Abstract
A large percentage of lymphocytes in the blood of cattle express the γδ T-cell receptor, but specific functions for these cells have not yet been clearly defined. There is evidence, however, that human, murine, and bovine γδ T cells have a role in the immune response to mycobacteria. This study investigated the ability of bovine γδ T cells to expand and produce gamma interferon (IFN-γ) in response to stimulation with mycobacterial products. Bovine γδ T cells, isolated from the peripheral blood of healthy cattle, expanded following in vitro stimulation with live mycobacteria, mycobacterial crude cell wall extract, and Mycobacterium bovis culture filtrate proteins. In addition, purified γδ T cells, cocultured with purified monocytes and interleukin-2, consistently produced significant amounts of IFN-γ in response to mycobacterial cell wall. The IFN-γ-inducing component of the cell wall was further identified as a proteolytically resistant, non-sodium dodecyl sulfate-soluble component of the mycolylarabinogalactan peptidoglycan.
The genes for the γ chain of the T-cell receptor (TCR) were discovered in 1984 in studies defining the TCR genes of the α and β chains (35). Two years later, the γδ T cell was identified (6). In the 15 years since their discovery, much research has been conducted to characterize the antigens that they recognize and to elucidate their functions. Populations of human and murine γδ T cells that have specific tissue tropisms and very limited diversity in TCRs, indicating restricted antigen recognition, have been defined (1, 20). Interestingly, γδ T-cell populations with extremely diverse junctional regions have also been identified, indicating an ability to recognize a wide range of antigens (3). The requirement of antigen presentation to γδ T cells remains unclear (23, 32, 34, 37, 38, 44). Moreover, γδ T cells have been demonstrated to recognize both protein antigens (4, 5, 15, 18, 36) and protease-resistant antigens (9, 14, 27, 31, 40, 41).
Few experiments have been conducted to phenotypically and functionally characterize bovine γδ T cells. Despite this, the experiments that have been performed have revealed interesting and significant information. Compared to humans and mice, cattle and other ruminants have a high percentage of circulating γδ T cells, representing as many as 75% of the total peripheral blood mononuclear cell (PBMC) population (19). Bovine γδ T cells demonstrate cytolytic activity and express interleukin-2 (IL-2), IL-4, IL-10, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) (7, 8). Additionally, a subset of bovine γδ T cells have been shown to efficiently migrate to sites of inflammation (46).
Several experiments have demonstrated the relevance of γδ T cells to mycobacterial diseases. Human, murine, and bovine γδ T cells have been shown to accumulate in either mycobacterial lesions or lymph nodes (11, 17, 25). Human γδ T cells recognize both peptide and nonpeptide mycobacterial antigens (16, 18, 30). γδ T cells from Mycobacterium bovis-infected cattle have also been shown to recognize M. bovis antigens (45). Additionally, altered granuloma formation was evident following mycobacterial challenge in γδ knockout mice and in γδ-depleted SCID-bo mice (12, 39).
IFN-γ production is essential for an effective response to mycobacterial challenges (10, 13), and γδ T cells have been shown to be a source of early IFN-γ. In vivo depletion of bovine γδ T cells results in a decreased production of early IFN-γ and a subsequent skewing of the Th1 immune response following experimental infection with virulent M. bovis (21). Given the large circulating population of bovine γδ T cells and the propensity of human, murine, and bovine γδ cells to recognize mycobacterial antigens, this study attempted to identify mycobacterial products that were recognized by γδ T cells from healthy cattle. This study demonstrates that while bovine γδ T cells expand following in vitro stimulation with various mycobacterial products, only a component of the mycobacterial cell wall subcellular fraction consistently elicited the production of IFN-γ from bovine γδ T cells.
MATERIALS AND METHODS
Animals.
All animals used in this study were between 6 months and 2 years old. All animals were cared for under the guidelines of the Colorado State University Animal Care and Use Committee. The animals were housed in outdoor pens and fed hay and grain once daily. Five of the animals used in this study (three Holstein steers, one Black Angus steer, and one Black Angus heifer) were purchased from a Johne's disease-free herd. The remaining 13 animals were nonpregnant Holstein heifers from XY Inc. (Fort Collins, Colo.). Colorado has accredited bovine tuberculosis-free status.
PBMC isolation.
