Abstract
Two inbred mouse strains, derived from feral founders, are susceptible to experimental leishmaniasis due to Leishmania major and support a disease of a severity intermediate between those observed in strains C57BL/6 and BALB/c. Mice of the MAI strain develop a severe, nonhealing, but nonfatal disease with no resistance to a secondary parasite challenge. The immunological responses showed a TH2 dominance characterized by an early peak of interleukin-4 (IL-4) and IL-13. However, neutralization of IL-4, which leads to a resistance phenotype in BALB/c mice, has no effect on disease progression in MAI mice. Mice of strain PWK develop a protracted but self-healing disease, characterized by a mixed TH1-plus-TH2 pattern of immune responses in which IL-10 plays an aggravating role, and acquire resistance to a secondary challenge. These features are close to those observed in human cutaneous leishmaniasis due to L. major and make PWK mice a suitable model for the human disease.
Leishmaniasis designates a large spectrum of diseases caused by a dimorphic protozoan parasite of the genus Leishmania. During their life cycle, parasites alternate between the midgut of the insect vector, where they develop as flagellated metacyclic promastigotes (53), and mammalian-host macrophages, where they differentiate and multiply as amastigotes (44).
Leishmaniases affect millions of people (9). Different Leishmania species induce various clinical presentations ranging from asymptomatic or localized infection to disseminated visceral disease (46). In humans, Leishmania major is the causative agent of zoonotic cutaneous leishmaniasis (ZCL), which is highly prevalent in North Africa, the Middle East, and Central Asia (12, 58).
Experimental infection of inbred BALB/c and C57BL/6 mice by L. major parasites constitutes one of the most studied models of parasitic disease (50). This model has been instrumental in the in vivo validation of the functional dichotomy of CD4+ T-helper cells and their involvement in determining the outcome of disease (34, 48, 54). Thus, resistant C57BL/6 mice infected with L. major develop a TH1 response resulting in gamma interferon (IFN-γ) production, macrophage activation, parasite killing, and resolution of the experimental lesion (19, 36, 55). In contrast, susceptible BALB/c mice mount a TH2 response and show macrophage deactivation leading to parasite dissemination and severe progressive disease (8, 19, 28, 38). The exquisite susceptibility of BALB/c mice to L. major infection has been ascribed to the occurrence, within the draining lymph nodes of these mice, of an early burst of interleukin 4 (IL-4), detectable at 16 h after parasite inoculation, which polarizes the immune response toward the TH2 pathway (20, 21, 32). This early IL-4 burst is produced by a highly restricted population of CD4+ T cells that express Vβ4 and Vα8 T-cell-receptor gene segments and are specific for a single epitope of the parasite LACK antigen (Leishmania homolog of receptor for activated C kinase) (23, 29, 31, 37, 45). Considering this very particular mechanism of BALB/c mouse susceptibility to L. major infection, conclusions drawn from disease-modulating experiments with this mouse strain can hardly be extrapolated to human disease. In fact, T-helper polarization is less sharply defined in humans than in mice. The immune response to the parasite is characterized by the production of a mixture of TH1 and TH2 cytokines, as observed in patients with visceral (11, 25) or cutaneous (35, 42, 47, 52) leishmaniasis. Characterization of additional models of experimental leishmaniasis reproducing more closely the pathogenic mechanisms of the human disease may help to develop prophylactic or therapeutic tools for humans.
In the present study, we investigated as experimental hosts nine new inbred strains derived from feral mice, and we identified two strains, named PWK and MAI, susceptible to infection with L. major parasites. Immunological investigations showed that the pathogenic mechanisms of disease in these strains differ from those classically reported for BALB/c and C57BL/6 mice and, in the case of PWK strain, appear closer to those described for human ZCL.
MATERIALS AND METHODS
Mice.
Female BALB/cJ Rj and C57BL/6J Rj mice were obtained from IFFA-CREDO-France and maintained in animal facilities at the Pasteur Institute of Tunis (Tunis, Tunisia). Eight- to 10-week-old males and females from inbred wild-mouse-derived strains (MAI/Pas, PWK/Pas, WLA/Pas, WMP/Pas, MBT/Pas, ZYD/Pas, BIK/g/Pas, STF/Pas, and SEG/Pas) that were bred by 20 to 50 brother-sister crosses were used in this study. All of the strains were raised and maintained at the Pasteur Institute in Paris (14, 27) (Table 1).
TABLE 1.
Wild-mouse-derived mouse strains used in this study
| Strains | Species | Geographical origin |
|---|---|---|
| WMP | Mus musculus domesticus | Morastir, Tunisia |
| WLA | Mus musculus domesticus | Issus, France |
| Bik/g | Mus musculus domesticus | Kefar Galim, Israel |
| MAI | Mus musculus musculus | Illmitz, Austria |
| PWK | Mus musculus musculus | Prague Zoo, Prague, Czech Republic |
| MBT | Mus musculus musculus | General Toshevo, Bulgaria |
| STF | Mus spretus | Foundouk, Tunisia |
| SEG | Mus spretus | Grenada, Spain |
| ZYD | Mus spicilegus | Yugoslavia |
Parasite and antigens.
L. major strain MHOM/TN94/GLC94, zymodeme MON25, isolated from a Tunisian patient with cutaneous leishmaniasis (35), was used in this study. The parasites were maintained by continuous passages in mice (26). Leishmanial total antigens (LTA) were prepared as described previously (41). LTA were used to assess the delayed-type hypersensitivity reaction (DTHR) in vivo and lymphoproliferative responses and cytokine production in vitro.
Infection of mice.
Amastigotes were isolated from L. major-harboring skin lesions of BALB/c mice as described previously (2). Briefly, L. major-loaded footpads were excised and disinfected, and the footpad skin was peeled away. Infected tissue was then minced. Amastigotes were purified by differential centrifugation and used to infect mice. Approximately 10 inbred mice of each strain were injected subcutaneously in the right hind footpad with 2 × 106 L. major amastigotes. Lesion development was monitored, for at least 15 weeks postinfection (p.i.), by measuring footpad swelling with a metric caliper, and lesion size was calculated by subtracting the size of the contralateral uninfected footpad.
