Abstract
This study describes a novel phage display method based on an iterative subtraction strategy to identify candidate vaccine antigens of Brugia malayi. A cDNA library of the infective larval stage of B. malayi expressed on the surface of T7 phage was sequentially screened with sera samples from human subjects showing different manifestations of the disease. Antigens that selectively and specifically bind to immune sera were then enriched using a multi-step panning procedure. This strategy identified five antigens, four of which were previously reported (ALT-2, TPX-2, VAH and COX-2) and the other one was a novel cuticular collagen (Col-4). Sera from immune individuals specifically recognized all the five antigens. However, ALT-2 appeared to be the most predominantly recognized antigen by the immune sera. Therefore, it was decided to evaluate the vaccine potential of recombinant ALT-2 (rALT-2) in a mouse and jird model. The results presented show that immunization with rALT-2 conferred over 73% protection against a challenge infection in the jird model and over 64% protection in the mouse model. The present study suggests that phage display-based cDNA screening may be a powerful tool to identify candidate vaccine antigens of infectious agents.
The technique of displaying peptides or proteins on the surface of bacteriophages is an approach to isolate genes that code for specific proteins of interest by screening appropriate ligands (44). One of the unique characteristics of displaying proteins on the surface of phage is the physical linkage between displayed protein and the genetic material that codes for it (7). Thus, the genes that code for the displayed protein can be easily cloned from phages that express even picomolar quantities of proteins (6).
Large repertoires of phage display libraries are now routinely used to isolate specific antibodies against targeted antigens. Conversely, random peptide phage libraries could also be used to identify linear epitopes or part of a larger antigenic determinant of infectious agents (15) and to identify new B-cell epitopes using serum antibodies that are important in autoimmune diseases (10). Folgori et al. (11) used this approach to screen a random peptide library with human sera containing antibodies against hepatitis B virus and identified mimotopes that elicited antigen-specific immune responses in mice. Another area that is rapidly developing is the screening of phage-displaying cDNA libraries of cancer cells using sera from cancer patients to identify potential antigens (44, 46). These reports suggest that the phage display technique may have great potential in identifying candidate antigens that are important in immunization or vaccine development.
Lymphatic filariasis caused by filarial parasites is a debilitating disease affecting over 120 million people in the tropical and sub-tropical countries (32). The causative agents of this condition are Brugia malayi and Wuchereria bancrofti. These two parasites are closely related and hence show significant antigenic overlap. Thus, antibodies generated against W. bancrofti in infected or immune individuals show significant cross reactivity with B. malayi antigens. In the areas of endemicity, some individuals are naturally immune to the disease (endemic normal; EN), whereas some carry the infection (microfilaremics; MF) and others exhibit chronic pathology (CP). Although the nature of protective immune responses is highly debated (38, 34), the general consensus is that the host immune responses play a major role in determining clinical manifestations of various groups (20, 13). In this respect, subjects in the “EN” group, which are constantly exposed to the infection without showing any symptoms of parasitemia (20, 13), are probably the most interesting group to study since they may carry circulating protective antibodies. If there are protective antibodies, it is important to identify unique antigens that induce their production. Therefore, in this study we used a phage display-based method to identify antigens recognized by sera of EN individuals.
Strategies to identify candidate vaccine antigens against Brugia or bancroftian filariasis have relied largely on screening expression libraries with immune sera (13), differential screening of abundantly expressed mRNAs (51, 18) or by the EST sequencing approach (3). Using these approaches several potential vaccine candidates have been identified and reported to have varying degrees of protection in animal models (1, 20, 24, 28, 29, 33, 37, 50). Although there are effective drugs available for the control of filariasis, developing a vaccine remains a promising strategy for mass control of this mosquito-borne infection in areas of endemicity (24, 29, 28, 50).
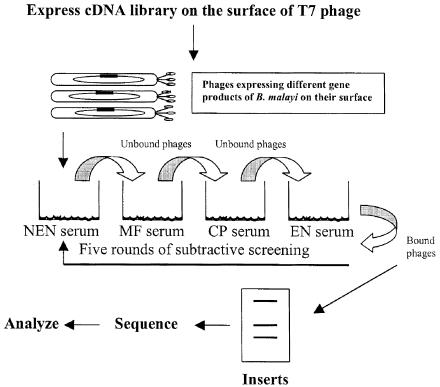
In this study, we used a phage-display based subtractive screening strategy (Fig. 1) to screen the cDNA library of B. malayi third stage larvae (L3) with serum from EN individuals. We hypothesized that this approach would identify novel antigens and/or antigens that have been described previously. As expected, our analysis identified five antigens, one of which was a novel antigen and four of which have been reported previously. We then selected the most abundantly recognized antigen by the immune sera and evaluated its immunization potential in a mouse and a jird infection model.
FIG. 1.
Schematic representation of phage display-based subtractive screening strategy to identify vaccine antigens from a cDNA library of B. malayi using sera samples from subjects showing various manifestations of the disease.
MATERIALS AND METHODS
Sera.
All serum samples used in this study were obtained from volunteers at Chennai, India. Reasons for collecting the blood in the night and the significance of the research were explained to the volunteers in local language. Informed consent was obtained from all patients in accordance with U.S. Department of Health and Human Services Human Experimentation Guidelines and Department of Public Health, Chennai, Tamil Nadu, India. All the procedures followed were in accordance with the guidelines issued by Department of Public Health, Government of Tamil Nadu, India for dealing with human subjects. The Institutional review board at the Center for Biotechnology, Anna University, India and College of Medicine at Rockford also approved the protocols.
Sera were classified into MF, CP or EN based on the detection of circulating parasites, parasite antigens or by evaluating clinical symptoms of lymphatic filariasis. Circulating microfilariae were detected in the blood of subjects as described previously (36). The presence of circulating antigen was detected using an Og4C3 kit (26) and a WbSXP-based enzyme-linked immunosorbent assay (ELISA) (36). Subjects with no circulating antigen or microfilariae were classified as EN, whereas subjects with circulating microfilariae and/or circulating antigen, as detected by ELISA, were considered as MF. Subjects showing lymphedema and other visible clinical symptoms of filariasis were grouped into CP. Control non-endemic normal (NEN) sera were obtained from Thomas Nutman (Laboratory of Parasitic Diseases, NIAID, NIH, Bethesda, Md.) or were collected at the University of Illinois Clinic at Rockford, Ill.
Display of B. malayi gene products on T7 bacteriophages.
