Abstract
Very low oral bioavailability due to extensive pre-systemic metabolism and P-gp efflux has constrained the oral metronomic chemotherapy of docetaxel (DTX). There is tremendous need of compounds facilitating oral delivery of DTX. The research was aimed to investigate the effect of piperlongumine (PPL) on human liver microsomal metabolism, Caco-2 permeability and cytotoxicity of DTX in triple negative breast cancer (TNBC) cell lines. Reduction in testosterone and DTX metabolism (2-fold increase in half-life) by piperlongumine was comparable to the standard CYP3A4 inhibitor, Cyclosporine A. P-gp efflux ratio of DTX across caco-2 monolayer was reduced from 2.37 to 1.52 on co-incubation with piperlongumine. The IC50 value of DTX was reduced 3-5 times and combination index values in all the cell lines were below 0.6. PPL at non-cytotoxic concentration showed significant enhancement of the anti-migration effect of DTX. Expression of tumor markers such as survivin, bcl2, C-myc and cyclin D1 were down regulated to a great extent with enhanced p53 expression when treated with combination instead of individual drug. Co-treatment with PPL led to 1.68 fold enhancement in DTX bioavailability in SD rats. Piperlongumine could be a potential candidate in overcoming the obstacles associated with oral docetaxel delivery with synergistic anticancer activity.
Introduction
Docetaxel (DTX) is a wide spectrum chemotherapeutic agent mostly used for the treatment of breast, ovarian, lung and prostate cancers.1 It is administered by intravenous (IV) infusion of Taxotere® injection which contains ethanol and polysorbate 80 as solubilizers. Due to very poor aqueous solubility and poor physical stability of DTX in saline, both the solubilizers are used in very high amount for its intravenous delivery. Premedication with corticosteroids and antihistamine is often necessary to avoid vehicle related hypersensitivity reactions. DTX has several dose dependent side effects such as bone marrow depression, alopecia, neutropenia, anemia, thrombocytopenia and neuropathy. Severities of the above mentioned side effects have imposed a stringent restriction on parenteral dose of DTX.1,2 Docetaxel, doxorubicin and cyclophosphamide based regimen is currently used as parenteral chemotherapy for triple negative breast cancer (TNBC).3 Vascular endothelial growth factor (VEGF) inhibitors, epidermal growth factor receptor (EGFR) inhibitors, poly-ADP ribose polymerase (PARP) inhibitors and epothilones are under investigation for their application in neoadjuvant chemotherapy for TNBC.4
TNBC is a type of breast cancer which does not show expression of estrogen, progesterone and HER2/neu receptors. TNBC is characterized by its unique molecular profile, distinct pattern of metastasis and resistance to conventional chemotherapy. TNBC is more likely to spread beyond the breast and recur after lumpectomy or chemotherapy. These risks appear to be greatest in the first few years after treatment. Less than 30 percent of women with metastatic TNBC survive for 5 years, and almost all of them die of their disease despite adjuvant chemotherapy, which is the mainstay of treatment. TNBC occurs more often in younger women, African American women and women with BRCA1 mutations. Treatment of patients with TNBC has been challenging due to the heterogeneity of the disease and the absence of well-defined molecular targets. This cancer type has a very poor therapeutic outcome (at least for the first five years after diagnosis) than estrogen receptor-positive tumors. TNBC has limited treatment options and poor prognosis following progression after standard chemotherapeutic regimens.3-6
Oral metronomic chemotherapy with DTX can be a promising approach for the treatment of TNBC, ovarian cancer, lung cancer and other solid tumors. Noteworthy, negligible oral absorption/bioavailability has severely limited the scope of oral delivery of many potential anticancer drugs. Oral administration of DTX will be highly beneficial for patients because it will reduce the inconvenience, pain, risks of complications associated with parenteral administration. It will facilitate the development of chronic treatment schedules which will decrease the cost of the therapy. Moreover, it will give an opportunity to investigate more schedule intensive treatment regimens. In such scenario, there is huge need of compounds which can facilitate oral absorption of DTX and selectively enhance its anticancer activity.
Finding a compound which can prevent or minimize its intestinal metabolism, reduce P-gp efflux and augment the cytotoxicity of DTX on cancerous cells is very important for its successful oral delivery. Intestinal cytochromal enzyme driven pre-systemic metabolism and P-gp mediated basolateral to apical efflux are the two major factors responsible for very poor oral bioavailability.7,8 A dual (CYP3A4 and P-gp) inhibitor will be helpful in achieving higher plasma concentration of DTX upon oral administration. Previous literature suggests the use of cyclosporin A, ritonavir and selective P-gp inhibitors for enhancing the docetaxel bioavailability.1,9-11 However, all of them have mere P-gp and/or CYP3A4 inhibitory effect without potent anticancer activity. Hence, there is a need to discover compounds that can positively modulate oral absorption and anticancer activity of DTX.
Plasma concentrations of drug attain after oral administration is usually much lower than the intravenous administration. Hence, it is extremely crucial to enhance the anti cancer efficacy of DTX. That means, a compound having synergizing cytotoxicity with DTX against various types of cancer cell lines could be appropriate for co-administration for oral delivery of DTX. Further, traditional approach for the introducing new cancer agents into cancer therapy is to give it in combination with established treatment regimens.12-15 In addition, combination with agents that sensitize cancer cell to chemotherapeutics has been recognized as an efficient strategy to overcome chemo resistance.
Natural compounds from medicinal plants are generally safe and are associated with low toxicity. Piperlongumine (PPL) is a very recently explored anticancer compound with cancer cell selective reactive oxygen species (ROS) inducing effect and inhibition of PI3K/Akt/Mtor pathways. PPL has shown anticancer activity at very low concentration (3-10 μM) in various cell lines. PPL has shown potential cytotoxic and antitumor properties on several types of cancer cells, including hematological, gastrointestinal, central nervous system, and other solid tumors. Its cytotoxicity was observed in the micromolar range in tumor cells, but not in normal cells.16-18
The present manuscript describes experiments to investigate the pharmacokinetic and pharmacodynamic interactions between PPL and DTX. Also, this study delineates the role of PPL as a P-gp and Cyp3A4 inhibitor.
Materials and methods
Chemicals and Drugs
PPL was purchased from Cayman Chemical (Ann Arbor, MI). Docetaxel, crystal violet and dimethyl sulfoxide (DMSO), Human liver microsomes (protein content 20 mg/ml) were purchased from Sigma-Aldrich (St. Louis, MO). DMEM, EMEM medium, fetal bovine serum and other cell culture materials were purchased from Lonza (Basel, Switzerland).
Cell Culture
Triple negative breast cancer cell lines; MDA-MB-231, HCC 70, HCC 1806, HS578T, MDAMB-468 and colon carcinoma cell line; caco-2 were obtained from American Type Culture Collection. All the cell lines were cultured in DMEM medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units per ml penicillin, and 100 mg/ml streptomycin. The caco-2 cells were cultured at 37°C in an atmosphere of 5% CO2 and 95% relative humidity with EMEM medium supplemented with fetal bovine serum (FBS) and penicillin-streptomycin antibiotic mixture, which was replaced every 2 days.
