Abstract
Objective
Connexin (Cx) 43 hemichannels play a role in mechanotransduction. This study was undertaken in order to determine if Cx43 hemichannels were activated in rat temporomandibular joint (TMJ) chondrocytes under mechanical stimulation.
Methods
Sprague-Dawley rats were stimulated dental-mechanically. Cx43 expression in rat TMJ cartilage was determined with immunohistochemistry and real-time PCR, and Cx43 hemichannel opening was evaluated by the extra- and intracellular levels of prostaglandin E2 (PGE2). Both primary rat chondrocytes and ATDC5 cells were treated with fluid flow shear stress (FFSS) to induce hemichannel opening. The Cx43 expression level was then determined by real-time PCR or western blotting, and the extent of Cx43 hemichannel opening was evaluated by measuring both PGE2 release and cellular dye uptake.
Results
Cx43 expression and intra- and extracellular PGE2 levels were increased in mechanically-stimulated rat TMJ cartilage compared to the unstimulated control. The FFSS treatment increased Cx43 expression and induced Cx43 hemichannel opening in primary rat chondrocytes and ATDC5 cells indicated by enhanced PGE2 release and dye uptake. Furthermore, the Cx43 hemichannel opening could be blocked by the addition of 18β-glycyrrhetinic acid, a connexin channel inhibitor, Cx43-targeting siRNA, or by withdrawal of FFSS stimulation. The migration of cytosolic Cx43 protein to the plasma membrane in ATDC5 cells was still significant after 8h post 2-h FFSS treatment, and the Cx43 protein level was still high at 48h which returned to control levels at 72h after treatment.
Conclusion
Cx43 hemichannels are activated and mediate small molecule exchange between TMJ chondrocytes and matrix under mechanical stimulation.
Keywords: connexin43, hemichannel, chondrocyte, osteoarthritis, mechanical loading, animal model
Introduction
Mechanical loading generates signals in articular chondrocytes, which trigger a cascade effect of anabolic and catabolic activities [1–3]. Normal biomechanical stimulation promotes bone physiological remodeling that ensures a balance between the synthesis and degradation of the matrix. Alternatively, abnormal, pathological loading disturbs this homeostasis and thus affects the functional integrity of the cartilage, for example, to increase the probability of cartilage degeneration and the development of osteoarthritis (OA) [4]. Behets et al. [5] reported that in canines with acute anterior cruciate ligament deficiency, cartilage loss was located predominantly at the medial tibial plateau, which generally bears more load than the lateral side.
The hemichannel is composed of six connexin (Cx) molecules that are localized at the cell surface and independent of physical contact with adjacent cells [6]. One protein capable forming hemichannels is Cx43, which is widely expressed in bone cells [7–11] and chondrocytes [12–16]. Cx43 hemichannels have been reported to be mechano-sensitive in osteocytes and osteoblasts, and display low substrate selectivity, permitting small molecules with molecular weight less than 1 kDa to pass through including small metabolites, ions, and intracellular signaling molecules [8–11]. Mechanical loading not only increases Cx43 expression in osteoblasts [8, 9] and osteocytes in vitro [10, 11], but also induces the opening of Cx43 hemichannels in osteocytes, leading to an increased release of PGE2 (molecular weight = 352.5 Da) into the bone matrix [11].
The mechanotransduction of chondrocytes was suggested to be mediated by PGE2 release [1, 17]. In a study of cartilage explants, compression stimulation was shown to increase PGE2 release [18]. As PGE2 is a charged anion that diffuses poorly across membrane bilayers, a transporter is necessary in order to regulate its travel to the extracellular space. Although a prostaglandin transporter has been identified in rat and bovine cells [19–21], this transporter mainly mediates the uptake of PGE2 into cells, not its release [22]. In osteocytes, the method by which PGE2 is rapidly released in response to mechanical loading is suggested to be through Cx43 hemichannels [11, 23]. Interestingly, elevated Cx43 expression was reported in human OA cartilage [14]. Considering mechanical stimulation has an important role in OA [4], it is likely that PGE2 release in OA cartilage induced by mechanical stimulation involves Cx43 hemichannels.
PGE2 plays a hormone-like role in mediating the metabolism of cartilage [24–26] and bone [27, 28] by transmitting signals in an autocrine and/or paracrine fashion. The synthesis of PGE2 is regulated by membrane PGE synthase1 (mPGES-1) and cyclooxygenase-2 (COX-2) [29], and, interestingly, increased expression of these enzymes as well as PGE2 production have been reported in cartilage and chondrocytes from OA patients [30–33]. In addition, a mouse model lacking mPGES1 treated by Freund’s adjuvant injection demonstrated an attenuation of synovitis, cartilage damage and bone erosion [34]. Reductions of prostaglandin synthesis have also been found to be efficacious in human OA where inhibition of COX by non-steroidal anti-inflammatory drugs is a cornerstone of pharmacologic therapy [35]. However, whether Cx43 hemichannels are involved in mechanically induced PGE2 release from chondrocytes into cartilage matrix requires clarification.
Temporomandibular joint (TMJ) OA is the most severe subtype of temporomandibular disorders (TMD) and is also one of the most widely spread orofacial afflictions [36]. Recently, we developed a rodent model with TMJ OA-like lesions by applying dental mechanical stimulation with aberrant prosthesis [37–39]. Mice exposed to a small-size diet, indicating that they had a lower level of functional loading compared to mice with a large-size diet, were associated with a reduced TMJ degradation induced by the mechanical stimulation from the designed aberrant prosthesis. With this model and in vitro experiments, the current study demonstrated a link between the mechanical stimulation-induced opening of Cx43 hemichannels and the release of small molecules such as PGE2 from TMJ chondrocytes. The data presented in this paper suggest a role for Cx43 hemichannels in the exchange of small molecules in TMJ cartilage when stimulated in a dental mechanical manner. In consideration of the catabolic role of PGE2 in cartilage reported in the literature [24–26], the current results support the etiological effect of aberrant mechanical stimulation on TMJ OA.