A Uni-ZAP XR cDNA library of the infective L3 of B. malayi was obtained from Filarial Genome Project Resource Center, Smith College, Northampton, Mass. This library was used as a template for generation of cDNA by PCR amplification using primers flanking the cDNA inserts. PCR parameters were 95°C of denaturation for 30 s, 55°C of primer annealing for 30 s, 72°C of primer extension for 3 min and cycled for 30 cycles. A final extension of 5 min was performed at 72°C before storing the samples at 4°C. PCR was performed with PfuTurbo DNA polymerase (Stratagene, La Jolla, Calif.) to enhance the fidelity, sensitivity and yield of PCR product. The forward primer for PCR was T3 primer with sequence 5′AATTAACCCTCACTAAAGGG3′ whereas the reverse primer was T7 promoter primer with sequence 5′GAAATACGACTCACTATAGGG 3′. PCR products were purified using the QIAquick PCR Purification kit (Qiagen, Valencia, Calif.) and size fractionated to select products of >300 bp length, using CHROMA SPIN columns (Clontech, Palo Alto, Calif.). The PCR products were digested with EcoRI and HindIII enzymes and ligated to similarly digested phage display vector T7Select 1-1 cloning system (Novagen, Madison, Wisc.). The library was packaged in vitro, titered and amplified as per the procedures outlined in T7Select system. Length and frequency of insertion was verified by PCR amplification of randomly selected clones.
Biopanning.
The strategy used for biopanning the cDNA library of B. malayi, to select EN specific clones that display B. malayi gene products on the surface of T7 bacteriophage, is shown in Fig. 1. Wells of a high binding microtiter plate (Pierce Chemicals, Rockford, Ill.) was coated overnight at 4°C with 1:100 dilutions of a pooled serum sample (from 10 individuals) from NEN, MF, CP or EN individuals. After washing the wells with phosphate buffered saline containing 0.1% Tween 20 (PBST), nonspecific sites were blocked with 5% bovine serum albumin (BSA) for 1 h at 37°C. For iterative screening, 100 μl of T7Select library (containing 1011 PFU/ml) was first added to wells coated with NEN sera and incubated for 1 h at room temperature. Unbound phages were removed from the wells and transferred to wells coated with CP sera. After 1 h of incubation at room temperature, unbound phages were removed and transferred to wells coated with MF sera and incubated for 1 h at room temperature. Unbound phages were again removed from the wells and transferred to wells coated with EN sera. After a final incubation for 1 h at room temperature, the unbound phages were discarded this time by washing the wells five times with PBST. The bound phages were then eluted with 200 μl of T7 elution buffer (TBS in 1% sodium dodecyl sulfate [SDS]) and amplified by infecting Escherichia coli host BLT5403. The amplified phages were then subjected to another three rounds of selective screening as above to enrich the clones that are highly specific for EN sera.
Sequencing and analysis.
After four rounds of biopanning, the final enriched EN specific clones were plated and single pure plaques were isolated. The cDNA inserts in these plaques were amplified by PCR using primers flanking the inserts. PCR primers were T7SelectUP with sequence of 5′GGAGCTGTCGTATTCCAGTC3′ and T7SelectDown primer 5′AACCCCTCAAGACCCGTTTA3′. PCR conditions were denaturation for 1 min at 94°C, primer annealing for 1 min at 50°C and primer extension for 1 min at 72°C for a total of 30 cycles. QIAquick columns (Qiagen) were then used to purify the PCR products after a final extension for 5 min at 72°C. Ends of the amplified PCR products were converted into blunt ends and cloned into pST Blue-1 Vector (Novagen). The nucleotide sequence of selected cDNA inserts in pST-Blue was determined at the DNA core facility of the University of Illinois Chicago. Sequences were analyzed by BLAST (www.ncbi.nlm.nih.gov) searches with the GenBank database. Further analysis by multiple sequence alignment was performed using the Clustalw (www.ebi.ac.uk) program.
Plaque lift hybridization analysis.
To determine the abundance and frequency of the clones, a plaque lift hybridization analysis was performed. Briefly, DNA probes were prepared by PCR amplifying the genes using insert specific primers and labeling with horseradish peroxidase (HRP) (Amersham). EN specific plaques were plated at a dilution of 10−6 and the plaques were transferred to five different nitrocellulose nylon membrane (Hybond N+) discs (Amersham) by replica plating for 5 min. The blots were then denatured on filter papers saturated with 0.5 M NaOH and rinsed twice with 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Following this the blots were transferred to hybridization buffer containing the probes and hybridized for 4 h at 42°C. After completion of the hybridization, blots were washed twice with primary wash buffer (6 M urea, 0.4% SDS and 0.5× SSC) at 42°C for 20 min followed by two secondary washes (2× SSC) at room temperature for 5 min. An ECL kit was then used to detect the hybridization signals (Amersham). Spots in each blot were then counted using SigmaScan Pro software and later confirmed by manual counting.
Serological assays.
An ELISA was performed using sera samples collected from individuals living in an area of endemicity to determine the levels of circulating total immunoglobulin G (IgG) antibodies against phage-displayed antigens. Serum samples were collected from the MF, CP, or EN group (24 subjects each) and compared with a group of NEN sera collected previously. All the analyses were performed in a double-blinded fashion. Briefly, 1 μg of the respective phage-displayed antigens suspended in 100 μl of coating buffer (NaHCO3/Na2CO3, 0.067 M [pH 9.6]) were added to the wells of a 96-well plate and incubated overnight at 4°C. After washing the wells four times with PBST, 100 μl of 3% BSA was added to block nonspecific sites and incubated for a further 2 h at 37°C. After washing the wells, serum samples were added (1:100 dilutions) to the wells and incubated for 2 h at 37°C. The wells were washed again four times with PBST and 100 μl of alkaline phosphatase labeled goat antihuman IgG (Sigma, St. Louis, Mo.) was added. Following incubation for one hour at 37°C color was developed using pNPP (P-nitrophenyl phosphate) substrate (1 mg/ml) in substrate buffer (100 mM Tris-Cl, pH 9.5, 100 mM NaCl, 5 mM MgCl2) and 100 μl of 3 N NaOH was added to the wells to stop the reaction. Absorbance was measured at 405 nm in a microplate reader. Antigen-specific IgG isotypes of antibodies were determined using kits purchased from Sigma. The protocol used in these ELISAs was the same as described above.
Expression and purification of recombinant ALT-2.
