Abstract
Purpose
To describe two cases of congenital corneal endothelial edema resulting from novel de novo mutations.
Methods
Case A patient was a 15 months old Caucasian infant and Case B patient was a 3 year old Hispanic child presenting with bilateral cloudy corneas since birth. Clinicopathological findings are presented. DNA samples were screened for mutations in candidate genes by Sanger sequencing.
Results
Slit-lamp examination of Case A patient revealed stromal edema and haze. Histology of keratoplasty button showed stromal thickening with loss of endothelium and thin Descemet’s membrane. Sanger sequencing established the diagnosis of congenital hereditary endothelial dystrophy (CHED) by detection of a compound heterozygous mutation in SLC4A11. The proband displayed a novel de novo frameshift mutation in one SLC4A11 allele, p.(Pro817Argfs*32), in conjunction with a maternally inherited missense mutation in SLC4A11, p.(Arg869His). Case B patient similarly presented with stromal edema and stromal haze. Histopathological analysis revealed a spongy epithelium, focal discontinuities in Bowman’s layer, stromal thickening with areas of compacted posterior stroma, variable thickness of Descemet’s membrane, and regional multilayered endothelium. Sanger sequencing found a novel de novo nonsense mutation in the first exon of ZEB1, p.(Cys7*).
Conclusions
To our knowledge, we present the earliest clinical presentation of posterior polymorphous corneal dystrophy resulting from a de novo mutation in ZEB1. Additionally, we present a CHED case with a thin Descemet’s membrane with a novel compound heterozygous SLC4A11 mutation. In the absence of a family history or consanguinity, de novo mutations may result in congenital corneal endothelial dystrophies.
Keywords: Congenital hereditary endothelial dystrophy, CHED, posterior polymorphous corneal dystrophy, PPCD, SLC4A11, ZEB1
INTRODUCTION
Corneal endothelial dystrophies are a subset of bilateral inherited diseases of the cornea that can result in a severe decline in visual acuity. Characterized by diffuse, non-inflammatory corneal edema and opacification arising from severe endothelial cell dysfunction, the disorders encompass several subtypes including congenital hereditary endothelial dystrophy (CHED; MIM #217700), posterior polymorphous corneal dystrophies (PPCD1; MIM #122000, PPCD3; MIM #609141), Fuchs’ endothelial corneal dystrophy (FECD), and X-linked endothelial corneal dystrophy (XECD). Despite their clinically unique features, many of the dystrophies still harbor similar histopathological hallmarks including structural aberrations in the individual layers of the cornea, degeneration of corneal endothelial cells, anomalous deposition of collagen on the posterior surface of the Descemet’s membrane, and loss of corneal deturgescence.1–3
In the current report, we describe two cases of congenital corneal edema without a family history of inherited corneal disease or consanguinity where candidate gene sequencing assisted in making the diagnosis by the identification of novel de novo mutations. We first describe a case of CHED with a de novo variant resulting in a compound heterozygous mutation in SLC4A11 gene. We further report a case initially presumed to have the clinical hallmarks of CHED which was subsequently re-diagnosed to be a case of PPCD3 following identification of a novel, de novo mutation in the ZEB1 gene. In this current era of human genomics with its promise of personalized patient care, the two cases reported here epitomize how genetic testing can complement careful clinical examination, and histopathological review, for the proper diagnosis, management, and counseling of the patients with congenital corneal endothelial dystrophies.
MATERIALS AND METHODS
The study protocol had the approval of the institutional review board of the University of Texas Southwestern Medical Center and was in compliance with the tenets of the Declaration of Helsinki. All study subjects were recruited at UT Southwestern after informed consent. The subjects underwent a complete eye examination including slit-lamp biomicroscopy and/or examination under anesthesia by cornea fellowship-trained ophthalmologists (VVM, CBB, WB).