In vivo cytokine neutralization.
Selected mice were treated intraperitoneally (i.p.) with 5 mg of an anti-IL-4 antibody (11B11; rat immunoglobulin G1 [IgG1]) or 5 mg of an anti-IL-10 antibody (JES-2A5; rat IgG1) 48 h before infection. Control mice were treated i.p. with irrelevant rat IgG1 antibodies.
Parasite detection and quantification.
Parasite load was quantified by a limiting-dilution in vitro culture adapted from the work of Laskay et al. (28). Briefly, the excised footpad or popliteal draining lymph nodes were homogenized, and serial 10-fold dilutions were plated in three replicates, at each dilution, in 96-well flat-bottom microtiter plates (Nunc, Roskilde, Denmark) containing Schneider's Drosophila medium supplemented with Grace's insect tissue culture medium (both from Gibco BRL, Paisley, Scotland) supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, and 10% heat-inactivated fetal calf serum. The number of viable parasites was determined microscopically from the reciprocal of the highest dilution at which promastigotes could be detected after 10 days of incubation at 26°C.
In order to assess the visceral dissemination of Leishmania parasites, splenic homogenates were cultured for 10 days at 26°C on a Leishmania growth medium based on coagulated rabbit serum (4).
DTHR to Leishmania antigens.
DTH was monitored by injecting LTA (equivalent to 2 × 106 promastigotes) in a final volume of 50 μl into the contralateral noninfected hind footpad (1). Swelling was measured with a metric caliper at 24, 48, and 72 h.
In vitro culture of lymph node cells and analysis of cytokine content in the supernatant.
Single-cell suspensions from lymph nodes were plated at a concentration of 4 × 106 per well in 24-well flat-bottom Costar tissue culture plates (Nunc) in a volume of 1 ml of complete RPMI medium containing 5% fetal calf serum. Cultures were incubated in medium alone or with LTA (equivalent to 2 × 106 promastigotes/well). Supernatants were collected after 48 or 72 h for subsequent cytokine determinations. Cytokines were measured by a monoclonal antibody (MAb)-based enzyme-linked immunosorbent assay (ELISA) using reagents purchased from PharMingen (San Diego, Calif.). Ninety-six-well flat-bottom microtiter plates (Nunc) were coated overnight at +4°C with purified rat MAbs to murine IL-2 (0.5 μg/ml), IL-4 (2 μg/ml), IL-10 (2 μg/ml), IL-12 (2 μg/ml), and IFN-γ (4 μg/ml) in 100 mM carbonate buffer, pH 8.2. Unsaturated binding sites were blocked with 0.5% gelatin in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T-G) for 2 h at room temperature. Culture supernatants were then incubated overnight at +4°C. For calibration of the assays, the respective recombinant cytokines (PharMingen) were incubated in serial dilutions. After washing, biotinylated rat MAbs to murine IL-2 (0.1 μg/ml), IL-4 (0.1 μg/ml), IL-10 (0.2 μg/ml), IL-12 (0.4 μg/ml), and IFN-γ (0.2 μg/ml) were added and incubated for 45 min at room temperature. The plates were washed and incubated with 1 μg of streptavidin-peroxidase conjugate (AMDEX; Amersham, Little Chalfont, Buckinghamshire, England)/ml in PBS-T-G for 30 min at room temperature. The reaction was developed by addition of 1 mg of orthophenylene diamine (Sigma, Saint Louis, Mo.)/ml prepared in 100 mM citrate buffer (pH 5) containing 0.03% hydrogen peroxide and was stopped, after 10 min, with 50 μl of 1.5 M sulfuric acid. The optical density was determined in a multiscan ELISA reader (Titerteck, Helsinki, Finland). Cytokine concentrations were calculated by using the respective standard curves. The detection limits in these assays were 20 pg/ml for IL-2, 40 pg/ml for IL-4, 1 ng/ml for IL-10, 5 pg/ml for IL-12, and 80 pg/ml for IFN-γ.
RNA isolation, reverse transcription, and real time quantitative PCR detection of cytokine mRNA.
Draining lymph nodes from each group of five mice were removed and immediately homogenized in TRI reagent (Euromedex, Souffel-Weyersheim, France), and RNA was extracted according to the manufacturer's recommendations. Approximately 5 μg of total RNA was reverse transcribed in a reaction mixture containing 20 IU of Moloney murine leukemia virus reverse transcriptase (Gibco BRL)/μl, 1× buffer (Gibco BRL), 250 μM each deoxynucleoside triphosphate (Amersham), 50 μg of random hexamers (Promega, Southampton, United Kingdom)/ml, and 1.7 IU of RNasin (Promega)/μl for 1 h at 42°C. Reverse transcriptase was then denatured at 95°C for 10 min. IL-2, IL-4, IL-10, IL-12 (p40), IL-13, and IFN-γ mRNA transcripts were quantified by quantitative real-time PCR (16). All experiments were performed according to the TaqMan procedure by using the Universal PCR Master Mix and Pre-developed Assay Reagent kits (Perkin-Elmer Applied Biosystems, Foster City, Calif.). PCRs were performed using the ABI PRISM 7700 sequence detection system (with version 1.6 software) with 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min. Real-time fluorescence measurements were taken, and a threshold cycle (CT) value for each sample was calculated. Data were normalized to the expression of an endogenous control (18S rRNA) and expressed as the ratio of target mRNA to 18S rRNA, given by the formula 2−ΔCT, where ΔCT is the difference in threshold cycles between the target and the reference. For each specific mRNA, results are expressed as the fold increase in infected mice compared with uninfected mice, given by the formula 2−ΔΔCT, where ΔΔCT is the difference in ΔCT between infected mice and saline-injected mice.
Statistical analysis.
Standard deviations (SD) were calculated, and the statistical significance of the results was analyzed by the InStat 2.03 test. A P value of ≤0.01 was considered statistically significant.
RESULTS
Clinical and parasitological heterogeneity of experimental infection with L. major in mice from feral inbred strains.