HPLC analysis
HPLC method was developed and validated to analyze DTX for CYP3A4, Pgp and pharmacokinetic study. Chromatographic separation was achieved on waters 717 instrument equipped with waters symmetry (250 mm/4.6mm/5μm) column using ACN:Phosphate buffer pH 3 (55:45) as mobile phase with flow rate of 0.8 ml/min, detected at 227 nm. Bioanalytical method to analyze DTX in plasma was developed and validated. Plasma DTX analysis was achieved at same chromatographic conditions. Retention time of DTX was 10.2±0.15 min respectively. Salting-out Liquid-Liquid Extraction (SALLE) method was used to extract DTX from plasma. Addition of 100 mg NaCl to 150 μl plasma samples followed by precipitation of plasma components and extraction of drug in acetonitrile. Samples were vigorously stirred and centrifuged at 5000 rpm for 10 min at 10 °C. Supernatant was mixed with 100 μl of Milli-Q water. Samples were directly loaded to HPLC auto-sampler for analysis. The accuracy study result showed percent recovery from 89 to 96 % at all the QC samples. Precision study showed that relative standard deviation (%RSD) was always <5 % at all the QC standards except lower limit of quantification (LLOQ, 40 ng/ml, %RSD<15). TST was analyzed using same HPLC system and column using ACN:Water (60:40) as mobile phase and 238 nm detection wavelength.
Microsomal enzyme assay
Microsomal enzyme assay was carried out by procedure described by Cheng Guo et al.19 Initially, the microsomal metabolism of testosterone (TST – a standard CYP3A4 substrate) in the presence of PPL and cyclosporine A (CYA - a standard CYP3A4 inhibitor) was evaluated. The same protocol was followed for evaluating the effect of PPL on DTX metabolism. Briefly, stock solutions of DTX, CYA, TST and PPL were prepared in methanol. For reaction samples, 20 μl of microsomal suspension was added to 430 μl HBSS solution and then 10 μl of PPL or CYA stock solution was added to achieve 50 μM final concentrations of PPL and CYA. Reaction was initiated by adding 30 μl of 50 mM NADPH. After 5 min of incubation at 37°C, 10 μl of DTX or TST stock solution was added to achieve 5 μM concentration of DTX or TST. For DTX or TST alone metabolism, 10 μl of HBSS was added in place of PPL and CYA stock solution. Sample of 100 μl was withdrawn and collected in microcentrifuge tube at specified time point and reaction was terminated by adding 200 μl of ACN followed by vigorous vortexing. Samples were centrifuged at 10000 rpm for 10 mins and supernatant was analyzed for drug content. Concentration of DTX or TST was analyzed by HPLC method.
Caco-2 uptake and permeability assay
Effect of PPL on caco-2 uptake and permeability of Pgp substrate was quantitatively and qualitatively analyzed using DTX and rhodamine 123 (R123), respectively.
For caco2 uptake studies, caco2 cells were plated in 6 well plates (Corning, NY, USA) at density of 50,000 cells/well. After 24 hr, cells were washed with PBS. Wells were treated with 1.5 ml of 50 μM PPL or 50 μM verapamil in HBSS. After 30 min, appropriate amount of DTX (10 mM in DMSO) and R123 (10 mM in DMSO) were added to individual well to achieve 5 μM of DTX and 1 μM rhodamine 123. For quantitative analysis, plates treated with DTX alone or with PPL/verapamil were incubated for 90 min and then washed twice with PBS. Cells were lysed using lysate buffer and DTX concentration was analyzed by HPLC. For qualitative analysis, plates were treated with R123 alone or with PPL/verapamil were incubated for 90 min and then washed twice with PBS. Fluorescence microscopic images were taken to observe the uptake of dye in the presence of PPL and verapamil.
Caco-2 permeability assay was carried out to analyze the efflux ratio of DTX as per the procedure described in our previous report.20,21 Briefly, the cells were harvested upon reaching approximately 80–90% confluence with 0.25% trypsin–EDTA and were grown on precoated 1.12 cm2, 0.4 μm pore polycarbonate membrane inserts in 12 mm × 12 transwell permeable support plates (Corning, NY, USA). The caco-2 cells were seeded onto the membranes at cell density of 50,000 cells/well. Each well was filled with 1.5 ml of media in the basolateral region and 0.5 ml in the apical region, was changed on alternate days. The confluent monolayers were used for the permeation studies at approximately 21 days post seeding. Monolayer integrity was accessed by measuring transepithelial electrical resistance (TEER) using the Millicell-ERS (Millipore, USA) and Lucifer yellow rejection assay. The transport studies were performed by removing the culture medium from the apical (A) and basolateral (B) regions of the cell monolayer. The cells were then washed with HBSS and replaced with HBSS (pH 6.4) in the apical region and HBSS (pH 7.4) in the basolateral region and equilibrated for 30 min. The monolayers were placed onto a plate shaker set at 30 rpm throughout the experiment to minimize stagnation of the boundary layer. For A-B transport analysis, 500 μl of 5 μM DTX with and without 50 μM PPL/verapamil was added to apical region while basolateral region was filled with 1.5 ml HBSS. For, B-A transport analysis, basolateral compartment was filled with 1.5 ml of DTX with and without PPL/verapamil at same concentration as above and apical region was filled with 0.5 ml HBSS. The plates were placed onto a shaker for continuous agitation (30 rpm) at 37°C. Samples were withdrawn from respective compartment after 90 min to analyze the amount of DTX permeated from A-B and B-A. Considering the surface area, initial concentration and time constant for A-B and B-A permeability study, the efflux ratio (ER) was calculated as per the equation given below.
Cytotoxicity assay
Cytotoxicity of individual drug and combination was evaluated at various concentrations. MDAMB-231 and MDA-MB-468 cells were seeded at a density of 10,000 cells/well in 96-well plates. Cells were allowed to adhere overnight and then exposed to different treatments for 48 h. The concentration range for DTX and PPL was 2-0.01 μM and 20-0.2 μM respectively. Stock solutions of DTX and PPL were prepared in DMSO. DTX: PPL molar ratio was varied from 1:1, 1:5 and 1:10. Cytotoxicity was assessed at the end of drug exposure using crystal violet assay as described previously.12,22 Absorbance was measured at 540 nm using a microplate reader (ChroMate 4300, FL, USA). Results were expressed as the % cell kill vs concentration of drug. IC50 value, the drug concentration at which 50 % growth inhibition is achieved, was calculated using Sigma Plot software, version 9.0 (Systat Software, San Jose, CA). Drug interactions were analyzed by calculating the combination index based on the analytical method of Chou and Talaly.23 Further the apoptosis inducing effect of DTX, PPL and combination was evaluated by acridine orange assay. Briefly, MDA-MB-231 cells were plated into 6 well plate (50,000/well) and treated with DTX (0.1 μM), PPL (1 μM) and combination in same concentration for 4 hr. Cells were washed with HBSS and incubated with acridine orange for 30 mins. Cells were thoroughly washed to remove extracellular dye and images were taken using fluorescence microscopy (Olympus, USA).
