Abstract
Sprague–Dawley rats were exposed via inhalation to vapor condensates of either gasoline or gasoline combined with various fuel oxygenates to assess potential neurotoxicity of evaporative emissions. Test articles included vapor condensates prepared from “baseline gasoline” (BGVC), or gasoline combined with methyl tertiary butyl ether (G/MTBE), ethyl t-butyl ether (G/ETBE), t-amyl methyl ether (G/TAME), diisopropyl ether (G/DIPE), ethanol (G/EtOH), or t-butyl alcohol (G/TBA). Target concentrations were 0, 2000, 10,000 or 20,000 mg/mg3 and exposures were for 6 h/day, 5 days/week for 13 weeks. The functional observation battery (FOB) with the addition of motor activity (MA) testing, hematoxylin and eosin staining of brain tissue sections, and brain regional analysis of glial fibrillary acidic protein (GFAP) were used to assess behavioral changes, traditional neuropathology and astrogliosis, respectively. FOB and MA data for all agents, except G/TBA, were negative. G/TBA behavioral effects resolved during recovery. Neuropathology was negative for all groups. Analyses of GFAP revealed increases in multiple brain regions largely limited to males of the G/EtOH group, findings indicative of minor gliosis, most significantly in the cerebellum. Small changes (both increases and decreases) in GFAP were observed for other test agents but effects were not consistent across sex, brain region or exposure concentration.
Keywords: Neurotoxicity, Glial fibrillary acidic protein (GFAP), Astrogliosis, Gasoline vapor condensates, Methyl tertiary butyl ether, Ethyl t-butyl ether, t-Amyl methyl ether, Diisopropyl ether, Ethanol, t-Butyl alcohol
1. Introduction
The 1990 amendments to the Clean Air Act (CAA) mandated the use of oxygenates in motor gasoline. In 1994, the U.S. Environmental Protection Agency (EPA) issued a final rule under the Act which added new health effects information and testing requirements to the Agency’s existing registration requirements. As described in more detail in a companion paper (Henley et al., 2014), requirements include inhalation exposures to evaporative emissions of the gasoline or additive in question. The health endpoints include assessments for standard subchronic toxicity, neurotoxicity, genotoxicity, immunotoxicity, developmental and reproductive toxicity, and chronic toxicity/carcinogenicity. The results of chronic toxicity testing of gasoline and gasoline combined with MTBE have already been reported (Benson et al., 2011). This paper describes the results of neurotoxicity testing submitted to EPA.
Test materials evaluated in the 13 week toxicity studies included vapor condensates prepared from an EPA described “baseline gasoline” (BGVC), as well as gasoline combined with methyl tertiary butyl ether (G/MTBE), ethyl t-butyl ether (G/ETBE), t-amyl methyl ether (G/TAME), diisopropyl ether (G/DIPE), ethanol (G/EtOH), or t-butyl alcohol (G/TBA). The goal of the studies was to provide information on the extent to which the use of oxygenates in gasoline might alter the hazard of evaporative emissions that are encountered during refueling of vehicles, compared to those from gasoline alone. The animals evaluated for neurotoxicity were exposed concurrently with animals involved in a subchronic inhalation toxicity study of the materials described above (Clark et al., 2014). Additional groups of animals were exposed to the test materials concurrently to evaluate the effects of exposure on immunotoxicity and genotoxicity, the results of which are described elsewhere (White et al., 2014; Schreiner et al., 2014).
Astrogliosis is the activated state of astrocytes, a characteristic feature of all types of CNS damage. A hallmark of astrogliosis, often termed “reactive gliosis,” is the intracellular accumulation of astroglial filaments, the major protein component of which is glial fibrillary acidic protein (GFAP). Thus, an increase in the brain concentration of GFAP serves as a biochemical indicator of astrogliosis and, as such, enhanced expression of GFAP is a biomarker of neurotoxicity. To validate the use of GFAP as a biomarker of neurotoxicity, prototype neurotoxicants were administered to experimental animals and the effects of these agents on the tissue content of GFAP was determined by immunoassay (O’Callaghan, 1991a, 2002; Norton et al., 1992; O’Callaghan and Sriram, 2005; O’Callaghan et al., 2014). Assays of GFAP were found to reveal dose-, time- and region-dependent patterns of neurotoxicity at toxicant dosages below those that cause light microscopic evidence of cell loss or damage (O’Callaghan, 1988; Norton et al., 1992; O’Callaghan and Sriram, 2005; O’Callaghan et al., 2014). Moreover, the temporal and regional increments in GFAP correspond to the temporal and regional patterns of neuronal damage, as revealed by sensitive silver stains (Balaban et al., 1988; Balaban, 1992). These findings indicate that assaying brain regional levels of GFAP represents a sensitive, simple and quantitative approach for evaluation of nervous system damage (O’Callaghan, 1991a, 2000; Norton et al., 1992; O’Callaghan and Sriram, 2005).
In this study, both standard neurobehavioral and motor toxicity assessments as well as the GFAP assay were used for assessing the potential neurotoxic effects of the fuel vapors described above. Although the EPA Guidelines for GFAP determination (US EPA, 1994a) specifies six regions to be analyzed, the analysis used in these studies was expanded to include an additional three areas of the brain to maximize the potential for detecting enhanced expression of GFAP due to exposure to the test substance.
2. Methods and materials
2.1. Test material exposures
Seven separate inhalation studies involving exposures to vapor condensates were conducted. Test materials included baseline gasoline (BGVC) and BGVC combined with methyl t-butyl ether (G/MTBE), ethanol (G/EtOH), t-amyl ethyl ether (G/TAME), ethyl t-butyl ether (G/ETBE), diisopropyl ether (G/DIPE), or t-butyl alcohol (G/TBA) at concentrations of 0, 2000, 10,000, 20,000 mg/m3/day, 5 days a for a total of 65 exposures over 13 weeks, performed at Huntingdon Life Sciences (East Millstone, NJ). Ten adult Sprague–Dawley rats (5 males and 5 females) were used in each of the investigations to examine neuropathology and to determine changes in glial fibrillary acidic protein (GFAP) levels in areas of the brain. Motor activity and performance in the functional observational battery (FOB) was evaluated in all 20 animals. The animals were satellite groups of a larger subchronic toxicity study, and the generation and composition of the vapor concentrations as well as additional details on the exposure methodology are reported in companion articles (Henley et al., 2014; Clark et al., 2014).