Penetrating keratoplasty specimens were fixed in formalin and embedded sections were processed for standard hematoxylin and eosin staining as described previously.4 Genomic DNA was extracted from leukocytes of peripheral blood using AutoGen FlexiGene by Qiagen (Valencia, CA). Exon coding regions were amplified by PCR with flanking primers and subsequently sequenced by Sanger sequencing. Primers for ZEB1 were selected using ExonPrimer and Primer3 (Helmholtz Center Munich, Institute of Human Genetics)5 while previously described primers for SLC4A11 were utilized.6
CASE REPORTS
The Case A patient was a 15 months old Caucasian toddler who presented to the pediatric ophthalmologist with a chief complaint of limited ambulation. Parents reported that the child “bumped into things” because of poor vision, noting that the onset was gradual and that the condition was worsening. Parents also reported that the child had bilateral cloudy corneas since birth. The family history was absent for eye disease or consanguinity. The patient’s ocular history was also remarkable for strabismus. The pediatric ophthalmologist noted mild stromal edema and stromal haze. A clinical diagnosis of CHED was made by a corneal specialist and future keratoplasty was advised. Pre-operative visual acuity was 20/80 OD and 20/80 OS as measured by Allen cards. Corneal pachymetry results showed thickened corneas at 800 µm OD and 800 µm OS. Intraocular pressures (IOPs) were deemed normal by tactile digital pressure and corneal diameters were also noted to be normal. The anterior and posterior segments of the eye were otherwise normal. At the age of 3 years and 4 months, the patient underwent penetrating keratoplasty (PKP) in both eyes over 2 months. Figure 1A shows 2 months status post PKP to the left eye at which point right eye was operated on. Five days post PKP to the right eye, the patient suffered a rupture of OD corneal transplant wound after a fall. Examination under anesthesia revealed that the dehiscence involved 270 degrees of the wound with lens and vitreous prolapse. The patient underwent emergent lensectomy, anterior vitrectomy, and repair of dehisced keratoplasty wound. At the most recent follow-up visit (17 months after initial transplant), both corneal grafts were noted to be clear and best corrected visual acuity was 20/60 in each eye.
