eukaryotic chaperonin cofactor GimC/prefoldin. The EMBO journal. 1999;18:6730–43. doi: 10.1093/emboj/18.23.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack EA, Altschuler GM, Dekker C, Filmore H, Willison KR. Yeast phosducin-like protein 2 acts as a stimulatory co-factor for the folding of actin by the chaperonin CCT via a ternary complex. Journal of molecular biology. 2009;391:192–206. doi: 10.1016/j.jmb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–8. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- 26.Camasses A, Bogdanova A, Shevchenko A, Zachariae W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol Cell. 2003;12:87–100. doi: 10.1016/s1097-2765(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 27.Vinh DB, Drubin DG. A yeast TCP-1-like protein is required for actin function in vivo. Proc Natl Acad Sci U S A. 1994;91:9116–20. doi: 10.1073/pnas.91.19.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yam AY, Xia Y, Lin HT, Burlingame A, Gerstein M, Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nature structural & molecular biology. 2008;15:1255–62. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braig K, Adams PD, Brunger AT. Conformational variability in the refined structure of the chaperonin GroEL at 2.8 A resolution. Nature structural biology. 1995;2:1083–94. doi: 10.1038/nsb1295-1083. [DOI] [PubMed] [Google Scholar]
- 30.Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, et al. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994;371:578–86. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 31.Huo Y, Hu Z, Zhang K, Wang L, Zhai Y, Zhou Q, et al. Crystal structure of group II chaperonin in the open state. Structure. 2010;18:1270–9. doi: 10.1016/j.str.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marco S, Urena D, Carrascosa JL, Waldmann T, Peters J, Hegerl R, et al. The molecular chaperone TF55. Assessment of symmetry. FEBS letters. 1994;341:152–5. doi: 10.1016/0014-5793(94)80447-8. [DOI] [PubMed] [Google Scholar]
- 33.Knapp S, Schmidt-Krey I, Hebert H, Bergman T, Jornvall H, Ladenstein R. The molecular chaperonin TF55 from the Thermophilic archaeon Sulfolobus solfataricus. A biochemical and structural characterization. Journal of molecular biology. 1994;242:397–407. doi: 10.1006/jmbi.1994.1590. [DOI] [PubMed] [Google Scholar]
- 34.Schoehn G, Quaite-Randall E, Jimenez JL, Joachimiak A, Saibil HR. Three conformations of an archaeal chaperonin, TF55 from Sulfolobus shibatae. Journal of molecular biology. 2000;296:813–9. doi: 10.1006/jmbi.2000.3505. [DOI] [PubMed] [Google Scholar]
- 35.Zhang K, Wang L, Liu Y, Chan KY, Pang X, Schulten K, et al. Flexible interwoven termini determine the thermal stability of thermosomes. Protein & cell. 2013;4:432–44. doi: 10.1007/s13238-013-3026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frydman J, Nimmesgern E, Erdjument-Bromage H, Wall JS, Tempst P, Hartl FU. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. The EMBO journal. 1992;11:4767–78. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubota H, Hynes G, Willison K. The eighth Cct gene, Cctq, encoding the theta subunit of the cytosolic chaperonin containing TCP-1. Gene. 1995;154:231–6. doi: 10.1016/0378-1119(94)00880-2. [DOI] [PubMed] [Google Scholar]
- 38.Klumpp M, Baumeister W, Essen LO. Structure of the substrate binding domain of the thermosome, an archaeal group II chaperonin. Cell. 1997;91:263–70. doi: 10.1016/s0092-8674(00)80408-0. [DOI] [PubMed] [Google Scholar]
- 39.Bosch G, Baumeister W, Essen LO. Crystal structure of the beta-apical domain of the thermosome reveals structural plasticity in the protrusion region. Journal of molecular biology. 2000;301:19–25. doi: 10.1006/jmbi.2000.3955. [DOI] [PubMed] [Google Scholar]
