Abstract
Purpose
The purpose was to 1) determine whether standard clinical muscle fatty infiltration and atrophy assessment techniques using a single image slice for patients with a rotator cuff tear (RCT) are correlated with three-dimensional measures in older individuals (60+ years), and 2) determine whether age-associated changes to muscle morphology and strength are compounded by a RCT.
Methods
Twenty older subjects were studied, 10 with a RCT of the supraspinatus (5M/5F) and 10 matched controls. Clinical imaging assessments (Goutallier, Fuchs scores; cross-sectional area ratio) were made for RCT subjects. Three-dimensional measurements of rotator cuff muscle and fat tissues were made for all subjects using MRI. Isometric joint moment was measured at the shoulder.
Results
There were no significant associations between single-image assessments and three-dimensional measurements of fatty infiltration for supraspinatus and infraspinatus. Compared to controls, RCT subjects had significantly increased fatty infiltration percentages for each rotator cuff muscle (all p≤0.023), reduced whole muscle volume for supraspinatus, infraspinatus, and subscapularis (all p≤0.038), and reduced fat-free muscle volume for supraspinatus, infraspinatus, and subscapularis (all p≤0.027). Only teres minor (p=0.017) fatty infiltration volume was significantly greater for RCT subjects. Adduction, flexion, and external rotation strength (all p≤0.021) were significantly reduced for RCT subjects, and muscle volume was a significant predictor of strength for all comparisons.
Conclusions
Clinical scores using a single image slice do not represent three-dimensional muscle measurements. Efficient methods are needed to more effectively capture three-dimensional information for clinical applications. RCT subjects had increased fatty infiltration percentages likely driven by muscle atrophy rather than increased fat volume. Muscle volume’s significant association with strength production suggests that treatments to preserve muscle volume should be pursued for older RCT patients.
Level of Evidence
Level II, diagnostic study, with development of diagnostic criteria on the basis of consecutive patients with universally applied reference “gold” standard.
Introduction
Rotator cuff tears (RCT) are a common musculoskeletal injury in older individuals. It is estimated that 20–50% of those 60+ years of age have a known RCT, up to 65% of those aged ≥70 have an asymptomatic RCT, and prevalence increases with age.1–4 Rotator cuff tears are associated with muscle atrophy and fatty infiltration,5–8 and fatty infiltration may affect muscle tissue recovery following a tear in older individuals.9 Clinicians evaluate atrophy and fatty infiltration when developing treatment plans for patients with a RCT. High levels of fatty infiltration are a contraindication for rotator cuff tendon repair, due to the high likelihood of a poor surgical outcome, increased likelihood of a re-tear, and no reversal of the fatty infiltration following surgery.5,10,11 Traditionally, computed tomography (CT) and magnetic resonance imaging (MRI) have been used to evaluate the muscle-to-fat ratio of rotator cuff muscles, as described by Goutallier et al.5 and Fuchs et al.,12 respectively. Atrophy is also frequently assessed from a single image slice, using muscle cross-sectional area as a surrogate.13 However, these clinical methods consider only a single image slice to view the rotator cuff muscle bellies, selected for consistency of anatomical landmarks. One image cannot fully capture morphological changes in other areas of the muscle,14 but little work has focused on the acquisition of three-dimensional measurements of muscle morphology to evaluate whether a single image is indicative of three-dimensional muscle information. Further, the Goutallier score does not have high reliability,15–18 suggesting that more objective methods should be used.15,16
Physiologic and morphologic changes accompanying rotator cuff injury are also observed during healthy aging,19 which may cloud injury presentation in older patients. Older adults exhibit muscle atrophy, fatty infiltration, and strength deficits even in the absence of musculoskeletal injury.19,20 Thus, it is difficult when working with older adults with a RCT to determine whether measured deficits are due to injury or age alone. Such an understanding is critical to creating an effective care plan and maintenance of function in older adults. For example, muscle atrophy may be an important contributor to strength deficits seen in RCT patients.10,11 Muscle volume is a significant predictor of upper limb strength21–23 and maintenance of a minimal strength threshold is necessary to perform functional upper limb tasks.20 Strength is critical to older adults’ ability to maintain independence,1 although the mechanisms relating muscle morphology to function in older adults with a RCT are not clear.
The purpose of this study was to 1) determine whether standard clinical muscle fatty infiltration and atrophy assessment techniques using a single image slice for patients with a rotator cuff tear (RCT) are correlated with three-dimensional measures in older individuals (60+ years), and 2) determine whether age-associated changes to muscle morphology and strength are compounded by a RCT. It was hypothesized that 1) single-image assessments would be correlated with muscle morphology, and 2) patients with a RCT would have less muscle volume, greater fat volume, and reduced strength compared to controls and that muscle volume would be correlated with strength.
Methods
This study was approved by the Wake Forest University Health Sciences Institutional Review Board and written informed consent was provided by all participants. A sample of 20 subjects (mean age 63.6±1.6 years), 10 with a degenerative supraspinatus tear and 10 age- and gender-matched controls (Table 1), were recruited to prospectively evaluate the applicability of measuring three-dimensional muscle morphology to identify the association between single-image assessments and three-dimensional measurements in an older adult group. Participants with a RCT were recruited from the outpatient clinics of CJT, GGP, and MTF from October 2011-September 2013; one patient (M05) approached the study team regarding participation. The presence of supraspinatus tearing was independently confirmed by all three orthopaedic surgeon authors. Following physician assessment, the medical record of potential participants was reviewed to ensure eligibility criteria was met (Figure 1) before recruitment. Control subjects were recruited from the community through a newsletter advertisement (Figure 1). Potential control participants were screened with a telephone questionnaire and a modified Jobe’s test.24 The injured side was evaluated for RCT subjects and the dominant side was evaluated for controls.
Table 1.
Participant demographics for rotator cuff tear and control subjects. The tear characteristics corresponding to the MRI in each rotator cuff tear participant’s medical record are also reported. Supraspinatus tears are described as either full- or partial-thickness tears, while remaining tendons are denoted as to whether or not tearing was observed. In cases where the radiologist’s report could not make a definitive determination regarding the tear status, in correspondence with the report, a possible tear is reported. Pain assessments from the pain category of the RAND 36-Item Short Form Health Survey (Medical Outcomes Study: 36-Item Short Form Survey Instrument, RAND Health, Santa Monica, CA) are reported; scores can range from 0–100, where a score of 100 is the best possible score, indicating no pain. Differences between groups were assessed with a t-test, with significance considered as p ≤ 0.05.