A recombinant construct of BmALT-2 in T7 expression vector was maintained in XL-1 Blue (Stratagene). For expression, the recombinant plasmid was transformed into BL21(DE3) pLysS (Invitrogen, Carlsabad, Calif.) to minimize toxicity due to the protein. When the cultures reached an optical density of 0.7 at 600 nm, 1 mM isopropyl-1-thio-β-d-galactopyranoside was added to the cultures to induce gene expression, and the cultures were incubated for an additional 3 hrs. Total proteins were separated in SDS-12% polyacrylamide gel electrophoresis, and the presence of histidine-tagged protein was confirmed using an anti-Xpress antibody (Invitrogen). Subsequently, the histidine-tagged recombinant proteins were purified using a TALON metal affinity resin (Clontech) as per the manufacturer's recommendations. The purified rBmALT-2 was passed through a polymyxin B-agarose column (Detoxi-gel; Pierce) to eliminate endotoxin (if any) in the preparation before use in protection studies. Analysis of the final purified rBmALT-2 protein by LAL assay confirmed that the endotoxin levels were comparable to background levels (1 ng/ml).
Immunization and challenge protection studies.
Male outbred Mangolian gerbils (jirds) weighing 35 to 40 g or male BALB/c mice weighing 15 to 20 g were purchased from Charles River Laboratories (Wilmington, Mass.), and five mice or jirds each were used per group. For immunization, mice or jirds were injected intraperitoneally with 10 μg of endotoxin-free rALT-2 protein (single band at 14 kDa) in Imject Alum (Pierce) followed by two booster doses at 2-week intervals. Control animals received equal amounts of adjuvants alone. Serum samples were collected from tail vein every 2 weeks to determine the titer of anti-ALT2 antibodies using an ELISA. Group of mice or jirds injected similarly with the adjuvant alone remained as negative controls.
Because the parasites do not develop into adults in mice, we used a micropore chamber method to determine the degree of protection conferred after immunization with ALT-2. Briefly, two weeks after the last booster dose, a micropore chamber (Millipore Corporations, Bedford, MA) containing 20 L3 of B. malayi was implanted into the peritoneal cavity of each mouse. The micropore chamber consisted of a Plexiglas ring (diameter, 13 mm; catalog no. PR0001401; Millipore) covered on either side with polycarbonate membranes (5-μm pore size). Chambers were sealed with cement (Millipore) after introduction of the larvae and checked for leakage. Strict aseptic conditions were followed for the surgical procedure. At 48 h after implantation, animals were sacrificed and the chambers were removed carefully from the peritoneum. Numbers of live and dead larvae in each chamber were then determined under a Nikon inverted microscope.
Jirds are permissive hosts for B. malayi. Therefore, the degree of protection in jirds immunized with ALT-2 was evaluated by calculating the percentage of adult worms established after a challenge infection. Jirds were infected intraperitoneally with 50 L3 of B. malayi and 68 days after infection, the number of adults worms present in the peritoneum was counted. Percent reduction in worm establishment was calculated using the following formula: average number of worms recovered from the control animals minus average number of worms recovered from the vaccinated animals ÷ average number of worms recovered from the control animals × 100.
Collection of peritoneal fluids.
After removing the micropore chamber, the peritoneal cavity of mice was washed with 5 ml of cold sterile phosphate-buffered saline. The fluid was centrifuged at 200 × g to remove the cells and supernatant was stored at −70°C. There was minimal contamination of blood in these wash fluids as determined by the presence of erythrocytes. Presence and titer of rBmALT-2 specific antibodies in the peritoneal wash fluid was then determined by an immunoblot analysis.
L3 worm homogenate and immunoblot analyses.
Approximately 1,000 L3 of B. malayi were homogenized using a glass tissue homogenizer and soluble proteins in the homogenate were collected by centrifugation (500 × g for 15 min). Reactivity of serum and peritoneal fluid samples to ALT-2 antigen in the worm homogenate and rALT-2 was determined using an immunoblot analysis. HRP-labeled rabbit anti-mouse IgG was used to detect both mouse and jird antibodies.
Statistical analysis.
Data from various clinical groups were compared using a Kruskal-Wallis one-way analysis of variance on ranks using the SigmaStat program (Jandel Scientific, San Rafel, Calif.). Data from protection studies were analyzed using a chi-square test and P values below 0.05 were considered statistically significant.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the genes identified in this study are as follows: (i) for B. malayi cuticular collagen (BmCol-4), AY347274; (ii) for B. malayi cytochrome c oxidase subunit 2 (BmCox-2), AY380811.
RESULTS
Display of the cDNA library of B. malayi on T7 bacteriophages.
Based on PFU after in vitro packaging it was calculated that the T7 phage display library of B. malayi L3 contained 3 × 108 independent clones. Amplification of inserts in randomly selected clones revealed that the library contained >90% recombinants with average insert size of >500 bp (data not shown). Taking into account the three possible reading frames and assuming that there are about 30,000 expressed genes in B. malayi, the number of clones required to achieve 90% probability would be 105 (40). Since the size of the generated phage display library exceeds the statistically required number it is highly likely that most of the expressed genes would be represented in this library.
Affinity selection of specific genes recognized by EN sera.
Major objective of this strategy was to enrich clones with high specificity to putative protective EN sera. Because of the diversity of the sera and the possibility of the presence of cross- reactive antibodies in the sera that could recognize the nonspecific clones we sought to subtract the phagotopes (phages expressing antigens) by first panning against sera from NEN, ME, and CP to remove all the nonspecific cross-reactive clones. This negative selection step was followed by a positive selection step to enrich clones that are specific to EN sera. The entire screening process was then repeated for another four rounds (Fig. 1). After each round of panning there was an increase in the number of clones, suggesting that the procedure did enrich EN-specific clones (Table 1). By the end of the fourth round of panning there was a 200-fold increase in EN specific clones compared to that obtained after the first round. Further rounds of panning did not increase the enrichment.
TABLE 1.
Selection and enrichment of EN serum specific clones by biopanning and subtractive screening of a phage display cDNA expression library of B. malayi
| Round of panning | Phage applied (PFU/ml) | Phage eluted (PFU/ml) | Enrichment (fold) |
|---|---|---|---|
| 1 | 1011 | 1 × 106 | 1 × 10−5 |
| 2 | 1011 | 5 × 106 | 5 × 10−5 |
| 3 | 1011 | 1 × 108 | 1 × 10−3 |
| 4 | 1011 | 2 × 108 | 2 × 10−3 |
| 5 | 1011 | 2 × 108 | 2 × 10−3 |
Sequence analysis of the EN-specific clones to identify the antigens.
About 90 clones were then randomly picked from individual plaques, and their DNA sequences were PCR amplified and analyzed on agarose gel to determine the size of the inserts. PCR analysis showed that 33% of the phage clones had an insert size of ∼210 bp, 30% of the phage clones had an insert size of ∼387 bp, 26% of the phage clones had an insert size of ∼400 bp, 6% of clones had an insert size of ∼129 bp and 5% of clones had an insert size of ∼198 bp. We then determined the sequences of five of these inserts.