Materials and Methods
Animals and mechanical stimulation
Thirty-six, 6-week-old female Sprague-Dawley rats (weight 140~160g) were provided by the Animal Center of Fourth Military Medical University. All procedures and the care administered to the animals were approved by the University Ethics Committee and performed according to institutional guidelines. The rats were randomly assigned to two control and two experimental groups with nine rats per group. For experimental groups, the aberrant prosthesis was applied as described previously [38]. Briefly, a section of a metal tube cutting down from a pinhead (Shinva Ande, Shangdong, China; length = 2.5 mm, inside diameter = 3 mm) was adhered to the left maxillary incisor and a curved section of a metal tube (length = 4.5 mm, inside diameter = 3.5 mm) was adhered to the left mandibular incisor. At the end of the tube adhered to the mandibular incisor, a 135 degree angle leaned to the labial side was performed in order to create a cross-bite relationship between the left incisors. In the control groups, rats received the same procedure, but no metal tube was adhered. The metal tube was tightened during the entire experimental period. All of the rats received the same standardized diet throughout the procedure.
Sample preparation
Animals were sacrificed at 2- and 8-weeks after the dental operation. Because no differences in degrading changes were found between the left- and right-side TMJs in the experimental mice in our previous report [37–39], the right-side TMJ cartilage tissues of nine rats in each group were removed and preserved at −70°C for mRNA extraction. The left-side TMJ tissue from five rats in each group were removed and embedded in paraffin. Sections of 5-μm-thick were prepared and used for hematoxylin–eosin (HE) and immunohistochemical staining. The left-side TMJ cartilage tissues of the other four rats in each group were used to isolate chondrocytes for ex vivo experiments.
HE and immunohistochemistry staining
The central sagittal sections of each joint stained with HE were imaged with a Leica DFC490 system (Solms, Hessen, Germany). A standard, three-step, avidin-biotin complex staining procedure as previously described [38] was performed using Cx43-antibody (dilution 1:400, Sigma, St. Louis, MO, USA) to detect changes in Cx43-positive cell numbers. In negative controls, non-immune serum was used instead of primary antibody. To ensure that the results were comparable amongst the various groups, the central sections of each joint were chosen and the samples in experimental and age-matched control groups were stained at the same time. The number of Cx43-positive cells and total cells were measured at the center third of the TMJ cartilage (Fig.1A). The percentage of Cx43-positive cells to the total cells was calculated and the mean of the percentage of positive cells in each group was used for further statistical analysis.
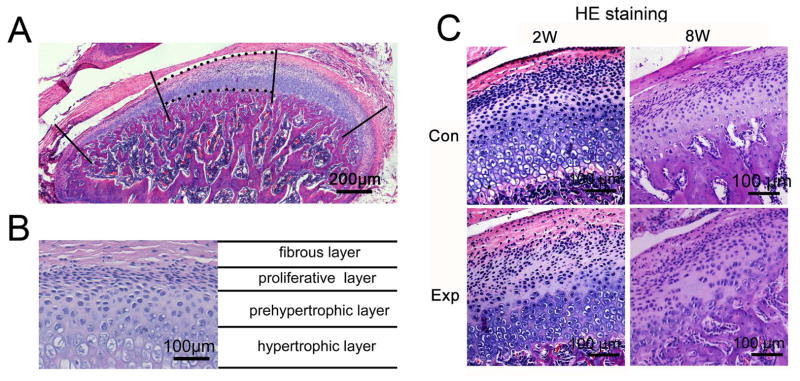
Fig. 1.
Histomorphology images of rat TMJ cartilage stained with hematoxylin and eosin (HE). A, An example of a sagittal central section of rat TMJ shows the center third of TMJ cartilage used to calculate the percentage of Cx43-positive chondrocytes. B, Cells are organized as four layers in rat TMJ cartilage as indicated. C, The representative sagittal central sections of TMJ shows the OA–like lesion, typically as a cell-free area, in 2-, and 8-week experimental groups. 2W, 2-week group; 8W, 8-week group; Con, control group; Exp, experimental group.
RNA extraction and real-time PCR assay
In each subgroup of in vivo experiments, three out of nine TMJs were pooled to create a single sample and three independent pooled samples in each of the experimental or control groups were prepared for statistical analysis. RNA was isolated by Trizol (Invitrogen, Carlsbad, CA, USA), and purified with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Gene expression was analyzed using the Applied Biosystems 7500 Real-time PCR machine and the primer sequences used are listed in Supplemental Table 1 and 2.
Primary rat chondrocytes isolation and culture
Primary rat chondrocytes were isolated as described previously [40]. In brief, chondrocytes were isolated from the condylar cartilage of 3-week-old female rat TMJs by digestion with 0.25% trypsin (Hyclone, Logan, UT, USA) for 20 min, followed by digestion with 0.2% type II collagenase (Invitrogen) for 2 h. Chondrocytes were resuspended in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone) containing 10% fetal bovine serum (Hyclone) and then cultured in a fully humidified atmosphere with 5% CO2 at 37°C for 3 days before use.
SiRNA transfection
ATDC5 cells were seeded at a density of 5×103/cm2 in DMEM-F12 medium (Hyclone) and transfected the following day using Lipofectamine 2000 (Invitrogen). The Cx43-targeting siRNA and negative control siRNA (GenePharma, Shanghai, China) were used at a final concentration of 50 nM. Six hours after transfection the culture medium was changed. Cells were used after 24 h for real-time PCR assay, and 72 h for western blotting or fluid flow shear stress (FFSS) experiments.
Cell shear stress induced by fluid flow
Primary rat chondrocytes and ATDC5 cells were subjected to FFSS treatment. The experiments were performed using Flexcell StreamerTM system (Flexcell, Hillsborough, NC, USA). Cells were cultured on the glass slides coated with collagen I (Flexcell) for 48 h before being exposed to steady laminar flow for 10 min or 2 h at intensities of 8, 16 or 24 dynes/cm2.