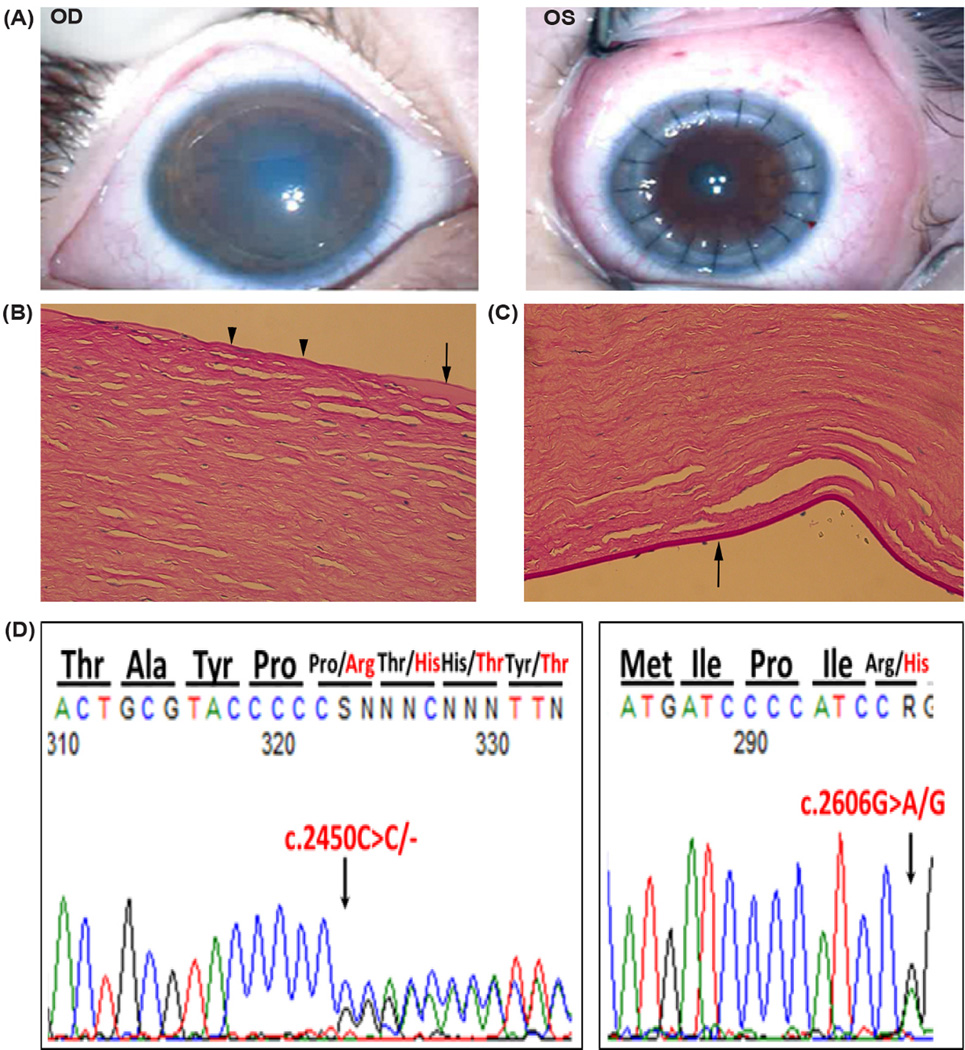
Figure 1.
Case A Patient. A. Photographs obtained with operating microscope 2 months status post penetrating keratoplasty to the left eye shows a cloudy edematous cornea in the right eye; and a clear and compact PKP graft in the left eye. Histopathological findings of keratoplasty button from Case A. Light microscopy showed features of CHED included B) focal loss in Bowman’s layer centrally (arrowheads), areas of thickened Bowman’s layer (arrow); and C) thin Descemet’s membrane with only a few remaining endothelial cells (arrow). D) DNA sequence chromatogram of proband revealing a novel de novo mutation (c.2450delC) in the paternal allele resulting in a frameshift mutation in exon 18 of the SLC4A11 gene. Co-inheritance of a maternal missense mutation (c.2606G>A) in exon 18 leads to the generation of the compound heterozygous mutation.
Histopathological analysis of keratoplasty button showed areas of thickening but also focal loss of Bowman’s layer centrally (Figure 1B), stromal thickening with edema, and sparse endothelial cells with a thin Descemet’s membrane (Figure 1C). The ocular pathologist’s report noted “some features of CHED are present; however, Descemet’s membrane is extremely thin. Only a few endothelial cells remain. Hence, clinical correlation is required.” Both parents were examined and had normal corneal examination by slit-lamp examination. Given the absence of consanguinity and absence of thickening of Descemet’s membrane, PCR amplification and traditional Sanger sequencing of the coding exons of SLC4A11 were performed on genomic DNA from the proband and parents. The mother was noted to have a pathogenic heterozygous single nucleotide polymorphism predicted to result in a missense mutation [SNP rs121909392: NM_032034.3:c.2606G>A, p.(Arg869His)]. In addition to the p.(Arg869His) mutation from his mother, the proband showed a novel de novo mutation in the father’s SLC4A11 allele (Figure 1D) resulting in a frameshift mutation [NM_032034.3:c.2450del, p.(Pro817Argfs*32)]. Paternity was confirmed by microsatellite loci genotyping using PowerPlex 1.2 System from Promega (Madison, WI).