Sequence analysis showed that four of the sequences were homologues of B. malayi abundant larval transcript-2 (ALT-2, 210 bp), thioredoxin peroxidase-2 (TPX-2, 400 bp), bee venom allergen homologue (VAH, 387 bp), and B. malayi cytochrome oxidase subunit 2 (BmCox-2, 150 bp). However, one of the clones, B. malayi cuticular collagen (BmCol-4, 129 bp), appeared to be novel. All the five clones were in the correct open reading frame (data not shown).
The cDNA clone of TPX-2 isolated by affinity panning encoded the N-terminal 133-amino-acid (aa) peptide of the putative full length TPX-2 homologue of B. malayi (accession no. [Acc.]: BMU47100) that has a total length of 200 aa. The cDNA clone of VAH isolated by affinity panning encoded the N-terminal 111 aa peptide of the putative full length VAH homologue of B. malayi (Acc: AF042088) that has a total length of 221 aa. The cDNA clone that corresponded to ALT-2 was of 99 aa long, similar to that of BmALT-2 (Acc: BMU84723). The cDNA clones BmCol-4 (Acc: AY347274) and BmCox-2 (Acc: AY380811) isolated by affinity panning were present as full-length genes.
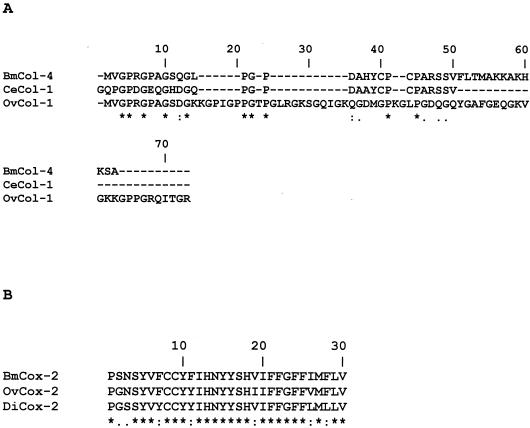
Multiple alignment of ALT-2 sequence homologues from W. bancrofti, Dirofilaria immitis and Onchocerca volvulus displayed 87, 60, and 46% homologies, respectively, whereas with TPX-2 the homologies ranged from 98% with Litmosoides sigmondoitis and D. immitis and 77% with O. volvulus and Ascaris suum. VAH showed 20% homology with the allergen from Diaulinopsis arenaria and 51 to 100% homology with other filariid worms. BmCol-4 showed 24% homology to Ovcol-1 and 70% homology to the c-terminal region of C. elegans cuticular collagen and BmCox-2 showed 87% homology to O. volvulus Cox-2 and 80% homology to D. immitis Cox-2 (Fig. 2).
FIG. 2.
(A) Multiple alignments (CLUSTAL) of the amino acid sequences of the novel cuticular collagen from Brugia malayi (BmCol-4, accession no. AY347274), Caenorhabditis elegans (CeCol-1, accession no. P34391), and Onchocerca volvulus (OvCol-1, accession no. Z69668). (B) Multiple alignments of the amino acid sequences of cytochrome c oxidase subunit 2 from B. malayi (BmCox-2, accession no. AY380811), from O. volvulus (OvCox-2, accession no. NP_008377), and from D. immitis (DiCox-2, accession no. NP_954724). The amino acid positions are numbered above the amino acid sequences.
BmALT-2 has no human homolog, whereas BmTPX-2 is 65% homologous to human thioredoxine peroxidase and BmVAH is 31% homologous to testis specific proteins and 29% homologous to cysteine-rich secretory protein-3 of human. Similarly, BmCox-2 showed 40% homology to the human Cox-2 proteins.
Frequency of individual antigens among EN-specific clones.
We then evaluated the frequency of these five antigens among the EN-specific phage plaques using a plaque lift hybridization analysis. Out of the total 1,219 EN-specific plaques analyzed, ALT-2 probe hybridized to 488 ± 35 plaques (∼40%), VAH probe hybridized to 270 ± 57 plaques (∼22%), TPX-2 probe hybridized to 263 ± 33 plaques (∼21%), BmCol-4 probe hybridized to 72 ± 2 plaques (∼6%) and BmCox-2 probe hybridized to 60 ± 1 plaques (∼5%). Thus, both the random PCR screening (∼89%) and the plaque lift hybridization analysis (∼83%) showed that the majority of the EN specific clones were of ALT-2, VAH, and TPX-2. Because of the low frequency of BmCol-4 and BmCox-2 clones, we did not further pursue these two proteins in this study.
Specificity of EN clones.
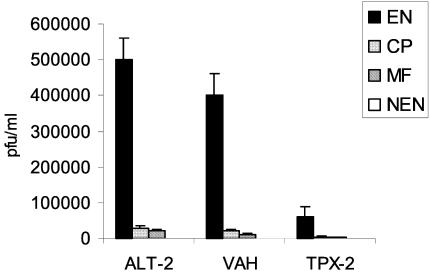
In order to confirm the specificity of the recombinant antigens displayed on the surface of phage, the amplified phages from individual clones were then analyzed for their reactivity to pooled sera from NEN, MF, CP, or EN. These studies showed that the clones were more highly specific (P < 0.01) to EN sera than MF, CP or NEN sera (Fig. 3).
FIG. 3.
Preferential binding of selected phage clones to EN sera. Microtiter plates were first coated with pooled sera (n = 10) from EN, MF, CP, or NEN subjects. Subsequently, 108 clones displaying ALT-2, TPX-2, or VAH were incubated and washed, and bound phage were eluted. The numbers of bound phages were calculated by determination of titers and plotted on the y axis. Results demonstrate that recombinant phage preferentially bound to EN sera. Data presented are representative of one of five similar experiments. *, significant (P < 0.001) compared to NEN subjects.
Serological reactivity of selected clones in ELISA.
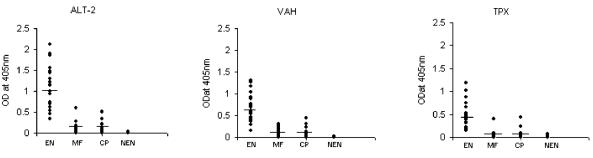
In order to further confirm the specificity of proteins expressed on the surface of EN specific phage clones, we tested their reactivity using a panel of fresh human serum samples obtained from an area of endemicity in India. This was a double-blinded study and consisted of 24 EN subjects, 24 CP patients, 24 MF patients, and 24 NEN subjects. These studies also confirmed that the three phage clones selected by the affinity selection were highly specific for sera from EN subjects (Fig. 4).