2.2. Neurobehavioral studies
FOB and motor activity evaluations were staggered over several sessions and conducted on non-exposure days at least 16 h post-exposure. With the exception of the pretest, evaluations were performed “blind”, i.e., the observer did not know the identity of the animal’s exposure group. Evaluations were conducted once before initiation of exposures and again during the 4th, 8th and 13th week of exposures. Time of testing was balanced across treatment groups. Noise level was maintained within a level of 55–65 decibels by a white noise generator. Temperature, humidity and illumination were measured and recorded to ensure that variations in environmental conditions are minimal during all evaluations. The functional observational battery (Moser, 1989) was performed for all animals before evaluation of motor activity and included:
Home Cage Evaluations: posture, vocalization and palpebral closure.
Handling Evaluations: reactivity to general stimuli (handling); assessment of signs of autonomic function: lacrimation, salivation, altered fur appearance, or red crusty deposits around eyes.
Open Field Evaluations: arousal level and gait; count of urination and defecation; convulsions, tremors, abnormal movements or behaviors, excessive or repetitive actions; piloerection and exophthalmos.
Reflex Assessments: response to visual (approach response) and auditory (finger snap) stimuli; response to a tail pinch; pupillary function.
Grip Strength (Meyer et al., 1979): grip strength was measured using a grip strength meter (Columbus Instruments International Corporation, Columbus, Ohio).
Landing Foot Splay: each animal was dropped into a pan of sand from a height of one foot. The distance between the marks left by the hind paws was measured in centimeters.
Hind limb Extensor Strength: animals were held in a vertical position facing the observer with a firm grasp around the thorax. The observer placed one finger against the bottom of each hind paw and pressed toward the animal. Muscular resistance and pressure exerted by the animals were scored.
Air Righting Ability: animals were held upside down and dropped from a height of one foot into a container of bedding. The landing position of each animal was recorded.
Body Weight: animals were removed from their cages and weighed using a Mettler Balance, Model PE4000 (Mettler Instrument Corporation, Hightstown, New Jersey).
Motor Activity: using a modified version of Schulze’s procedures (Schulze, 1990), the locomotor activity of all animals was monitored using an automated Photobeam Activity System (San Diego Instruments, Inc., San Diego, California). Sessions were 60 min in length; each session was divided into 12 intervals of 5 min.
2.3. Neuropathology
Following 13 weeks of exposures, designated animals (5/sex/group) were anesthetized with an IP injection of sodium pentobarbital and transcardially perfused with phosphate buffered saline followed by 1% glutaraldehyde and 4% paraformaldehyde in the same buffer. After perfusion, the tissues listed in Table 1 were obtained. Measurement of the size (length and width) and weight of the whole brain (cerebrum, cerebellum and ponsmedulla) were made. All tissues were then placed into a fresh solution of the same fixative prior to processing.
Table 1.
Tissues examined microscopically.
| No.a | Tissues for neuropathology | Exposure groups
|
||
|---|---|---|---|---|
| Preserved | Microscopic examination | |||
|
| ||||
| All | Control, high | Low and mid | ||
| 6 | Brain (forebrain, central cerebrum, hippocampus, basal ganglia, midbrain, cerebellum and pons, medulla) | X | X | |
| 2 | Eye with optic nerve | X | X | |
| 6 | Spinal cord (cervical, thoracic, lumbar, cross and longitudinal sections) | X | X | |
| 2 | Sciatic nerve (cross and longitudinal sections) | X | X | |
| 2 | Tibial nerve (cross and longitudinal sections) | X | X | |
| 2 | Sural nerve (cross and longitudinal sections) | X | X | |
| 2 | Trigeminal ganglia | X | X | |
| 4 | Dorsal root ganglia (from C3–C6 and L4–L6) | X | X | |
| 4 | Dorsal root fibers (from C3–C6 and L4–L6) | X | X | |
| 4 | Ventral root fibers (from C3-Cs and L4–L6) | X | X | |
| 6 | Lungs and trachea | X | X | X |
| Tissues with macroscopic findings | X | X | X | |
Number of organs/sections that were preserved/examined per animal.
Peripheral nerves were post-fixed in 1% osmium tetroxide, processed and embedded in epoxy resin, sectioned at approximately 2 microns and stained with toluidine blue. All other tissues, including the brain, eye with optic nerve, spinal cord, trigeminal ganglia, dorsal root ganglia, dorsal and ventral root fibers, lungs and trachea were processed by standard techniques, embedded in paraffin and sectioned at approximately 6 microns.
The tissues listed in Table 1 were examined microscopically for all animals as indicated. Tissues with macroscopic lesions were examined in all animals. Any abnormalities not noted during macroscopic postmortem examinations which were seen during histological processing were recorded.
2.4. Immunoassay of GFAP
Personnel from the United States Center for Disease Control and Prevention – NIOSH (CDC) Health Effects Laboratory Division sacrificed (carbon dioxide inhalation) the animals (5 males, 5 females) after the 13 week exposure period, and removed, weighed and dissected the brains. The samples were shipped on dry ice to the CDC Health Effects Laboratory, Morgantown, WV, and upon receipt, were further processed for determination of GFAP response according to EPA guidelines (US EPA, 1994a). Brains were dissected free-hand on a cold plate. Analysis was limited to cerebellum, cortex, hippocampus, hypothalamus, olfactory bulbs, pituitary, striatum, thalamus and the rest of the brain. Each brain region was homogenized in 10 volumes of hot (85–95 °C) 1% sodium dodecyl sulfate (SDS) and stored at −75 °C until use. Total protein was determined by the bicinchonic acid (BCA) method (Smith et al., 1985) using bovine serum albumin as standard. GFAP was assayed by detergent-based Sandwich ELISA (Enzyme-linked immunosorbant assay) employing the 96 well microplate format (O’Callaghan, 1991a,b, 2002). This method is based on the binding of GFAP to antibodies (i.e. rabbit anti-GFAP) that have been coated onto individual wells of microtiter plates which is then bound in “sandwich” fashion by a second antibody to GFAP. The second antibody is bound by an enzyme-linked antibody directed toward it but not toward the first anti-GFAP antibody (i.e. monoclonal anti-GFAP and alkaline phosphatase conjugated anti-mouse IgG). Quantification is achieved by addition of a substrate (i.e. p-nitro-phenylphosphate) for the antibody bound enzyme, followed by spectrophotometry of the colored reaction product using a microtiter plate reader. The amount of GFAP was expressed as μg GFAP/mg total homogenate protein. The GFAP standard was prepared from rat spinal cord as described previously (O’Callaghan, 1991a).