- 40.Pereira JH, Ralston CY, Douglas NR, Kumar R, Lopez T, McAndrew RP, et al. Mechanism of nucleotide sensing in group II chaperonins. The EMBO journal. 2012;31:731–40. doi: 10.1038/emboj.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ditzel L, Lowe J, Stock D, Stetter KO, Huber H, Huber R, et al. Crystal structure of the thermosome, the archaeal chaperonin and homolog of CCT. Cell. 1998;93:125–38. doi: 10.1016/s0092-8674(00)81152-6. [DOI] [PubMed] [Google Scholar]
- 42.Tracy CM, Gray AJ, Cuellar J, Shaw TS, Howlett AC, Taylor RM, et al. Programmed cell death protein 5 interacts with the cytosolic chaperonin containing tailless complex polypeptide 1 (CCT) to regulate beta-tubulin folding. The Journal of biological chemistry. 2014;289:4490–502. doi: 10.1074/jbc.M113.542159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llorca O, Martin-Benito J, Gomez-Puertas P, Ritco-Vonsovici M, Willison KR, Carrascosa JL, et al. Analysis of the interaction between the eukaryotic chaperonin CCT and its substrates actin and tubulin. Journal of structural biology. 2001;135:205–18. doi: 10.1006/jsbi.2001.4359. [DOI] [PubMed] [Google Scholar]
- 44.Llorca O, McCormack EA, Hynes G, Grantham J, Cordell J, Carrascosa JL, et al. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature. 1999;402:693–6. doi: 10.1038/45294. [DOI] [PubMed] [Google Scholar]
- 45.Llorca O, Martin-Benito J, Ritco-Vonsovici M, Grantham J, Hynes GM, Willison KR, et al. Eukaryotic chaperonin CCT stabilizes actin and tubulin folding intermediates in open quasi-native conformations. The EMBO journal. 2000;19:5971–9. doi: 10.1093/emboj/19.22.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munoz IG, Yebenes H, Zhou M, Mesa P, Serna M, Park AY, et al. Crystal structure of the open conformation of the mammalian chaperonin CCT in complex with tubulin. Nature structural & molecular biology. 2011;18:14–9. doi: 10.1038/nsmb.1971. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Benito J, Boskovic J, Gomez-Puertas P, Carrascosa JL, Simons CT, Lewis SA, et al. Structure of eukaryotic prefoldin and of its complexes with unfolded actin and the cytosolic chaperonin CCT. The EMBO journal. 2002;21:6377–86. doi: 10.1093/emboj/cdf640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin-Benito J, Bertrand S, Hu T, Ludtke PJ, McLaughlin JN, Willardson BM, et al. Structure of the complex between the cytosolic chaperonin CCT and phosducin-like protein. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17410–5. doi: 10.1073/pnas.0405070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuellar J, Martin-Benito J, Scheres SH, Sousa R, Moro F, Lopez-Vinas E, et al. The structure of CCT-Hsc70 NBD suggests a mechanism for Hsp70 delivery of substrates to the chaperonin. Nature structural & molecular biology. 2008;15:858–64. doi: 10.1038/nsmb.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yebenes H, Mesa P, Munoz IG, Montoya G, Valpuesta JM. Chaperonins: two rings for folding. Trends in biochemical sciences. 2011;36:424–32. doi: 10.1016/j.tibs.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Booth CR, Meyer AS, Cong Y, Topf M, Sali A, Ludtke SJ, et al. Mechanism of lid closure in the eukaryotic chaperonin TRiC/CCT. Nature structural & molecular biology. 2008;15:746–53. doi: 10.1038/nsmb.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cong Y, Baker ML, Jakana J, Woolford D, Miller EJ, Reissmann S, et al. 4.0-A resolution cryo-EM structure of the mammalian chaperonin TRiC/CCT reveals its unique subunit arrangement. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4967–72. doi: 10.1073/pnas.0913774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cong Y, Schroder GF, Meyer AS, Jakana J, Ma B, Dougherty MT, et al. Symmetry-free cryo-EM structures of the chaperonin TRiC along its ATPase-driven conformational cycle. The EMBO journal. 2012;31:720–30. doi: 10.1038/emboj.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, et al. Structural and Functional Analysis of the Middle Segment of Hsp90: Implications for ATP Hydrolysis and Client Protein and Cochaperone Interactions. Molecular cell. 2003;11:647–58. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 55.Dekker C, Roe SM, McCormack EA, Beuron F, Pearl LH, Willison KR. The crystal structure of yeast CCT reveals intrinsic asymmetry of eukaryotic cytosolic chaperonins. The EMBO journal. 2011;30:3078–90. doi: 10.1038/emboj.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin-Benito J, Grantham J, Boskovic J, Brackley KI, Carrascosa JL, Willison KR, et al. The inter-ring arrangement of the cytosolic chaperonin CCT. EMBO reports. 2007;8:252–7. doi: 10.1038/sj.embor.7400894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leitner A, Joachimiak LA, Bracher A, Monkemeyer L, Walzthoeni T, Chen B, et al. The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Structure. 2012;20:814–25. doi: 10.1016/j.str.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalisman N, Adams CM, Levitt M. Subunit order of eukaryotic TRiC/CCT chaperonin by cross-linking, mass spectrometry, and combinatorial homology modeling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2884–9. doi: 10.1073/pnas.1119472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reissmann S, Joachimiak LA, Chen B, Meyer AS, Nguyen A, Frydman J. A gradient of ATP affinities generates an asymmetric power stroke driving the chaperonin TRIC/CCT folding cycle. Cell reports. 2012;2:866–77. doi: 10.1016/j.celrep.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian G, Vainberg IE, Tap WD, Lewis SA, Cowan NJ. Specificity in chaperonin-mediated protein folding. Nature. 1995;375:250–3. doi: 10.1038/375250a0. [DOI] [PubMed] [Google Scholar]
- 61.Spiess C, Meyer AS, Reissmann S, Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends in cell biology. 2004;14:598–604. doi: 10.1016/j.tcb.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bigotti MG, Clarke AR. Chaperonins: The hunt for the Group II mechanism. Archives of biochemistry and biophysics. 2008;474:331–9. doi: 10.1016/j.abb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 63.Pereira JH, Ralston CY, Douglas NR, Meyer D, Knee KM, Goulet DR, et al. Crystal structures of a group II chaperonin reveal the open and closed states associated with the protein folding cycle. The Journal of biological chemistry. 2010;285:27958–66. doi: 10.1074/jbc.M110.125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J, Baker ML, Schroder GF, Douglas NR, Reissmann S, Jakana J, et al. Mechanism of folding chamber closure in a group II chaperonin. Nature. 2010;463:379–83. doi: 10.1038/nature08701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Ma B, DiMaio F, Douglas NR, Joachimiak LA, Baker D, et al. Cryo-EM structure of a group II chaperonin in the prehydrolysis ATP-bound state leading to lid closure. Structure. 2011;19:633–9. doi: 10.1016/j.str.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilak O, Harrop SJ, Siddiqui KS, Chong K, De Francisci D, Burg D, et al. Chaperonins from an Antarctic archaeon are predominantly monomeric: crystal structure of an open state monomer. Environmental microbiology. 2011;13:2232–49. doi: 10.1111/j.1462-2920.2011.02477.x. [DOI] [PubMed] [Google Scholar]
- 67.Reissmann S, Parnot C, Booth CR, Chiu W, Frydman J. Essential function of the built-in lid in the allosteric regulation of eukaryotic and archaeal chaperonins. Nature structural & molecular biology. 2007;14:432–40. doi: 10.1038/nsmb1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sekiguchi H, Nakagawa A, Moriya K, Makabe K, Ichiyanagi K, Nozawa S, et al. ATP dependent rotational motion of group II chaperonin observed by X-ray single molecule tracking. PloS one. 2013;8:e64176. doi: 10.1371/journal.pone.0064176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bigotti MG, Bellamy SR, Clarke AR. The asymmetric ATPase cycle of the thermosome: elucidation of the binding, hydrolysis and product-release steps. Journal of molecular biology. 