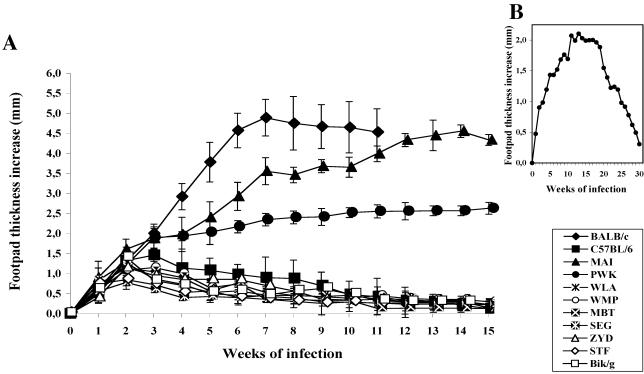
Mice from nine different inbred strains derived from feral parents were challenged in the right hind footpad with 2 × 106 L. major amastigotes and monitored for lesion development for at least 15 weeks. BALB/c and C57BL/6 mice were used as controls. The results of one out of three reproducible experiments are shown in Fig. 1A.
FIG. 1.
Lesion progression in mice from inbred strains who were inoculated with L. major amastigotes. (A) Results for mice of nine inbred strains inoculated with 2 × 106 parasites. (B) Lesion progression over 30 weeks of observation in PWK mice inoculated with 2 × 106 parasites. Results are expressed as increases in footpad thickness (in millimeters) and are means ± SD for 8 to 10 individual mice per group.
Most of the wild-mouse-derived strains (7 of 9) were highly resistant to experimental infection. However, two strains, MAI and PWK, both belonging to the Mus musculus musculus species, supported the development of lesions of significant size after L. major infection (Table 1). Mice of the MAI strain developed large cutaneous nodules that progressed continuously over time and reached a size similar to that observed in BALB/c mice, although ulceration was never observed. PWK mice also developed nonulcerated lesions that ran a protracted course but which, in contrast to those of BALB/c (P = 0.00063) and MAI (P = 0.0027) mice, ultimately healed spontaneously (Fig. 1B). The lesion reached a maximum size after 10 weeks, stabilized at a plateau phase until week 20 p.i., and then started regression; total recovery was attained around week 30 p.i. Globally, lesions were always larger than those observed in the resistant C57BL/6 mice (P = 0.0138). When mice were challenged with lower parasite doses (2 × 105), the patterns of disease progression were unchanged. However, at 2 × 104 parasites, only BALB/c mice still developed a large, nonhealing lesion, confirming that BALB/c mice are more susceptible to L. major than MAI or PWK mice (data not shown).
Histological examination of the inoculation site was performed 3, 6, and 8 weeks after injection of 2 × 106 L. major amastigotes. Only BALB/c, MAI, and PWK mice developed a granuloma composed mainly of parasite-loaded macrophages, lymphocytes, and some polynuclear leukocytes. The dermal cellular infiltrate was extensive in MAI and BALB/c mice and was associated with epidermis ulceration only in the latter strain. The granuloma was smaller in PWK mice and, interestingly, contained numerous plasma cells. A central necrosis of the granuloma was massive in BALB/c mice, important in MAI mice, and moderate in PWK mice.
Parasites infiltrating infected footpads and draining lymph nodes were enumerated by limiting dilution 5, 15, and 30 weeks postinoculation (Table 2). There was a good correlation between the sizes of the nodules or lesions at the inoculation sites and the parasite loads (data not shown). In mice of the resistant strains MBT and SEG, very few or no viable parasites could be recovered from the infected footpads and popliteal nodes at week 5 or 15. The parasite load in mice of the susceptible MAI strain reached high levels, similar to those observed in BALB/c mice. In PWK mice, the parasite load also increased significantly in footpad lesions (P < 0.005) and in popliteal draining lymph nodes (P < 0.005). However, at week 30, when lesions had almost completely healed, the parasite load regressed by 80% in the infected footpads (P < 0.0001), and parasites were no longer detected within the draining lymph nodes. These data indicate that, in contrast to MAI and BALB/c mice, PWK mice are able to control parasite multiplication. Interestingly, at week 15 p.i., parasites could be recovered from the spleens of MAI and PWK mice, showing that these strains allow visceral dissemination of L. major parasites. However, parasites were no longer detected in the spleens of PWK mice at week 30, confirming the effective control of the experimental infection by these mice (P < 0.00001).
TABLE 2.
Parasite burden following L. major infection of different wild-mouse-derived mouse strainsa
| Strains | −Log10 parasite titer/organ in:
|
|||||
|---|---|---|---|---|---|---|
| Infected footpad
|
Draining lymph node
|
|||||
| Wk 5 | Wk 15 | Wk 30 | Wk 5 | Wk 15 | Wk 30 | |
| BALB/c | 7.2 ± 0.8 | 7.3 ± 0.7 | NDb | 5.1 ± 0.9 | 5.8 ± 1.1 | 10.4 ± 0.4 |
| C57BL/6 | 3.2 ± 0.7 | 1.7 ± 0.4 | 0.2 ± 0.3 | 0.7 ± 0.4 | 0.3 ± 0.4 | 0.0 ± 0.0 |
| MAI | 4.8 ± 0.9 | 9.0 ± 0.2 | 11.1 ± 0.5 | 2.6 ± 0.9 | 4.3 ± 0.9 | 6.8 ± 0.7 |
| PWK | 6.3 ± 0.4 | 6.0 ± 0.8 | 1.3 ± 0.6 | 4.6 ± 0.4 | 2.5 ± 0.6 | 0.0 ± 0.0 |
| MBT | 1.0 ± 0.6 | 0.0 ± 0.0 | ND | 0.2 ± 0.4 | 0.0 ± 0.0 | ND |
| SEG | 0.4 ± 0.6 | 0.3 ± 0.0 | ND | 0.0 ± 0.0 | 0.0 ± 0.0 | ND |
Parasite quantitation was performed in the indicated groups at weeks 5, 15, and 30 after L. major infection. Results are expressed as the negative of the mean (± SD) of the log10 dilution of infected footpad or draining popliteal lymph node tissue positive for L. major promastigotes. Results were obtained in three replicates from three to four individual mice in each group.
ND, not determined.