2.5. Statistical analyses
The following parameters were analyzed statistically: mean body weight values and body weight changes (from pretest), mean motor activity counts, mean FOB data including forelimb and hind limb grip strength measurements, and mean landing foot splay measurements. In analyzing the FOB and motor activity data, all analyses included sex as an independent variable. In those instances where there were significant effects of sex, separate analyses by sex may have been done to explain the nature of the effect.
Some of the sensorimotor and observational battery variables were measured on a continuous scale and some were measured on a nominal or count scale. All measures were classified as either continuous or nominal. The analysis of the continuous variables was conducted by a mixed model analysis of covariance with a first order autoregressive error structure on the time points. The pretest response was used as the covariate. The residuals from the model were tested for normality at the p < 0.01 level of significance. Those variables that did not exhibit normally distributed residuals at the 0.01 level were transformed by Blom’s normalized rank transformation and reanalyzed (Blom, 1958). The nominal data of the functional observational battery was analyzed by a cumulative logit repeated measures analysis with contrast terms to test for differences between control and dosed groups (Agresti, 1989). For the measures that were count data (such as number of rears or number of urinations), the count responses may have been grouped into several data ranges to allow sufficient data in the cells. For example, the count of urinations might have been separated into three groups of 0, 1 to 4, 5 or more, and analyzed.
The analysis of the motor activity used a mixed model analysis of covariance with an unstructured error relationship among the 5-min periods, and a first order autoregressive error structure on weeks. The pretest response was used as the covariate. The residuals from the model were tested for normality at the p < 0.01 level of significance by the Kolmogorov–Smirnov test for normality. If the residuals were not normally distributed, the dependent variable (number of beam breaks) was converted to a function of its percentile rank, then transformed by the inverse normal distribution (the transformation is known as Blom’s transformation where the rank order is replaced by [rank − 0.375]/[n + 0.25]), and reanalyzed (Blom, 1958).
Statistical methods for the GFAP assay employed separate one-way analysis of variance (ANOVA) for each of the brain areas from male and female rats (JMP®, SAS Institute, 1995). The significance level was set at p < 0.05 and, to ensure detection of between group treatment effects, the least significance-difference test (Keppel, 1973) was used for post hoc analyses.
2.6. Compliance
These studies were conducted in accordance with the United States Environmental Protection Agency’s (EPA) Good Laboratory Practice Standards (US EPA, 1994b), and complied with all appropriate parts of the Animal Welfare Act Regulations USDA, 1989, 1991). The study also met the requirements of EPA’s guidelines for neurotoxicity toxicity screening and GFAP determination (US EPA, 1994a, 1994c, 1998).
3. Results
3.1. Motor activity
For the BGVC and G/MTBE exposure groups, the results of the analyses do not indicate a statistically significant exposure-related effect for either the original data or the Blom transformed data and the data plots do not suggest an exposure-related response (data not shown).
G/EtOH and G/ETBE – the residuals from the motor activity data analysis and those from the Blom transformed data were not normally distributed. A possible explanation for the non-normal distribution of the residuals is that approximately 32% of the observations in the EtOH group and 19% in the G/ETBE group were zero (due to acclimatization). It is difficult to have normally distributed residuals when a large percentage of the data have the same value. However, inspection of the residuals shows a reasonably symmetric distribution with slightly heavier tails than a normal distribution. Overall, these departures of the residuals from normality are not thought to affect the analysis conclusions which do not indicate a statistically significant exposure related effect for either the original or Blom transformed data.
G/TAME – based on a preliminary non-statistical evaluation of the data in which a possible increase in motor activity was noted in the high dose females during the 4th and 13th week of exposure, motor activity was also evaluated during the 17th week of study (recovery). However, the subsequent statistical evaluation of the data did not confirm this initial determination. The results of the analyses indicate a statistically significant differences in response by exposure, time, sex, exposure by sex by time interaction, and exposure by time by replicate interaction for the Blom transformed data (similar results were seen for the untransformed data).
In order to understand the interactions, analyses were performed separately by sex. The residuals from the untransformed data were not normally distributed for either sex. The residuals Blom transformed analyses were normally distributed for the males and not normally distributed for the residuals from the female analysis. There were no statistically significant exposure related effects for the data from the males. Analysis of the Blom transformed female data indicated there was a statistically significant exposure related response with the high exposure group showing more beam breaks compared to the control group (p < 0.03). The least squares means are the means accounting for the initial activity (pretest), study week, replicates, and their interactions. There was no linear relationship between exposure levels and response.
The residuals from the motor activity data analysis and those from the Blom transformed data were not normally distributed. Approximately 19% of the observations were zero. As explained above for G/EtOH and G/ETBE, these departures of the residuals from normality are not thought to affect the analysis conclusions.
G/DIPE – the results of the analysis of motor activity data indicated statistically significant differences in responses in exposure levels for both the original data and the Blom transformed data (p < 0.02). Specifically, contrast analysis of the Blom transformed data showed that responses from low and medium exposure groups were significantly different from the control group. However, there was no linear relationship between exposure levels and response, or any consistent change in activity related to exposure. The data plots do not suggest a consistent exposure related response, but show an increase in activity in the female control group and a decrease in activity in the female mid exposure group only during study week 4 (there was not a statistically significant time by exposure effect).
G/TBA – the results of the analyses indicated statistically significant differences in responses in exposure levels for both the original data and the Blom transformed data (p < 0.01). Analysis of contrast on Blom transformed data shows that the response of high exposure group is significantly more active compared to the control group (p < 0.01).
Recovery – because only the control and high exposure groups had recovery animals only these two groups were used in the recovery analysis. The results indicated statistically significant differences in responses in exposure levels for both the original and the Blom transformed data (p < 0.02 for original, and p < 0.05 for the Blom transformed data). There were more beam breaks averaged over time periods, sex, and replicates in the high exposure group (mean = 54.71 breaks) compared to the control group (mean = 50.33 breaks).
Overall, there was a statistically significant increase in motor activity seen in the high exposure G/TBA group relative to the control group during both the exposure and recovery periods.
3.2. Functional observational battery
3.2.1. Continuous measures
None of the four continuous measures in the FOB (i.e., fore- and hindlimb grip strength, foot splay, and body weight) indicated statistically significant exposure-related differences in any of the seven exposure groups. The residuals from body weight were not normally distributed (neither the observed data nor Blom transformed data) in most of the exposure groups (Table 2). Because the significant levels were not near the 0.05 level of significance the deviation from normality was not considered crucial in these analyses.