Dye uptake assay
To verify that hemichannels were induced to open in response to FFSS stimulation, we conducted dye uptake experiments following the methods used for osteocytes [11]. Primary rat chondrocytes or ATDC5 cells were stimulated with shear stress at 16 dynes/cm2 for 10 min and then incubated for 0 or 8 h. Before the shear stress stimulation, ATDC5 cells were transfected with Cx43 siRNA or negative control siRNA. During the shear stress treatment primary rat chondrocytes were exposed in the absence or presence of 100 μM 18 β-glycyrrhetinic acid (18β-GA, Sigma), an inhibitor of connexin channels. After stimulation and incubation, dye uptake experiments were conducted for 5 min, in the presence of Ca2+-free medium (Hyclone), 0.4% Lucifer yellow (LY, molecular weight = 547 Da, Sigma), and 0.4% rhodamine dextran (RD, molecular weight = 10 kDa, Sigma). LY (Excitation wavelength = 425nm, Emission wavelength = 515nm) was used as a tracer for Cx43 hemichannel activity, and RD (Excitation wavelength = 540nm, Emission wavelength = 625nm) was used as a negative control because its molecular mass is 10kD which is too large to pass through the Cx43 hemichannels. After the dye uptake experiments, cells were washed with medium containing 1.8 mM Ca2+ to close the hemichannels, fixed with 1% paraformaldehyde and incubated with DAPI for 3 min at room temperature. Similar fields were observed under the fluorescence microscope (Olympus, Tokyo, Japan), and dye uptake was presented as a percentage of LY fluorescence cells.
Quantification of PGE2
For the ex vivo study, chondrocytes were isolated from rat TMJ cartilage with and without dental mechanical stimulation and cultured for 72 h. The conditioned medium was collected, and the extracellular PGE2 released into the medium was measured using a PGE2 EIA kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer’s instructions. The cultured chondrocytes were thoroughly washed three times with PBS and lysed to measure intracellular PGE2.
For the in vitro study, primary rat chondrocytes and ATDC5 cells were stimulated with shear stress at 16 dynes/cm2 for 2 h. The medium was collected immediately after stress stimulation for extracellular PGE2 measurement, whereas intracellular PGE2 was detected after the cells were thoroughly washed and lysed.
Confocal microscopic observation
Primary rat chondrocytes and ATDC5 cells were treated with 2-h FFSS at 16 dynes/cm2 and then immediately fixed by 4% paraformaldehyde. In addition, another set of ATDC5 cells were incubated for 8, 48 or 72 h post-FFSS and then fixed in order to observe cell responses during recovery. Both types of the cells were incubated overnight with the Cx43-antibody (dilution 1:500, Sigma) at 4°C, and with CY3-conjugated antibody (dilution 1:1000, Invitrogen) for 1 h, and, lastly, with DAPI for 3 min at room temperature. Samples were observed under a confocal microscopic system (Olympus, Tokyo, Japan). The integral optical density (IOD) of Cx43 fluorescence (Excitation wavelength = 553nm, Emission wavelength = 565nm) was measured with Image Pro Plus 6.0 (Olympus) and the results were represented as the ratio of the IOD of FFSS groups to the IOD of control groups.
Cell surface protein isolation and western blotting
ATDC5 cells were treated with and without 2-h FFSS at 16 dynes/cm2 and incubated for 8, 48 and 72 h. After the incubation, the surface proteins of ATDC5 were biotinylated and isolated using the Cell Surface Protein Isolation Kit (Thermo Scientific, Rockford, IL, USA). The total protein was obtained by lysing the cells. Total protein and isolated surface protein of ATDC5 cells were fractionated by SDS-PAGE and transferred onto a nitrocellulose membrane. The nitrocellulose sheet was blocked with 5% nonfat milk and incubated with the Cx43-antibody (dilution 1:2000, Sigma). The blots were developed using a horseradish peroxidase–conjugated secondary antibody (Zhong Shan Goldenbridge Biotechnology) and enhanced chemiluminescence detection.
Statistical Analysis
Values are presented as the mean and 95% confidence intervals (CI) (lower and upper limits). Statistical analysis was performed using SPSS software, version 11.0 (SPSS). All data acquisition and analysis was performed blindly. For comparison of two groups, the Student’s t-test was used. For multiple comparisons of three groups or more than three groups, one-way ANOVA followed by Bonferroni’s post hoc test was used. The statistical significance was defined as P < 0.05.
Results
Histological changes in rat TMJ cartilage with dental mechanical stimulation
In TMJ cartilage of control groups, four layers were typically identified as the fibrous, proliferative, prehypertrophic and hypertrophic layers (Fig.1 B and C). In 2- and 8-week experimental groups, OA–like lesions were observed in the cartilage, characterized as reduced number and size of chondrocytes, pyknotic nuclei, irregularity of cellular arrangement, and cell free areas within the cartilage (Fig. 1C), which were similar to lesion characteristics we previously reported [37, 38].
Expression of Cx43, and the synthesis and release PGE2 were increased in TMJ cartilage exposed to dental mechanical stimulation
In control groups, Cx43-positive cells could be observed in all layers of TMJ cartilage, but were most obvious in the prehypertrophic layer and the superficial zone of the hypertrophic layer (Fig. 2A). Although the size of chondrocytes was smaller than their lacuna (Fig. 2A), this was merely an artifact as the dehydration procedure induces chondrocyte shrinkage [41].
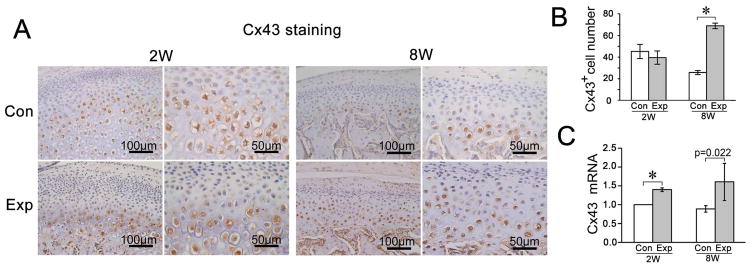
Fig. 2.