| Subject | Age (years) | Height (cm) |
Body mass (kg) |
Dominant arm |
Injured arm |
Supraspinatus | Infraspinatus | Subscapularis | Teres minor | SF-36 Pain Score |
|---|---|---|---|---|---|---|---|---|---|---|
| RF01 | 64 | 162.6 | 58.5 | Right | Right | Partial-thickness tear | No tear | No tear | No tear | 20 |
| RF02 | 65 | 165.1 | 83.9 | Right | Right | Full-thickness tear | No tear | No tear | No tear | 40 |
| RF03 | 65 | 149.9 | 53.5 | Right | Left | Full-thickness tear | Tear | Tear | No tear | 40 |
| RF04 | 63 | 160 | 73.5 | Right | Right | Full-thickness tear | Tear | No tear | No tear | 40 |
| RF05 | 65 | 162.6 | 65.8 | Right | Left | Partial-thickness tear | No tear | Tear | No tear | 40 |
| RM01 | 64 | 175.3 | 73 | Right | Left | Partial-thickness tear | No tear | Possible tear | No tear | 40 |
| RM02 | 61 | 167.6 | 83.9 | Right | Left | Full-thickness tear | Tear | No tear | No tear | 40 |
| RM03 | 64 | 177.8 | 108 | Left | Left | Full-thickness tear | Tear | No tear | No tear | 40 |
| RM04 | 64 | 182.9 | 88.5 | Right | Left | Full-thickness tear | Tear | Tear | No tear | 40 |
| RM05 | 62 | 177.8 | 95.3 | Left | Left | Partial-thickness tear | Possible tear | Possible tear | No tear | 40 |
| CF01 | 64 | 152.4 | 74.8 | Left | - | - | - | - | - | 40 |
| CF02 | 63 | 172.7 | 54.4 | Right | - | - | - | - | - | 80 |
| CF03 | 67 | 172.7 | 70.8 | Right | - | - | - | - | - | 80 |
| CF04 | 65 | 162.6 | 65.8 | Right | - | - | - | - | - | 100 |
| CF05 | 64 | 160 | 60.3 | Right | - | - | - | - | - | 100 |
| CM01 | 64 | 172.7 | 70.3 | Right | - | - | - | - | - | 20* |
| CM02 | 61 | 177.8 | 99.8 | Right | - | - | - | - | - | 60 |
| CM03 | 64 | 182.9 | 86.2 | Right | - | - | - | - | - | 100 |
| CM04 | 62 | 172.7 | 73.5 | Right | - | - | - | - | - | 100 |
| CM05 | 61 | 175.3 | 70.3 | Right | - | - | - | - | - | 60 |
|
Rotator Cuff Tear Mean ± SD |
63.7 ± 1.3 | 168.1 ± 10.1 | 78.4 ± 16.8 | 38.0 ±6.3 | ||||||
|
Control Mean ± SD |
63.5 ± 1.8 | 170.2 ± 9.1 | 72.6 ± 12.8 | 74.0 ±28.4 | ||||||
|
Difference between groups |
0.2 (p = 0.66) | 2.1 (p = 0.32) | 5.8 (p = 0.18) | 36.0 (p < 0.01) |
R=rotator cuff tear group; C=control group; F=female; M=male.
Participant reported unrelated lower back pain
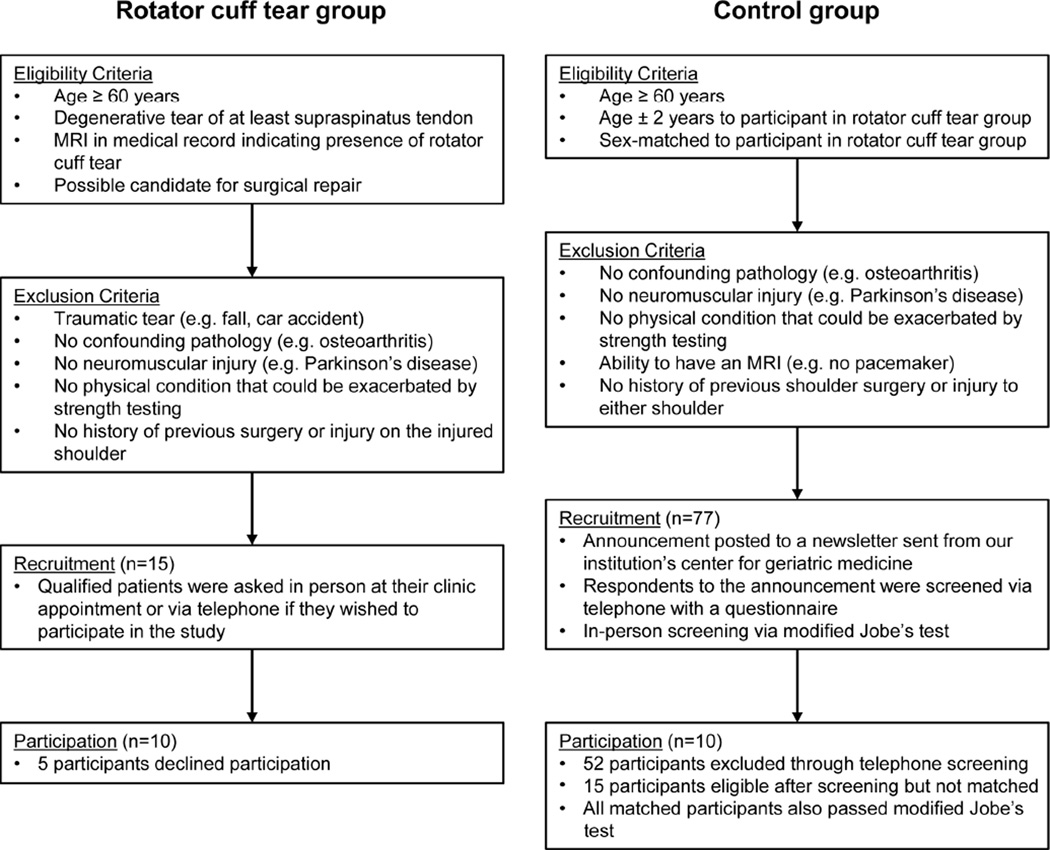
Figure 1.
Study participant recruitment flow chart. The left side of the chart shows the recruitment criteria and process for participants with a rotator cuff tear, while the right side of the chart shows the recruitment criteria and process for participants in the control group.
Single-image assessments
Three orthopaedic surgeons (CJT, GGP, MTF) independently evaluated each RCT subject to assign a Goutallier score. Evaluations were performed by each surgeon twice, with reviews separated by 1 week. Surgeons were blinded to patient identity and the order in which patients were reviewed was randomized. Assessment was made using a standard T1-weighted MRI available from each patient’s medical record. For one participant, a T1-weighted scan was not available, so that subject was excluded from these analyses. A Goutallier score was assigned to each rotator cuff muscle according to the methods of Goutallier5, as modified by Fuchs12, in which the image slice immediately lateral to the scapular spine’s attachment to the body of the scapula was evaluated. Assessment of Goutallier score, which ranges from 0 (no fat visible in the muscle) to 4 (more fat than muscle tissue is visible), was made for the supraspinatus, infraspinatus, subscapularis, and teres minor. Since it is thought to improve inter-observer reliability,12 Goutallier scores were translated into the corresponding Fuchs score, which includes three stages (Fuchs stage 0 = Goutallier scores 0 and 1; Fuchs stage 1 = Goutallier score 2; Fuchs stage 2 = Goutallier scores 3 and 4). Each physician’s score from both reviews was used to calculate a mean Goutallier score and a mean Fuchs score, which were used in subsequent analyses. The range of assigned scores is shown in Table 2.
Table 2.
Range of Goutallier score and Fuchs score, and mean cross-sectional area ratios (CSA-ratio) for each participant with a rotator cuff tear. Goutallier scores were assigned individually by three orthopaedic surgeons at two reviews separated by at least one week. Goutallier scores were converted into their corresponding Fuchs score. Cross-sectional area ratios were calculated by taking the mean measurements from two reviewers.
| RF01 | RF02 | RF03 | RF04 | RF05 | RM01 | RM02 | RM03 | RM04 | |
|---|---|---|---|---|---|---|---|---|---|
| Goutallier Score | |||||||||
| Supraspinatus score range | 1–3 | 2 | 1–4 | 0–2 | 1–2 | 1–2 | 1–4 | 1–3 | 1–2 |
| Infraspinatus score range | 0–1 | 2 | 2–4 | 0–1 | 0–2 | 0–1 | 0–2 | 0–2 | 0–1 |
| Teres minor score range | 0 | 2–4 | 0–3 | 0–3 | 0–1 | 0 | 0–2 | 0–3 | 0–1 |
| Subscapularis score range | 0–1 | 1 | 2–4 | 0–1 | 0–2 | 0–1 | 0–1 | 0–2 | 0–1 |
| Fuchs Score | |||||||||
| Supraspinatus score range | 0–2 | 1 | 0–2 | 0–1 | 0–1 | 0–1 | 0–2 | 0–2 | 0–1 |
| Infraspinatus score range | 0 | 1 | 1–2 | 0 | 0–1 | 0 | 0–1 | 0–1 | 0 |
| Teres minor score range | 0 | 1–2 | 0–2 | 0–2 | 0 | 0 | 0–1 | 0–2 | 0 |
| Subscapularis score range | 0 | 0 | 1–2 | 0 | 0–1 | 0 | 0 | 0–1 | 0 |
| Cross-sectional area ratio | |||||||||
| Supraspinous fossa area | 5.1 | 5.8 | 5.2 | 4.2 | 4.8 | 5.7 | 3.8 | 8.6 | 6.4 |
| Supraspinatus CSA-ratio | 0.5 | 0.5 | 0.5 | 1.2 | 0.6 | 0.7 | 1.0 | 0.6 | 1.1 |
| Infraspinatus CSA-ratio | 1.2 | 1.4 | 0.6 | 1.7 | 1.1 | 1.0 | 2.7 | 1.6 | 1.2 |
| Teres minor CSA-ratio | 0.9 | 0.4 | 0.4 | 0.1 | 0.3 | 1.0 | 1.5 | 0.8 | 0.5 |
| Subscapularis CSA-ratio | 3.0 | 1.5 | 0.5 | 1.5 | 2.0 | 2.2 | 3.4 | 1.4 | 1.9 |
R=rotator cuff tear group; F=female; M=male.