Table 2.
Functional observational battery (FOB) findings.
| FOB areas and measures | Exposure group
|
||||||
|---|---|---|---|---|---|---|---|
| BGVC | G/MTBE | G/EtOH | G/TAME | G/ETBE | G/DIPE | G/TBA | |
| Home cage evaluations | |||||||
| Posture vocalization | X | X | X | X | X | X | X |
| Palpebral closure | X | X | X | X | |||
| Handling evaluations | |||||||
| Ease of removal | X | X | X | X | X | ||
| Ease of handling | X | X | |||||
| Chromodacryorrhea | X | X | X | X | |||
| Lacrimation | X | X | |||||
| Salivation | |||||||
| Altered fur appearance | |||||||
| Open field evaluations | |||||||
| Gait and posture | |||||||
| Locomotion | X | X | X | ||||
| Arousal level | X | X | X | ||||
| Exophthalmia | |||||||
| Piloerection | X | X | |||||
| Defecation count | X | X | X | X | X | X | X |
| Urination count | X | X | X | X | X | X | X |
| Fasciculation | |||||||
| Tremors | |||||||
| Convulsions | |||||||
| Reflex assessments | |||||||
| Response to visual stimuli | X | X | X | X | X | ||
| Response to auditory stimuli | |||||||
| Pain perception | X | X | |||||
| Pupillary response | X | X | |||||
| Sensitivity to light touch | X | X | X | X | X | X | |
| Proprioception | |||||||
| Other | |||||||
| Forelimb grip strength* | O | ||||||
| Hindlimb grip strength* | O,B | ||||||
| Air righting ability | |||||||
| Landing foot splay* | B | ||||||
| Body weight* | O, B | O, B | O, B | O, B | O | O, B | |
X – demonstrated non-statistically significant variation.
O = observed data not normally distributed; B = Blom transformed data not normally distributed.
A blank box indicates there was not a statistically significant exposure-related difference in the endpoint evaluated.
Analyzed as a continuous variable.
In the G/TBA group, analysis of Blom transformed foot splay data indicated a significant exposure by time interaction (p < 0.04). Contrast analysis on Blom transformed data of foot splay indicated the week 4 to week 8 mean response of the control group was significantly different from the corresponding change in the mid dose group. A reasonable interpretation is that the week 8 control exposure group mean landing foot splay is higher than expected.
3.2.2. Discrete measures
For the measures evaluated in the FOB on a nominal or count scale, only between 6 and 11 of the measures showed any practical level of variation in the seven exposure groups (Table 2). None of these demonstrated statistically significant exposure-related differences. For the remaining variables all animals, or at most three to four responses, showed the same response in all groups at all time points and were not analyzed because of the lack of meaningful variation in response.
3.3. Brain measurements
No toxicologically significant differences in brain measurements or weights were observed in any of the test substance exposed animals in any of the exposure groups. A statistically significant decrease in brain weight was noted in the high dose male BGVC animals at the terminal interval. However, a similar difference was not seen at the same interval in the main study animals (Clark et al., 2014) and this difference was not considered toxicologically significant. The brain weights in perfused neuropathology animals were lower in general than those in non-perfused main study animals since the perfusion fixatives desiccate the brain tissue.
3.4. Neuropathology
No microscopic changes attributable to test substance effect were observed in brain, spinal cord, eyes, peripheral nerves, or ganglia among the high dose exposed animals in any of the exposure studies.
3.5. GFAP results
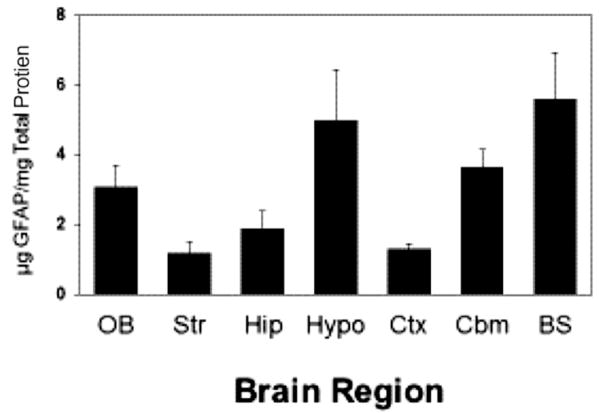
Results of the GFAP analyses are presented in a series of tables and figures. Table 3 presents GFAP levels from BGVC-exposed animals and serves as a baseline comparison for the other exposure groups. GFAP levels from the gasoline–ether and gasoline–alcohol exposed animals are shown in Table 4 (G/MTBE), Table 5 (G/TBA), Table 6 (G/ETBE), and Table 7 (G/EtOH), respectively. Control levels for GFAP vary markedly across different brain regions seen historically (Fig. 1) and in the present studies. Mean GFAP levels were reported for striatum, hippocampus, olfactory bulb, thalamus, hypothalamus, cerebellum, and the rest of the brain. Assays of pituitary samples gave variable results for BGVC and G/MTBE and were generally below levels of detection by this method for other vapor condensate samples and thus are not reported.
Table 3.
Mean GFAP levels on specific regions of rat brains following a 13 week whole body inhalation exposure to baseline gasoline (BGVC) vapor condensate.