The comparison of Cx43 expression levels in the rat TMJ cartilage between groups exposed to dental mechanical stimulation (Exp) and controls (Con). A, Immunohistochemical staining of Cx43. Both lower and higher magnification images are provided to better exhibit the layers of the joint cartilage and the plasma membrane and cytoplasm localization of Cx43 protein. B and C, Comparison of the percentage of Cx43-positive chondrocytes (n=5) and mRNA expression levels of Cx43 (n=3) detected by a real-time PCR assay in rat TMJ cartilage between the control and experimental groups. Values are represented as the mean with lower and upper limits of 95% confidence intervals (CI). *P < 0.0001. 2W, 2-week group; 8W, 8-week group; Con, control group; Exp, experimental group.
Compared to age-matched controls, the percentage of Cx43-positive cells was significantly increased in the 8-week experimental group whereas there was no difference at 2-weeks (Fig. 2 A and B). However, the Cx43 mRNA expression was significantly increased in both the 2- and 8-week experimental groups versus their age-matched controls (Fig. 2C).
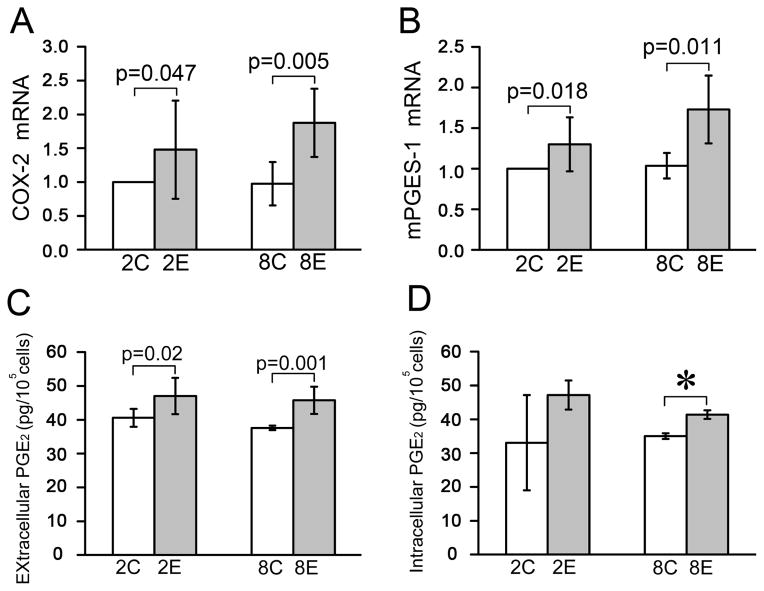
Next, the mRNA expression of COX-2 and mPGES-1 were found to be significantly higher in TMJ cartilage from the 2- and 8-week experimental groups compared to the age-matched controls (Fig. 3 A and B). Furthermore, the amount of extracellular PGE2 was significantly increased in both the 2- and 8-week experimental groups (Fig. 3C), but the intracellular PGE2 was increased only at 8-weeks and not at 2-weeks (Fig. 3D).
Fig. 3.
The synthesis and release of PGE2 were increased in the rat TMJ cartilage exposed to dental mechanical stimulation. A and B, Comparison of the mRNA expression levels of COX-2 (n=3) and mPGES-1 (n=3) detected by a real-time PCR assay in rat TMJ cartilage between the control and experimental groups. C and D, Comparison of the amount of extracellular (n=4) and intracellular (n=4) PGE2 between the control and experimental groups. Chondrocytes were isolated from rat TMJ cartilage with and without dental mechanical stimulation and cultured for 72 h. The medium was collected for measurement of the extracellular PGE2 using a PGE2 EIA kit (Cayman) and the cultured chondrocytes were used for detecting the intracellular PGE2 level via thoroughly washing and lysing procedures. Values are represented as the mean with lower and upper limits of 95% CI. *P <0.0001. 2W, 2-week group; 8W, 8-week group; Con, control group; Exp, experimental group.
Cx43 hemichannels opened under FFSS but closed after withdrawal
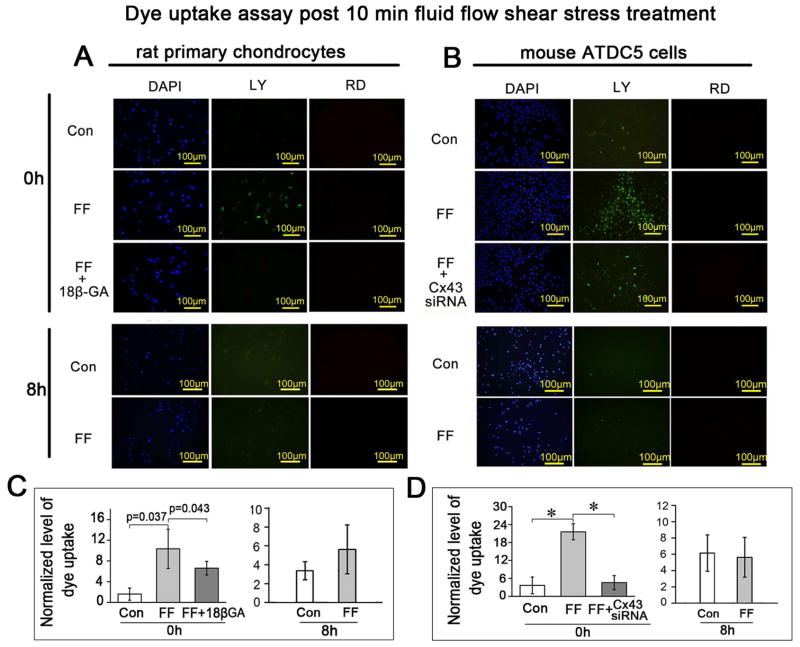
To assess the effect of FFSS on Cx43 hemichannels, dye uptake assays were performed on primary rat chondrocytes (Fig. 4 A and C) and ATDC5 cells (Fig. 4 B and D) at 0 and 8 h after 10 min FFSS treatment at 16 dynes/cm2. The amount of LY uptake in primary rat chondrocytes (Fig. 4 A and C) or ATDC5 cells (Fig. 4 B and D) was significantly increased at 0 h after the FFSS for 10 min, indicating hemichannel opening. This increase of LY uptake was blocked by the connexin channel inhibitor 18β-GA (Fig. 4 A and C) or by Cx43 siRNA (Fig. 4 B and D). Cx43 siRNA transfection in ATDC5 cells knocked down the expression of Cx43 at both the mRNA and protein levels (Supplemental Fig. 1). However, the LY uptake was completely reversed after 8 h incubation in the same solution post 10 min FFSS treatment, indicating that the Cx43 hemichannels closed after the withdrawal of FFSS.