DTHR to LTA in L. major-infected mice from wild-mouse-derived strains.
As an indicator of the induction of a TH1 response, the specific DTHR was evaluated in L. major-infected mice derived from wild strains and controls. LTA was injected into the contralateral footpad 10 weeks after infection, and footpad swelling was measured at 24, 48, and 72 h (Fig. 2A). As expected, BALB/c mice did not develop any DTHR, whereas C57BL/6 mice exhibited a strong reaction (P < 0.01). Interestingly, mice belonging to the two susceptible wild-mouse-derived strains showed contrasting results: no DTHR could be detected in MAI mice, whereas PWK mice developed a significant DTHR similar (P = 0.00365) to that observed in the highly resistant MBT and SEG strains.
FIG. 2.
Investigation of the DTHR and resistance to reinfection developed by inbred mice inoculated with a primary challenge of 2 × 106 L. major amastigotes. (A) DTHR footpad test. Groups of MAI, PWK, MBT, SEG, BALB/c, and C57BL/6 mice were injected at week 10 p.i. with LTA in the uninfected contralateral hind footpad. Footpad swelling was measured (in millimeters) with a metric caliper at 24, 48, and 72 h. Results are means ± SD. (B) Course progression of secondary lesions in MAI and PWK mice (derived from wild strains) compared to BALB/c and C57BL/6 mice. Mice were inoculated subcutaneously with 2 × 106 L. major amastigotes and were challenged at week 10 p.i. with the same number of parasites in the contralateral footpad. The progression of the secondary lesion was monitored for 14 additional weeks. Results are expressed as mean lesion sizes for five mice per group (in millimeters) ± SD.
Primary infection induces strong resistance to a secondary parasite challenge in the PWK but not the MAI strain.
Mice were first injected in one footpad with 2 × 106 amastigotes and then challenged at week 10 p.i. with the same number of parasites into the contralateral footpad. The progression of the secondary lesion was monitored for 14 additional weeks. As expected, BALB/c mice developed a secondary lesion not significantly different from the primary lesion (P = 0.1627), whereas C57BL/6 mice fully controlled the secondary parasite challenge. Like BALB/c mice, MAI mice were fully susceptible to the secondary challenge. However, PWK mice were able to control the secondary challenge and developed only a small lesion that regressed completely within 6 weeks (Fig. 2B).
Cytokine patterns displayed by MAI and PWK mice after L. major infection.
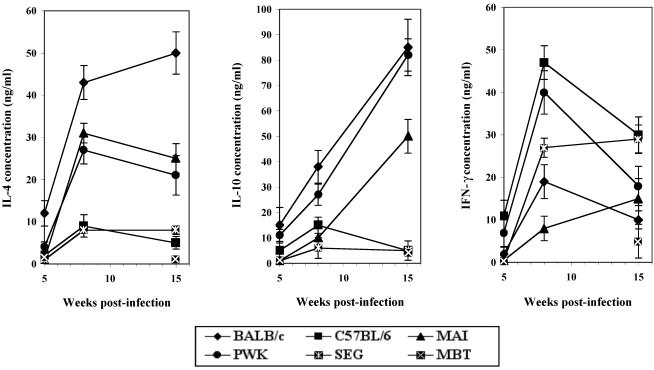
In order to better define the immunological parameters associated with resistance or susceptibility to Leishmania infection in mice of wild-mouse-derived inbred strains, IL-4, IL-10, and IFN-γ levels in the supernatants of LTA-stimulated mononuclear cells of draining lymph nodes were assessed 8 and 15 weeks after infection with L. major, time points at which the disease was fully established. In response to infection with Leishmania, mice could be classified as high, intermediate, or low cytokine producers (Fig. 3). As previously reported (18, 30), BALB/c mice produced high levels of IL-4 and IL-10 and low levels of IFN-γ. In contrast, C57BL/6 mice produced a high level of IFN-γ and low levels of IL-4 and IL-10. A similar profile was observed in mice of the resistant SEG strain. Low levels of IFN-γ and high levels of IL-4 characterized MAI mice at weeks 8 and 15. Levels of IL-10 were elevated only at week 15. In contrast, PWK mice showed parallel increases in the three cytokines IFN-γ, IL-10, and IL-4, giving a mixed pattern of TH1 and TH2 responses.
FIG. 3.
Cytokine expression in mice from wild-mouse-derived inbred strains inoculated with 2 × 106 L. major amastigotes. Shown are levels of IL-4 (left), IL-10 (center), and IFN-γ (right) expressed in the supernatants of in vitro LTA-stimulated draining lymph node cells obtained from mice during the established phase of disease at 5, 8, and 15 weeks p.i. Cytokines were analyzed by ELISA. Values are expressed as nanograms per milliliter and are means from four separate experiments. Spontaneous cytokine production was less than 10% when cells were left unstimulated. Diamonds, BALB/c; triangles, MAI; circles, PWK; large squares, MBT; small squares, SEG.
Since early cytokine production was shown to be critical for initiation of the T-helper response, the kinetics of IL-4 transcripts, as well as of IL-2, IL-10, IL-12, IL-13, and IFN-γ transcripts, were monitored within the draining lymph nodes of MAI and PWK wild-mouse-derived mice and of control BALB/c and C57BL/6 mice. mRNA quantification was performed at 16 and 48 h after L. major infection by using reverse transcription and real-time quantitative PCR amplification. For each cytokine, and for each time point, the results are expressed as the fold increases in specific mRNA levels detected in infected mice over those in saline-injected mice (Table 3).
TABLE 3.
Early expression of cytokine transcripts in mice from inbred strains infected with L. major
| Cytokine | Fold increasea in cytokine expression in the following strain:
|
||||
|---|---|---|---|---|---|
| MBT | C57BL/6 | PWK | MAI | BALB/c | |
| IL-4 | ND | 1 | 1 | 10 | 20 |
| IL-10 | ND | 1 | 20 | 3.5 | 15 |
| IL-13 | ND | 1 | 1 | 10 | 4 |
| IL-2 | ND | 1 | 1 | 5 | 7 |
| IL-12 | ND | 1 | 1 | 10 | 3 |
| IFN-γ | ND | 100 | 2 | 7 | 20 |
Over basal levels expressed by uninfected animals. Quantitation of cytokine transcripts was performed on mRNA extracted from cells of draining lymph nodes collected 16 h after parasite inoculation. ND, not determined. Boldfaced values represent the most significant alterations in cytokine expression.