Migration assay
In vitro migration (scratch) assay was carried out as per the protocol with modification in the method of analyzing the results to improve accuracy and precision.24 MDA-MB-231 cells (10,000 cells per well) were plated 96 well plate in complete media, and cultured overnight. A uniform scratch was made in the center of well with a cell-scraper. Cells were treated with DTX, PPL and combination in different molar ratio. After 48 h cells were fixed with 0.5 % gluteraldehyde and stained with crystal violet for 15 minutes. The images of scratch area were taken using Olympus microscope to calculate the area using ImageJ software. Areas of gap (scratch) before and after treatment were analyzed for calculating the % bridging of migration area.
Western Blot Analysis
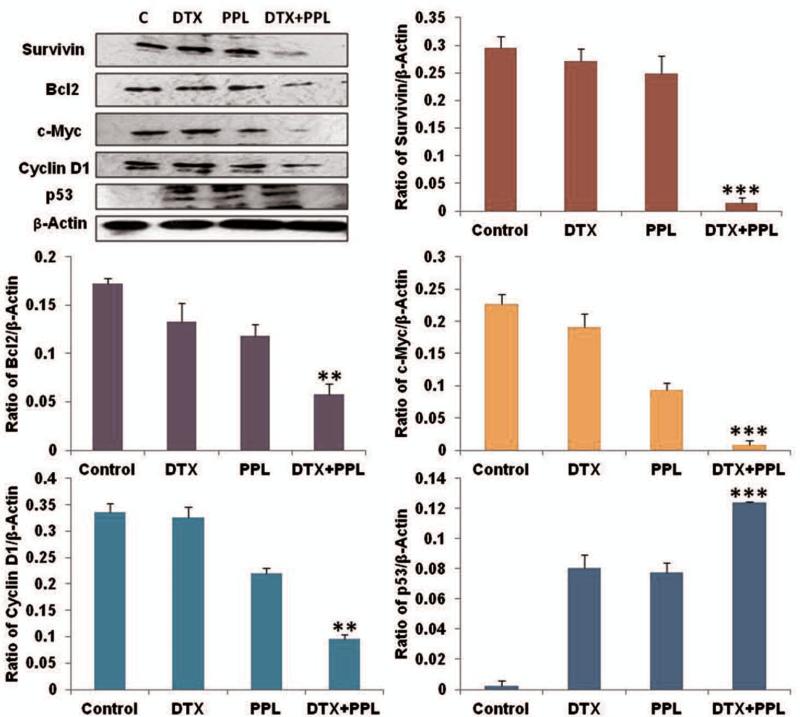
For western blot analysis MDA-MB-231 cells were seeded and cultured into flask and exposed to DTX, PPL and combination in 1:10 molar ratio. Whole-cell protein lysates were prepared according to standard protocol using RIPA buffer (Cell Signaling, Danvers, MA). Protein (50 mg) was loaded per well of a 10% SDS-PAGE gel using electrophoresis buffer (0.192 M glycine, 25 mM Tris and 0.1% SDS). After electrophoresis, the gel was transferred onto a nitrocellulose membrane (Bio-Rad Laboratorie, Hercules, CA) using transfer buffer (0.192 M glycine, 25 mM Tris, 0.025% SDS and 10% methanol). After the proteins were electrotransferred to nitrocellulose membranes, membranes were blocked in PBS-T with 5% BSA and probed with specific primary antibodies overnight. The primary antibodies such as Bcl-2, cyclin D1, survivin, p53 (1:1000) purchased from cell signaling technologies and c-Myc (1:400) from Santa Cruz, CA,USA were used and β-actin was used as a housekeeping protein. Proteins were detected with HRP conjugated secondary antibodies using SuperSignal West pico chemiluminescent solution (PIERCE, Rockford, IL). Quantification and analysis of the results were carried out with Image J software (v1.33u, NIH, USA).
In vivo pharmacokinetic in rats
In vivo pharmacokinetic studies of DTX with and without co-administration of PPL were carried out in Sprague Dawley rats. Rats were purchased from Harlan Inc. (Indianapolis, IN). The rats were housed and maintained in specific pathogen-free conditions in a facility approved by the American Association for Accreditation of Laboratory Animal Care. Food and water were provided ad libitum to the animals in standard cages. Animals were maintained at standard conditions of 37 °C and 60% humidity. All experiments were done in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Florida A&M University. After 1 week of acclimatization animals were divided into 3 groups (n=6); 1. Oral Taxotere® 2. Oral Taxotere® + PPL and 3. IV Taxotere®. For oral administration dose of DTX and PPL 10 mg/kg and 50 mg/kg, respectively while for IV dosing DTX was administered at 5 mg/kg dose. For oral groups, blood samples were collected at 0.5, 1, 2, 4,8, 12 and 24 hr, while for IV group blood samples were collected at 0.066, 0.5, 1, 2, 4 and 8 h. Blood samples were collected via tail vein. Blood samples were centrifuged at 10,000 rpm at 4 °C for 10 min to separate plasma. DTX plasma concentration data was analyzed by noncompartmental analysis using Kinetica 5® (Thermofisher, USA).
Statistical Analysis
Data are presented as mean±SEM. Comparisons were carried out using one-way analysis of variance - bonferroni test and student t-test. Statistical significance was acceptable to a level of P<0.05. All statistical analyses were performed using GraphPad prism V3.05 (GraphPad Software, La Jolla, CA) and microsoft excel.
Results
Microsomal metabolism of DTX
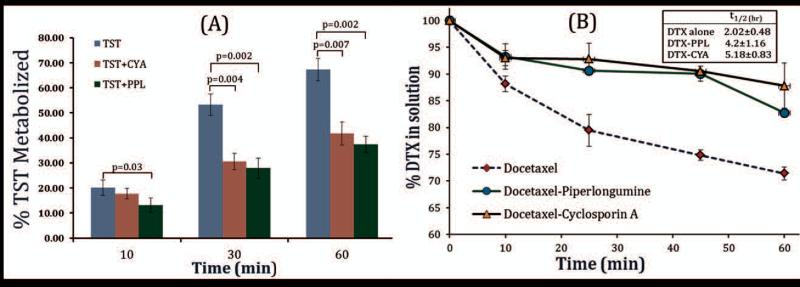
Results of microsomal metabolism of TST and DTX in the presence of PPL are depicted in Figure 1, which showed that at all the time points, PPL caused significant and comparable inhibition of TST metabolism to standard inhibitor CYA. Results of percent TST metabolized over 60 min, showed no statistical significant difference between CYA and PPL group. Both PPL and CYA showed nearly 50 percent reductions in TST metabolism. After confirming the CYP3A4 inhibitory effect of PPL on standard substrate, effect of PPL on DTX metabolism was evaluated. As shown in Figure 1, percent reduction DTX in solution over the period of 60 mins was analyzed by HPLC. DTX alone showed drastic decline in DTX concentration in solution while DTX co-incubated with PPL or CYA showed a slight initial decline followed by sustained the same DTX concentration for 45 mins. This suggests that PPL has effectively prevented the DTX metabolism in the presence of cytochrome enzymes. Rate of elimination and half-life of DTX were calculated from logC vs time graph. PPL increased the half-life of DTX by 2 fold which is expected to have a substantial contribution in improving its bioavailability. CyA caused 2.5 fold enhancements in DTX half-life but potent immunosuppression effect with negligible cytotoxicity strongly disfavors its use as DTX bioavailability enhancer.