Fig. 4.
The Cx43 hemichannels opened under the FFSS but closed after fluid flow withdrawal. Ten minutes of FFSS treatment at 16 dynes/cm2 stimulated the uptake of dye in primary rat chondrocytes (A and C) and ATDC5 cells (B and D) in contrast to the untreated control cells. This increased dye uptake was blocked by 18β-GA (A and C) or Cx43 siRNA (B and D). The dye uptake capability was diminished when cells were tested after 8 h incubation following the 10 min shear stress stimulation, indicating the closure of Cx43 hemichannels after withdrawal of the FFSS stimulation. n=3 for each experiment. Values are the mean with lower and upper limits of 95% CI. *P < 0.0001. Con, control groups; FF, FFSS group.
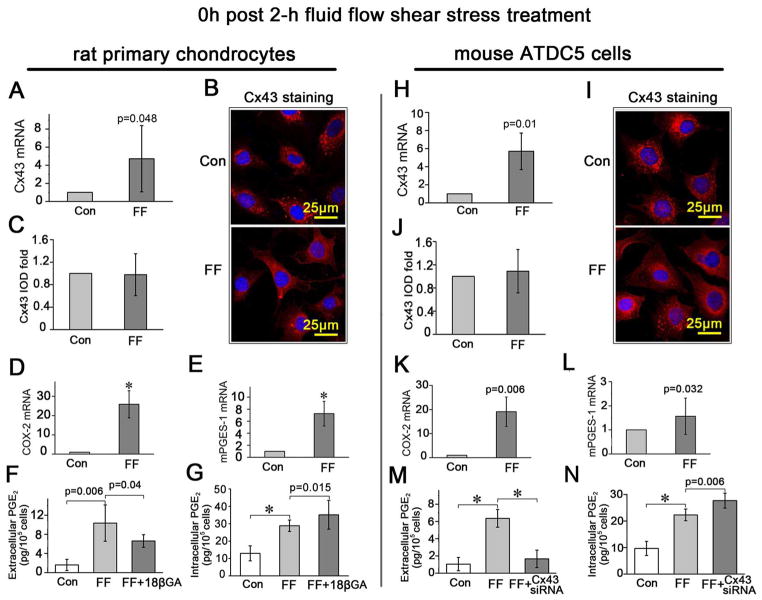
The expression of Cx43, and the synthesis and release of PGE2 were all increased upon FFSS
The mRNA expression of Cx43 in primary rat chondrocytes (Fig. 5A) and ATDC5 cells (Fig. 5H) was increased immediately after 2-h of FFSS. Although the increased Cx43 protein level was not significant according to the IOD value evaluated on fluorescent images observed under confocal microscopy (Fig. 5 B and C for primary rat chondrocytes, Fig. 5 I and J for ADTC5 cells), there was a redistribution of Cx43 from the cytosol to the plasma membrane, making the polygonal cell body more visible compared to those that did not receive shear stress treatment (Fig. 5 B and I). Furthermore, the Cx43 signals close to the nuclei revealed by fluorescent staining were much regularly arranged after 2-h FFSS stimulation (Fig. 5 B and I, lower panels) while cells without stress stimulation demonstrated a more scattered Cx43 signal pattern (Fig. 5 B and I, upper panels). Combined, these data indicated a redistribution of Cx43 induced by FFSS treatment.
Fig. 5.
The expression of Cx43, and the synthesis and release of PGE2 were all increased upon FFSS. A, D, E and H, K, L, Comparison of the Cx43, COX-2 and mPGES-1 mRNA expression levels in primary rat chondrocytes (A, D and E) and ATDC5 cells (H, K and L) between those treated with 2-h FFSS stimulation and those without. B and I, Confocal microscopy images showed the expression of Cx43 in primary rat chondrocytes (B) and ATDC5 cells (I) with (FF) and without (Con) 2-h FFSS treatment. C and J, Quantitative analysis of integral optical density (IOD) of Cx43 expression according to the confocal images. F and M: The amount of the released PGE2 from primary rat chondrocytes with/without 18β-GA (F) or from ATDC5 cells with/without Cx43 siRNA (M), under 2-h FFSS treatment in comparison with the blank controls (Con) determined using a PGE2 EIA kit. G and N: The amount of the intracellular PGE2 from primary rat chondrocytes with/without 18β-GA (G) or from ATDC5 cells with/without Cx43 siRNA (N), under 2-h FFSS treatment in comparison with the blank controls using a PGE2 EIA kit. All samples were prepared immediately after FFSS treatment. n=3 for each experiment. Values are the mean with lower and upper limits of 95% CI. *P < 0.0001. Con, control groups; FF, FFSS treatment group.