FIG. 4.
Specificity of the recombinant antigens displayed on the surface of T7 phage. Phages displaying ALT-2, TPX-2, or VAH were coated onto a microtiter plate and their reactivity to a panel of sera samples collected from EN (n = 24), CP (n = 24), MF (n = 24), or NEN (n = 24) subjects was evaluated using an ELISA. Each spot on the scatter plot represent an individual. Data presented are representative of one of three similar experiments using 10 to 24 patient sera.
Isotype of anti-ALT-2 IgG antibodies.
Majority of the IgG responses in EN subjects appeared to be towards ALT-2. Previous studies with BmALT-1 (a homologue of BmALT-2 antigen) showed that the EN normal individuals carry predominantly IgG1 and IgG3 isotypes of antibodies Therefore, to test whether BmALT-2 also elicits a similar response, we analyzed the isotypes of anti-BmALT-2 IgG antibodies (IgG1, IgG2, IgG3, and IgG4) in the serum samples of EN individuals. These studies showed that nearly all EN subjects had significantly higher levels of circulating IgG1 and IgG3 antibodies against BmALT-2 in their sera compared to MF, CP or NEN samples. Levels of IgG2 were slightly increased in EN subjects but were not statistically significant. Similarly, levels of anti-ALT-2 IgG4 antibodies were barely detectable in all the samples tested.
Effect of immunization with ALT-2 on worm establishment.
Over 40% of the EN-specific clones expressed BmALT-2. Therefore, in these studies we decided to test the vaccine potential of ALT-2 in mice and jirds. Recombinant BmALT-2 was purified to >99% purity by immobilized metal affinity chromatography and endotoxin free purified recombinant BmALT-2 was used to immunize mice and jirds. Following immunization, blood samples were collected at 2-week intervals to determine the titer of ALT-2 antibodies. These studies showed that the antibody titer peaked (1:8,000) at 8 weeks after immunization in jirds and reached a titer of 1:4,000 at 6 weeks in mice.
At 8 weeks after immunization, 20 L3 of B. malayi were introduced into the peritoneal cavity of mice in a micropore chamber. Examination of the micropore chamber contents at 48 h after implantation showed that only 6.4 ± 1.3 worms were alive in mice immunized with ALT-2 compared to the control group, which had 18 ± 1.5 worms alive. Thus, there was a 64.4% reduction in worm survival in rBmALT-2 immunized animals (P < 0.0001) compared to adjuvant controls. Several of the dead worms recovered from the micropore chambers were surrounded by a mass of infiltrated cells. Similarly, jirds immunized with rBmALT-2 and challenged 8 weeks postimmunization with 50 L3 had significantly fewer worms (5 ± 2) in their peritoneal cavity on day 68 compared to control jirds (19 ± 8). Percentage of worm reduction in jirds was 73.7%.
Analysis of the peritoneal fluid and sera from immunized mice showed the presence of BmALT-2 specific antibodies (Fig. 5). The titer of anti-BmALT-2 antibodies in the peritoneal fluid of mice was 1:100. These antibodies in the sera and peritoneal fluids recognized a single band at 14 kDa in the L3 worm homogenates (Fig. 5). Similarly, serum samples collected from jirds immunized with ALT-2 also recognized the rBmALT-2 protein (Fig. 5). Purified rBmALT-2 was shown to form oligomers in solution and these oligomers, especially the forms at 14 and 24 kDa, were highly recognized by both serum and peritoneal fluids (Fig. 5). Since we did not have access to good antijird antibodies we had to use an anti-mouse cross-reacting antibody to detect jird antibodies. When serum from control jirds was used there was no reactivity, suggesting that the vaccination induced ALT-2-specific antibodies in jirds (Fig. 5).
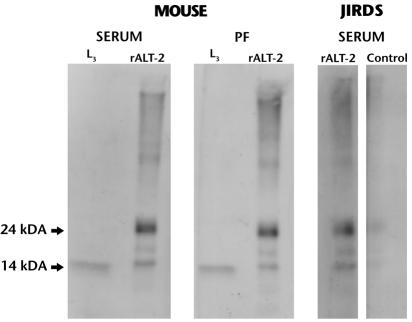
FIG. 5.
Antibody responses in the serum and peritoneal fluids (PF) of mice and jirds immunized with rBmALT-2. Samples were collected 6 weeks after immunization from mice and 8 weeks after immunization from jirds. Reactivities of the antibodies against L3 homogenate (L3) and rBmALT-2 (rALT-2) were determined by an immunoblot analysis. The antibodies recognized a single band around 14 kDa in the L3 worm homogenate and mainly two bands (oligomers) at 14 and 24 kDa of the rBmALT-2. Data are representative of two experiments with similar results.
DISCUSSION
In the present study, we have developed an iterative immunoscreening method to identify putative protective antigens of B. malayi expressed on the surface of T7 bacteriophage. To our knowledge this is the first study that demonstrates the potential use of a phage display-based cDNA library and iterative screening to identify vaccine candidate antigens from filarial parasites. Phage display selection strategies mainly depend on the physical link between DNA encoding displayed protein ligands. In the present study, we used a T7 bacteriophage system to display B. malayi antigens as fusion to the capsid protein. The T7 display system was selected because of its several advantages over the filamentous phage display system. Although several peptides (9) and antibodies (2) have been successfully displayed on the surface of filamentous phage as N-terminal fusions to gene III or gene VIII, display of cDNA libraries on the surface of filamentous phage has been limited because of the presence of potential translational stop codons (4, 21). Some of these problems were subsequently circumvented by introducing modifications to the filamentous phage expression system such as development of expression libraries with fusions to gene VI protein (22, 45) or addition of a Jun-fos Leucine zipper interaction (8). Despite these modifications there are still some major limitations to this system, which include poor expression of gene VI and/or low-efficiency transformation of large cDNA libraries (14). In this respect the high-efficiency in vitro packaging of T7 bacteriophage with cDNA facilitates the construction of large-size libraries (47). The fact that T7 bacteriophage can grow faster and can withstand harsh elution conditions makes it an ideal choice for biopanning. Furthermore, proteins displayed on the surface of T7 phage do not have to be a secretory protein (47). In our hands, we were able to display a cDNA library of B. malayi L3 containing 3 × 108 independent clones on the surface of T7 phage. Over 90% of these clones had inserts with size above 500 bp. Since the size of the phage display library generated was three log above the estimated number (∼26,215) of expressed genes in B. malayi, it is possible that most of the expressed genes would be represented in the T7 expression library that we constructed (40).