2006;362:835–43. doi: 10.1016/j.jmb.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 70.Machida K, Masutani M, Kobayashi T, Mikami S, Nishino Y, Miyazawa A, et al. Reconstitution of the human chaperonin CCT by co-expression of the eight distinct subunits in mammalian cells. Protein expression and purification. 2012;82:61–9. doi: 10.1016/j.pep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Meyer AS, Gillespie JR, Walther D, Millet IS, Doniach S, Frydman J. Closing the folding chamber of the eukaryotic chaperonin requires the transition state of ATP hydrolysis. Cell. 2003;113:369–81. doi: 10.1016/s0092-8674(03)00307-6. [DOI] [PubMed] [Google Scholar]
- 72.Douglas NR, Reissmann S, Zhang J, Chen B, Jakana J, Kumar R, et al. Dual action of ATP hydrolysis couples lid closure to substrate release into the group II chaperonin chamber. Cell. 2011;144:240–52. doi: 10.1016/j.cell.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iizuka R, Yoshida T, Ishii N, Zako T, Takahashi K, Maki K, et al. Characterization of archaeal group II chaperonin-ADP-metal fluoride complexes: implications that group II chaperonins operate as a “two-stroke engine”. The Journal of biological chemistry. 2005;280:40375–83. doi: 10.1074/jbc.M506785200. [DOI] [PubMed] [Google Scholar]
- 74.Weissman JS, Hohl CM, Kovalenko O, Kashi Y, Chen S, Braig K, et al. Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83:577–87. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 75.Terada TP, Kuwajima K. Thermodynamics of nucleotide binding to the chaperonin GroEL studied by isothermal titration calorimetry: evidence for noncooperative nucleotide binding. Biochimica et biophysica acta. 1999;1431:269–81. doi: 10.1016/s0167-4838(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 76.Gutsche I, Mihalache O, Baumeister W. ATPase cycle of an archaeal chaperonin. Journal of molecular biology. 2000;300:187–96. doi: 10.1006/jmbi.2000.3833. [DOI] [PubMed] [Google Scholar]
- 77.Jiang Y, Douglas NR, Conley NR, Miller EJ, Frydman J, Moerner WE. Sensing cooperativity in ATP hydrolysis for single multisubunit enzymes in solution. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16962–7. doi: 10.1073/pnas.1112244108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Todd MJ, Viitanen PV, Lorimer GH. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994;265:659–66. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- 79.Cliff MJ, Limpkin C, Cameron A, Burston SG, Clarke AR. Elucidation of steps in the capture of a protein substrate for efficient encapsulation by GroE. The Journal of biological chemistry. 2006;281:21266–75. doi: 10.1074/jbc.M601605200. [DOI] [PubMed] [Google Scholar]
- 80.Horowitz PM, Lorimer GH, Ybarra J. GroES in the asymmetric GroEL14-GroES7 complex exchanges via an associative mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2682–6. doi: 10.1073/pnas.96.6.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee MJ, Stephenson DA, Groves MJ, Sweeney MG, Davis MB, An SF, et al. Hereditary sensory neuropathy is caused by a mutation in the delta subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct4 ) gene. Hum Mol Genet. 2003;12:1917–25. doi: 10.1093/hmg/ddg198. [DOI] [PubMed] [Google Scholar]
- 82.Bouhouche A, Benomar A, Bouslam N, Chkili T, Yahyaoui M. Mutation in the epsilon subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct5) gene causes autosomal recessive mutilating sensory neuropathy with spastic paraplegia. J Med Genet. 2006;43:441–3. doi: 10.1136/jmg.2005.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feldman DE, Frydman J. Protein folding in vivo: the importance of molecular chaperones. Curr Opin Struct Biol. 2000;10:26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 84.Feldman DE, Spiess C, Howard DE, Frydman J. Tumorigenic mutations in VHL disrupt folding in vivo by interfering with chaperonin binding. Mol Cell. 2003;12:1213–24. doi: 10.1016/s1097-2765(03)00423-4. [DOI] [PubMed] [Google Scholar]