The 2-h FFSS treatment increased the synthesis of PGE2 indicated by enhanced mRNA expression of COX-2 and mPGES-1 in both the primary rat chondrocytes (Fig. 5 D and E) and ATDC5 cells (Fig. 5 K and L). In addition, FFSS increased the release of PGE2, which could be significantly inhibited by addition of the connexin channel inhibitor 18 β-GA (Fig. 5F) or Cx43 siRNA (Fig. 5M) although though the intracellular PGE2 level was still significantly upregulated compared to controls (Fig. 5 G and N).
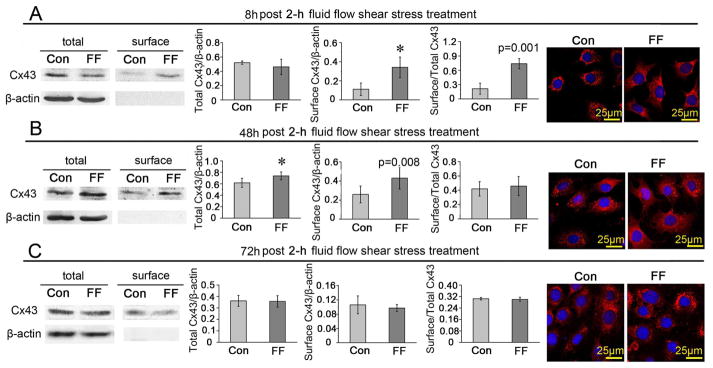
The long-term effects of FFSS on the expression and distribution of Cx43 protein in ATDC5 cells
At 8 h after 2-h FFSS treatment, the cell surface level, but not the total level of Cx43 protein level was higher than those did not receive stress stimulation, leading to an increased ratio of surface/total Cx43 protein (Fig. 6A). The Cx43 expression observed under the confocal microscope was similar in the 8 h group compared to cells fixed immediately after FFSS as shown in Fig. 5I. The difference in the ratio of surface/total Cx43 protein between the FFSS treated group and the control was not significant at 48 h after fluid flow stress although both the total and cell surface expressed Cx43 protein levels were still higher (Fig. 6B) and the Cx43 immunofluorescent signals were much stronger than control (Fig. 6B). However, at 72 h after fluid flow stress, the total and cell surface expressed Cx43 protein levels, the surface/total Cx43 ratio (Fig. 6C) and the Cx43 immunofluorescent signal intensity returned to control levels (Fig. 6C).
Fig. 6.
The long-term effects of FFSS on the expression and distribution of Cx43 protein in ATDC5 cells. ATDC5 cells were incubated for 8 h (A), 48 h (B) and 72 h (C) after being treated with and without 2-h shear stress at 16 dynes/cm2, and then tested with western blotting assays or observed under a confocal microscope. n=3 for each experiment. Values are the mean with lower and upper limits of 95% CI. *P < 0.0001. Con, control groups; FF, FFSS treatment group.
Effects of different intensities of FFSS on the expression of Cx43, SOX-9, collagen type II and aggrecan
The mRNA expression level of Cx43 increased under shear stress at all levels tested including 8, 16 and 24 dynes/cm2 (Supplemental Fig. 2A). The mRNA expression of SRY (sex determining region Y)-box 9 (SOX-9) was upregulated (Supplemental Fig. 2B) while that of collagen type II (Supplemental Fig. 2C) was decreased at all three levels. The mRNA expression of aggrecan was increased only at the level of 8 dynes/cm2 and showed no significant changes at levels of 16 and 24 dynes/cm2 (Supplemental Fig. 2D).
Discussion
In the present study, we demonstrated that dental mechanical stimulation increased not only Cx43 expression, but also the synthesis and release of PGE2 in rat TMJ cartilage. Consistently, FFSS treatment promoted the expression and membrane transferring of Cx43 of primary rat chondrocytes and ATDC5 cells, and increased the synthesis and release of PGE2. FFSS also induced an opening of Cx43 hemichannels in chondrocytes evaluated by dye uptake and PGE2 release, which could be blocked by 18β-GA or Cx43 siRNA. Furthermore, the opening of Cx43 hemichannels, revealed by dye uptake, closed when the mechanical loading was withdrawn, indicating that the opening of Cx43 hemichannels required the persistence of mechanical loading. However, the increased Cx43 expression and its membrane transferring induced by 2-h FFSS treatment persisted until 48 h and returned to control levels at 72 h post-treatment.
The present data indicated, as has been described in osteocytes [11, 42], that Cx43 hemichannels play a role in exchanging small molecules (e.g. PGE2) between chondrocytes and the extracellular matrix in response to mechanical stimulation. As Cx43 expression and membrane localization were reported as chronic responses to mechanical stimulation [43], the dental loading conditions presented here appear to take on the characteristics of chronic biomechanical effects on TMJ cartilage. In osteocytes under shear stress, the intracellular PGE2 did not increase when Cx43 hemichannels were inhibited. Unlike the osteocytes, however, the intracellular level of PGE2 was higher in primary rat chondrocytes and ATDC5 cells when Cx43 hemichannels activity was blocked with 18β-GA or Cx43 siRNA. Besides the possible influences from the cell type differences, one of the potential reasons for this discrepancy could be that the Cx43 hemichannels are the principal pathway for the release of PGE2 from chondrocytes, which means that when the hemichannels are blocked, there is no outlet for PGE2 release in chondrocytes. Another difference between the data presented here and that from osteocytes regards the expression level of Cx43 after FFSS withdrawal. In this study, expression level of Cx43 persisted in the ATDC5 cells at a higher level 48 h after withdrawal of FFSS stimulation, which was greater than the 24 h reported in the osteocytes although that study did not look at any longer time points. [10]. The data in chondrocytes suggested that the reduction of the Cx43 expression in chondrocyte lagged behind the closing of Cx43 hemichannels, which was not the case in osteocytes [43]. The earlier recovery of Cx43 expression and redistribution in osteocytes was explained as a potential feedback inhibition of biosynthesis by the substrate [11], while in chondrocytes, the effect of this potential inhibition mechanism seemed weak. In other words, the current chondrocyte response to the present chronic mechanical loading, i.e. the increased Cx43 hemichannel activity, is recognized as a principle component of the biomolecular communication between chondrocytes and the cartilage matrix.