The susceptible BALB/c mice were characterized, as previously described, by an early burst of IL-4 (29). Interestingly, this early burst also involved IL-10, IFN-γ, and, to a lesser extent, IL-2. In the resistant C57BL/6 mice, only IFN-γ mRNA was detected at 16 h postinoculation. Contrasting results were obtained with the two susceptible wild-mouse-derived mouse strains. MAI mice displayed an early cytokine pattern different from that observed for BALB/c mice, i.e., strong increases in IL-4, IL-13, and IL-12 levels and, to a lesser extent, in IFN-γ levels. Increases in IL-10 and IL-2 mRNA levels were only modest in this strain. PWK mice also displayed a pattern of early cytokine burst that included only IL-10 transcripts.
Treatment with anti-IL-4 does not alter disease evolution in MAI or PWK mouse strains, but anti-IL-10 treatment significantly reduces disease progression in PWK mice.
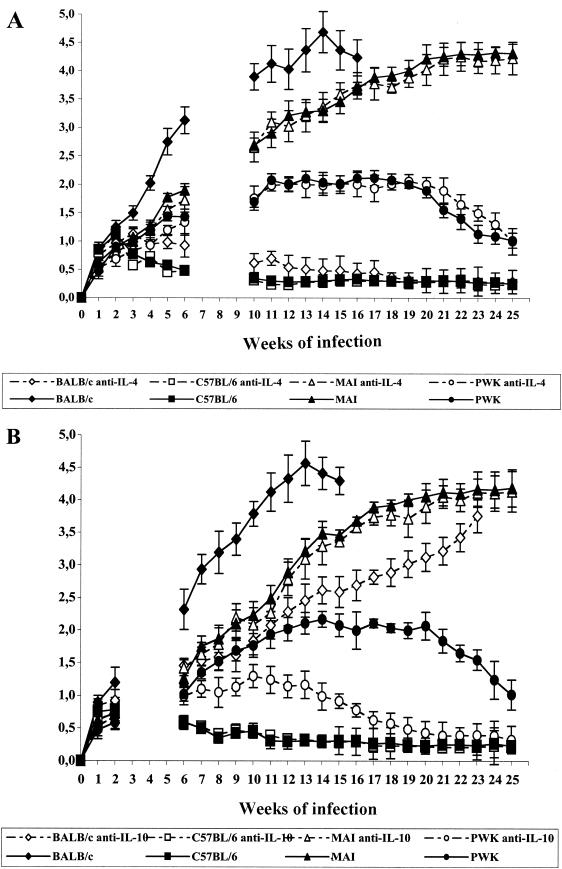
It is well documented that the early burst of IL-4 skews the immune response toward TH2 dominance and that its neutralization by anti-IL-4 treatment abolishes the susceptibility of BALB/c mice to L. major infection (51). Therefore, we investigated whether neutralization of IL-4 or IL-10 alters the pattern of disease progression of MAI or PWK mice. Mice were first treated with either anti-IL-4 or anti-IL-10 antibodies or with an irrelevant antibody of the same isotype; then they were infected with L. major and monitored for disease progression. BALB/c and C57BL/6 mice were treated similarly and served as controls (Fig. 4).
FIG. 4.
Effect of early treatment with an anti-IL-4 or anti-IL-10 MAb on disease progression in MAI and PWK mice(wild-mouse-derived strains). BALB/c and C57BL/6 mice were used as well. Mice (five per group) were treated i.p. with 5 mg of the anti-IL-4 (11B11; rat IgG1) (A) or anti-IL-10 (JES-2A5; rat IgG1) (B) antibody 48 h before infection with L. major GLC94. Control mice of each strain were treated i.p. with control antibodies. Results are expressed as mean sizes of lesions (in millimeters) ± SD.
In contrast to the response of BALB/c mice, which resist parasite challenge after IL-4 depletion, we found that anti-IL-4 treatment did not alter the course of experimental disease in MAI and PWK mice (Fig. 4A).
Interestingly, when treated with anti-IL-10 antibodies, mice of the PWK strain showed a significant reduction in disease progression, in terms of both lesion size and time limit for healing. In contrast, administration of anti-IL-10 antibodies did not affect disease evolution in mice of the MAI strain (Fig. 4B) and had only a minor effect (delay in disease progression without healing) in BALB/c mice.
DISCUSSION
Experimental models of infectious diseases using inbred mouse strains invariably suffer from the limitation introduced by their restricted genetic background compared to the large genetic polymorphism which characterizes naturally occurring outbred animals and humans. This holds particularly true for leishmaniasis, where caution should be exercised in extrapolating data collected from the canonical pair of resistant and susceptible strains (i.e., C57BL/6 and BALB/c) to the human disease. The present study was undertaken in order to identify additional models of experimental leishmaniasis by investigating new inbred strains derived from feral mouse founders caught directly in nature (14, 27) (Table 1). Such models based on new genetic backgrounds may hopefully unveil new pathogenic mechanisms of host-pathogen interaction more relevant to human disease.
Of the nine newly investigated strains, only two (MAI and PWK) appeared definitely susceptible to L. major, confirming that resistance to this parasite species is the rule among mice and susceptibility is the exception (7). Of note, these two new susceptible strains belong to the M. musculus species, as do strains BALB/c and C57BL/6.
Mice of the MAI strain are definitely susceptible to L. major; they develop a lesion at the site of inoculation that grows continuously and that, at week 15 p.i., reaches a size and parasite load similar to those observed in BALB/c mice. MAI and BALB/c mice support visceral localization of the parasite. Histologically, lesions in both strains show large granulomas with an important central necrosis.
MAI mice, like BALB/c mice, do mount a DTHR to parasite antigens and do not develop resistance to reinfection. In addition, during established disease, both strains are characterized by a clear TH2 dominance: high levels of IL-4 and IL-10, and low levels of IFN-γ and IL-2.