The Case B patient was a Hispanic child who presented with corneal clouding at the age of 3 to a pediatric ophthalmologist. Per history by mother, the corneas were “hazy since birth.” Family history was unremarkable for eye disease and without consanguinity. At 4 years of age, best spectacle corrected visual acuity was 20/70 in the OD and 20/80 OS. Exam under anesthesia showed evidence of stromal edema and haze with band keratopathy (Figure 2A). Central corneal pachymetry measured 763 µm OD and 734 µm OS using a Corneo-gage Plus ultrasonic pachymeter (Sonomed, Cleveland, OH). IOPs were 15 mmHg OD and 18 mmHg OS. Corneal diameters were normal. No signs of anterior chamber or intra-ocular inflammation were noted. The anterior and posterior segments of the eye were otherwise normal. Treatment of band keratopathy was done by a pediatric ophthalmologist at the time of the examination under anesthesia. A clinical diagnosis of CHED was made and PKP was advised upon referral to a corneal specialist. At the age of 4, patient underwent bilateral PKP - with 3 months separation between procedures. At 8 years and 3 months of age, on the most recent follow-up visit, the patient’s visual acuity was 20/25 in each eye and both corneal grafts were noted to be clear.
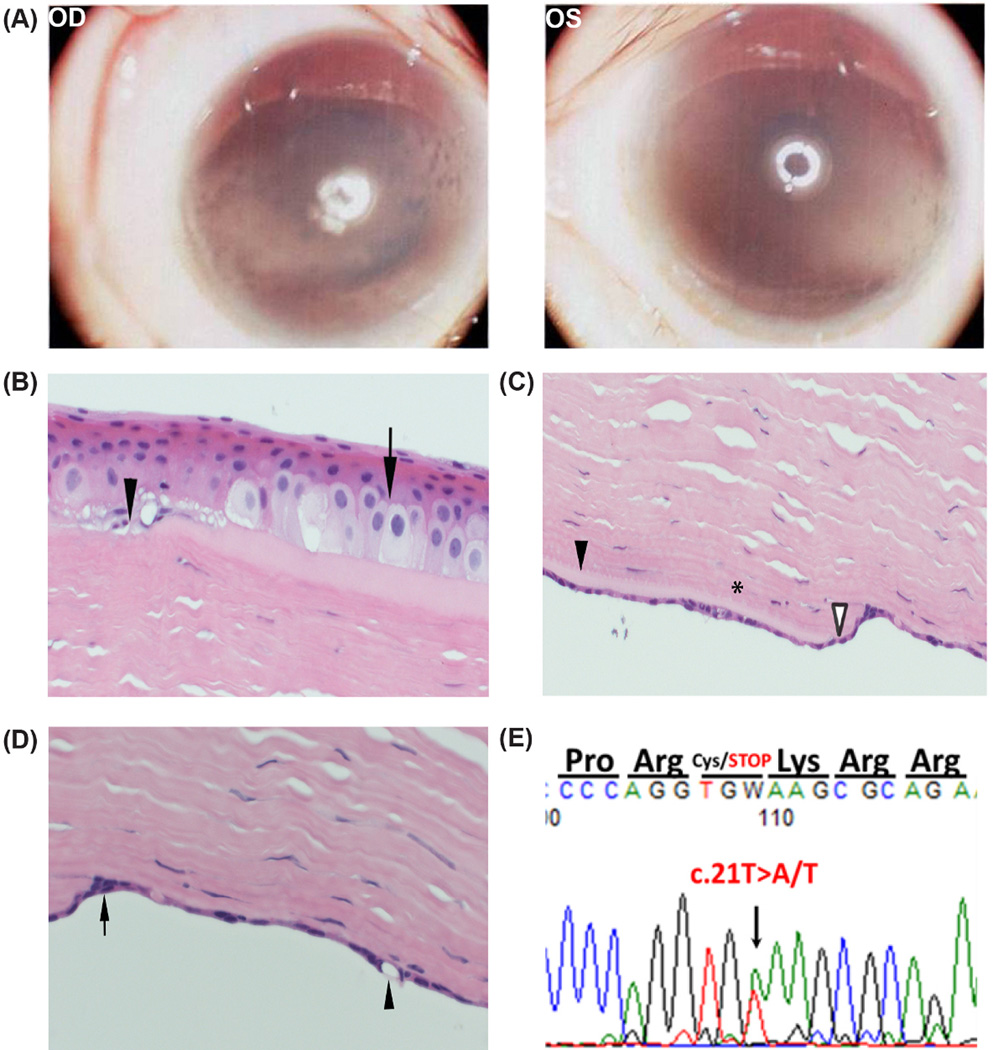
Figure 2.