In the current work, we also detected the effects of different intensities of FFSS on the mRNA expression levels of SOX-9, aggrecan and type II collagen in ATDC5 cells. We demonstrated a consistent increase of SOX-9, and a temporary increase of aggrecan but a decrease of collagen type II at the mRNA level. These results are in accordance with what were reported in the literature, which showed that the expression of SOX-9, collagen type II and aggrecan changed in response to mechanical loading [44–47]. In the in vitro experiments of this study, we chose FFSS stimulation because the incisal movement was disturbed by the aberrant prosthesis applied in our rodent model with TMJ OA-like lesions [37–39]. It has been reported that both articular chondrocyte metabolism and extracellular matrix integrity-associated biological factors are directly modulated by fluid flow shear forces that act on the cells through mechanotransduction processes [1]. Studies have shown that the shear stress increased the expression of osteoarthritic markers in chondrocytes, such as PGE2, IL-6, and nitric oxide which promoted glycosaminoglycan synthesis [1, 17]. Similarly, mechanical compression was also reported to be capable of increasing the production of PGE2 and nitric oxide in cartilage [18]. Both the shear- and compression-stimulation were reported to induce chondrocyte transcription via the MAPK pathway [18, 46]. Based on previous reports, PGE2 has a positive impact on the expression of SOX-9 [48] but a negative impact on cartilage matrix proteins including aggrecan and collagen type II [26, 49]. However, our results indicated a catabolic effect of the increased release of PGE2 through the Cx43 hemichannels.
In summary, the present study provides evidence that Cx43 hemichannels play an important role in chondrocyte mechanotransduction. The function of Cx43 hemichannels is likely to offer a general mechanism for the rapid exit of small molecules such as PGE2 when stimulated mechanically. As such, it provides a solution to the initial event of mechanically induced degradation of cartilage because PGE2, as reported in literature, is involved in the catabolic metabolism of chondrocytes during the progression of OA [50].
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Natural Science Foundation of China (No. 81271169) for Meiqing Wang, a Welch grant (AQ-1507) and NIH EY012085 for Jean X. Jiang.
We gratefully acknowledge Shujing Cai for assistance in histology procedure and Danielle Callaway for carefully editing the text.
Footnotes
Author contributions
Conception and design of the study: JZ, MW, JXJ.
Sample and data collection: JZ, HZ, and MZ.
Statistical analysis: ZQ.
Drafting of manuscript: JZ, MW, YW and JXJ.
Critical revision of manuscript: YW, JXJ, LL, LJ, TY and DAC.
Approving final manuscript version: JZ, HZ, MZ, ZQ, YW, DAC, JXJ, LL, LJ and MW.
Competing interests
The authors do not have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lane Smith R, Trindade MC, Ikenoue T, Mohtai M, Das P, Carter DR, et al. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37:95–107. [PubMed] [Google Scholar]
- 2.Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1:4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TM, Grodzinsky AJ, Hunziker EB, Sandy JD. Effects of injurious compression on matrix turnover around individual cells in calf articular cartilage explants. J Orthop Res. 1998;16:490–499. doi: 10.1002/jor.1100160415. [DOI] [PubMed] [Google Scholar]
- 4.Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 5.Behets C, Williams JM, Chappard D, Devogelaer JP, Manicourt DH. Effects of calcitonin on subchondral trabecular bone changes and on osteoarthritic cartilage lesions after acute anterior cruciate ligament deficiency. J Bone Miner Res. 2004;19:1821–1826. doi: 10.1359/JBMR.040609. [DOI] [PubMed] [Google Scholar]
- 6.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 7.Ilvesaro J, Vaananen K, Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J Bone Miner Res. 2000;15:919–926. doi: 10.1359/jbmr.2000.15.5.919. [DOI] [PubMed] [Google Scholar]
- 8.Romanello M, Veronesi V, D’Andrea P. Mechanosensitivity and intercellular communication in HOBIT osteoblastic cells: a possible role for gap junction hemichannels. Biorheology. 2003;40:119–121. [PubMed] [Google Scholar]
- 9.Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Miner Res. 1998;13:218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]
- 10.Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 11.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwab W, Hofer A, Kasper M. Immunohistochemical distribution of connexin 43 in the cartilage of rats and mice. Histochem J. 1998;30:413–419. doi: 10.1023/a:1003220225670. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Pritchard MR, Middleton FA, Horton JA, Damron TA. Microarray analysis of perichondral and reserve growth plate zones identifies differential gene expressions and signal pathways. Bone. 2008;43:511–520. doi: 10.1016/j.bone.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayan MD, Carpintero-Fernandez P, Gago-Fuentes R, Martinez-de-Ilarduya O, Wang HZ, Valiunas V, et al. Human articular chondrocytes express multiple gap junction proteins: differential expression of connexins in normal and osteoarthritic cartilage. Am J Pathol. 2013;182:1337–1346. doi: 10.1016/j.ajpath.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gago-Fuentes R, Carpintero-Fernandez P, Goldring MB, Brink PR, Mayan MD, Blanco FJ. Biochemical evidence for gap junctions and Cx43 expression in immortalized human chondrocyte cell line: a potential model in the study of cell communication in human chondrocytes. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Donahue HJ, Guilak F, Vander Molen MA, McLeod KJ, Rubin CT, Grande DA, et al. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. J Bone Miner Res. 1995;10:1359–1364. doi: 10.1002/jbmr.5650100913. [DOI] [PubMed] [Google Scholar]
- 17.Smith RL, Donlon BS, Gupta MK, Mohtai M, Das P, Carter DR, et al. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J Orthop Res. 1995;13:824–831. doi: 10.1002/jor.1100130604. [DOI] [PubMed] [Google Scholar]