- 85.Feldman DE, Thulasiraman V, Ferreyra RG, Frydman J. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Molecular cell. 1999;4:1051–61. doi: 10.1016/s1097-2765(00)80233-6. [DOI] [PubMed] [Google Scholar]
- 86.Trinidad AG, Muller PA, Cuellar J, Klejnot M, Nobis M, Valpuesta JM, et al. Interaction of p53 with the CCT complex promotes protein folding and wild-type p53 activity. Molecular cell. 2013;50:805–17. doi: 10.1016/j.molcel.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kasembeli M, Lau WC, Roh SH, Eckols TK, Frydman J, Chiu W, et al. Modulation of STAT3 folding and function by TRiC/CCT chaperonin. PLoS biology. 2014;12:e1001844. doi: 10.1371/journal.pbio.1001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freund A, Zhong FL, Venteicher AS, Meng Z, Veenstra TD, Frydman J, et al. Proteostatic control of telomerase function through TRiC-mediated folding of TCAB1. Cell. 2014;159:1389–403. doi: 10.1016/j.cell.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kubota S, Kubota H, Nagata K. Cytosolic chaperonin protects folding intermediates of Gbeta from aggregation by recognizing hydrophobic beta-strands. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8360–5. doi: 10.1073/pnas.0600195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyata Y, Shibata T, Aoshima M, Tsubata T, Nishida E. The molecular chaperone TRiC/CCT binds to the Trp-Asp 40 (WD40) repeat protein WDR68 and promotes its folding, protein kinase DYRK1A binding, and nuclear accumulation. J Biol Chem. 2014;289:33320–32. doi: 10.1074/jbc.M114.586115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Willardson BM, Howlett AC. Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding. Cellular signalling. 2007;19:2417–27. doi: 10.1016/j.cellsig.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yi C, Li S, Wang J, Wei N, Deng XW. Affinity purification reveals the association of WD40 protein constitutive photomorphogenic 1 with the hetero-oligomeric TCP-1 chaperonin complex in mammalian cells. Int J Biochem Cell Biol. 2006;38:1076–83. doi: 10.1016/j.biocel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 93.Behrends C, Langer CA, Boteva R, Bottcher UM, Stemp MJ, Schaffar G, et al. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Molecular cell. 2006;23:887–97. doi: 10.1016/j.molcel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 94.Kitamura A, Kubota H, Pack CG, Matsumoto G, Hirayama S, Takahashi Y, et al. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat Cell Biol. 2006;8:1163–70. doi: 10.1038/ncb1478. [DOI] [PubMed] [Google Scholar]
- 95.Tam S, Geller R, Spiess C, Frydman J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nature cell biology. 2006;8:1155–62. doi: 10.1038/ncb1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tam S, Spiess C, Auyeung W, Joachimiak L, Chen B, Poirier MA, et al. The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat Struct Mol Biol. 2009 doi: 10.1038/nsmb.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khabirova E, Moloney A, Marciniak SJ, Williams J, Lomas DA, Oliver SG, et al. The TRiC/CCT chaperone is implicated in Alzheimer’s disease based on patient GWAS and an RNAi screen in Abeta-expressing Caenorhabditis elegans. PloS one. 2014;9:e102985. doi: 10.1371/journal.pone.0102985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inoue Y, Aizaki H, Hara H, Matsuda M, Ando T, Shimoji T, et al. Chaperonin TRiC/CCT participates in replication of hepatitis C virus genome via interaction with the viral NS5B protein. Virology. 2011;410:38–47. doi: 10.1016/j.virol.