Cross-sectional area ratio, which is a clinical measure used to describe muscle atrophy, was calculated for RCT patients using the segmentation methods of Zanetti et al.13 Briefly, these methods entail calculation of cross-sectional area of the supraspinatus, total infraspinatus, total subscapularis, teres minor, and supraspinatus fossa by tracing their boundaries using the measurement tool included with the MR viewing software (iSite PACS, Philips Healthcare Informatics, Foster City, CA). Two reviewers (MEV, ACS) calculated cross-sectional areas and the mean value between reviewers was used. Cross- sectional area ratio was calculated separately for each muscle by dividing the mean muscle cross-sectional area by the supraspinatus fossa area. Muscle cross-sectional areas were assessed from the same T1-weighted image slice used to assess the Goutallier score.
Three-dimensional assessments
Three-dimensional measurements of rotator cuff muscle volume and fatty infiltration were acquired using MRI. Subjects were imaged supine with either a 1.5T (GE Healthcare, Milwaukee, WI) or 3T (Siemens Medical Solutions USA, Malvern, PA) scanner due to an institutional system upgrade. Images of the muscles crossing the glenohumeral joint were acquired with a flexed array long bone coil (1.5T; Invivo, Orlando, FL) or an 18-channel body matrix coil (3T; Siemens Medical Solutions USA, Malvern, PA). Scanning was performed using the clinically available pulse sequence for each machine, the Three Point Dixon (1.5T) or the Two Point Dixon (3T) method,25,26 which enables quantification of water (muscle) and fat within the region of interest. Images were acquired in 3mm (1.5T) or 1mm (3T) slices. Scan parameters were configured on each machine to allow for collection of analogous images across machines (1.5T Scanner: TE=4.200, 6.581, 8.962ms; TR=13.5ms; Flip angle=18°; Matrix size=320×256; Bandwidth=±62.5kHz; Field of view=320mm; Slice thickness=3mm; 3T Scanner: TE=2.46, 3.69ms; TR=7.0ms; Flip angle=9°; Matrix size=256×256; Bandwidth=±248.3kHz; Field of view=224mm (read), 120.5% (phase); Slice thickness=1mm). Total scan time was ~13 minutes (1.5T) or ~15 minutes (3T). Post-scan processing to produce the fat and water images associated with the Dixon method was conducted using the software supplied by each scanner manufacturer. Accuracy of the Dixon method was assessed with a fat-water phantom of known composition. Findings were consistent between 1.5T and 3T scanners, with a mean difference in calculated fat volume percentage of 0.54%.
Three-dimensional measurements of whole muscle volume of supraspinatus, infraspinatus, subscapularis, and teres minor were calculated using manual segmentation as previously described.22,23 Briefly, muscle boundaries were traced on each image slice (3D Doctor, Able Software Corp., Lexington, MA) and a 3-dimensional polygonal surface was created from the boundaries. Individual muscle volumes were calculated from the polygonal surfaces. Supraspinatus muscle volume was calculated using 1mm slices, when available. All other muscle volumes were calculated using a 3mm slice thickness. For scans acquired with 1mm slices, every third image was used to achieve a 3mm slice thickness.
Three-dimensional measurements of fatty infiltration were calculated for each muscle using a custom Matlab (Rev. 2012b, The MathWorks, Natick, MA) program and Equation 1, where SIfat and SIwater are the signal intensities for fat and water images of the Dixon method, respectively. This calculation was performed on a voxel-by-voxel basis, then averaged across all voxels in the volume to determine the percentage of fatty infiltration (%fat) within each muscle. T1 corrections were applied using the scaling coefficients described by Gold et al. which accounted for signal-to-noise bias across scanners; noise correction was performed using magnitude discrimination, described by Liu et al., which was implemented using Equation 1.
| [Equation 1] |
Percentage of fatty infiltration was converted to fatty infiltration volume by multiplying %fat by whole muscle volume. Fat-free muscle volume was calculated by subtracting fatty infiltration volume from whole muscle volume measurements.
Strength assessment
Strength was evaluated by measuring the maximal isometric joint moment with a Biodex dynamometer (Biodex Medical Systems, Shirley, NY). Three 5-second trials were recorded for abduction/adduction (shoulder abducted to 30°, elbow braced in full extension, forearm in neutral posture, wrist braced), flexion/extension (shoulder flexed to 30°, elbow braced in full extension, forearm pronated to 90°, wrist braced), and internal/external rotation (shoulder abducted to 30°, elbow flexed to 90°, forearm in neutral posture, wrist braced). Sixty seconds of rest were given between trials and 2 minutes of rest were given between tests to reconfigure the dynamometer. Subjects were instructed to stop a trial if they felt any pain. During each trial, participants were given verbal encouragement to elicit maximal performance. A custom Matlab program was used to determine the maximal joint moment for each trial. The program consisted of a search window which identified the maximum joint moment that was maintained for at least 0.5 seconds.21 The maximal value across the 3 trials was considered the subject’s maximum isometric strength.
Statistical Analysis
Descriptive statistics were used to describe the demographic characteristics of the subjects in RCT and control groups. Linear regression was used to evaluate the relationships between single-image assessments and three-dimensional measurements. Specifically, the associations between mean Goutallier and Fuchs scores relative to percentage of fatty infiltration, and muscle cross-sectional area ratio relative to whole muscle volume measurements were examined. Kappa statistics were calculated for inter- and intra-rater repeatability measures for Goutallier score and Fuchs score. Kappas are reported for each pair-wise comparison for each combination of reviewers to assess inter- rater repeatability; intra-rater reliability was evaluated by calculating a kappa statistic for each reviewer across the two reviews. A higher positive kappa indicates better agreement, a kappa equal to zero is agreement due to chance, and a negative kappa indicates worse agreement than chance. Exact tests were used to calculate statistical significance of the kappa statistics.
Analysis of covariance (ANCOVA) with adjustments for age and sex was used to separately evaluate mean differences between RCT and control groups for three- dimensional measurements of muscle volume, fatty infiltration volume, percent fatty infiltration, fat-free muscle volume, and isometric joint moment. The four failed joint moment trials due to pain were excluded from statistical analyses. Linear regression analyses were used to evaluate the relationship between three-dimensional measurements of muscle volume and the isometric joint moment of each muscle’s primary movement; parallel lines ANCOVA demonstrated that there were no differences between RCT and control groups, so subjects were considered as a single cohort for these analyses, with adjustments for group and sex. Significance was set to p<0.05. Due to the exploratory nature of the analyses, Type I error was not accounted for by adjusting for multiple measurements. Analyses were performed with SAS software (version 9.3, SAS Institute, Inc., Cary, NC).
Results
Six RCT subjects had full-thickness tears, while 4 subjects had marked partial-thickness (>50% tendon thickness) tears. All recruited patients volunteered to participate and no participants withdrew from the study. Each participant completed all study evaluations within 2 weeks.