Figure 1.
Effect of PPL and CYA on TST and DTX metabolism by human liver microsome. Final concentration of DTX/TST and PPL/CYA in reaction medium was 5 μM and 50 μM, respectively. Reactions were carried out in triplicate. PPL showed CYP3A4 inhibition comparable to standard inhibitor CYA. p values were calculated using t-test and p<0.05 considered as significant.
Caco-2 uptake study
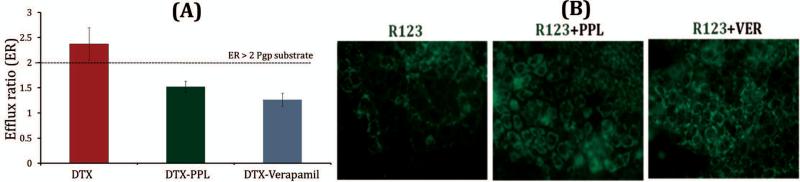
Caco-2 cellular uptake and Caco-2 monolayer permeability assay were performed to evaluate the effect of PPL on DTX and Rhodamine 123 (R123 - A fluorescent P-gp substrate) transport. Verapamil was taken as standard P-gp inhibitor. PPL showed 1.34 fold higher intracellular accumulation of DTX while 1.6 fold higher DTX uptake was observed with verapamil. Similar kind of results was observed for uptake study of R123. As shown in Figure 2, green fluorescence intensity was higher with R123+PPL group as compared to R123 only group. However, standard P-gp inhibitor verapamil showed highest intracellular concentration of R123 as depicted by high fluorescence intensity in Figure 2.
Figure 2.
P-gp efflux inhibition by PPL in Caco-2 cell line (A) Efflux ratio of DTX (B) Intracellular uptake of P-gp substrate fluorescent dye. Permeation was carried out at 5 μM DTX concentration with or without 50 μM PPL/Verapamil (n=3). PPL has significantly lowered the ER of DTX (t-test, p<0.05). Intracellular green fluorescence intensity was increased with co-incubation of R123 with PPL
Further, caco-2 monolayer permeability of DTX was evaluated using transwell. Efflux ratio (ER) of DTX alone and in combination with PPL or verapamil was calculated using A-B and B-A permeability co-efficient of DTX. Usually, drugs having ER > 2 are considered as P-gp substrate which severely limits their bioavailability.21 Interestingly, DTX ER was remarkably reduced from 2.37 to 1.52 when incubated with PPL (p<0.05) as shown in Figure 2. Verapamil as a standard P-gp inhibitor showed further reduction in ER but it is not a suitable candidate for this purpose because of its cardiovascular pharmacological profile. No statistically significant difference was observed between PPL and verapamil group (p>0.05). This P-gp inhibitory effect is not only important in enhancing oral bioavailability of anticancer drugs but also facilitates drug uptake in multidrug resistance tumors (studies under evaluation).
PPL Synergistically Enhanced DTX cytotoxicity
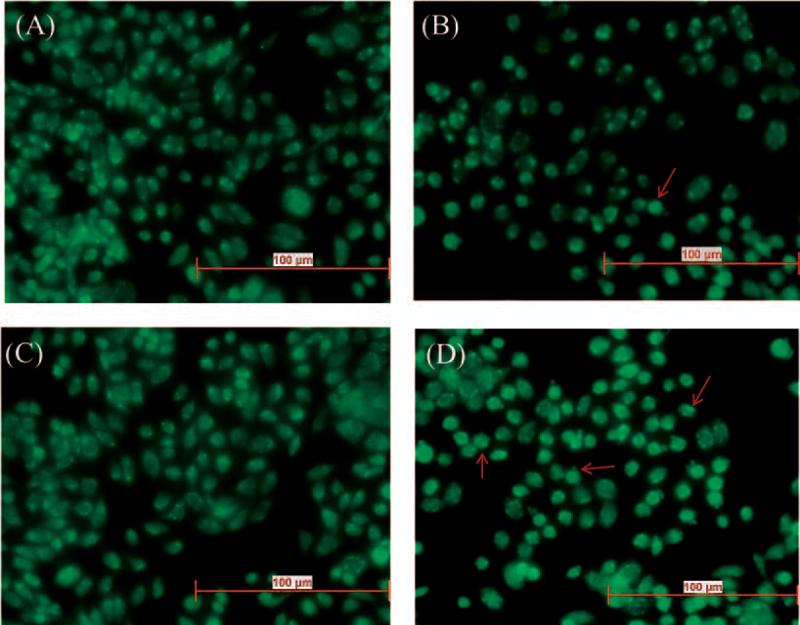
Cytotoxicity of DTX was found to be enhanced on co-incubation with PPL. Calculation of IC50 values and combination index suggested a synergistic interaction between DTX and PPL. IC50 value for DTX only, its combination with PPL (1:10 molar ratio) and combination index in various TNBC cell lines are given in Table 1. Significant reduction in IC50 value of DTX was observed with PPL. Surprisingly, DTX-PPL combination index values were much lower in African American origin TNBC cell lines compared to Caucasian cell lines. Interestingly, DTX cytotoxicity was found to be higher even at non-cytotoxic concentration of PPL. PPL has not shown a significant cell killing below 4 μM. However, even at non-cytotoxic concentrations, PPL augmented the activity of DTX. This suggests that PPL reduces the threshold for the DTX induced apoptosis. Images of acridine orange apoptosis assay have been shown in Figure 3. Cellular and nuclear morphology were observed to evaluate the apoptosis in DTX, PPL and combination treated cells. As shown by acridine orange assay (Figure 3), number of cells with compromised chromatin is higher in combination treated group compared to control or single drug groups.
Table 1.