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 99.Lingappa JR, Martin RL, Wong ML, Ganem D, Welch WJ, Lingappa VR. A eukaryotic cytosolic chaperonin is associated with a high molecular weight intermediate in the assembly of hepatitis B virus capsid, a multimeric particle. The Journal of cell biology. 1994;125:99–111. doi: 10.1083/jcb.125.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J, Wu X, Zan J, Wu Y, Ye C, Ruan X, et al. Cellular chaperonin CCTgamma contributes to rabies virus replication during infection. Journal of virology. 2013;87:7608–21. doi: 10.1128/JVI.03186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou H, Xu M, Huang Q, Gates AT, Zhang XD, Castle JC, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008;4:495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Jager S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, et al. Global landscape of HIV-human protein complexes. Nature. 2012;481:365–70. doi: 10.1038/nature10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hong S, Choi G, Park S, Chung AS, Hunter E, Rhee SS. Type D retrovirus Gag polyprotein interacts with the cytosolic chaperonin TRiC. J Virol. 2001;75:2526–34. doi: 10.1128/JVI.75.6.2526-2534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thulasiraman V, Yang CF, Frydman J. In vivo newly translated polypeptides are sequestered in a protected folding environment. The EMBO journal. 1999;18:85–95. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tam S, Geller R, Spiess C, Frydman J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nature cell biology. 2006;8:1155–62. doi: 10.1038/ncb1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tam S, Spiess C, Auyeung W, Joachimiak L, Chen B, Poirier MA, et al. The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nature structural & molecular biology. 2009;16:1279–85. doi: 10.1038/nsmb.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buckle AM, Zahn R, Fersht AR. A structural model for GroEL-polypeptide recognition. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3571–5. doi: 10.1073/pnas.94.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joachimiak LA, Walzthoeni T, Liu CW, Aebersold R, Frydman J. The structural basis of substrate recognition by the eukaryotic chaperonin TRiC/CCT. Cell. 2014;159:1042–55. doi: 10.1016/j.cell.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villebeck L, Moparthi SB, Lindgren M, Hammarstrom P, Jonsson BH. Domain-specific chaperone-induced expansion is required for beta-actin folding: a comparison of beta-actin conformations upon interactions with GroEL and tail-less complex polypeptide 1 ring complex (TRiC) Biochemistry. 2007;46:12639–47. doi: 10.1021/bi700658n. [DOI] [PubMed] [Google Scholar]
- 110.Villebeck L, Persson M, Luan SL, Hammarstrom P, Lindgren M, Jonsson BH. Conformational rearrangements of tail-less complex polypeptide 1 (TCP-1) ring complex (TRiC)-bound actin. Biochemistry. 2007;46:5083–93. doi: 10.1021/bi062093o. [DOI] [PubMed] [Google Scholar]
- 111.Russmann F, Stemp MJ, Monkemeyer L, Etchells SA, Bracher A, Hartl FU. Folding of large multidomain proteins by partial encapsulation in the chaperonin TRiC/CCT. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21208–15. doi: 10.1073/pnas.1218836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends in genetics : TIG. 2000;16:276–7. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 113.Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.3r1. 2010. [Google Scholar]
- 114.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10037–41. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lerner MGCHA. APBS plugin for PyMOL. University of Michigan; Ann Arbor: 2006. [Google Scholar]
- 116.Eastman P, Friedrichs MS, Chodera JD, Radmer RJ, Bruns CM, Ku JP, et al. OpenMM 4: A Reusable, Extensible, Hardware Independent Library for High Performance Molecular Simulation. Journal of chemical theory and computation. 2013;9:461–9. doi: 10.1021/ct300857j. [DOI] [PMC free article] [PubMed] [Google Scholar]