The experimental disease in strain PWK reveals salient differences with that observed in strain BALB/c or C57BL/6. PWK mice develop nonulcerated nodules of larger size and more protracted evolution than those in C57BL/6 mice, and these nodules ultimately heal by week 30 p.i. The parasite load also reaches higher levels in PWK mice than in C57BL/6 mice at all disease stages (1,000-fold increase). However, the parasite load has regressed by 80% within the lesion, and has totally disappeared from the draining lymph node and spleen, at week 30, coincident with clinical recovery. Histologically, the tissular granuloma in PWK mice shows only very limited spontaneous necrosis, in contrast to those in BALB/c and MAI mice.
PWK mice also develop a strong DTHR to parasite antigens and resist a secondary parasite challenge.
The patterns of cytokines secreted in vitro by lymph node cells during the established phase of disease further distinguish PWK mice from BALB/c or C57BL/6 mice. PWK mice express a mixed pattern of TH1 and TH2 cytokines characterized by high levels of IL-10, IL-4, and IL-2 and intermediate levels of IFN-γ.
Table 3 summarizes the salient features of the experimental disease in MBT, C57BL/6, PWK, MAI, and BALB/c mice. The three feral inbred strains complete the spectrum of disease phenotypes and define a gradient of increasing disease severity from the highly resistant MBT strain to the highly susceptible BALB/c strain.
A salient feature of the disease in PWK and MAI mice is the absence, even after a long period of observation, of any ulceration of the nodules at the site of parasite inoculation. Nonulcerating nodules have been reported to be a characteristic feature of L. major-infected SCID mice (15, 57), and recently it was suggested that the occurrence of ulceration is dependent on the presence of CD4+ T cells (56). Interestingly, preliminary experiments show that F1 hybrid mice issued from MAI × PWK crosses regularly develop an ulcerative nodule. Studies are in progress to investigate the cellular basis of this phenotype.
Several reports have stressed the crucial role of early cytokine expression in the development of the subsequent immune response and disease progression. Our investigation extends the previous studies in that it includes a larger panel of cytokines and two new models in strains of feral origin, PWK and MAI. We show that each strain actually expresses a specific pattern of early cytokine bursts. Considering the complex interactions, either synergistic or antagonistic, between some of these cytokines, it is likely that their actual net effect on T-lymphocyte priming differs from strain to strain and accounts, at least in part, for the observed differences in clinical evolution and disease outcome.
A strong early peak in IL-4 transcript levels (>10-fold increase) was detected only in the nonhealer strains BALB/c and MAI; it was absent in the healer strains PWK and C57BL/6. However, although anti-IL-4 antibody treatment of BALB/c mice could cause the phenotype to revert to resistance (51), the same treatment has no effect in MAI mice, suggesting that disease susceptibility in the latter strain may indicate the intervention of an IL-4-independent mechanism, possibly involving the IL-4-like cytokine IL-13 (43), to shape the immune response toward TH2 dominance. Actually, MAI is the only strain expressing an early peak of IL-13 transcripts. This hypothesis is in keeping with recent reports emphasizing the role of IL-13 as a factor in susceptibility to experimental leishmaniasis (38). Thus, animals lacking the IL-12 gene or genetically resistant mice, receiving anti-IL-12 antibodies, are unable to control the disease, whereas such treatment of BALB/c mice during the first 2 weeks after infection rendered these animals resistant (36, 39). Strain MAI is unique in exhibiting a significant increase in IL-12 transcript levels at 16 h p.i. Actually, IL-12 is the key determinant of resistance to Leishmania parasites (17, 39, 55). Considering that MAI mice develop a nonhealing disease without evidence of DTHR to parasite antigens, our results stress the capacity of concomitant negative cytokines (i.e., IL-4, IL-13) to interrupt TH1 development even when IL-12 is produced. This negative effect may occur through down-regulation of the expression of the IL-12 receptor β-chain (20).
PWK mice present a unique pattern of early cytokine response characterized by a robust (20-fold increase) and isolated burst of IL-10 transcripts. IL-10 is likely involved in disease progression in PWK mice, since anti-IL-10 treatment reduces lesion size and accelerates healing. The negative role of IL-10 in experimental leishmaniasis was only recently fully evaluated; it was found that transgenic mice of a resistant genetic background that express IL-10 under the control of the major histocompatibility complex class II Ea promoter are susceptible to L. major infection, despite the concomitant IFN-γ production (13). In contrast, BALB/c mice in which the IL-10 gene has been inactivated develop relatively small lesions containing 1,000-fold fewer parasites (24). The role of IL-10 in maintaining chronic disease and in preventing sterile cure, even in genetically resistant C57BL/6 mice, was recently demonstrated, in that sterile cure could be achieved only after IL-10 gene inactivation or administration of anti-IL-10 receptor antibodies (4).
Several characteristics of the PWK model of experimental leishmaniasis are reminiscent of those observed in human leishmaniasis. L. major infection in humans is characterized by cutaneous sores of variable number and severity, lasting several months, that ultimately heal spontaneously and are associated with the development of a strong DTHR and resistance to reinfection. Intralesional cytokine expression indicates a mixed TH1-plus-TH2 pattern. High levels of IL-10 transcripts have been shown to correlate with an unfavorable clinical evolution and larger intralesional parasite loads (35). These features are closer to those observed in PWK mice than to those in C57BL/6 mice. Expression of the cytokine IL-10 is also a salient feature of other forms of human leishmaniasis: high plasma IL-10 levels and expression of IL-10 by keratinocytes are predictive of the development of post-kala azar dermal leishmaniasis (10). Similarly, visceral leishmaniasis in humans is characterized not by TH2 dominance but rather by the coexpression of high levels of IFN-γ and IL-10 (22, 23). Finally, high intralesional IL-10 mRNA expression in American cutaneous leishmaniasis is associated with unresponsiveness to treatment (6). Together, these data suggest that the experimental disease in PWK mice, characterized by high early IL-10 levels, may be a suitable model for human leishmaniasis and may offer a valuable tool for testing of the protective effect of potential vaccine preparations.