Single-image assessments
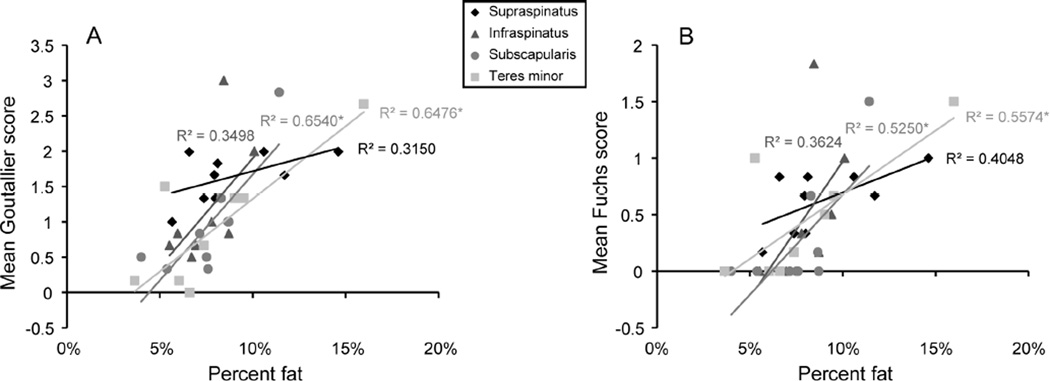
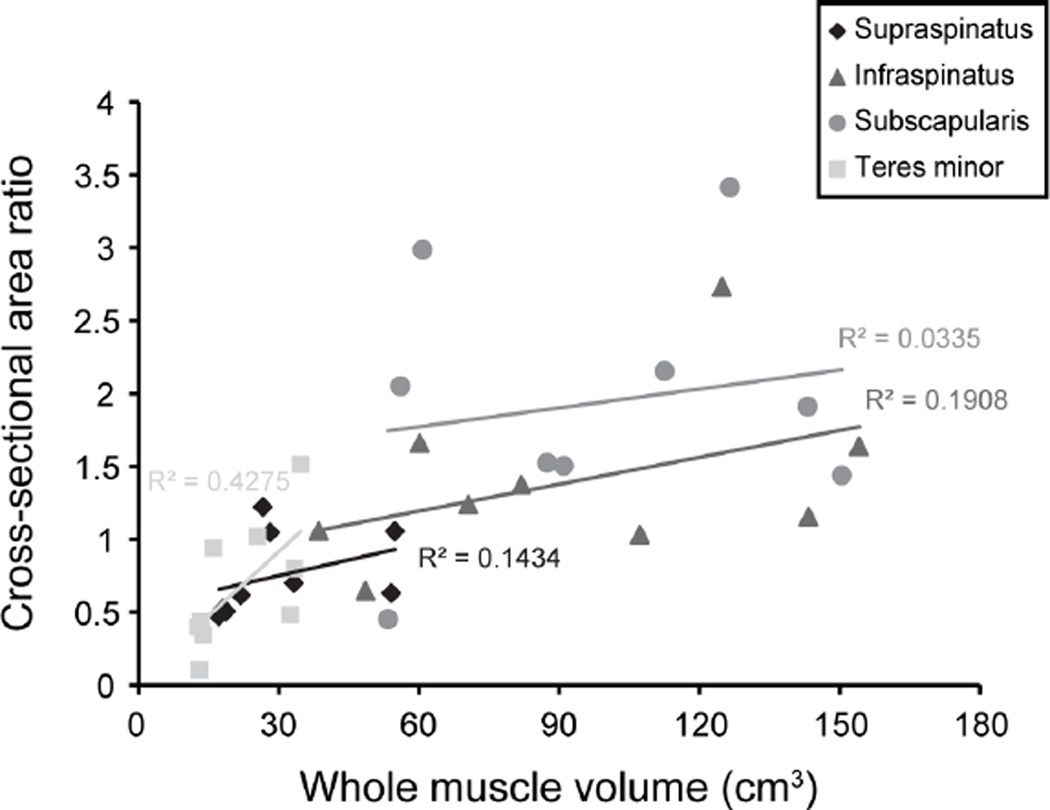
The linear relationship between three-dimensional measurements of percent fatty infiltration and mean Goutallier score (Figure 2A) and mean Fuchs score (Figure 2B) was not significant for supraspinatus or infraspinatus but was significant for subscapularis and teres minor. There was no significant linear relationship between cross-sectional area ratio for any of the rotator cuff muscles and three-dimensional measures of whole muscle volume (Figure 3). Few of the inter- and intra-rater agreements for Goutallier score or Fuchs score reached statistical significance (Table 3), indicating that there was not statistical agreement across raters or reviews.
Figure 2.
Mean Goutallier score (A) and mean Fuchs score (B) versus percentage of fatty infiltration for rotator cuff muscles. The injured arm (3 right /6 left) was assessed for participants with a rotator cuff tear and the dominant (9 right/1 left) was assessed for controls. * denotes a significant linear relationship, whereby significance indicates that the Goutallier score or Fuchs score captures three-dimensional measures of fatty infiltration. The relationship between mean Goutallier score (A) and percentage of fatty infiltration was significant for subscapularis and teres minor (supraspinatus, p=0.116; infraspinatus, p=0.094; subscapularis, p=0.008; teres minor, p=0.009). The relationship between Fuchs score (B) and percentage of fatty infiltration was significant for subscapularis and teres minor (supraspinatus, p=0.065; infraspinatus, p=0.086; subscapularis, p=0.027; teres minor, p=0.021).
Figure 3.
Three-dimensional muscle volume measurements versus cross-sectional area ratio (muscle cross-sectional area/supraspinatus fossa area) for supraspinatus (p=0.315), infraspinatus (p=0.240), subscapularis (p=0.637), and teres minor (p=0.056) muscles. The injured arm (3 right /6 left) was assessed for participants with a rotator cuff tear and the dominant arm was assessed for controls. The presence of a significant correlation would have indicated that the cross-sectional area ratio effectively estimates three-dimensional muscle volume.
Table 3.
Kappa statistics of Goutallier score and Fuchs score for each of the three reviewers at two separate reviews. Kappas are shown for each pair-wise comparison of all combinations of reviewers. Exact tests were used to determine the statistical significance of each kappa statistic.
| Supraspinatus | Total Infraspinatus |
Total Subscapularis |
Teres Minor | |
|---|---|---|---|---|
| Goutallier score | ||||
| Inter-rater kappa (κ) | ||||
| Review 1 | 0.05, −0.03, 0.06 | 0.44, 0.10, 0.06 | 0.36, 0.26, 0.13 | 0.20, 0.37, 0.12 |
| Review 2 | 0.11, 0.05, 0.61 | 0.45, 0.18, 0.16 | 0.02, 0.17, 0.35 | −0.03, 0.13, 0.27 |
| Intra-rater kappa (κ) | ||||
| Reviews 1 and 2 | 0.49*, 0.37, 0.63 | 0.75*, 0.45, 0.40* | 0.80*, 0.65*, 0.34 | 0.31, 0.68*, 0.42 |
| Fuchs score | ||||
| Inter-rater kappa (κ) | ||||
| Review 1 | 0.05, 0.09, 0.18 | 0.44, 1.0*, 0.44 | 0.70*, 0.61, 0.25 | 0.14, 0.25, 0.10 |
| Review 2 | 0.11, 0.05, 0.61 | 0.58*, 0.75*, 0.36 | 0.50, 0.18, 0.63 | 0.12, 0.63, 0.36 |
| Intra-rater kappa (κ) | ||||
| Reviews 1 and 2 | 0.80*, 0.37, 1.0 | 0.75*, 0.60, 1.0* | 0.73, 1.0*, 0.47 | 0.25, 0.80*, 0.63 |
Indicates statistical significance.