Combination index of DTX-PPL on various TBNC cell lines
| Cell lines | IC50 (μM) | Combination index | |
|---|---|---|---|
| DTX only | DTX in combination | ||
| MDA-MB-231 | 0.458±0.081 | 0.12±0.03 | 0.57 |
| HS578T | 0.077±0.013 | 0.039±0.009 | 0.6 |
| MDA-MB-468 | 0.36±0.069 | 0.00046±0.0002 | 0.0022 |
| HCC1806 | 0.0022±0.001 | 0.00029±0.0002 | 0.16 |
| HCC70 | 0.019±0.008 | 0.001074±0.0008 | 0.058 |
Figure 3.
Acridine orange apoptosis assay (a) Control (b) DTX (c) PPL (d) DTX+PPL in MDAMB-231 cell line after 4 hrs of drug exposure (n=6 wells per sample). Red arrows indicate apoptotic cells. Combination showed significantly higher number of apoptotic cells compared to individual drug.
Migration assay
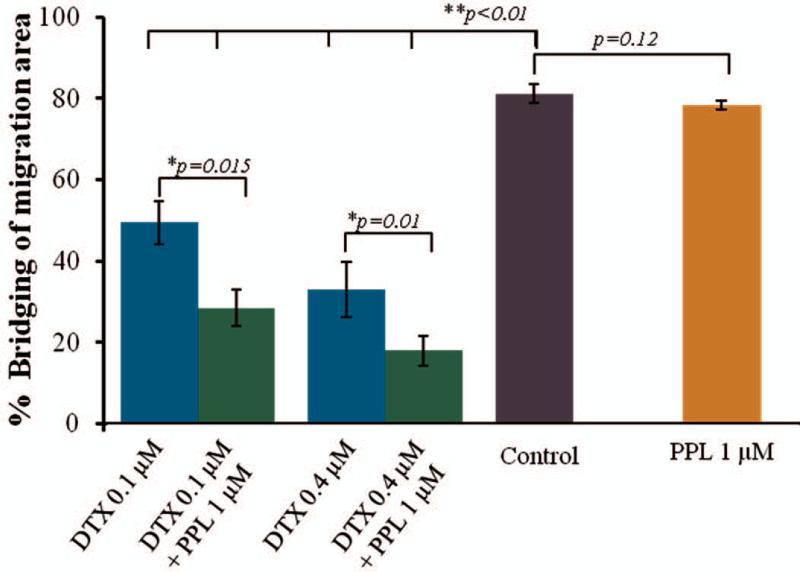
Results of migration assay further confirmed the enhancement of DTX activity in the presence of PPL. The in vitro scratch assay is a simple method to study cell migration in vitro. As shown in Figure 4, control group showed > 80 % bridging of scratch within 48 hr while DTX and PPL combination treated group showed significant inhibition of proliferation and migration of cells within the scratched area. PPL has no cytotoxcity at 1 μM as shown in cytotoxic studies above. Migration assay also showed similar behavior at 1 μM PPL concentration. PPL failed to show inhibition of cell migration therefore percent bridging of migration area was comparable to control. However as anticipated, PPL synergistically enhanced the anti-migration effect of DTX. DTX at 0.1 μM and 0.4 μM concentrations showed 49.3 and 33.8 percent bridging of scratch area, respectively. However, combination of DTX with PPL showed just 28.5 and 17.95 percent bridging of scratch area at 0.1 μM and 0.4 μM DTX concentrations, respectively. The results clearly indicated that even at non-cytotoxic concentration, PPL promoted the DTX anticancer activity. Calculation of area of scratch or gap is more accurate than calculating length of scratch because gap was found to be uneven and showed very high standard deviation.
Figure 4.
In vitro migration assay. Cells were treated with DTX (0.1 and 0.4 μM) with 1 μM PPL in 96 well plate for 48 hrs after scratch formation (n=6). PPL showed significant reduction in % bridging of migration area. ANOVA-bonferroni test was used to calculated the p values and p<0.05 considered as significant.
PPL Augmented DTX Induced Alterations protein expression
We examined the expression of various markers like growth promoting (Cyclin D1), pro-survival (Bcl2 and Survivin) and tumor suppressor (p53) and proto-oncogene (c-Myc) by western blot. The expression of survivin, Bcl2, Cyclin D1 and c-Myc decreased significantly as compared to control lysates (Figure 5). Bcl-2 expression was found to be reduced by 2.59, 2.74 and 3.85 fold respectively in DTX, PPL and DTX+PPL groups compared to untreated control lysates. The DTX+PPL produced repression in the Bcl-2 expression compared to DTX and PPL only, supporting the superior anticancer activity of combination in TNBC. Similarly, the cell survival marker survivin expression was also significantly down regulated (1.03 and 1.19 fold and 3.89 fold) in DTX, PPL and DTX+PPL compared to control groups. The cell proliferating related cyclin D1 expression was found to be higher in control tumor lysates. Treatment with DTX, PPL and DTX+PPL resulted in significantly down regulation of cyclin D1. The relative expressions were found to be reduced 0.8, 2.10 and 4.41 fold significantly in DTX, PPL and DTX+PPL, respectively compared to untreated control tumor lysates. These results indicated that PPL and DTX alone as well as in combination act as anti-tumor agents. Compared to untreated control cells, DTX and PPL treated cells had significantly increased expression of tumor suppressor protein p53, however, DTX and PPL combination was more effective in terms of increasing the expression of p53 (3.9 fold) than DTX (1.2 fold) and PPL (1.1 fold), further confirming the superior anticancer effects of DTX+PPL combination in breast cancer.
Figure 5.
Western blot and densitometry analysis of proliferative and apoptotic markers. Flask were treated with DTX 0.1 μM and/or 1 μM PPL for 48 hr (n=3). ANOVA-bonferroni test was used to calculated the p values and p<0.05 considered as significant.
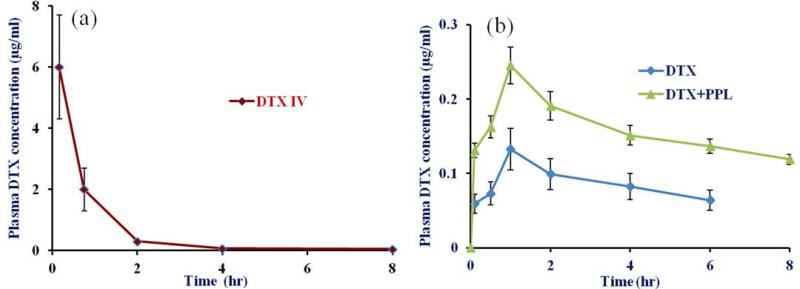
In vivo pharmacokinetic in rats
Pharmacokinetic profile of DTX after IV injection and oral administration with and without concomitant administration of PPL is shown in Figure 6 (a) and (b), respectively. Mean pharmacokinetic parameters are summarized in Table 2. DTX plasma concentration after oral administration was found to be higher at all the time points in DTX-PPL group. Further, DTX alone group showed non-detectable plasma concentration after 8 hr while DTX-PPL groups showed non-detectable concentration after 12 hr. All parameters have contributed to higher AUC of DTX in combination. Combination group showed 1.68 fold higher bioavailability compared to single drug.