| Brain area | Control | 2000 mg/m3 | 10,000 mg/m3 | 20,0000 mg/m3 |
|---|---|---|---|---|
| Males (N = 5) | ||||
| Striatum | 1.01 ± 0.09a | 0.89 ± 0.18 | 0.84 ± 0.17 | 0.93 ± 0.09 |
| Hippocampus | 2.88 ± 0.24 | 3.08 ± 0.27 | 2.63 ± 0.21 | 2.76 ± 0.12 |
| Cortex | 1.31 ± 0.14 | 1.44 ± 0.07 | 1.43 ± 0.13 | 1.20 ± 0.06 |
| Olfactory bulb | 2.49 ± 0.21 | 2.73 ± 0.47 | 2.26 ± 0.23 | 2.36 ± 0.12 |
| Thalamus | 2.15 ± 0.23 | 2.17 ± 0.29 | 1.71 ± 0.19 | 1.88 ± 0.10 |
| Hypothalamus | 8.62 ± 0.81 | 7.46 ± 0.84 | 6.82 ± 0.74 | 6.66 ± 0.90 |
| Cerebellum | 4.63 ± 0.37 | 4.09 ± 0.49 | 3.72 ± 0.28 | 3.93 ± 0.25 |
| Rest of brain | 4.70 ± 0.34 | 5.48 ± 0.58 | 3.81 ± 0.39 | 5.11 ± 0.38 |
| Females (N = 5) | ||||
| Striatum | 0.90 ± 0.13 | 0.82 ± 0.05 | 0.95 ± 0.04 | 0.87 ± 0.11 |
| Hippocampus | 2.83 ± 0.18 | 2.46 ± 0.06 | 3.06 ± 0.20 | 2.89 ± 0.36 |
| Cortex | 1.57 ± 0.14 | 1.50 ± 0.12 | 1.84 ± 0.28 | 1.60 ± 0.22 |
| Olfactory bulb | 2.34 ± 0.26 | 2.49 ± 0.28 | 2.70 ± 0.27 | 2.50 ± 0.26 |
| Thalamus | 2.12 ± 0.23 | 1.64 ± 0.12 | 2.02 ± 0.23 | 1.88 ± 0.23 |
| Hypothalamus | 8.71 ± 0.90 | 6.13 ± 0.36 | 8.36 ± 1.30 | 7.71 ± 0.90 |
| Cerebellum | 3.98 ± 0.36 | 3.04 ± 0.35 | 4.29 ± 0.54 | 4.23 ± 0.40 |
| Rest of brain | 4.59 ± 0.37 | 4.87 ± 0.23 | 4.93 ± 0.30 | 4.91 ± 0.37 |
Each value represents the mean ± SEM for the concentration of GFAP (μg/mg total protein).
Table 4.
Mean GFAP levels on specific regions of rat brains following a 13 week whole body inhalation exposure to gasoline MTBE (G/MTBE) vapor condensate.
| Brain areaA1:E20 | Control | 2000 mg/m3 | 10,000 mg/m3 | 20,0000 mg/m3 |
|---|---|---|---|---|
| Males (N = 5) | ||||
| Striatum | 0.94 ± 0.14a | 0.97 ± 0.13 | 1.08 ± 0.16 | 0.83 ± 0.07 |
| Hippocampus | 2.62 ± 0.23 | 3.45 ± 0.30b | 3.18 ± 0.28 | 3.02 ± 0.12 |
| Cortex | 1.37 ± 0.06 | 1.66 ± 0.20 | 1.65 ± 0.10 | 1.28 ± 0.11 |
| Olfactory bulb | 2.14 ± 0.14 | 2.45 ± 0.23 | 2.06 ± 0.17 | 2.36 ± 0.15 |
| Thalamus | 1.90 ± 0.08 | 2.19 ± 0.20 | 2.04 ± 0.08 | 2.13 ± 0.08 |
| Hypothalamus | 6.04 ± 0.99 | 6.64 ± 0.93 | 6.63 ± 0.79 | 6.09 ± 0.71 |
| Cerebellum | 5.97 ± 0.17 | 6.53 ± 0.38 | 5.99 ± 0.56 | 6.23 ± 0.43 |
| Rest of brain | 4.19 ± 0.42 | 5.81 ± 0.51b | 5.58 ± 0.27b | 5.08 ± 0.49 |
| Females (N = 5) | ||||
| Striatum | 0.93 ± 0.06 | 0.96 ± 0.09 | 0.83 ± 0.06 | 0.78 ± 0.05 |
| Hippocampus | 2.67 ± 0.08 | 3.12 ± 0.20 | 2.64 ± 0.16 | 2.60 ± 0.14 |
| Cortex | 1.23 ± 0.05 | 1.26 ± 0.10 | 1.18 ± 0.10 | 1.04 ± 0.07 |
| Olfactory bulb | 1.91 ± 0.16 | 1.92 ± 0.16 | 2.01 ± 0.09 | 2.01 ± 0.15 |
| Thalamus | 1.58 ± 0.13 | 1.96 ± 0.25b | 1.64 ± 0.08 | 1.43 ± 0.06 |
| Hypothalamus | 5.67 ± 0.65 | 6.59 ± 0.61 | 4.86 ± 0.11 | 5.08 ± 0.52 |
| Cerebellum | 5.22 ± 0.49 | 5.18 ± 0.78 | 4.94 ± 0.26 | 4.16 ± 0.36 |
| Rest of brain | 4.62 ± 0.51 | 4.54 ± 0.30 | 4.04 ± 0.22 | 4.29 ± 0.26 |
Each value represents the mean ± SEM for the concentration of GFAP (μg/mg total protein).
Statistically different from control (p < 0.05).
Table 5.
Mean GFAP levels on specific regions of rat brains following a 13 week whole body inhalation exposure to gasoline TBA (G/TBA) vapor condensate.
| Brain area | Control | 2000 mg/m3 | 10,000 mg/m3 | 20,0000 mg/m3 |
|---|---|---|---|---|
| Males (N = 5) | ||||
| Striatum | 1.22 ± 0.12a | 1.25 ± 0.12 | 1.18 ± 0.07 | 1.32 ± 0.10 |
| Hippocampus | 2.52 ± 0.17 | 2.76 ± 0.18 | 3.00 ± 0.30 | 2.62 ± 0.07 |
| Cortex | 1.17 ± 0.07 | 1.42 ± 0.10b | 1.23 ± 0.13 | 1.10 ± 0.06 |
| Olfactory bulb | 2.12 ± 0.21 | 2.11 ± 0.18 | 1.92 ± 0.19 | 2.28 ± 0.20 |
| Thalamus | 2.29 ± 0.11 | 2.73 ± 0.38 | 2.18 ± 0.22 | 2.30 ± 0.15 |
| Hypothalamus | 7.08 ± 0.70 | 7.40 ± 0.96 | 6.48 ± 0.45 | 4.97 ± 0.70b↓ |
| Cerebellum | 5.21 ± 0.47 | 4.66 ± 0.22 | 4.93 ± 0.44 | 4.67 ± 0.34 |
| Rest of brain | 4.91 ± 0.33 | 5.17 ± 0.43 | 4.45 ± 0.20 | 4.09 ± 0.16 |
| Females (N = 5) | ||||
| Striatum | 1.32 ± 0.20 | 1.47 ± 0.12 | 1.41 ± 0.10 | 1.05 ± 0.11 |
| Hippocampus | 2.72 ± 0.12 | 2.74 ± 0.07 | 2.87 ± 0.18 | 2.36 ± 0.22 |
| Cortex | 1.17 ± 0.06 | 1.22 ± 0.07 | 1.37 ± 0.07 | 1.12 ± 0.06 |
| Olfactory bulb | 1.80 ± 0.24 | 1.91 ± 0.10 | 2.07 ± 0.17 | 1.80 ± 0.07 |
| Thalamus | 2.16 ± 0.15 | 2.08 ± 0.07 | 2.49 ± 0.12 | 1.96 ± 0.12 |
| Hypothalamus | 7.01 ± 0.72 | 6.27 ± 0.75 | 6.05 ± 0.47 | 5.18 ± 0.53 |
| Cerebellum | 3.96 ± 0.12 | 4.42 ± 0.34 | 4.81 ± 0.22 | 3.96 ± 0.22 |
| Rest of brain | 4.51 ± 0.32 | 5.00 ± 0.65 | 5.18 ± 0.33 | 3.95 ± 0.12 |
Each value represents the mean ± SEM for the concentration of GFAP (μg/mg total protein).