Finally, the new strains of feral origin offer an opportunity to investigate the genetic basis of susceptibility or resistance to L. major parasites and hopefully to identify novel genes. Such studies have been conducted with BALB/c and C57BL/6 mice (3, 33, 34, 49) but have not yet contributed significantly to the identification of the human counterpart genes (5, 40). Our preliminary results indicate that F1 generation hybrids issued from MAI × BALB/c and PWK × BALB/c crosses express resistant phenotypes, showing that the genes associated with susceptibility in strains MAI and PWK are likely different from those acting in strain BALB/c. Experiments to resolve this issue are in progress.
Acknowledgments
This work was partially supported by grants from the Tunisian Ministry of Research and Technology. B. E. C. Babay was supported by fellowships from the “Agence Universitaire de la Francophonie,” from the “Réseau International des Instituts Pasteur et Instituts Associés,” and from “Fonds Yersin” (Pasteur Institute).
We are grateful to Geneviève Milon for hosting several experiments and for helpful discussions. We thank Isabelle Lanctin and the staff of the Pasteur Institute Animal Facility for excellent animal husbandry.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Babay, B., H. Louzir, P.-A. Cazenave, and K. Dellagi. 1999. Depletion of peritoneal CD5+ B cells has no effect on the course of Leishmania major infection in susceptible and resistant mice. Clin. Exp. Immunol. 117:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barral, A., E. A. Peterson, D. L. Sacks, and F. A. Neva. 1983. Late metastatic leishmaniasis in the mouse. A model for mucocutaneous disease. Am. J. Trop. Med. Hyg. 32:277-285. [DOI] [PubMed] [Google Scholar]
- 3.Beebe, A. M., S. Mauze, N. J. Schork, and R. L. Coffman. 1997. Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity 6:551-557. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell, J. M., G. F. Black, C. S. Peacock, E. N. Miller, D. Sibthorpe, D. Gnananandha, J. J. Shaw, F. Silveira, Z. Lins-Lainson, F. Ramos, A. Collins, and M. A. Shaw. 1997. Immunogenetics of leishmanial and mycobacterial infections: the Belem Family Study. Philos. Trans. R. Soc. Lond. B 352:1331-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourreau, E., G. Prevot, J. Pradinaud, and P. Launois. 2001. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J. Infect. Dis. 184:1628-1630. [DOI] [PubMed] [Google Scholar]
- 7.Bradley, D. J. 1987. Genetics of susceptibility and resistance in the vertebrate host, p. 551-581. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine, vol. II. Academic Press, London, United Kingdom. [Google Scholar]
- 8.Chatelain, R., K. Varkila, and R. L. Coffman. 1992. IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 148:1182-1187. [PubMed] [Google Scholar]
- 9.Desjeux, P. 1996. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 14:417-423. [DOI] [PubMed] [Google Scholar]
- 10.Gasim, S., A. M. Elhassan, E. A. Khalil, A. Ismail, A. M. Kadaru, A. Kharazmi, and T. G. Theander. 1998. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala-azar dermal leishmaniasis. Clin. Exp. Immunol. 111:64-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghalib, H. W., M. R. Piuvezam, Y. A. W. Skeiley, M. Siddig, F. A. Hashim, D. M. Russo, and S. G. Reed. 1993. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J. Clin. Investig. 92:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimaldi, G., Jr., R. B. Tesh, and D. McMahon-Pratt. 1989. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am. J. Trop. Med. Hyg. 41:687-725. [DOI] [PubMed] [Google Scholar]
- 13.Groux, H., F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M. G. Roncarolo, and R. L. Coffman. 1999. A transgenic model to analyse the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162:1723-1729. [PubMed] [Google Scholar]
- 14.Guenet, J. L. 1998. Wild mice as a source of genetic polymorphism. Pathol. Biol. 46:685-688. [PubMed] [Google Scholar]
- 15.Guy, R. A., and M. Belosevic. 1995. Reponse of scid mice to establishment of Leishmania major infection. Clin. Exp. Immunol. 100:440-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Methods 6:986-994. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin-12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzel, F. P., M. D. Sadick, S. S. Mutha, and R. M. Locksley. 1991. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. USA 88:7011-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himmelrich, H., C. Parra-Lopez, F. Tacchini-Cottier, J. A. Louis, and P. Launois. 1998. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor β2 chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 161:6156-6163. [PubMed] [Google Scholar]
- 20.Himmelrich, H., P. Launois. I. Maillard, T. Biedermann, F. Tacchini-Cottier, R. M. Locksley, M. Röcken, and J. A. Louis. 2000. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164:4819-4825. [DOI] [PubMed] [Google Scholar]
- 21.Julia, V., and N. Glaichenhaus. 1999. CD4+ T cells which react to the Leishmania major LACK antigen rapidly secrete interleukin-4 and are detrimental to the host in resistant B10.D2 mice. Infect. Immun. 67:3641-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julia, V., M. Rassoulzadegan, and N. Glaichenhaus. 1996. Resistance to Leishmania major induced by tolerance to a single antigen. Science 274:421-423. [DOI] [PubMed] [Google Scholar]
- 23.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 24.Karp, C. L., S. H. El-Safi, T. A. Wynn, M. M. H. Satti, A. M. Kordofani, F. A. Hashim, M. Hag-Ali, F. A. Neva, T. B. Nutman, and D. L. Sacks. 1993. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J. Clin. Investig. 91:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kébaïer, C., H. Louzir, M. Chenik, A. Ben Salah, and K. Dellagi. 2001. Heterogeneity of wild Leishmania major isolates in experimental murine pathogenicity and specific immune response. Infect. Immun. 69:4906-4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koide, T., K. Moriwaki, K. Ikeda, H. Niki, and T. Shiroishi. 2000. Multi-phenotype behavioral characterization of inbred strains derived from wild stocks of Mus musculus. Mammalian Genome 11:664-670. [DOI] [PubMed] [Google Scholar]