Three-dimensional assessments
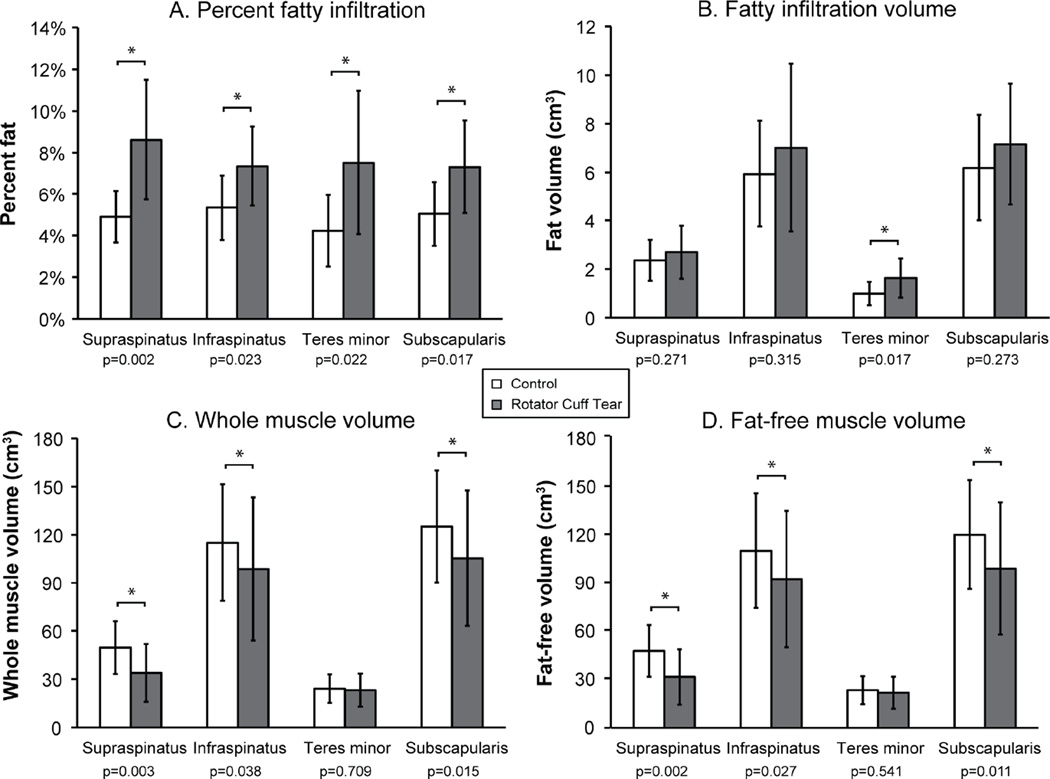
The mean difference between three-dimensional muscle volumes calculated using segmented images of 1mm versus 3mm slice thickness was 0.28%. Based on three-dimensional assessments of muscle morphology (Table 4), patients with a RCT had increased percentages of fatty infiltration compared to controls, which was driven by muscle atrophy. The RCT group had a significantly larger percentage of fatty infiltration compared to controls for supraspinatus, infraspinatus, subscapularis, and teres minor (Figure 4A). However, except for teres minor, the RCT group did not have larger volumes of fatty infiltration (Figure 4B). The RCT group had smaller whole muscle volume for supraspinatus, infraspinatus, and subscapularis muscles (Figure 4C). Fat-free muscle volume was significantly less for the RCT group than the control group for supraspinatus, infraspinatus, and subscapularis (Figure 4D).
Table 4.
Three-dimensional muscle morphology measurements for each participant for rotator cuff muscles and maximum isometric joint moment measurements.
| RF01 | RF02 | RF03 | RF04 | RF05 | RM01 | RM02 | RM03 | RM04 | RM05 | CF01 | CF02 | CF03 | CF04 | CF05 | CM01 | CM02 | CM03 | CM04 | CM05 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle morphology | ||||||||||||||||||||
| Supraspinatus | ||||||||||||||||||||
| Whole volume (cm3) | 18.7 | 18.1 | 17.2 | 26.6 | 21.8 | 33.3 | 28.1 | 54.1 | 54.8 | 66.2 | 31.2 | 39.4 | 44.5 | 29.8 | 37.5 | 77.7 | 44.8 | 61.3 | 65.3 | 64.2 |
|
Fatty infiltration volume (cm3) |
2.2 | 2.7 | 1.1 | 1.5 | 1.7 | 2.7 | 3.0 | 4.4 | 4.0 | 3.6 | 1.6 | 1.6 | 1.6 | 2.1 | 2.0 | 2.1 | 2.2 | 3.2 | 3.1 | 4.1 |
| Fat-free volume (cm3) | 16.5 | 15.5 | 16.1 | 25.1 | 20.1 | 30.6 | 25.2 | 49.7 | 50.8 | 62.6 | 29.6 | 37.9 | 42.9 | 27.8 | 35.5 | 75.6 | 42.6 | 58.2 | 62.2 | 60.1 |
| % Fat | 11.7% | 14.6% | 6.6% | 5.7% | 7.9% | 8.0% | 10.6% | 8.1% | 7.4% | 5.5% | 5.1% | 4.0% | 3.7% | 7.0% | 5.2% | 2.7% | 5.0% | 5.2% | 4.8% | 6.4% |
| Infraspinatus | ||||||||||||||||||||
| Whole volume (cm3) | 70.5 | 81.9 | 48.6 | 60.2 | 38.5 | 107.3 | 124.9 | 154.2 | 143.4 | 157.6 | 89.1 | 84.2 | 96.9 | 67.5 | 77.6 | 172.8 | 133.4 | 146.3 | 153.8 | 129.3 |
|
Fatty infiltration volume (cm3) |
4.9 | 8.3 | 4.1 | 3.3 | 3.4 | 7.2 | 9.7 | 14.5 | 8.6 | 6.1 | 3.9 | 4.1 | 5.0 | 4.7 | 4.9 | 4.7 | 11.1 | 6.3 | 7.4 | 7.3 |
| Fat-free volume (cm3) | 65.6 | 73.7 | 44.5 | 56.8 | 35.2 | 100.1 | 115.1 | 139.7 | 134.8 | 151.4 | 85.2 | 80.1 | 92.0 | 62.8 | 72.7 | 168.2 | 122.4 | 140.0 | 146.4 | 122.1 |
| % Fat | 7.0% | 10.1% | 8.5% | 5.5% | 8.7% | 6.7% | 7.8% | 9.4% | 6.0% | 3.9% | 4.4% | 4.8% | 5.1% | 7.0% | 6.3% | 2.7% | 8.3% | 4.3% | 4.8% | 5.6% |
| Teres Minor | ||||||||||||||||||||
| Whole volume (cm3) | 16.1 | 13.3 | 12.7 | 13.0 | 13.9 | 25.4 | 34.7 | 33.4 | 32.4 | 36.6 | 16.7 | 13.2 | 29.9 | 11.7 | 22.0 | 28.0 | 38.5 | 25.1 | 34.1 | 21.6 |
|
Fatty infiltration volume (cm3) |
1.1 | 2.1 | 1.1 | 0.7 | 0.8 | 1.7 | 2.6 | 3.2 | 1.2 | 1.9 | 0.7 | 0.4 | 0.8 | 0.5 | 0.7 | 0.7 | 1.4 | 1.7 | 1.4 | 1.6 |
| Fat-free volume (cm3) | 15.0 | 11.2 | 11.6 | 12.3 | 13.0 | 23.7 | 32.2 | 30.2 | 31.3 | 34.8 | 15.9 | 12.8 | 29.0 | 11.2 | 21.4 | 27.4 | 37.1 | 23.4 | 32.8 | 19.9 |
| % Fat | 6.6% | 16.0% | 9.0% | 5.3% | 6.0% | 6.6% | 7.4% | 9.5% | 3.6% | 5.1% | 4.4% | 3.3% | 2.8% | 4.4% | 3.0% | 2.4% | 3.6% | 6.8% | 4.0% | 7.6% |
| Subscapularis | ||||||||||||||||||||