Statistically different from control (p < 0.05).
Table 6.
Mean GFAP levels on specific regions of rat brains following a 13 week whole body inhalation exposure to gasoline ETBE (G/ETBE) vapor condensate.
| Brain area | Control | 2000 mg/m3 | 10,000 mg/m3 | 20,0000 mg/m3 |
|---|---|---|---|---|
| Males (N = 5) | ||||
| Striatum | 1.04 ± 0.11a | 0.82 ± 0.07 | 1.01 ± 0.05 | 0.93 ± 0.07 |
| Hippocampus | 2.43 ± 0.15 | 2.32 ± 0.08 | 2.26 ± 0.23 | 2.45 ± 0.09 |
| Cortex | 2.15 ± 0.12 | 1.75 ± 0.11b↓ | 1.88 ± 0.11 | 1.72 ± 0.07b↓ |
| Olfactory bulb | 2.21 ± 0.15 | 2.04 ± 0.15 | 2.22 ± 0.13 | 2.26 ± 0.16 |
| Thalamus | 2.76 ± 0.29 | 2.18 ± 0.13 | 2.67 ± 0.12 | 2.40 ± 0.17 |
| Hypothalamus | 7.12 ± 0.95 | 8.17 ± 1.45 | 9.49 ± 1.82 | 6.54 ± 0.93 |
| Cerebellum | 4.78 ± 0.32 | 4.11 ± 0.30 | 4.28 ± 0.27 | 4.11 ± 0.36 |
| Rest of brain | 6.46 ± 0.84 | 4.91 ± 0.28b↓ | 5.61 ± 0.32 | 5.36 ± 0.37 |
| Females (N = 5) | ||||
| Striatum | 0.88 ± 0.06 | 1.12 ± 0.07b | 0.99 ± 0.09 | 1.05 ± 0.12 |
| Hippocampus | 2.33 ± 0.16 | 2.69 ± 0.33 | 2.36 ± 0.20 | 2.24 ± 0.10 |
| Cortex | 1.77 ± 0.09 | 1.98 ± 0.13 | 1.75 ± 0.17 | 1.76 ± 0.06 |
| Olfactory bulb | 2.04 ± 0.17 | 2.43 ± 0.21 | 2.30 ± 0.30 | 2.33 ± 0.08 |
| Thalamus | 1.95 ± 0.15 | 2.41 ± 0.22 | 2.37 ± 0.30 | 2.24 ± 0.17 |
| Hypothalamus | 4.89 ± 1.06 | 7.21 ± 0.69 | 8.50 ± 1.54 | 7.50 ± 1.58 |
| Cerebellum | 4.02 ± 0.37 | 4.44 ± 0.18 | 4.41↓ ± 0.51 | 4.25 ± 0.32 |
| Rest of brain | 4.61 ± 0.23 | 6.17 ± 0.23b | 5.18 ± 0.58 | 5.73 ± 0.43 |
Each value represents the mean ± SEM for the concentration of GFAP (μg/mg total protein).
Statistically different from control (p < 0.05).
Table 7.
Mean GFAP levels on specific regions of rat brains following a 13 week whole body inhalation exposure to gasoline ethanol (G/EtOH) vapor condensate.
| Brain area | Control | 2000 mg/m3 | 10,000 mg/m3 | 20,0000 mg/m3 |
|---|---|---|---|---|
| Males (N = 5) | ||||
| Striatum | 0.94 ± 0.10a | 1.28 ± 0.18 | 1.44 ± 0.29b | 1.10 ± 0.14 |
| Hippocampus | 2.91 ± 0.15 | 3.46 ± 0.39 | 3.40 ± 0.31 | 2.91 ± 0.14 |
| Cortex | 1.06 ± 0.07 | 1.40 ± 0.12b | 1.33 ± 0.12 | 1.18 ± 0.08 |
| Olfactory bulb | 2.28 ± 0.13 | 3.03 ± 0.36b | 2.88 ± 0.15 | 2.48 ± 0.22 |
| Thalamus | 1.53 ± 0.09 | 2.30 ± 0.18b | 1.97 ± 0.30b | 1.73 ± 0.14 |
| Hypothalamus | 7.52 ± 1.03 | 8.53 ± 0.27 | 7.34 ± 0.78 | 7.44 ± 1.09 |
| Cerebellum | 3.49 ± 0.14 | 4.52 ± 0.25b | 4.62 ± 0.36b | 4.42 ± 0.32b |
| Rest of brain | 3.53 ± 0.27 | 4.54 ± 0.51b | 3.92 ± 0.19 | 3.89 ± 0.32 |
| Females (N = 5) | ||||
| Striatum | 0.94 ± 0.12 | 1.24 ± 0.19 | 1.00 ± 0.09 | 1.04 ± 0.14 |
| Hippocampus | 2.76 ± 0.13 | 3.13 ± 0.13 | 2.91 ± 0.21 | 2.90 ± 0.21 |
| Cortex | 1.10 ± 0.06 | 1.26 ± 0.15 | 1.13 ± 0.08 | 1.16 ± 0.10 |
| Olfactory bulb | 2.57 ± 0.15 | 3.15 ± 0.31 | 2.53 ± 0.26 | 2.96 ± 0.19 |
| Thalamus | 1.54 ± 0.09 | 1.61 ± 0.14 | 1.77 ± 0.12 | 1.57 ± 0.06 |
| Hypothalamus | 7.34 ± 0.73 | 8.21 ± 1.03 | 9.62 ± 1.05 | 8.48 ± 0.44 |
| Cerebellum | 3.65 ± 0.15 | 3.93 ± 0.22 | 4.17 ± 0.34 | 3.97 ± 0.41 |
| Rest of brain | 3.65 ± 0.13 | 3.92 ± 0.44 | 3.92 ± 0.29 | 3.91 ± 0.31 |
Each value represents the mean ± SEM for the concentration of GFAP (μg/mg total protein).