An earlier attempt to subtract phage clones that reacted with normal sera was unsuccessful (30). It is possible that selection of phagotopes using sera depends largely on the frequency with which they are recognized and their affinity for different patients sera. Furthermore, it is possible that the negative selection followed by the positive selection and the three amplification steps in our immunoscreening procedure helped the selective amplification of specific clones. All the five clones were in the right reading frame, indicating that phage display screening identified only the right clones (data not shown). An ELISA using sera samples collected from an area of endemicity further confirmed that only sera from EN individuals recognized the three antigens (ALT-2, TPX-2, and VAH) identified by iterative screening. We did not test the other two antigens in this study. Isotype analysis suggested that the majority of the anti-ALT2 antibodies in EN subjects were of IgG1 and IgG3 isotype with very little IgG4. These findings were similar to those reported by Gregory et al. (18) for ALT-1 in EN subjects from Indonesia. Thus, our studies also confirmed the previous observation that these larval antigens apparently elicit an IgG1 or IgG3 protective response, whereas adult and microfilarial antigens predominantly elicit an IgG4 response.
The ALT family of proteins was first identified in Dirofilaria immitis, a dog parasite, by their reactivity with immune sera (12). Subsequently ALT homologues were identified in Onchocerca volvulus (23) and B. malayi (18). BmALT exists as ALT-1 or ALT-2 and in terms of weight per gram of protein per worm ALT-2 is more abundant than ALT-1. Biological functions of these ALT proteins are not known but are localized to the esophageal glands of the parasite. ALT-1 and ALT-2 proteins share 79% identity and cross-reactivity (18). Although EN and MF individuals carry antibodies against BmALT-1, significant protection (∼70%) is achieved against B. malayi in jirds after immunization with BmALT-1 (18). A similar immunization with OvAlt-1, however, conferred only partial protection (23). Interestingly, both ALT-1 and ALT-2 genes are abundantly expressed in B. malayi (18), yet the biopanning procedure seemed to have identified only the ALT-2 clones. This may be because the negative selection procedure in our biopanning must have eliminated all the cross-reactive clones including ALT-1.
Thioredoxin peroxidase family of genes code for antioxidant enzymes that are involved in the detoxification of host products or immune effector cells. TPX genes have been cloned from B. malayi (16), O. volvulus (3), Schistosoma mansoni (52), D. immitis (25), and Plasmodium falciparum (35). Studies using TPX of D. immitis suggest that in addition to its antioxidant property D. immitis TPX may be a protective antigen (25). Therefore, the finding that the EN individuals carry circulating antibodies against BmTPX-2 might be significantly relevant for the development of a protective response against B. malayi in human.
VAH (ASP) was initially cloned from secretions of hookworm Ancylostoma caninum (19) and subsequently identified in B. malayi (31) and O. volvulus larval secretions. Functional analysis shows that VAH can induce angiogenesis (49). Immune animals develop strong antibodies against VAH and protection studies in A. caninum (17, 43) and O. volvulus suggest that VAH is a potential vaccine candidate. Similarly, BmVAH (VAL-1) has also been found to give partial protection in jirds against challenge infection (31). Interestingly, over 95% of microfilaraemic individuals were seropositive for BmVAL-1 antibodies. At present, we do not know why only sera from EN individuals in our study recognized BmVAH clones.
Similarly, the EN sera selectively recognized BmCol-4. These findings were similar to those reported by Stewart et al. (48) for OvCol-1, where IgG3 antibodies in the sera of putative immune individuals appeared to significantly recognize OvCol-1. Collagen is a major component of the parasite cuticle protecting the internal structures of the parasite from adverse conditions of the host environment. Furthermore, cuticular collagen is also essential in the process of molting from L3 to L4 (5, 39, 42, 41). Given the essential function of cuticular collagen and the fact that EN sera specifically recognize the BmCol-4, there is a great potential for developing BmCol-4 as a vaccine target.
BmCox-2 shares 40% sequence similarity with human Cox-2. The importance of Cox-2 enzymes in oxidative metabolism suggests that BmCox-2 may be a target for impaired development of filarial parasites.
The basic concept of a multivalent vaccine emanates from the fact that more than one antigen or epitope is required for the generation of a protective immune response against a pathogen. This is especially true for multicellular parasites of human and animals. To develop such a multivalent vaccine it is important that we identify all the potential immunoprotective antigens or epitopes for each pathogen. The present study shows that multiple antigens for human filarial parasites can be potentially obtained by screening phagotopes with sera from individuals who are naturally immune to the parasite. To begin to analyze the potential of these three antigens as vaccine candidates, we first decided to evaluate the vaccine potential of ALT-2 mainly because over 40% of the clones recognized by EN sera were ALT-2. Moreover, there are no ALT-2 homologous genes in human, so there will be minimal autoimmune responses when ALT-2 is used as a vaccine antigen in human. Other than primates, the closest animal models to study the pathology and immune responses to lymphatic filarial parasites are the mice and jirds (27). Therefore, in these studies, we immunized mice and jirds with rBmALT-2 and evaluated the degree of protection to a challenge infection. These studies showed that over 73% protection could be achieved in jirds and over 64% protection could be achieved in mice after a challenge infection. These findings are similar to those reported by Gregory et al. (18) for ALT-1, where they also observed a high protection rate after immunizing jirds with rALT-1. Further studies will determine whether a combination of all three antigens could increase the rate of protection.
The findings presented in this study demonstrate that screening phage display cDNA libraries of an infectious agent with immune sera can preferentially select antigens with potential for vaccine development. The fact that the displayed peptide on the surface of the phage is linked to the gene makes it easy for cloning the gene of interest. Furthermore, this technology could also be potentially used for identifying antigens that are potentially important in the immunodiagnosis or as drug targets against several infectious agents.
Acknowledgments
This work was supported by Public Health Service grant AI-39066 from the NIAID and an internal grant (C.R.B.) from the University of Illinois at Chicago.
We thank Sandra J. Laney and Steven A. Williams, Smith College, for the cDNA libraries. B. malayi life cycle stages used in this study were provided by an NIAID supply contract (AI #02642).