| Whole volume (cm3) | 60.8 | 91.0 | 53.4 | 87.4 | 56.0 | 112.6 | 126.5 | 150.5 | 143.2 | 172.4 | 100.2 | 80.6 | 106.1 | 84.0 | 96.4 | 168.2 | 161.3 | 155.7 | 158.6 | 141.4 |
|
Fatty infiltration volume (cm3) |
4.6 | 7.9 | 6.1 | 4.7 | 4.9 | 8.0 | 9.5 | 12.5 | 5.7 | 7.6 | 5.5 | 3.2 | 4.1 | 7.0 | 4.7 | 5.5 | 10.9 | 7.6 | 6.0 | 7.3 |
| Fat-free volume (cm3) | 56.2 | 83.1 | 47.3 | 82.7 | 51.2 | 104.5 | 117.0 | 138.0 | 137.5 | 164.8 | 94.7 | 77.3 | 102.0 | 77.0 | 91.7 | 162.7 | 150.4 | 148.1 | 152.6 | 134.1 |
| % Fat | 7.6% | 8.7% | 11.4% | 5.4% | 8.7% | 7.1% | 7.5% | 8.3% | 4.0% | 4.4% | 5.5% | 4.0% | 3.8% | 8.3% | 4.9% | 3.3% | 6.8% | 4.9% | 3.8% | 5.2% |
| Isometric joint moment | ||||||||||||||||||||
| Abduc tion (Nm) | 3.8 | 16.4 | 2.2 | 12.0 | - | 4.7 | 38.7 | 58.9 | 21.5 | 35.1 | 29.6 | 16.3 | 19.0 | 15.0 | 16.5 | 54.4 | 27.1 | 28.4 | 42.4 | 34.1 |
| Adduc tion (Nm) | 18.5 | 24.7 | 38.5 | 34.6 | 36.3 | 65.9 | 34.1 | 66.8 | 84.6 | 48.6 | 33.7 | 41.0 | 51.1 | 42.0 | 46.6 | 97.7 | 48.5 | 61.7 | 86.6 | 75.0 |
| Flexion (Nm) | 1.2 | 4.0 | - | 11.1 | - | - | 32.9 | 28.6 | 21.8 | 26.6 | 14.8 | 16.8 | 20.2 | 9.0 | 16.5 | 38.8 | 24.8 | 24.2 | 38.9 | 41.5 |
| Extension (Nm) | 31.0 | 42.4 | 41.3 | 41.4 | 37.5 | 83.7 | 64.6 | 77.5 | 88.4 | 26.6 | 45.3 | 48.5 | 59.6 | 43.1 | 48.3 | 64.1 | 69.0 | 72.0 | 84.0 | 64.3 |
| Internal Rotation (Nm) | 16.8 | 33.4 | 15.1 | 28.5 | 12.7 | 42.0 | 37.0 | 44.9 | 57.8 | 28.8 | 25.3 | 21.0 | 24.5 | 22.9 | 29.7 | 57.7 | 36.3 | 35.9 | 54.6 | 38.4 |
| External Rotation (Nm) | 0.5 | 7.4 | 3.8 | 5.5 | 0.8 | 6.9 | 18.2 | 14.3 | 12.3 | 14.7 | 5.6 | 12.6 | 16.8 | 12.6 | 13.0 | 35.2 | 11.0 | 16.4 | 24.7 | 20.7 |
R=rotator cuff tear group; C=control group; F=female; M=male.
Figure 4.
Mean±SD for quantitative measurements of muscle and fatty infiltration volume for rotator cuff muscles for older adults with a rotator cuff tear (gray bars) and healthy controls (white bars). For participants with a rotator cuff tear, the injured arm (3 right /7 left) was assessed and for controls, the dominant arm (9 right/1 left) was assessed. (A) There was a significantly greater percentage of fatty infiltration for all muscles (supraspinatus, p=0.002; infraspinatus, p=0.023; teres minor, p=0.022; subscapularis, p=0.017) for the rotator cuff tear group. (B) Only teres minor (p=0.017) had a significantly greater volume of fatty infiltration for the rotator cuff tear subjects. (C) The rotator cuff tear group had significantly reduced whole volume measurements for supraspinatus (p=0.003), infraspinatus (p=0.038), and subscapularis (p=0.015) muscles. (D) Fat-free muscle volume was significantly reduced for the rotator cuff tear group for supraspinatus (p=0.002), infraspinatus (p=0.027), and subscapularis (p=0.011) muscles.
Strength vs. volume
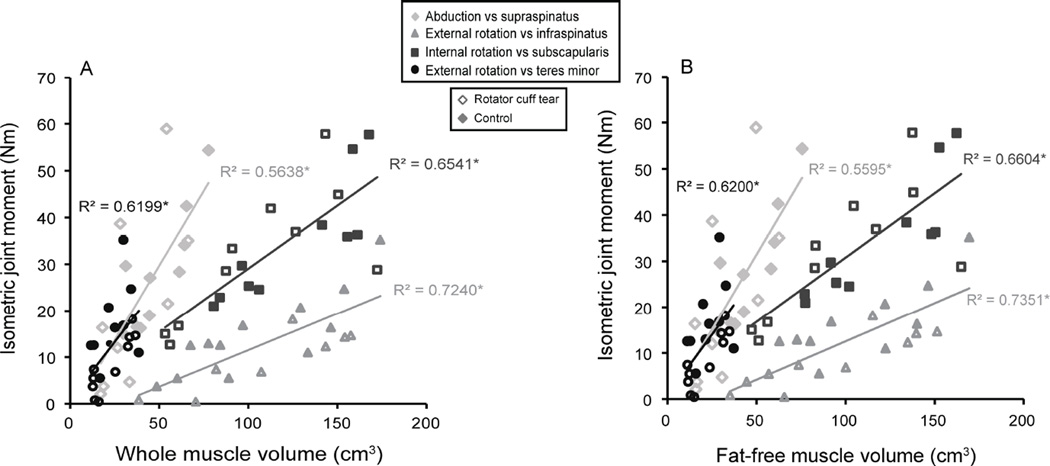
One RCT subject could not perform the abduction trials and three RCT subjects could not perform the flexion trials due to pain (Table 4). Isometric joint moment was less for the RCT group in adduction, flexion, and external rotation, but the other joint moments were similar between groups (Figure 5). There were significant associations between whole muscle volume and the joint moment of a muscle’s primary action for supraspinatus, infraspinatus, subscapularis, and teres minor (Figure 6A). Similarly, the relationship between strength and fat-free muscle volume was significant for all comparisons (Figure 6B).
