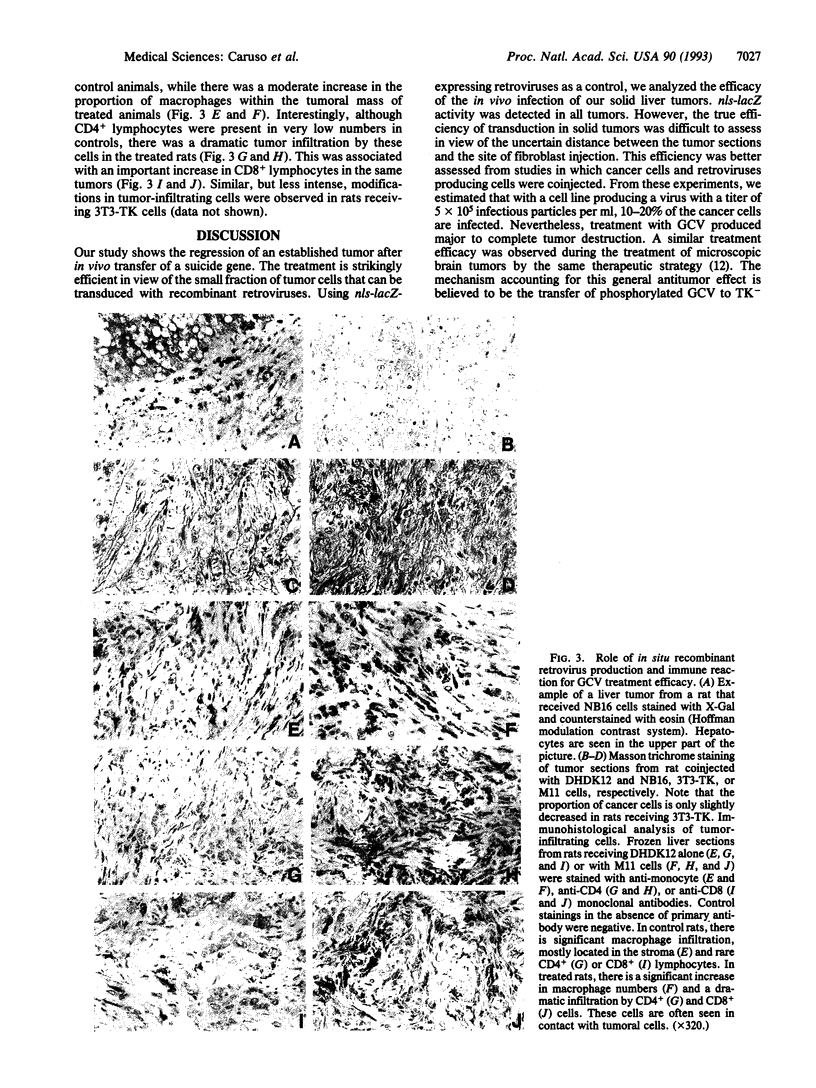
Abstract
The herpes simplex virus type 1 thymidine kinase (HSV1-TK) converts nontoxic nucleoside analogs such as ganciclovir into phosphorylated compounds that act as chain terminators and specifically kill dividing cells. This property could be exploited for the treatment of tumors that are made up of rapidly dividing cells invading a nonproliferating tissue. For this purpose, specific expression of the suicide gene into dividing tumor cells can be further targeted by using retroviral-mediated gene transfer. We investigated whether the direct intratumoral transduction of a suicide gene might induce the elimination of malignant solid tumors. Rats with established macroscopic liver metastases were given an intratumoral injection of packaging cells producing either HSV1-TK- or lacZ-expressing recombinant retroviral particles. All rats were next treated with ganciclovir. A dramatic regression of the tumor volume was observed in the HSV1-TK-treated animals. The residual tumors were mostly made up of a massive fibrotic reaction, with the mean cancer cell mass being reduced approximately 60-fold compared to controls. In some animals, the residual tumors were devoid of cancer cells. This treatment efficacy appears in part due to a "bystander effect" in which phosphorylated ganciclovir could be transferred from cell to cell and to an active local immune reaction evidenced by massive infiltration of the tumors by macrophages and both CD4+ and CD8+ lymphocytes. This efficient therapeutic approach might be an ultimate treatment for disseminated liver metastases in humans and could also be applied to treatment of a large variety of solid tumors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigner K., Walther H., Tonn J., Wenzl A., Hechtel R., Merker G., Schwemmle K. First experimental and clinical results of isolated liver perfusion with cytotoxics in metastases from colorectal primary. Recent Results Cancer Res. 1983;86:99–102. doi: 10.1007/978-3-642-82025-0_18. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Heyman R., Hsi M., Evans R. M. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson G., Gullberg B., Hafström L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105(1):20–23. doi: 10.1007/BF00391826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso M., Klatzmann D. Selective killing of CD4+ cells harboring a human immunodeficiency virus-inducible suicide gene prevents viral spread in an infected cell population. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):182–186. doi: 10.1073/pnas.89.