- 27.Kopf, M., F. Brombacher, G. Köhler, G. Kienzle, K.-H. Widmann, K. Lefrang, C. Humborg, B. Ledermann, and W. Solbach. 1996. IL-4-deficient BALB/c mice resist infection with Leishmania major. J. Exp. Med. 184:1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laskay, T., M. Diefenbach, M. Rollinghof, and W. W. Solbach. 1995. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 25:2220-2227. [DOI] [PubMed] [Google Scholar]
- 29.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, L. Xénarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by Vβ4 Vα8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 30.Launois, P., T. Ohteki, K. Swihart, H. R. MacDonald, and J. A. Louis. 1995. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1−. Eur. J. Immunol. 25:3298-3307. [DOI] [PubMed] [Google Scholar]
- 31.Lipoldova, M., M. Svobodova, M. Krulova, H. Havelkova, J. Badalova, E. Nohynkova, V. Holan, A. A. M. Hart, P. Volf, and P. Demant. 2000. Susceptibility to Leishmania major infection in mice: multiple loci and heterogeneity of immunopathological phenotypes. Genes Immun. 1:200-206. [DOI] [PubMed] [Google Scholar]
- 32.Locksley, R. M., F. P. Heinzel, B. J. Holaday, S. S. Reiner, and M. D. Sadick. 1991. Induction of Th1 and Th2 CD4+ subsets during murine Leishmania major infection. Res. Immunol. 14:28-32. [DOI] [PubMed] [Google Scholar]
- 33.Louzir, H., P. C. Melby, A. Ben Salah, H. Marrakchi, K. Aoun, R. Ben Ismail, and K. Dellagi. 1998. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J. Infect. Dis. 177:1687-1695. [DOI] [PubMed] [Google Scholar]
- 34.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C.-Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 35.Maillard, I., P. Launois, H. Himmelrich, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, and J. A. Louis. 2001. Functional plasticity of the LACK-reactive Vβ4 Vα8 CD4+ T cells normally producing the early IL-4 instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Eur. J. Immunol. 31:1288-1296. [PubMed] [Google Scholar]
- 36.Matthews, D. J., C. L. Emson, G. J. McKenzie, M. E. Jolin, J. M. Blackwell, and A. N. McKenzie. 2000. IL-13 is a susceptibility factor for Leishmania major infection. J. Immunol. 164:1458-1462. [DOI] [PubMed] [Google Scholar]
- 37.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M. K. Gately, J. A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 38.Meddeb-Garnaoui, A., S. Gritli, S. Garbouj, M. Ben Fadhel, R. El Kares, L. Mansour, B. Kaabi, L. Chouchane, A. Ben Salah, and K. Dellagi. 2001. Association analysis of HLA-class II and class III gene polymorphisms in the susceptibility to Mediterranean visceral leishmaniasis. Hum. Immunol. 62:509-517. [DOI] [PubMed] [Google Scholar]
- 39.Melby, P. C., F. A. Neva, and D. L. Sacks. 1989. Profile of human T cell response to leishmanial antigens: analysis by immunoblotting. J. Clin. Investig. 83:1868-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melby, P. C., F. J. Andrade-Narvaez, B. J. Darnell, G. Valencia-Pacheco, V. V. Tryon, and A. Palomo-Cetina. 1994. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect. Immun. 62:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohrs, M., B. Ledermann, G. Kohler, A. Dorfmuller, A. Gessner, and F. Brombacher. 1999. Differences between IL-4- and IL-4 receptor α-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 162:7302-7308. [PubMed] [Google Scholar]
- 42.Mosser, D. M., and A. Brittingham. 1997. Leishmania, macrophages and complement: a tale of subversion and exploitation. Parasitology 115:9-23. [DOI] [PubMed] [Google Scholar]
- 43.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z.-E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 44.Peters, W., and R. Killick-Kendrick. 1987. The leishmaniases in biology and medicine. Clinical aspects and control, 2nd ed. Academic Press, London, United Kingdom.
- 45.Pirmez, C., M. Yamamura, K. Uyemura, M. Paes-Oliveira, F. Conceicao-Silva, and R. L. Moddin. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 51:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 47.Roberts, L. J., T. M. Baldwin, J. M. Curtis, E. Handman, and S. J. Foote. 1997. Resistance to Leishmania major is linked to the H2 region on chromosome 17 and chromosome 9. J. Exp. Med. 185:1705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 49.Sadick, M. D., F. P. Heinzel, B. J. Holaday, R. T. Pu, R. S. Dawkins, and R. M. Locksley. 1990. Cure of murine leishmaniasis with anti-IL-4 monoclonal antibody. Evidence for a T cell-dependent, interferon-gamma-independent mechanism. J. Exp. Med. 171:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sassi, A., H. Louzir, A. Ben Salah, M. Mokni, A. Ben Osman, and K. Dellagi. 1999. Leishmanin skin test lymphoproliferative response and cytokine production after symptomatic or asymptomatic Leishmania major infection in Tunisia. Clin. Exp. Immunol. 116:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlein, Y. 1993. Leishmania and sandflies: interactions in the life cycle and transmission. Parasitol. Today 9:255-258. [DOI] [PubMed] [Google Scholar]
- 52.Scott, P., and J. P. Farrell. 1998. Experimental cutaneous leishmaniasis: induction and regulation of T cells following infection of mice with Leishmania major. Chem. Immunol. 70:60-80. [DOI] [PubMed] [Google Scholar]
- 53.Sypek, J. P., C. L. Chung, S. E. H. Mayor, S. J. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin-12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terabe, M., T. Kuramochi, M. Ito, T. Hatabu, C. Sanjoba, K.-P. Chang, T. Onodera, and Y. Matsumoto. 2000. CD4+ cells are indispensable for ulcer development in murine cutaneous leishmaniasis. Infect. Immun. 68:4574-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varkila, K., R. Chatelain, L. M. Leal, and R. L. Coffman. 1993. Reconstitution of C.B-17 scid mice with BALB/c T cells initiates a T helper type 1 response and renders them capable of healing Leishmania major infection. Eur. J. Immunol. 23:262-268. [DOI] [PubMed] [Google Scholar]
- 56.WHO Expert Committee. 1990. Control of leishmaniasis, p. 158. WHO Technical Report Series. World Health Organization, Geneva, Switzerland.