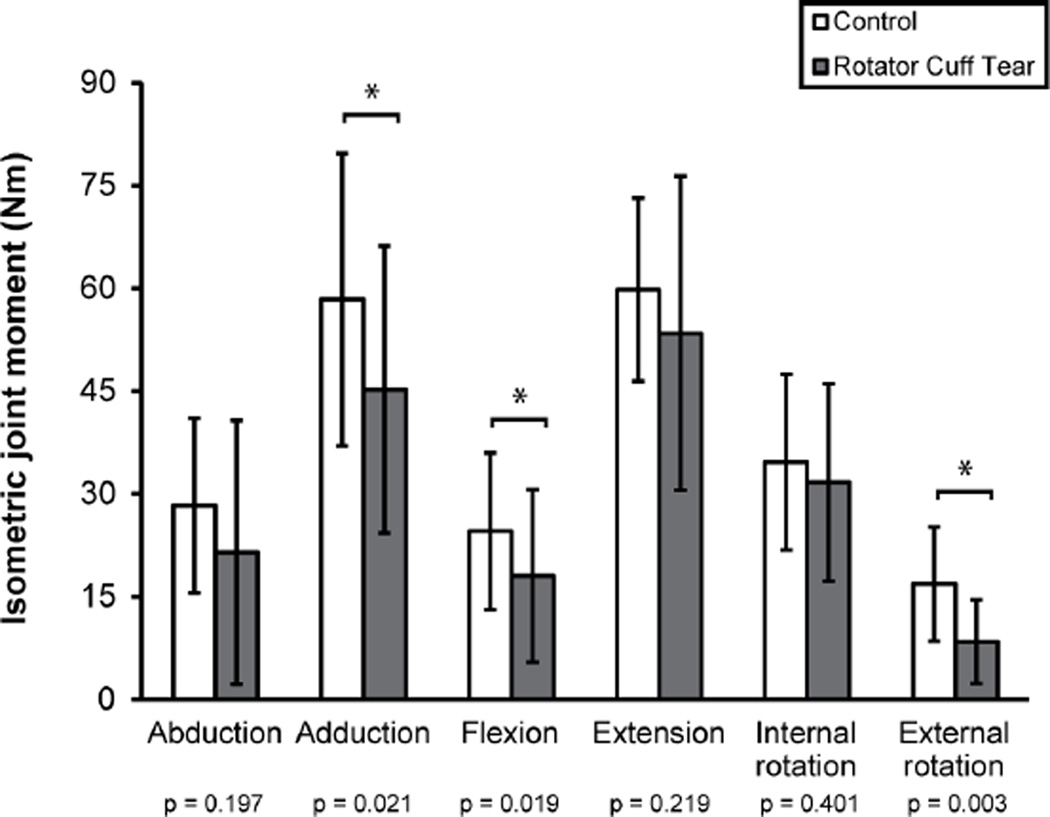
Figure 5.
Mean±SD isometric joint moment for the rotator cuff tear group (gray bars) and healthy controls (white bars). The injured arm (3 right/7 left) was evaluated for participants with a rotator cuff tear and the dominant arm (9 right/1 left) was assessed for controls. One participant could not perform abduction and three participants could not perform flexion due to pain; these trials were not included in the statistical analyses. Isometric joint moment was significantly reduced for adduction (p=0.021), flexion (p=0.019), and external rotation (p=0.003).
Figure 6.
Isometric joint moment versus whole muscle volume (A) and fat-free muscle volume (B) for subjects with a rotator cuff tear (hollow markers) and healthy controls (filled markers). The injured arm (3 right/7 left) was assessed for participants with a rotator cuff tear and the dominant arm (9 right/1 left) was evaluated for controls. * denotes a significant linear relationship, which indicates that strength production is dependent on three-dimensional muscle volume measures. There are significant relationships for (A) whole muscle volume and the joint moment of each muscle’s primary action (supraspinatus, p=0.005; infraspinatus, p<0.001; subscapularis, p=0.006; teres minor, p=0.001) and for (B) fat-free muscle volume and isometric joint moment (supraspinatus, p=0.005; infraspinatus, p<0.001; subscapularis, p=0.001; teres minor, p=0.001).
Discussion
The results from this study demonstrate that single-image assessments did not capture three-dimensional measures of fatty infiltration or muscle volume. These three- dimensional assessments of muscle morphology indicate that muscle atrophy, not increased fatty infiltration volume, drives the increased fat percentage in these RCT patients. Muscle volume measurements are significant predictors of strength in older adults with and without a RCT, highlighting the need for clinicians to consider the amount of muscle tissue in older RCT patients, as strength capacity is known to have important functional implications.20
This study presents evidence that assessments from a single image are insufficient for estimating the amount of muscle tissue or fatty infiltration in the entire muscle. The Goutallier and Fuchs scores were originally developed to estimate the proportion of fatty infiltration using a single image slice. While this single image has a reliable bony landmark, it does not capture morphological changes at other locations, particularly the musculo-tendinous junction. Fatty infiltration has been shown to be distributed non- uniformly throughout the muscle belly,8,29 challenging the rationale of using a single image to evaluate fatty infiltration.14 Jo and Shin30 showed that new baseline images are needed following surgical repair because surgery moves the muscle and changes its appearance in the MR image. Additionally, the large image slices (e.g. 5mm slices with 1.5mm inter-slice gap12) of clinical scans average signal intensity across the slice thickness, potentially masking valuable morphological information.
The three-dimensional measurements acquired in this study represent a quantitative technique applied to the entire muscle. Recently, Nardo et al.31 quantified fatty infiltration for rotator cuff muscles using MRI, but only 4 image slices of the muscle belly that were within 8mm of the traditional slice were used in their evaluation; three- dimensional muscle volumes were not assessed and important morphological information was likely missed. The three-dimensional measurements of percent fatty infiltration calculated here were less than those traditionally associated with Goutallier scores (e.g. Goutallier score of 3 is defined as 50% fatty infiltration), which is consistent with the findings of Nardo and colleagues. This discrepancy may be due to the tendency of individuals to visually identify image voxels as either muscle or fat.31 Nardo and colleagues reported a significant linear relationship between percent fatty infiltration and Goutallier scores when all rotator cuff muscles were considered together, including muscles graded 3–4. However, they did not identify significant differences between grades 0 and 1 or between grades 1 and 2, which are the grades typically considered for surgical repair.32,33 This suggests that quantitative measurements of fat percentage are not as well correlated among lower Goutallier scores, which is consistent with the results of the current study, where mean Goutallier scores were primarily low scores, but ranged from 0–3. The three-dimensional assessments reported here suggest that important information about the amount of muscle and fat tissue is missed with single image assessment techniques, especially for lower Goutallier stages. These results motivate future work to develop efficient methods to calculate three-dimensional measurements of muscle morphology and to characterize the spatial localization of fatty infiltration within the whole muscle, which is possible using the technique described here.
As in previous reports,12,15–18 inconsistency in assignment of Goutallier scores was observed. It has been suggested that condensing the 5 Goutallier score categories to the 3 Fuchs score categories improves reliability,16 but results from this study do not support that conclusion. The Goutallier score has appeal because it can be assessed quickly in a busy clinical setting, although consistent reports describing a lack of agreement between raters is problematic. Nevertheless, these assessment techniques are applied to identify surgical candidates.5,7,11,14 Single-image assessments may mislead clinicians because they do not account for variation in three-dimensional muscle morphology. Both atrophy and fatty infiltration occur with aging but are separate processes6 and the results of increased muscle atrophy without marked increases in fatty infiltration volume following a RCT support that report. Muscle atrophy without increased fatty infiltration volume may cause fat proportions to appear larger. Development of an efficient and objective method to quantify three-dimensional fat and muscle tissue would improve reliability, supply clinicians with a more comprehensive muscle morphology assessment for older patients, and provide a better foundation to determine treatment.
Similar to previous work,8,12 muscle atrophy was identified in older adults with a RCT. Older adults have reduced upper limb muscle volume compared to healthy young adults,23 and the current study suggests that a RCT may be associated with muscle atrophy exceeding age- associated atrophy. This work demonstrated that three-dimensional muscle volume is an important predictor of strength after a RCT, which is consistent with previous studies in healthy adults.21,23 Strength is a factor in functional ability, and marked strength decreases following RCT may affect the ability of older individuals to successfully perform daily activities necessary to maintain independence1 because of strength losses below the minimum threshold necessary to complete the tasks.20 Conservative treatment for older RCT patients may further compound the muscle atrophy shown here, and arthroscopic repair, which has been shown to be successful in older patients,32–34 should be proactively considered to avoid further atrophy and strength loss.
The RCT group had reduced strength for adduction, flexion, and external rotation. The large inter-subject variability in strength measurements (cf. Figure 5), likely reflects the natural variability inherent to the population. The lack of difference between the RCT and control groups for some strength measures may be due to the large variability or may be the result of age-associated strength decreases20 in the absence of a musculoskeletal injury in the control group. Importantly, there were RCT patients who could not perform flexion and abduction strength trials due to pain. Repeating the analysis to include failed trials (0 Nm) resulted in larger mean differences between groups, but did not change the outcome of the analysis. Although three RCT subjects failed flexion strength assessments due to pain, these measurements were not included in regression analyses because flexion was not the primary movement direction of any rotator cuff muscles.