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R., Crumpacker C. S., Schnipper L. E. Inhibition of herpes simplex virus transformed and nontransformed cells by acycloguanosine: mechanisms of uptake and toxicity. Virology. 1981 Aug;113(1):9–19. doi: 10.1016/0042-6822(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Elion G. B. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Ezzeddine Z. D., Martuza R. L., Platika D., Short M. P., Malick A., Choi B., Breakefield X. O. Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biol. 1991 Jun;3(6):608–614. [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., McGuirt P. V., Keller P. M., Fyfe J. A., Elion G. B. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980 Apr 30;102(2):420–430. doi: 10.1016/0042-6822(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Martin M. S., Martin F., Michel M. F., Hammann A. Growth characteristics and metastatic potential of seven intestinal carcinoma lines serially passaged in syngeneic rats. Virchows Arch A Pathol Anat Histopathol. 1991;418(3):193–199. doi: 10.1007/BF01606056. [DOI] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolten F. L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986 Oct;46(10):5276–5281. [PubMed] [Google Scholar]
- Moolten F. L., Wells J. M. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990 Feb 21;82(4):297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Rapp F. Anticellular effects of 9-(2-hydroxyethoxymethyl) guanine against herpes simplex virus-transformed cells. J Gen Virol. 1979 Oct;45(1):227–230. doi: 10.1099/0022-1317-45-1-227. [DOI] [PubMed] [Google Scholar]
- Panis Y., Caruso M., Houssin D., Andreoletti M., Khayat D., Salzmann J. L., Klatzmann D. Traitement de tumeurs hépatiques expérimentales par transfert de gène suicide in vivo chez le rat. C R Acad Sci III. 1992;315(13):541–544. [PubMed] [Google Scholar]
- Panis Y., Ribeiro J., Chrétien Y., Nordlinger B. Dormant liver metastases: an experimental study. Br J Surg. 1992 Mar;79(3):221–223. doi: 10.1002/bjs.1800790309. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A. Immunotherapy and gene therapy of cancer. Cancer Res. 1991 Sep 15;51(18 Suppl):5074s–5079s. [PubMed] [Google Scholar]
- Salzmann J. L., Peltier-Koch F., Bloch F., Petite J. P., Camilleri J. P. Morphometric study of colonic biopsies: a new method of estimating inflammatory diseases. Lab Invest. 1989 Jun;60(6):847–851. [PubMed] [Google Scholar]
- Scheele J., Stangl R., Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990 Nov;77(11):1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- Short M. P., Choi B. C., Lee J. K., Malick A., Breakefield X. O., Martuza R. L. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990 Nov;27(3):427–439. doi: 10.1002/jnr.490270322. [DOI] [PubMed] [Google Scholar]