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Abraham, D., R. B. Grieve, J. M. Holy, and B. M. Chrinstensen. 1989. Immunity to larval Brugia malayi in BALB/c mice: protective immunity and inhibition of larval development. 40:598-604. [DOI] [PubMed]
- 2.Barbas, C. F., III, A. S. Kang, R. A. Lerner, and S. J. Benkovic. 1991. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. USA 88:7978-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaxter, M. L., N. Raghavan, I. Ghosh, D. Guilliano, W. Lu, S. A. Williams, B. Slatko, and A. L. Scott. 1996. Genes expressed in Brugia malayi infective third stage larvae. Mol. Biochem. Parasitol. 77:77-93. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R. 1995. Phage display. Immunotechnology 1:87-94. [DOI] [PubMed] [Google Scholar]
- 5.Caulagi, V. R., and T. V. Rajan. 1995. The structural organization of an alpha 2 (type IV) basement membrane collagen gene from the filarial nematode Brugia malayi. Mol. Biochem. Parasitol. 70:227-229. [DOI] [PubMed] [Google Scholar]
- 6.Crameri, R., and K. Blaser. 1996. Cloning Aspergillus fumigatus allergens by the pJuFo filamentous phage display system. Int. Arch. Allergy Immunol. 110:41-45. [DOI] [PubMed] [Google Scholar]
- 7.Crameri, R., R. Jaussi, G. Menz, and K. Blaser. 1994. Display of expression products of cDNA libraries on phage surfaces. A versatile screening system for selective isolation of genes by specific gene-product/ligand interaction. Eur. J. Biochem. 226:53-58. [DOI] [PubMed] [Google Scholar]
- 8.Crameri, R., and M. Suter. 1995. Display of biologically active proteins on the surface of filamentous phages: a cDNA cloning system for the selection of functional gene products linked to the genetic information responsible for their production. Gene 160:139. [DOI] [PubMed] [Google Scholar]
- 9.Cwirla, S. E., E. A. Peters, R. W. Barrett, and W. J. Dower. 1990. Peptides on phage: a vast library of peptides for identifying ligands. Proc. Natl. Acad. Sci. USA 87:6378-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dybwad, A., O. Forre, J. Kjesldsen-Kragh, J. B. Natvig, and M. Sioud. 1993. Identification of new B cell epitopes in the sera of rheumatoid arthritis patients using a random nanopeptide phage library. Eur. J. Immunol. 23:3189-3193. [DOI] [PubMed] [Google Scholar]
- 11.Folgori, A., R. Tafi, A. Meola, F. Felici, G. Galfre, R. Cortese, P. Monaci, and A. Nicosia. 1994. A general strategy to identify mimotopes of pathological antigens using only random peptide libraries and human sera. EMBO J. 13:2236-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank, G. R., C. A. Tripp, and R. B. Grieve. 1995. Molecular cloning of a developmentally regulated protein isolated from excretory-secretory products of larval Dirofilaria immitis. Mol. Biochem. Parasitol. 75:231-240. [DOI] [PubMed] [Google Scholar]
- 13.Freedman, D. O., T. B. Nutman, and E. A. Ottesen. 1989. Protective immunity in bancroftian filariasis. Selective recognition of a 43-kD larval stage antigen by infection-free individuals in an endemic area. J. Clin. Investig. 83:14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuh, G., and S. S. Sidhu. 2000. Efficient phage display of polypeptides fused to the carboxy-terminus of the M13 gene-3 minor coat protein. FEBS Lett. 480:231-234. [DOI] [PubMed] [Google Scholar]
- 15.Germashewski, V., and K. Murray. 1996. Identification of polyclonal serum specificities with phage-display libraries. J. Virol. Methods 58:21-32. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh. I., S. W. Eisinger, N. Raghavan, and A. L. Scott. 1998. Thioredoxin peroxidases from Brugia malayi. Mol. Biochem. Parasitol. 91:207-220. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, K., and P. J. Hotez. 1999. Antibody-dependent reductions in mouse hookworm burden after vaccination with Ancylostoma caninum secreted protein 1. J. Infect. Dis. 180:1674-1681. [DOI] [PubMed] [Google Scholar]
- 18.Gregory, W. F., A. K. Atmadja, J. E. Allen, and R. M. Maizels. 2000. The abundant larval transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect. Immun. 68:4174-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawdon, J. M., B. F. Jones, D. R. Hoffman, and P. J. Hotez. 1996. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J. Biol. Chem. 271:6672-6678. [DOI] [PubMed] [Google Scholar]
- 20.Helmy, H., G. J. Weil, R. Faris, A. M. Gad, R. Chandrashekar, A. Ashour, and R. M. Ramzy. 2000. Human antibody responses to Wuchereria bancrofti infective larvae. Parasite Immunol. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 21.Jefferies, D. 1998. Selection of novel ligands from phage display libraries: an alternative approach to drug and vaccine discovery. Parasitol. Today 14:202-206. [DOI] [PubMed] [Google Scholar]
- 22.Jespers, L. S., J. H. Messens, A. De Keyser, D. Eeckhout, I. Van den Brande, Y. G. Gansemans, M. J. Lauwereys, G. P. Vlasuk, and P. E. Stanssens. 1995. Surface expression of ligand-based selection of cDNAs fused to filamentous phage gene VI. Biotechnology 13:378-382. [DOI] [PubMed] [Google Scholar]
- 23.Joseph, G. T., T. Huima, and S. Lustigman. 1998. Characterization of an Onchocerca volvulus L3-specific larval antigen, Ov-ALT-1. Mol. Biochem. Parasitol. 96:177-183. [DOI] [PubMed] [Google Scholar]
- 24.Kazura, J. W., P. A. Maroney, E. Pearlman, and T. W. Nilsen. 1990. Protective efficacy of a cloned Brugia malayi antigen in a mouse model of microfilaremia. J. Immunol. 145:2260-2264. [PubMed] [Google Scholar]
- 25.Klimowski, L., R. Chandrashekar, and C. A. Tripp. 1997. Molecular cloning, expression and enzymatic activity of a thioredoxin peroxidase from Dirofilaria immitis. Mol. Biochem. Parasitol. 90:297-306. [DOI] [PubMed] [Google Scholar]
- 26.Lalitha, P., M. Ravichandran, S. Suba, P. Kaliraj, R. B. Narayanan, and K. Jayaraman. 1998. Quantitative assessment of circulating antigens in human lymphatic filariasis: a field evaluation of monoclonal antibody-based ELISA using blood collected on filter strips. Trop. Med. Int. Health 3:41-45. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence, R. A., and E. Devaney. 2001. Lymphatic filariasis: parallels between the immunology of infection in humans and mice. Parasite Immunol. 23:353-361. [DOI] [PubMed] [Google Scholar]