Limitations
This study had a small sample of 20 subjects, which may be underpowered, and we did not adjust for type I error. While manual segmentation methods are impractical for a busy clinical setting, these results motivate further work to develop efficient techniques to effectively capture three-dimensional muscle information. Although other studies have evaluated changes in muscle atrophy and fatty infiltration following successful and failed surgical repairs,10,11 the objectives of this study were to obtain baseline assessments of muscle morphology and strength in the context of aging. MR images were acquired with one of two scanners. A control group was used rather than the contralateral shoulder because of the increased incidence of an asymptomatic tear on the contralateral side when a symptomatic tear presents.35
Conclusions
Clinical scores using a single image slice do not represent three-dimensional muscle measurements. Efficient methods are needed to more effectively capture three-dimensional information for clinical applications. RCT subjects had increased fatty infiltration percentages likely driven by muscle atrophy rather than increased fat volume. Muscle volume’s significant association with strength production suggests that treatments to preserve muscle volume should be pursued for older RCT patients.
Acknowledgements
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (#F31 AG040921 (MEV)), the Wake Forest University Claude D. Pepper Older Americans Independence Center (#P30 AG021332), the National Science Foundation (#1405246 (KRS)), and the Wake Forest University Center for Biomolecular Imaging and the Wake Forest School of Medicine Translational Science Institute Clinical Research Unit. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Science Foundation, or Wake Forest University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration: We declare that abstracts describing small portions of this manuscript have been presented at the Gait and Clinical Movement Analysis Society Conference, the International Society of Biomechanics Conference, the American Society of Biomechanics Conference, the World Congress of Biomechanics, the Wake Forest Graduate Student and Post-doc Research Symposium, and the Virginia Tech-Wake Forest School of Biomedical Engineering and Sciences Symposium. An abstract describing a portion of this work was awarded 3rd Place (certificate and $80 award to MEV) for Outstanding Podium Presentation by a PhD Student at the 13th Annual Virginia Tech-Wake Forest School of Biomedical Engineering and Sciences Symposium. We declare that author MTF serves as a consultant for Smith and Nephew, it does not represent a conflict of interest and no financial remuneration was received related to the work described in this manuscript. Author CJT has an ownership interest in a medical device measuring tension in rotator cuff tendon repairs with research applications; participation with device development and testing is outside the scope of this manuscript and does not represent a conflict of interest.
References
- 1.Lin JC, Weintraub N, Aragaki DR. Nonsurgical treatment for rotator cuff injury in the elderly. J Am Med Dir Assoc. 2008;9:626–632. doi: 10.1016/j.jamda.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699–1704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 3.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 4.Matsen FA., 3rd Clinical practice. Rotator-cuff failure. N Engl J Med. 2008;358:2138–2147. doi: 10.1056/NEJMcp0800814. [DOI] [PubMed] [Google Scholar]
- 5.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994:78–83. [PubMed] [Google Scholar]
- 6.Barry JJ, Lansdown DA, Cheung S, Feeley BT, Ma CB. The relationship between tear severity, fatty infiltration, and muscle atrophy in the supraspinatus. J Shoulder Elbow Surg. 2013;22:18–25. doi: 10.1016/j.jse.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Mellado JM, Calmet J, Olona M, et al. Surgically repaired massive rotator cuff tears: MRI of tendon integrity, muscle fatty degeneration, and muscle atrophy correlated with intraoperative and clinical findings. AJR Am J Roentgenol. 2005;184:1456–1463. doi: 10.2214/ajr.184.5.01841456. [DOI] [PubMed] [Google Scholar]
- 8.Nakagaki K, Ozaki J, Tomita Y, Tamai S. Alterations in the supraspinatus muscle belly with rotator cuff tearing: Evaluation with magnetic resonance imaging. J Shoulder Elbow Surg. 1994;3:88–93. doi: 10.1016/S1058-2746(09)80115-8. [DOI] [PubMed] [Google Scholar]
- 9.Gumucio JP, Korn MA, Saripalli AL, et al. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. 2013 doi: 10.1016/j.jse.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007;16:691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 11.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 13.Zanetti M, Gerber C, Hodler J. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Invest Radiol. 1998;33:163–170. doi: 10.1097/00004424-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kuzel BR, Grindel S, Papandrea R, Ziegler D. Fatty Infiltration and Rotator Cuff Atrophy. J Am Acad Orthop Surg. 2013;21:613–623. doi: 10.5435/JAAOS-21-10-613. [DOI] [PubMed] [Google Scholar]
- 15.Lippe J, Spang JT, Leger RR, Arciero RA, Mazzocca AD, Shea KP. Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy. 2012;28:154–159. doi: 10.1016/j.arthro.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468:1558–1564. doi: 10.1007/s11999-009-0818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer EE, Jr, Dunn WR, Wright RW, et al. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging. Am J Sports Med. 2008;36:99–103. doi: 10.1177/0363546507307504. [DOI] [PubMed] [Google Scholar]
- 18.Khazzam M, Kuhn JE, Mulligan E, et al. Magnetic resonance imaging identification of rotator cuff retears after repair: interobserver and intraobserver agreement. Am J Sports Med. 2012;40:1722–1727. doi: 10.1177/0363546512449424. [DOI] [PubMed] [Google Scholar]
- 19.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 20.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 21.Holzbaur KR, Delp SL, Gold GE, Murray WM. Moment-generating capacity of upper limb muscles in healthy adults. J Biomech. 2007;40:2442–2449. doi: 10.1016/j.jbiomech.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Holzbaur KR, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. J Biomech. 2007;40:742–749. doi: 10.1016/j.jbiomech.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Vidt ME, Daly M, Miller ME, Davis CC, Marsh AP, Saul KR. Characterizing upper limb muscle volume and strength in older adults: a comparison with young adults. J Biomech. 2012;45:334–341. doi: 10.1016/j.jbiomech.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillooly JJ, Chidambaram R, Mok D. The lateral Jobe test: A more reliable method of diagnosing rotator cuff tears. Int J Shoulder Surg. 2010;4:41–43. doi: 10.4103/0973-6042.70822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 26.Glover GH. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging. 1991;1:521–530. doi: 10.1002/jmri.1880010504. [DOI] [PubMed] [Google Scholar]
- 27.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343. [DOI] [PubMed] [Google Scholar]
- 28.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 29.Beeler S, Ek ET, Gerber C. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg. 2013;22:1537–1546. doi: 10.1016/j.jse.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Jo CH, Shin JS. Changes in appearance of Fatty infiltration and muscle atrophy of rotator cuff muscles on magnetic resonance imaging after rotator cuff repair: establishing new time-zero traits. Arthroscopy. 2013;29:449–458. doi: 10.1016/j.arthro.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Nardo L, Karampinos DC, Lansdown DA, et al. Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. Journal of Magnetic Resonance Imaging. 2014;39:1178–1185. doi: 10.1002/jmri.24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charousset C, Bellaiche L, Kalra K, Petrover D. Arthroscopic repair of full-thickness rotator cuff tears: is there tendon healing in patients aged 65 years or older? Arthroscopy. 2010;26:302–309. doi: 10.1016/j.arthro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 33.Verma NN, Bhatia S, Baker CL, 3rd, et al. Outcomes of arthroscopic rotator cuff repair in patients aged 70 years or older. Arthroscopy. 2010;26:1273–1280. doi: 10.1016/j.arthro.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Moraiti C, Valle P, Maqdes A, et al. Comparison of functional gains after arthroscopic rotator cuff repair in patients over 70 years of age versus patients under 50 years of age: a prospective multicenter study. Arthroscopy. 2015;31:184–190. doi: 10.1016/j.arthro.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Liem D, Buschmann VE, Schmidt C, et al. The prevalence of rotator cuff tears: is the contralateral shoulder at risk? Am J Sports Med. 2014;42:826–830. doi: 10.1177/0363546513519324. [DOI] [PubMed] [Google Scholar]