Figure 6.
Pharmacokinetic profile of DTX after (a) IV and (b) oral administration of DTX with and without concomitant administration of PPL in SD rats (n=6). Oral dose for DTX and PPL were 10 mg/kg and 50 mg/kg, respectively while for IV dosing DTX was administered at 5 mg/kg via tail vein.
Table 2.
Pharmcokinetic parameters of DTX after oral administration in SD rats in the presence and absence of PPL (mean±SD, n=6)
| Parameters | DTX alone | DTX with PPL | DTX IV |
|---|---|---|---|
| Cmax (μg/ml) | 0.13±0.028 | 0.24±0.026 | 6.1±1.7 |
| AUC (μg.h/ml) | 0.98±0.35 | 1.65±0.48 | 5.29±1.1 |
| T1/2 (h) | 5.2±0.93 | 6.62±1.3 | 6.67±1.6 |
| MRT (h) | 8.11±1.8 | 9.88±2.8 | 1.99±0.36 |
| Absolute BA (%) | 9.26 | 15.2 | - |
| Relative BA (%) | - | 168 | - |
Discussion
Parenteral delivery of paclitaxel and docetaxel is associated with severe dose dependent and excipients induced side effects.1,25 Extensive drug metabolism by gut and liver microsomal enzymes is a major bioavailability limiting factor for taxane.1,7,8 The objective of this study was to investigate the ability of PPL to improve the bioavailability and anticancer activity of DTX through its interaction with pre-systemic and hepatic metabolic enzymes as well as the classical efflux pump proteins. The cytochromal enzyme inhibitory effect of PPL was first evaluated using a standard CYP3A4 substrate testosterone (TST) in comparison to standard inhibitor cyclosporine A (CYA) and then confirmed using DTX. The human liver microsomal assay was carried out using pooled human liver microsomes from 3 different sources and no difference in metabolism of TST was observed. Inhibitory effect of PPL was firstly evaluated on standard probe substrate, testosterone (TST) and then on DTX. Further, P-glycoprotein dependent efflux of DTX from the basolateral to apical side is another major obstacle that limits for oral absorption of drugs. Importantly, many of the CYP3A4 substrate is also affected by P-gp mediated transport because there are some overlapping substrate specificities between CYP3A4 and P-gp.26 It was therefore, important to investigate if PPL is a P-gp substrate or not.
The enhanced uptake of R123 and DTX and reduced efflux ratio of DTX (to<2) in caco-2 monolayer confirmed the P-gp inhibitory effect of PPL; suggestive of possible consideration for use in the treatment of multi drug resistance tumors. A study by Hendrikx et al. demonstrated that P-gp inhibitors have more pronounced effect on paclitaxel bioavailability while CYP3A4 inhibitors are more effective in enhancing docetaxel bioavailability.11 Additionally, oral co-administration of the taxanes with elacridar (potent third generation P-gp inhibitor) increased plasma concentrations of paclitaxel by 10.7 fold and docetaxel by 4 fold11, respectively. Co-administration with ritonavir (potent CYP3A4 inhibitor) resulted in 2.5 fold and 7.3 fold enhancement in paclitaxel and docetaxel plasma concentrations, respectively.11 Cyclosporine A (CYA) is a strong P-gp and CYP3A4 inhibitor and has been extensively studied for enhancing oral bioavailability of taxanes in animals and humans. The absolute bioavailability of oral docetaxel was 8% ± 6% and 90% ± 44% without and with CYA, respectively.27
There are synthetic or natural CYP3A4 substrates and/or P-gp inhibitors like morin, curcumin, silymarin, tesmilifene which have been reported to enhance oral bioavailability of paclitaxel or docetaxel from negligible to moderate extent by virtue of their CYP3A4 or P-gp inhibitory effect.28-31 However, PPL has proven to be a far better candidate for oral delivery of taxanes and has displayed a distinct edge over aforementioned compounds because of its anticancer and DTX synergistic activity. We can expect that the combination will be more effective in vivo in pharmacodynamics studies because inhibition of liver microsomal metabolism of DTX will possibly lead to reduced clearance which will enhance mean residence time (MRT) in the body.
Manipulation of apoptosis has become one of the popular strategies for cancer treatment. Apoptosis studies demonstrated the higher cell killing by DTX-PPL combination compared to individual drugs. PPL has been demonstrated to selectively enhance the accumulation of ROS in cancer cells, which could be the major contributing factor for its potentiating activity of DTX.16 Further, our data demonstrated that the combination of PPL and DTX induced apoptotic cell death as indicated by typical apoptotic morphology i.e. nuclear morphological alterations (membrane blabbing, chromatin condensation and nuclear fragmentation) and positive AO staining. Number of apoptotic cells was higher in combination treated group. Rapid induction of apoptosis in co-treated group could possibly play a role in synergistic activity. PPL mediated sensitization of cancer cells for DTX lead to reduction in IC50 of DTX in various TNBC cell line. This will lower the minimum therapeutic concentration required for anti-cancer activity of DTX which will help in successful oral DTX chemotherapy. There are several reports on a similar kind of alkaloid, piperine, which is obtained from both piper nigrum and piper longum. Piperine has been reported to act as bioenhancer for boosting the oral bioavailability of various CY3A4 substrates. However, our in vitro studies with piperine did not show potent anticancer and synergistic effect with docetaxel as was shown by PPL (data not shown). Therefore, PPL is a better option for promoting oral docetaxel than similarly related piperine.
Cancer cell migration is fundamentally required for breast tumor invasion and metastasis. MDA-MB-231 cells are more migratory than MCF-7 cells. Aberrantly activated migratory machinery and signaling contributing to invasive phenotype of MDA-MB-231 making them highly malignant.32 Significant synergism of PPL and DTX was also observed in migration assay, which again supports its synergistic role in anti-proliferative activity of DTX. Therefore, we are anticipating that in vivo TNBC studies will show a reduction in metastatic burden which will eventually result in prolonged life expectancy. Further, a recent report by Chanvorachote et al., showed that superoxide anion and hydrogen peroxide down-regulated Cav-1 expression and inhibited cell migration and invasion.33 However, in our study we found that PPL showed no anti-migration effect at concentration below 4 μM. We have used 1 μM PPL concentration for migration assay with DTX. Though, PPL could not exert anti-migration effect at such low concentration, it was found to promote the anti-migration effect of DTX. We have also performed clonogenic assay of DTX PPL combination as described previously34 but no synergism was observed in it (data not shown) suggesting that PPL only plays role in facilitating the early apoptosis in DTX treated cells but has insignificant role to play in its clonogenic effect. TNBC cell lines evaluated in current research paper have different origin. MDA-MB-231 and HS578T are of Caucasian origin while HCC 70, HCC 1806 and MDA-MB-468 are of African American origin. African American triple-negative breast cancers are more aggressive in nature, occurring at a younger age, are larger, rapidly proliferative and induce more frequent deaths due to lack of appropriate therapy. The lifetime risk of TNBC is highest in African American women (1.98%), compared with 0.77% for Asians, 1.04 % for Hispanics and 1.25% for whites.35 Interestingly, the IC50 vlaues of DTX and combination index of DTX-PPL were found to be significantly lower in African American origin TNBC cell lines. However, IC50 of PPL was found to be similar in all the cell lines. This suggests that DTX-PPL combination could be very successful in treating TNBC in African American populations. Detailed investigation in this direction is sought to understand the cytotoxic disparities of DTX-PPL in TNBC. There is no such report available on disparity in cytotoxicity of a drug in Caucasian and African American cell lines. In this context, current finding of profound DTX-PPL synergism in African American cell line versus Caucasian TNBC cell lines could be helpful in proposing an effective chemotherapeutic approach for the treatment of TNBC. This aspect is currently under investigation in our laboratory and we are aware that it needs more exploration.