- 28.Li, B. W., R. Chandrashekar, and G. J. Weil. 1993. Vaccination with recombinant filarial paramyosin induces partial immunity to Brugia malayi infection in jirds. J. Immunol. 150:1881-1885. [PubMed] [Google Scholar]
- 29.Li, B. W., S. Zhang, K. C. Curtis, and G. J. Weil. 1999. Immune responses to Brugia malayi paramyosin in rodents after DNA vaccination. Vaccine 18:76-81. [DOI] [PubMed] [Google Scholar]
- 30.Meola, A., P. Delmastro, P. Monaci, A. Luzzago, A. Nicosia, F. Felici, R. Cortese, and G. Galfre. 1995. Derivation of vaccines from mimotopes. J. Immunol. 154:3162-3172. [PubMed] [Google Scholar]
- 31.Murray, J., W. F. Gregory, N. Gomez-Escobar, A. K. Atmadja, and R. M. Maizels. 2001. Expression and immune recognition of Brugia malayi VAL-1, a homologue of vespid venom allergens and Ancylostoma secreted proteins. Mol. Biochem. Parasitol. 118:89-96. [DOI] [PubMed] [Google Scholar]
- 32.Ottesen, E. A., and C. P. Ramachandran. 1995. Lymphatic filariasis. Infection and disease control stratagies. Parasitol. Today 11:129-131. [Google Scholar]
- 33.Pearlman, E., W. K. Kroeze, F. E. Hazlett, Jr., S. S. Chen, S. D. Mawhorter, W. H. Boon, and J. W. Kazura. 1993. Brugia malayi: acquired resistance to microfilariae in BALB/c mice correlates with local Th2 responses. Exp. Parasitol. 76:200-208. [DOI] [PubMed] [Google Scholar]
- 34.Peralta M. E., K. A. Schmitz, and T. V. Rajan. 1999. Failure of highly immunogenic filarial proteins to provide host-protective immunity. Exp. Parasitol. 91:334-340. [DOI] [PubMed] [Google Scholar]
- 35.Rahlfs, S., and K. Becker. 2001. Thioredoxin peroxidases of the malarial parasite Plasmodium falciparum. Eur. J. Biochem. 268:1404-1409. [DOI] [PubMed] [Google Scholar]
- 36.Rao, K. V. N., D. Eswaran, V. Ravi, M. Gnanasekar, R. B. Narayanan, P. Kaliraj, K. Jayaraman, A. Marson, N. Raghavan, and A. L. Scott. 2000. The Wuchereria bancrofti orthologue of Brugia malayi SXP1 and the diagnosis of bancroftian filariasis. Mol. Biochem. Parasitol. 107:71-80. [DOI] [PubMed] [Google Scholar]
- 37.Rao, U. R., G. Salinas, K. Mehta, and T. R. Klei. 2000. Identification and localization of glutathione-S-transferase as a potential target enzyme in Brugia species. Parasitol. Res. 86:908-915. [DOI] [PubMed] [Google Scholar]
- 38.Ravindran, B., A. K. Satapathy, P. K. Sahoo, and J. J. Babu Geddam. 2000. Protective immunity in human Bancroftian filariasis: inverse relationship between antibodies to microfilarial sheath and circulating filarial antigens. Parasite Immunol. 22:633-637. [DOI] [PubMed] [Google Scholar]
- 39.Rhoads, M. L., R. H. Fetterer, and J. F. Urban, Jr. 2001. Cuticular collagen synthesis by Ascaris suum during development from the third to fourth larval stage: identification of a potential chemotherapeutic agent with a novel mechanism of action. J. Parasitol. 87:1144-1149. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Scott, A. L., P. Yenbutr, S. W. Eisinger, and N. Raghavan. 1995. Molecular cloning of the cuticular collagen gene Bmcol-2 from Brugia malayi. Mol. Biochem. Parasitol. 70:221-225. [DOI] [PubMed] [Google Scholar]
- 42.Selkirk, M. E., L. Nielsen, C. Kelly, F. Partono, G. Sayers, and R. M. Maizels. 1989. Identification, synthesis and immunogenicity of cuticular collagens from the filarial nematodes Brugia malayi and Brugia pahangi. Mol. Biochem. Parasitol. 15:229-246. [DOI] [PubMed] [Google Scholar]
- 43.Sen, L., K. Ghosh, Z. Bin, S. Qiang, M. G. Thompson, J. M. Hawdon, R. A. Koski, X. Shuhua, and P. J. Hotez. 2000. Hookworm burden reductions in BALB/c mice vaccinated with recombinant Ancylostoma secreted proteins (ASPs) from Ancylostoma duodenale, Ancylostoma caninum and Necator americanus. Vaccine 18:1096-1102. [DOI] [PubMed] [Google Scholar]
- 44.Sioud, M, and M. H. Hansenn. 2001. Profiling the immune response in patients with breast cancer by phage-display cDNA libraries. Eur. J. Immunol. 31:716-725. [DOI] [PubMed] [Google Scholar]
- 45.Smith, G. P. 1985. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315-1317. [DOI] [PubMed] [Google Scholar]
- 46.Somers, V. A., R. J. Brandwijk, B. Joosten, P. T. Moerkerk, and J. W. Arends. 2002. A panel of candidate tumor antigens in colorectal cancer revealed by the serological selection of a phage displayed cDNA expression library. J. Immunol. 169:2772-2780. [DOI] [PubMed] [Google Scholar]
- 47.Son, M., and P. Serwer. 1992. Role of exonuclease in the specificity of bacteriophage T7 DNA packaging. Virology 190:824-833. [DOI] [PubMed] [Google Scholar]
- 48.Stewart, G. R., Y. Zhu, W. Parredes, T. I. M. Tree, R. Guderian, and J. E. Bradley. 1997. The novel cuticular collagen Ovcol-1 of Onchocerca volvulus is preferentially recognized by immunoglobulins G3 from putatively immune individuals. Infect. Immun. 65:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tawe, W., E. Pearlman, T. R. Unnasch, and S. Lustigman. 2000. Angiogenic activity of Onchocerca volvulus recombinant proteins similar to vespid venom antigen 5. Mol. Biochem. Parasitol. 109:91-99. [DOI] [PubMed] [Google Scholar]
- 50.Wang, S. H., H. J. Zheng, S. Dissanayake, W. F. Cheng, Z. H. Tao, S. Z. Lin, and W. F. Piessens. 1997. Evaluation of recombinant chitinase and SXP1 antigens as antimicrofilarial vaccines. Am. J. Trop. Med. Hyg. 56:474-481. [DOI] [PubMed] [Google Scholar]
- 51.Werner, C., G. I. Higashi, J. A. Yates, and T. V. Rajan. 1989. Differential recognition of two cloned Brugia malayi antigens by antibody class. Mol. Biochem. Parasitol. 35:209-218. [DOI] [PubMed] [Google Scholar]
- 52.Williams, D. L., H. Asahi, D. Botkin, and M. J. Stadecker. 2001. Schistosome infection stimulates host CD4+ T-helper-cell and B-cell responses against a novel egg antigen, thioredoxin peroxidase. Infect. Immun. 69:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]