Statistically different from control (p < 0.05).
Fig. 1.
Levels of GFAP found in different regions of rat brain. OB, olfactory bulbs; Str, striatum; Hip, hippocampus; Hypo, hypothalamus; Ctx. Cortex; Cbm, cerebellum; BS, brain stem. Adapted from Martin and O’Callaghan, 1995.
Exposure to BGVC (Table 3) G/DIPE and G/TAME did not elevate GFAP levels in any brain region above controls in male or female rats.
Exposure to G/MTBE (Table 4) caused slight statistically significant increases in GFAP levels in the hippocampus in male rats of the 2000 mg/m3 group and in the rest of the brain in male rats in the 2000 mg/m3 and 10,000 mg/mg3 group. Increases in GFAP in the thalamus were also seen in 2000 mg/m3 exposed females. Effects were small in magnitude and not concentration-related.
G/TBA (Table 5) exposed male rats had a slight statistically significant increase in GFAP in the cortex of the 2000 mg/m3 group and a decrease in GFAP in the hypothalamus of 20,000 mg/m3 males.
No changes in GFAP levels were seen in females. G/ETBE exposure (Table 6) was associated with slight statistically significant decreases in GFAP levels in the cortex of males in the 2000 and 20,000 mg/m3 groups and rest of the brain in 2000 mg/m3 males. Slight statistically significant increases in GFAP levels were seen in 2000 mg/m3 females in the striatum and rest of the brain. The increases in the striatum of females at the lowest concentration were small in magnitude.
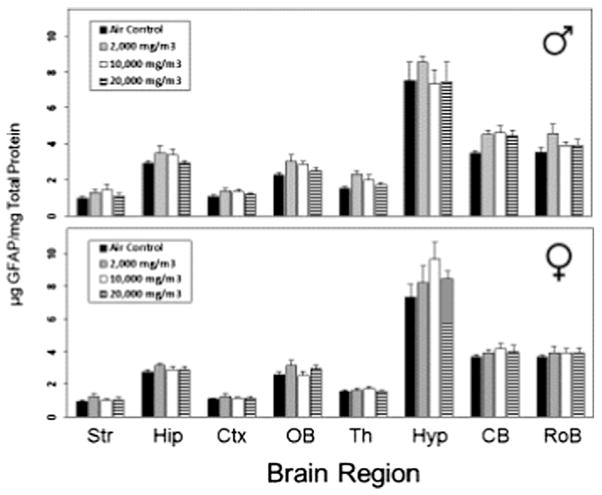
Statistically significant changes in GFAP levels were seen in male rats exposed to G/EtOH (Table 7, Fig. 2). In the cerebellum statistically significant increases in GFAP were seen at all dose levels although at the highest concentration (20,000 mg/m3) the increase was below that seen at lower dose levels. GFAP increases in the thalamus were statistically significant but not concentration related at 2000 and 10,000 mg/m3 and increases were seen only at 2000 mg/m3 in the cortex, olfactory bulbs and the rest of the brain and only in the striatum in the 10,000 mg/m3 group where the increase was 150% of control. None of the G/EtOH exposed females showed statistically significantly increases in GFAP in any brain area.
Fig. 2.
Levels of GFAP found in different regions of rat brain for rats treated with EtOH. Str: striatum; Hip: hippocampus; Ctx: cortex; OB: olfactory bulbs; TH: thalamus; Hyp: hypothalamus; Cb: cerebellum; RoB: rest of brain. Each value represents the mean ± SEM for the concentration of GFAP (μg/mg total protein). *Statistically different from air control, p < 0.05.
4. Conclusions and discussion
Overall, administration of the gasoline substances at the concentrations tested were not associated with changes in motor activity or with any change in counts of the 25 nominal FOB measures, or the 4 continuous FOB measures. The foot splay differences in the G/TBA group occurred within an interaction and did not appear to be part of an exposure related change.
Three of the test materials (BGVC, G/DIPE and G/TAME) showed no statistically significant increases in GFAP levels in male or female rats. Under the exposure conditions employed, treatment-induced astrogliosis i.e. an induction in brain region levels of GFAP, did not occur in the representative areas of the adult rat brain for these materials. When male rats showed statistically significant changes in various brain regions, effects in females exposed to the same materials were either not present (G/TBA, G/EtOH) or minimal and not-concentration related (G/MTBE, G/ETBE). Decreases in GFAP levels seen in a few regions in male rats (G/TBA, G/ETBE) were not interpreted as adverse because the neurotoxicological significance of a decrease in GFAP is unknown. For these gasoline/oxygenate blend vapor condensates exposure by inhalation for 13 weeks did not appear to result in toxicologically significant gliosis in the brain regions examined. Statistical differences observed in males and females were attributed to non-concentration related variations in response and in some cases inconsistent dissection of difficult brain regions.
The exception is response of rats exposed to G/EtOH. Increases in GFAP levels were seen in several brain regions in male rats with a concentration-related increase seen over all doses in the cerebellum although the high dose dropped back below the increases at lower dose levels. These increases in the cerebellum were in the 30% range and indicated minor gliosis This finding is consistent with positive GFAP results reported from oral treatment of ethanol alone (Franke et al., 1997).
It can be concluded that inhalation exposures for 13 weeks to gasoline vapors and vapors from gasoline combined with the fuel oxygenates studied is largely without effect on morphological, functional and biochemical indices of neurotoxicity. Although some changes in GFAP levels were reported, in general, neuropathologically relevant induction of astrogliosis was not observed. Induction of the expression of GFAP reveals underlying neuropathology regardless of the cause, dose or location of the damage to the CNS (O’Callaghan and Sriram, 2005). Only the G/EtOH results were considered indicative of compound induced gliosis damage which was not reflected in neurobehavioral or neuropathologic study results. Even in this case, however, the levels of GFAP observed were barely above baseline and did not approach values for known neurotoxicants that produce only subtle damage to the affected brain area (e.g. see O’Callaghan and Sriram, 2005; O’Callaghan et al., 2014). Thus, while the current data on G/EtOH are of note, they deserve a more comprehensive evaluation and validation in future studies.