Case B Patient. A) Operating microscope photographs at age 3 prior to any surgical intervention shows stromal edema and haze with associated band keratopathy in the right and left corneas. B) Light microscopy demonstrated characteristic features of PPCD including a spongy epithelium (arrow) with minimal basal epithelium, discontinuities in Bowman's layer (arrowhead), C) a compacted posterior stroma (*) with regional thinning (open arrowhead) and thickening (closed arrowhead) of the Descemet’s membrane, and D) a focal multilayered (arrow) and vacuolated endothelium (arrowhead). E) DNA sequence chromatogram of proband revealing a de novo nonsense mutation resulting in a premature stop in exon 1 of the ZEB1 gene.
The preliminary report by a general pathologist remarked on the keratoplasty button findings (Figures 2B, 2C, 2D) “as consistent with an endothelial dystrophy.” The patient’s parents and siblings were examined and all were noted to have normal corneal examinations by slit-lamp examination. PCR amplification and traditional Sanger sequencing of the coding sequence of SLC4A11 revealed no pathogenic variants in proband, siblings or parents. Sanger sequencing of the exons of the ZEB1 gene was performed in the proband, siblings, and parents. A novel de novo ZEB1 mutation was found in the proband [NM_001174093.1:c.21T>A, p.(Cys7*)] (Figure 2E) which was absent in both parents and siblings. Paternity was confirmed genetically. This single nucleotide substitution is predicted to result in a nonsense mutation and a premature stop codon. The pathology slides were subsequently reviewed with an ocular pathologist (RNH). Light microscopy analysis of the excised left cornea showed a spongy epithelium with minimal basal epithelium (Figure 2B). Discontinuities in Bowman's layer were noted. The stroma was thickened but areas of the posterior stroma were compacted. Descemet’s membrane displayed regional thinning and thickening and abnormal endothelium (Figure 2C). The ocular pathologist noted that the focal multi-layered endothelium was consistent with posterior polymorphous corneal dystrophy (PPCD) (Figure 2D).
DISCUSSION
The presented cases illustrate the importance of genetic testing in conjunction with meticulous clinical examination and histopathological review for the diagnosis, management, and counseling of patients with congenital corneal edema. Additionally, these cases highlight that de novo mutations should be considered in the etiology of congenital corneal endothelial dystrophies in the absence of a family history of corneal disease or presence of consanguinity.
Manifesting at birth or early childhood, CHED (MIM #217700) distinguishes itself by its histological hallmarks which include dystrophic endothelial cells, a typically thickened Descemet’s membrane, and diffuse stromal edema that give a distinct ‘‘ground glass’’ appearance to the corneas. The affected gene for CHED has been identified as SLC4A11.7 The protein product from the SLC4A11 transcript was originally described as a sodium borate cotransporter 1 (NaBC1).8 More recent work investigating the role of the protein specifically in bovine corneal endothelial cells suggests that the protein does not have any significant affinity for borate and instead appears to function as a sodium-dependent intracellular pH modulator transporting mainly hydroxyl anions.9 Additional observations from exogenous expression and knockout of SLC4A11 in multiple experimental animal models and human corneas suggest that the protein may also promote basolateral entry of water molecules into corneal endothelium.10 These observations provide a mechanistic basis for the loss of deturgescence and progressive disorganization of the endothelial layer seen in CHED. Numerous homozygous or compound heterozygous SLC4A11 mutations have been described in CHED individuals.1,11–14 To our knowledge, de novo mutations in SLC4A11 have not been previously described in the literature prior to this report.