Lastly, the combination of PPL and DTX in the current study depicted potential anticancer activities due to significant suppression of various proliferative and apoptotic molecular markers associated with triple negative breast cancer. We observed that the combination of PPL and DTX downregulated the expression of Bcl2 and survivin. These observations are in agreement with the previous studies regarding the apoptotic inducing effect of PPL in other cancer cell types.16-18 Survivin is highly expressed in most cancers and is expected to play a significant role in chemotherapeutic resistance, increased tumor recurrence, and shorter patient survival. Recently, Gong et al. reported that PPL along with cisplatin or paclitaxel induces apoptosis in ovarian cancer cells.18 They observed that treatment with PPL led to cell cycle arrest in G2/M phase and accumulation of the intracellular ROS in a dose and time dependent manner. Moreover, in our study PPL synergistically enhanced the anticancer effect of DTX suggesting that PPL might be a potential chemosensitizer for breast cancer chemotherapy. Hence we construe from this study that lower concentrations of PPL and DTX used in combination may produce a synergistic anticancer effect that warrants further investigation for its potential clinical applications. The decreased c-Myc and cyclin D1 levels further supported the anti-apoptotic effects of these drugs because these are important components in the cell cycle progression, apoptosis inhibition and cellular transformation. Further, increased level of tumor suppressor protein p53 also suggests the promising synergistic anticancer effects of PPL-DTX combination. While PPL alone displayed anti-cancer activity, PPL-DTX combination displayed enhanced growth inhibition compared to DTX or PPL only, which might be due to the synergism by this combination.
Taken together, we are reporting for the first time a compound, PPL, meeting all the desired characteristics for enhancing the oral delivery of DTX. Based on encouraging in vitro data collected from DTX-PPL combination experiments, our lab is currently working on in vivo anti-cancer efficacy studies. There are severe limitations on achieving and sustaining high DTX plasma concentration upon oral administration. We anticipate overcoming those limitations by combining oral DTX regimen with PPL.
As described in pharmacokinetic studies, PPL successfully enhanced the plasma concentration of DTX in SD rats. CYP3A4 is major limiting factor in compromised oral bioavailability of DTX. Based on results of in vitro caco-2 permeation and microsomal inhibition studies, we have anticipated improved bioavailability of DTX when given with PPL in vivo. Higher plasma concentration of DTX following oral administration is mostly depend on reduced interaction with metabolizing enzyme and efflux pump in GIT. Peak plasma concentration was reached at similar time in both groups but DTX concentration was 1.84 fold higher in combination group. Interestingly, DTX concentration was maintained above 100 ng/ml upto 8 hrs in combination groups while DTX alone showed significantly lower concentration at all the time points. Due to higher bioavailability, amount of unabsorbed DTX in GIT would be reduced, which might be helpful in minimizing GIT related toxicities. Further, higher and prolong DTX plasma concentration and AUC with combination group will significantly enhance its anti-cancer efficacy at same dose. Similar to in vitro, PPL could have potentially inhibited the intestinal brush border microsomal enzyme and P-gp. Further studies are now being conducted by using nanoformulation of both drugs to improve the oral bioavailability of DTX.
Conclusion
PPL could be a promising candidate satisfying crucial requirements for enhancing oral delivery of DTX. Human liver microsome and Caco-2 permeability studies provided the strong evidence for dual inhibitory effect of PPL, which will help in reducing metabolism and efflux of DTX. On the other hand, promising anticancer activity and strong synergism with DTX in TNBC cell lines will facilitate reduction in therapeutic dose of DTX. Thus, oral DTX-PPL could be a very promising approach for neoadjuvant or adjuvant chemotherapy for the treatment of TNBC and other cancers.
Supplementary Material
Acknowledgement
This research was supported with funding from: the National Institutes of Health's Minority Biomedical Research Support (MBRS)-SC1 program [Grant#SC1 GM092779-01]; the National Institute on Minority Health and Health Disparities (NIMHD) P20 program [Grant#1P20 MD006738-03]; and the Department of Defense (DOD) Breast Cancer Program [Grant # W81XWH-11-1-0211]
Abbreviations
- CI
Combination Index
- CYA
Cyclosporine A
- DTX
Docetaxel
- EGFR
Epidermal Growth Factor Receptor
- ER
Efflux ratio
- PARP
Poly-ADP Ribose Polymerase
- PPL
Piperlongumine
- R123
Rhodamine 123
- TEER
Transepithelial Electrical Resistance
- TNBC
Triple Negative Breast Cancer
- TST
Testosterone
- VEGF
Vascular Endothelial Growth Factor
References
- 1.Jibodh RA, Lagas JS, Nuijen B, Beijnen JH, Schellens JH. Taxanes: old drugs, new oral formulations. European journal of pharmacology. 2013;717(1-3):40–46. doi: 10.1016/j.ejphar.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 2.Engels FK, Mathot RA, Verweij J. Alternative drug formulations of docetaxel: a review. Anti-cancer drugs. 2007;18(2):95–103. doi: 10.1097/CAD.0b013e3280113338. [DOI] [PubMed] [Google Scholar]
- 3.Rodler E, Korde L, Gralow J. Current treatment options in triple negative breast cancer. Breast disease. 2010;32(1-2):99–122. doi: 10.3233/BD-2010-0304. [DOI] [PubMed] [Google Scholar]
- 4.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clinical breast cancer. 2009;9(Suppl 2):S73–81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. The oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of clinical investigation. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrikx JJ, Lagas JS, Rosing H, Schellens JH, Beijnen JH, Schinkel AH. P-glycoprotein and cytochrome P450 3A act together in restricting the oral bioavailability of paclitaxel. International journal of cancer Journal international du cancer. 2013;132(10):2439–2447. doi: 10.1002/ijc.27912. [DOI] [PubMed] [Google Scholar]