Footnotes
Conflict of interest
None declared.
Contributor Information
James P. O’Callaghan, Email: jdo5@cdc.gov.
Wayne C. Daughtrey, Email: wayne.c.daughtrey@exxonmobil.com.
Charles R. Clark, Email: okietox@gmail.com.
Ceinwen A. Schreiner, Email: castox@comcast.net.
Russell White, Email: whiter@api.org.
References
- Agresti A. A survey of models for repeated ordered categorical response data. Stat Med. 1989;8:1209–1224. doi: 10.1002/sim.4780081005. [DOI] [PubMed] [Google Scholar]
- Balaban CD, O’Callaghan JP, Billingsley ML. Trimethyltin-induced neuronal damage in the rat brain: comparative studies using silver degeneration stains, immunocytochemistry and immunoassay for neuronotypic and gliotypic proteins. Neuroscience. 1988;26:337–361. doi: 10.1016/0306-4522(88)90150-9. [DOI] [PubMed] [Google Scholar]
- Balaban CD. The use of selective silver degebeation stains in neurotoxicology: Lessions from studies of selective neurotoxicants. In: Issacson RL, Jensen KF, editors. The Vulnerable Brain and Environmental Risks, Vol 1. Malnutrition and Hazard Assessment. Plenum Press; New York: 1992. pp. 223–238. [Google Scholar]
- Benson JM, Gigliotti AP, March TH, Barr EB, Tibbetts BM, Skipper BJ, Clark CR, Twerdok L. Chronic carcinogenicity study of gasoline vapor condensate (GVC) and GVC containing methyl tertiary-butyl ether in F344 rats. J Toxicol Environ Health. 2011;74:638–657. doi: 10.1080/15287394.2011.538837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom G. Statistical Estimates and Transformed Beta Variables. J. Wiley and Sons; NY: 1958. [Google Scholar]
- Clark CR, Schreiner CA, Parker CM, Gray T, Hoffman G. Health assessment of gasoline and fuel oxygenate vapors: subchronic inhalation toxicity. Regul Toxicol Pharmacol. 2014;70(2S):S18–S28. doi: 10.1016/j.yrtph.2014.07.003. http://dx.doi.org/10.1016/j.yrtph.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Franke H, Kittner H, Wirkner K, Schramek J. The reaction of astrocytes and neurons in the hippocampus of adult rats during chronic ethanol treatment and correlation with behavioral impairment. Alcohol. 1997;14(5):445–454. doi: 10.1016/s0741-8329(96)00209-1. [DOI] [PubMed] [Google Scholar]
- Henley M, Letinsky DJ, Carr J, Caro M, Daughtrey W, White R. Health assessment of gasoline and fuel oxygenate vapors: generation and characterization of test materials. Regul Toxicol Pharmacol. 2014;70(2S):S13–S17. doi: 10.1016/j.yrtph.2014.05.012. http://dx.doi.org/10.1016/j.yrtph.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. Prentice-Hall Inc; Engelwood Cliffs, NJ: 1973. [Google Scholar]
- Martin P, O’Callaghan J. Biochemical immunohistology. In: Ohnishi ST, Ohnishi T, editors. Central Nervous System Trauma: Research Techniques. CRC Press; Baca Raton, FL: 1995. pp. 509–516. [Google Scholar]
- Meyer OA, Tillson HA, Byrd WC, Riley MT. A method for the routine assessment of fore- and hind-limb grip strength of rats and mice. Neurobehav Toxicol. 1979;1:233–236. [PubMed] [Google Scholar]
- Moser VC. Screening approaches to neurotoxicity: a functional observational battery. J Am Coll Toxicol. 1989;8(1):85–94. [Google Scholar]
- Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP. Neurotypic and gliotypic proteins as biochemical markers of neurotoxicity. Neurotoxicol Teratol. 1988;10:445–452. doi: 10.1016/0892-0362(88)90006-2. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP. Quantification of glial fibrillary acidic protein: comparison of slot-immunobinding assays with a novel sandwich ELISA. Neurotoxicol Teratol. 1991a;13:275–281. doi: 10.1016/0892-0362(91)90073-6. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP. The use of glial fibrillary acidic protein in first-tier assessments of neurotoxicity. J Am Coll Toxicol. 1991b;10(6):719–726. [Google Scholar]
- O’Callaghan JP. Measurement of glial fibrillary acidic protein. In: Costa LG, editor. Current Protocols in Toxicology. John Wiley and Sons; New York: 2002. pp. 12.8.1–12.8.12. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. GFAP and other glial proteins as biomarkers of neurotoxicity. Exp Opin Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Van Gilder RL, Sofroniew MV, Miller DB. Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PlosOne. 2014 doi: 10.1371/journal.pone.0102003. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute, Inc. JMP Statistics and Graphics Guide. Version 3. 1995. [Google Scholar]
- Schreiner C, Hoffman G, Mason C, Gudi R, Clark CR. Health assessment of gasoline and fuel oxygenate vapors: micronucleus and sister chromatid exchange tests by inhalation exposure. Regul Toxicol Pharmacol. 2014;70(2S):S29–S34. doi: 10.1016/j.yrtph.2014.05.014. http://dx.doi.org/10.1016/j.yrtpr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Schulze Gene E. Large-scale assessment of motor activity in rodents: procedures for routine use in toxicology studies. Journal of American College of Toxicology. 1990;9:455–463. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenket DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. http://dx.doi.org/10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- US EPA. GFAP Assay 79.67, CFR. 1994a Jun 2759(122) [Google Scholar]
- US EPA. Good Laboratory Practice Standards 79.60, CFR. 1994b Jun 2759(122) [Google Scholar]
- US EPA. GLP for Neuropathology Assessments 79.66, CFR. 1994c Jun 2759(122) [Google Scholar]
- US EPA. OPPTS Health Effects Test Guidelines 870.6200. Neurotoxicity Screening Battery. 1998 Aug [Google Scholar]
- White KL, Peachee VL, Armstrong SR, Twerdok LE, Clark CR, Schreiner CA. Health assessment of gasoline and fuel oxygenate vapors: immunotoxicity evaluation. Regul Toxicol Pharmacol. 2014;70(2S):S43–S47. doi: 10.1016/j.yrtph.2014.04.010. http://dx.doi.org/10.1016/j.yrtph.2014.04.010. [DOI] [PubMed] [Google Scholar]