Case A patient had clinical features of CHED but the pathology showed marked loss of endothelium without a thickened Descemet’s membrane precluding a definitive diagnosis. Additionally as an autosomal recessive disorder, CHED occurs primarily in patients with a history of consanguinity. Sanger sequencing of the subject and his parents helped make the diagnosis of CHED by the detection of a novel de novo mutation in the paternal SLC4A11 allele combining with a maternal missense mutation p.(Arg869His) to result in a compound heterozygous mutation in the gene. The maternally inherited missense mutation has been previously implicated in CHED and is specifically thought to interrupt the interaction between a specific domain (the transmembrane surface of helix 12) of the cotransporter encoded by SLC4A11 transcript and the cytoplasmic membrane.6 Interestingly, recent detailed light and electron microscopic studies of the Descemet’s membrane of CHED cases have revealed that marked thickening of this layer is not a universal finding of the disorder.15,16 Moreover, a majority, of the reported homozygous mutations in exon 18 were associated with only moderate if any thickening of the Descemet’s membrane.15,16
Although the Case B patient also had presented with clinical features of CHED, the pathological findings and detection of a de novo mutation in ZEB1 helped establish the diagnosis of PPCD3. In contrast to CHED, corneal changes in PPCD usually occur in the second or third decade of life. PPCD also features a more expansive array of clinical manifestations encompassing grouped vesicles, scalloped bands, geographic lesions, and opacities.2,3 Although band keratopathy may be a rare association with PPCD,17 it is a landmark finding of late X-linked endothelial dystrophy presenting in the form of endothelial changes resembling “moon craters”.18 Mutations in ZEB1 (a zinc finger transcription factor) (MIM #609141) are found in nearly one third of all PPCD probands.2,3 De novo mutations in ZEB1 have been described in several families.3,19 By modulating genes responsible both for maintenance of the epithelial phenotype and the epithelial to mesenchymal (EMT) transition, ZEB1 has been shown to regulate neurodevelopment and tumor metastasis.1,20
Interestingly, the Case B patient was only 3 years old but presented all the hallmarks of PPCD3 including corneal edema and an abnormal multilayered endothelial cell layer. To our knowledge, our study is the first to report such an early onset PPCD3 from a de novo mutation. Familial PPCD3 cases of congenital onset15 and early onset at 3 months of age21 have been reported previously.
In this era of genomics and promise of personalized medicine, these cases highlight the important role that gene sequencing may play in the diagnosis of congenital corneal endothelial dystrophies with atypical clinical and/or histopathological presentations. Genetic counseling at the appropriate age is warranted for the case B patient with the de novo heterozygous mutation in ZEB1 is at risk of passing on the deleterious allele to half of his offspring. Meticulous correlation of mutations to clinicopathological findings may improve our clinical understanding of these endothelial disorders and also shed light on their underlying molecular biology.
ACKNOWLEDGEMENT
We thank the subjects for their participation. This study was supported by grants R01 EY022161 and P30 EY020799 from the National Eye Institute, National Institutes of Health, Bethesda, MD, an unrestricted grant from Research to Prevent Blindness, New York and support from the UT Southwestern Summer Medical Student Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflict of interest or financial interest in any of the issues contained in this article.