- 8.Patel K, Patil A, Mehta M, Gota V, Vavia P. Oral delivery of paclitaxel nanocrystal (PNC) with a dual Pgp-CYP3A4 inhibitor: preparation, characterization and antitumor activity. International journal of pharmaceutics. 2014;472(1-2):214–223. doi: 10.1016/j.ijpharm.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Koolen SL, Oostendorp RL, Beijnen JH, Schellens JH, Huitema AD. Population pharmacokinetics of intravenously and orally administered docetaxel with or without co-administration of ritonavir in patients with advanced cancer. British journal of clinical pharmacology. 2010;69(5):465–474. doi: 10.1111/j.1365-2125.2010.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helgason HH, Koolen SL, Werkhoven E, Malingre MM, Kruijtzer CM, Huitema AD, Schot ME, Smit WM, Beijnen JH, Schellens JH. Phase II and pharmacological study of oral docetaxel plus cyclosporin A in anthracycline pre-treated metastatic breast cancer. Current clinical pharmacology. 2014;9(2):139–147. doi: 10.2174/1574884708666131111193403. [DOI] [PubMed] [Google Scholar]
- 11.Hendrikx JJ, Lagas JS, Wagenaar E, Rosing H, Schellens JH, Beijnen JH, Schinkel AH. Oral co-administration of elacridar and ritonavir enhances plasma levels of oral paclitaxel and docetaxel without affecting relative brain accumulation. British journal of cancer. 2014;110(11):2669–2676. doi: 10.1038/bjc.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes A, Shaik MS, Chatterjee A, Singh M. Evaluation of an aerosolized selective COX-2 inhibitor as a potentiator of doxorubicin in a non-small-cell lung cancer cell line. Pharmaceutical research. 2003;20(9):1485–1495. doi: 10.1023/a:1025774630993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Ichite N, Singh M. 15-Deoxy-Delta12,14-prostaglandin J2 enhances docetaxel anti-tumor activity against A549 and H460 non-small-cell lung cancer cell lines and xenograft tumors. Anti-cancer drugs. 2007;18(1):65–78. doi: 10.1097/CAD.0b013e3280101006. [DOI] [PubMed] [Google Scholar]
- 14.Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Singh M. Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharmaceutical research. 2006;23(9):2094–2106. doi: 10.1007/s11095-006-9074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chougule MB, Patel A, Sachdeva P, Jackson T, Singh M. Enhanced anticancer activity of gemcitabine in combination with noscapine via antiangiogenic and apoptotic pathway against non-small cell lung cancer. PloS one. 2011;6(11):e27394. doi: 10.1371/journal.pone.0027394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475(7355):231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Makhov P, Golovine K, Teper E, Kutikov A, Mehrazin R, Corcoran A, Tulin A, Uzzo RG, Kolenko VM. Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. British journal of cancer. 2014;110(4):899–907. doi: 10.1038/bjc.2013.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong LH, Chen XX, Wang H, Jiang QW, Pan SS, Qiu JG, Mei XL, Xue YQ, Qin WM, Zheng FY, Shi Z, Yan XJ. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxidative medicine and cellular longevity. 2014;2014:906804. doi: 10.1155/2014/906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Han Y, Meng X, Sun X, Yu Q, Li Y, Wan L, Huo Y, Guo C. Effect of regular organic solvents on cytochrome P450-mediated metabolic activities in rat liver microsomes. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(11):1922–1925. doi: 10.1124/dmd.110.033894. [DOI] [PubMed] [Google Scholar]
- 20.Godugu C, Patel AR, Doddapaneni R, Somagoni J, Singh M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PloS one. 2014;9(3):e89919. doi: 10.1371/journal.pone.0089919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X, Skolnik S, Chen X, Wang J. Attenuation of intestinal absorption by major efflux transporters: quantitative tools and strategies using a Caco-2 model. Drug metabolism and disposition: the biological fate of chemicals. 2011;39(2):265–274. doi: 10.1124/dmd.110.034629. [DOI] [PubMed] [Google Scholar]
- 22.Chougule MB, Patel AR, Jackson T, Singh M. Antitumor activity of Noscapine in combination with Doxorubicin in triple negative breast cancer. PloS one. 2011;6(3):e17733. doi: 10.1371/journal.pone.0017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chougule M, Patel AR, Sachdeva P, Jackson T, Singh M. Anticancer activity of Noscapine, an opioid alkaloid in combination with Cisplatin in human non-small cell lung cancer. Lung cancer. 2011;71(3):271–282. doi: 10.1016/j.lungcan.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature protocols. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 25.Patel K, Patil A, Mehta M, Gota V, Vavia P. Medium chain triglyceride (MCT) rich, paclitaxel loaded self nanoemulsifying preconcentrate (PSNP): a safe and efficacious alternative to Taxol. Journal of biomedical nanotechnology. 2013;9(12):1996–2006. doi: 10.1166/jbn.2013.1710. [DOI] [PubMed] [Google Scholar]
- 26.Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clinical pharmacokinetics. 2003;42(1):59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Malingre MM, Richel DJ, Beijnen JH, Rosing H, Koopman FJ, Ten Bokkel Huinink WW, Schot ME, Schellens JH. Coadministration of cyclosporine strongly enhances the oral bioavailability of docetaxel. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(4):1160–1166. doi: 10.1200/JCO.2001.19.4.1160. [DOI] [PubMed] [Google Scholar]
- 28.Ganta S, Devalapally H, Amiji M. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. Journal of pharmaceutical sciences. 2010;99(11):4630–4641. doi: 10.1002/jps.22157. [DOI] [PubMed] [Google Scholar]
- 29.Choi YH, Suh JH, Lee JH, Cho IH, Lee MG. Effects of tesmilifene, a substrate of CYP3A and an inhibitor of P-glycoprotein, on the pharmacokinetics of intravenous and oral docetaxel in rats. The Journal of pharmacy and pharmacology. 2010;62(8):1084–1088. doi: 10.1111/j.2042-7158.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 30.Park JH, Park JH, Hur HJ, Woo JS, Lee HJ. Effects of silymarin and formulation on the oral bioavailability of paclitaxel in rats. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2012;45(3):296–301. doi: 10.1016/j.ejps.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Choi BC, Choi JS, Han HK. Altered pharmacokinetics of paclitaxel by the concomitant use of morin in rats. International journal of pharmaceutics. 2006;323(1-2):81–85. doi: 10.1016/j.ijpharm.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Miller EM, Gallo KA. MLK3 is critical for breast cancer cell migration and promotes a malignant phenotype in mammary epithelial cells. Oncogene. 2010;29(31):4399–4411. doi: 10.1038/onc.2010.198. [DOI] [PubMed] [Google Scholar]
- 33.Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, Chanvorachote P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. The Journal of biological chemistry. 2010;285(50):38832–38840. doi: 10.1074/jbc.M110.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh M, Ghose T, Faulkner G, Kralovec J, Mezei M. Targeting of methotrexate-containing liposomes with a monoclonal antibody against human renal cancer. Cancer research. 1989;49(14):3976–3984. [PubMed] [Google Scholar]
- 35.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.