- 18.Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Cavadias S, et al. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology. 2008;45:301–320. [PubMed] [Google Scholar]
- 19.Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68–69:633–647. doi: 10.1016/s0090-6980(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 20.Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- 21.Banu SK, Arosh JA, Chapdelaine P, Fortier MA. Molecular cloning and spatio-temporal expression of the prostaglandin transporter: a basis for the action of prostaglandins in the bovine reproductive system. Proc Natl Acad Sci U S A. 2003;100:11747–11752. doi: 10.1073/pnas.1833330100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan BS, Satriano JA, Pucci M, Schuster VL. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter “PGT”. J Biol Chem. 1998;273:6689–6697. doi: 10.1074/jbc.273.12.6689. [DOI] [PubMed] [Google Scholar]
- 23.Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10:259–264. doi: 10.1080/cac.10.4-6.259.264. [DOI] [PubMed] [Google Scholar]
- 24.Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 25.Mathy-Hartert M, Burton S, Deby-Dupont G, Devel P, Reginster JY, Henrotin Y. Influence of oxygen tension on nitric oxide and prostaglandin E2 synthesis by bovine chondrocytes. Osteoarthritis Cartilage. 2005;13:74–79. doi: 10.1016/j.joca.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513–523. doi: 10.1002/art.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baylink TM, Mohan S, Fitzsimmons RJ, Baylink DJ. Evaluation of signal transduction mechanisms for the mitogenic effects of prostaglandin E2 in normal human bone cells in vitro. J Bone Miner Res. 1996;11:1413–1418. doi: 10.1002/jbmr.5650111007. [DOI] [PubMed] [Google Scholar]
- 28.Harada SI, Balena R, Rodan GA, Rodan SB. The role of prostaglandins in bone formation. Connect Tissue Res. 1995;31:279–282. doi: 10.3109/03008209509010823. [DOI] [PubMed] [Google Scholar]
- 29.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Kojima F, Naraba H, Miyamoto S, Beppu M, Aoki H, Kawai S. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther. 2004;6:R355–365. doi: 10.1186/ar1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuko-Hongo K, Berenbaum F, Humbert L, Salvat C, Goldring MB, Thirion S. Up-regulation of microsomal prostaglandin E synthase 1 in osteoarthritic human cartilage: critical roles of the ERK-1/2 and p38 signaling pathways. Arthritis Rheum. 2004;50:2829–2838. doi: 10.1002/art.20437. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Afif H, Cheng S, Martel-Pelletier J, Pelletier JP, Ranger P, et al. Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes. J Rheumatol. 2005;32:887–895. [PubMed] [Google Scholar]
- 33.Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, Rediske J, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest. 1997;99:1231–1237. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herndon CM. Topical delivery of nonsteroidal anti-inflammatory drugs for osteoarthritis. J Pain Palliat Care Pharmacother. 2012;26:18–23. doi: 10.3109/15360288.2011.653600. [DOI] [PubMed] [Google Scholar]
- 36.Zarb GA, Carlsson GE. Temporomandibular disorders: osteoarthritis. J Orofac Pain. 1999;13:295–306. [PubMed] [Google Scholar]
- 37.Zhang X, Dai J, Lu L, Zhang J, Zhang M, Wang Y, et al. Experimentally created unilateral anterior crossbite induces a degenerative ossification phenotype in mandibular condyle of growing Sprague-Dawley rats. J Oral Rehabil. 2013 doi: 10.1111/joor.12072. [DOI] [PubMed] [Google Scholar]
- 38.Wang YL, Zhang J, Zhang M, Lu L, Wang X, Guo M, et al. Cartilage degradation in temporomandibular joint induced by unilateral anterior crossbite prosthesis. Oral Dis. 2013 doi: 10.1111/odi.12112. [DOI] [PubMed] [Google Scholar]
- 39.Liu YD, Liao LF, Zhang HY, Lu L, Jiao K, Zhang M, et al. Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage. 2013 doi: 10.1016/j.joca.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao K, Zhang J, Zhang M, Wei Y, Wu Y, Qiu ZY, et al. The identification of CD163 expressing phagocytic chondrocytes in joint cartilage and its novel scavenger role in cartilage degradation. PLoS One. 2013;8:e53312. doi: 10.1371/journal.pone.0053312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papadopoulou AK, Papachristou DJ, Chatzopoulos SA, Pirttiniemi P, Papavassiliou AG, Basdra EK. Load application induces changes in the expression levels of Sox-9, FGFR-3 and VEGF in condylar chondrocytes. FEBS Lett. 2007;581:2041–2046. doi: 10.1016/j.febslet.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Zhao Q, Wang E, Zhang C, Wang G, Yuan Q. Dynamic compression of rabbit adipose-derived stem cells transfected with insulin-like growth factor 1 in chitosan/gelatin scaffolds induces chondrogenesis and matrix biosynthesis. J Cell Physiol. 2012;227:2003–2012. doi: 10.1002/jcp.22927. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald JB, Jin M, Chai DH, Siparsky P, Fanning P, Grodzinsky AJ. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 2008;283:6735–6743. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
- 47.Correia C, Pereira AL, Duarte AR, Frias AM, Pedro AJ, Oliveira JT, et al. Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A. 2012;18:1979–1991. doi: 10.1089/ten.tea.2012.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierzchalska M, Grabacka M, Michalik M, Zyla K, Pierzchalski P. Prostaglandin E2 supports growth of chicken embryo intestinal organoids in Matrigel matrix. Biotechniques. 2012;52:307–315. doi: 10.2144/0000113851. [DOI] [PubMed] [Google Scholar]
- 49.Vo NV, Sowa GA, Kang JD, Seidel C, Studer RK. Prostaglandin E2 and prostaglandin F2alpha differentially modulate matrix metabolism of human nucleus pulposus cells. J Orthop Res. 2010;28:1259–1266. doi: 10.1002/jor.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attur M, Al-Mussawir HE, Patel J, Kitay A, Dave M, Palmer G, et al. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J Immunol. 2008;181:5082–5088. doi: 10.4049/jimmunol.181.7.5082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.