REFERENCES
- 1.Aldave AJ, Han J, Frausto RF. Genetics of the corneal endothelial dystrophies: an evidence-based review. Clin Genet. 2013;84:109–119. doi: 10.1111/cge.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss JS, Møller HU, Aldave AJ, et al. IC3D Classification of Corneal Dystrophies - Edition 2. Cornea. 2015;34:117–159. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 3.Liskova P, Palos M, Hardcastle AJ, et al. Further genetic and clinical insights of posterior polymorphous corneal dystrophy 3. JAMA Ophthalmol. 2013;131:1296–1303. doi: 10.1001/jamaophthalmol.2013.405. [DOI] [PubMed] [Google Scholar]
- 4.Kim SY, Muftuoglu O, Hogan RN, et al. Histopathology and spectral domain OCT findings of pneumatic-assisted dissection in DALK. Cornea. 2012;31:1288–1293. doi: 10.1097/ICO.0b013e31824d0d23. [DOI] [PubMed] [Google Scholar]
- 5.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 6.Jiao X, Sultana A, Garg P, et al. Autosomal recessive corneal endothelial dystrophy (CHED2) is associated with mutations in SLC4A11. J Med Genet. 2007;44:64–68. doi: 10.1136/jmg.2006.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vithana EN, Morgan P, Sundaresan P, et al. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2) Nat Genet. 2006;38:755–757. doi: 10.1038/ng1824. [DOI] [PubMed] [Google Scholar]
- 8.Park M, Li Q, Shcheynikov N, et al. NaBC1 is a ubiquitous electrogenic Na+ -coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell. 2004;16:331–341. doi: 10.1016/j.molcel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Jalimarada SS, Ogando DG, Vithana EN, et al. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci. 2013;54:4330–4340. doi: 10.1167/iovs.13-11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilas GL, Loganathan SK, Liu J, et al. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet. 2013;22:4579–4590. doi: 10.1093/hmg/ddt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SH, Jeong HJ, Kim M, et al. A novel nonsense mutation of the SLC4A11 gene in a Korean patient with autosomal recessive congenital hereditary endothelial dystrophy. Cornea. 2013;32:181–182. doi: 10.1097/ICO.0b013e31828d9ffd. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui S, Zenteno JC, Rice A, et al. Congenital hereditary endothelial dystrophy caused by SLC4A11 mutations progresses to Harboyan syndrome. Cornea. 2014;33:247–251. doi: 10.1097/ICO.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodaganur SG, Kapoor S, Veerappa AM, et al. Mutation analysis of the SLC4A11 gene in Indian families with congenital hereditary endothelial dystrophy 2 and a review of the literature. Mol Vis. 2013;19:1694–1706. [PMC free article] [PubMed] [Google Scholar]
- 14.Puangsricharern V, Yeetong P, Charumalai C, et al. Two novel mutations including a large deletion of the SLC4A11 gene causing autosomal recessive hereditary endothelial dystrophy. Br J Ophthalmol. 2014;98:1460–1462. doi: 10.1136/bjophthalmol-2014-305584. [DOI] [PubMed] [Google Scholar]
- 15.Sultana A, Garg P, Ramamurthy B, et al. Mutational spectrum of the SLC4A11 gene in autosomal recessive congenital hereditary endothelial dystrophy. Mol Vis. 2007;13:1327–1332. [PubMed] [Google Scholar]
- 16.Paliwal P, Sharma A, Tandon R, et al. Congenital hereditary endothelial dystrophy - mutation analysis of SLC4A11 and genotype-phenotype correlation in a North Indian patient cohort. Mol Vis. 2010;16:2955–2963. [PMC free article] [PubMed] [Google Scholar]
- 17.Lam HY, Wiggs JL, Jurkunas UV. Unusual presentation of presumed posterior polymorphous dystrophy associated with iris heterochromia, band keratopathy, and keratoconus. Cornea. 2010;29:1180–1185. doi: 10.1097/ICO.0b013e3181d007e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid E, Lisch W, Philipp W, et al. A new, X-linked endothelial corneal dystrophy. Am J Ophthalmol. 2006;141:478–487. doi: 10.1016/j.ajo.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Krafchak CM, Pawar H, Moroi SE, et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am J Hum Genet. 2005;77:694–708. doi: 10.1086/497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans CJ, Liskova P, Dudakova L, et al. Identification of six novel mutations in ZEB1 and description of the associated phenotypes in patients with posterior polymorphous corneal dystrophy3. Ann Hum Genet. 2015;79:1–9. doi: 10.1111/ahg.12090. [DOI] [PubMed] [Google Scholar]
- 21.Liskova P, Filipec M, Merjava S, et al. Variable ocular phenotypes of posterior polymorphous corneal dystrophy caused by mutations in the ZEB1 gene. Ophthalmic Genet. 2010;31:230–234. doi: 10.3109/13816810.2010.518577. [DOI] [PubMed] [Google Scholar]