Depending on the experiment, between 120 and 250 ml of whole blood was collected into 8-ml CPT Vacutainer tubes containing sodium heparin (Becton Dickinson, Franklin Lakes, N.J.). The tubes were centrifuged at 1,800 relative centrifugal force for 30 min at 20°C. PBMCs were removed and washed once in phosphate-buffered saline (PBS). Residual red blood cells were removed by resuspending the cell pellet in ACK lysis solution and incubating on ice for 5 min. PBMCs were then washed twice in PBS, counted, and resuspended in complete RPMI 1640 (RPMI 1640 supplemented with 10% heat inactivated fetal calf serum, 0.12% β-mercaptoethanol, 5% HEPES, 5% l-glutamine [200 mM], and 50 mg of ampicillin).
Antigen preparation and stimulation.
Mycobacterium tuberculosis H37Rv mannose-capped lipoarabinomannan (ManLAM) (John Belisle, Colorado State University, Fort Collins) (National Institutes of Health [NIH] contract AI-75320) and Mycobacterium sp. noncapped lipoarabinomannan (NIH contract) were used at a concentration of 1 μg/ml. Mycobacterium avium subsp. paratuberculosis >10-kDa culture filtrate proteins (CFP), Mycobacterium bovis 862422 >10-kDa CFP, M. tuberculosis H37Rv subcellular cell wall (NIH contract), isopentenyl pyrophosphate (IPP) (Sigma), M. bovis 862422 subcellular cell wall, M. tuberculosis H37Rv total lipid extract (NIH contract), M. tuberculosis H37Rv mycolylarabinogalactan peptidoglycan (mAGP) (NIH contract), soluble cell wall fraction of M. bovis 862422, and proteinase K-treated M. bovis 862422 subcellular cell wall were all used at a concentration of 10 μg/ml. Live M. bovis 862422 and live M. avium subsp. paratuberculosis were used at a multiplicity of infection of 0.1.
CFP from M. bovis 862422 and M. avium subsp. paratuberculosis were isolated from the culture supernatants of log-phase bulk cultures by passage through a 0.2-μm-pore-size filter. A membrane with a 10,000-Da cutoff was then used to concentrate the culture filtrate by Amicon ultrafiltration. The concentrated culture filtrate was dialyzed against 10 mM ammonium bicarbonate, lyophilized, and stored at −80°C.
M. bovis 862422 subcellular cell wall was prepared from a gamma-irradiated whole-cell pellet of M. bovis 862422. The cells were resuspended at 2 g/ml in PBS containing DNase, RNase, phenylmethylsulfonyl fluoride, pepstatin A, and leupeptin. The cells were then broken by passing the suspension through a French pressure cell 10 times. The unbroken cells were removed by centrifugation at 3,000 × g for 5 min at 4°C. The supernatant was centrifuged at 27,000 × g for 1 h at 4°C. The cell wall pellet was then washed in PBS twice. The protein concentration in the crude cell wall was determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.). The crude cell wall was resuspended in PBS at 0.5 mg/ml and stored at −80°C.
The sodium dodecyl sulfate (SDS)-soluble cell wall fraction of M. bovis was prepared from the subcellular cell wall extract. The subcellular cell wall pellet was resuspended in PBS with 2% SDS (1 mg/ml) and stirred at 37°C for 12 h. The soluble cell wall was collected from the supernatant after centrifugation at 27,000 × g for 1 h. Another 2% SDS extraction was performed on the pellet for an additional 4 h at 37°C. The soluble cell wall was collected from the supernatant after centrifugation at 27,000 × g for 1 h. A final 2% SDS extraction was performed on the pellet for an additional 12 h at 37°C. The soluble cell walls from the three individual 2% SDS extractions were pooled. SDS was removed from the soluble cell wall fraction by using Extracti gel columns (Pierce) and the SDS-Out (Pierce) SDS precipitation kit. The protein concentration was determined by the bicinchoninic acid assay (Pierce) and adjusted to 0.5 mg/ml with PBS. The soluble cell wall (10 μg/ml) was incubated with bovine PBMCs for 60 h to ensure that any residual SDS would not be toxic to the cells.
The proteinase K-treated M. bovis cell wall was prepared from the M. bovis 862422 subcellular cell wall extract. The crude cell wall was digested with 50, 100, 200, and 1,000 μg of proteinase K at 65°C for 1 h, and the proteinase K was heat inactivated at 90°C for 5 min. Protein digestion was assessed by one-dimensional gel electrophoresis. All four concentrations of proteinase K resulted in complete digestion of proteins. For in vitro stimulation, the crude cell wall sample that was digested with 1,000 μg of proteinase K was used.
In vitro expansion of bovine γδ T cells.
PBMCs were obtained from five healthy cattle, as described above. The PBMCs were resuspended in complete RPMI 1640 and seeded at 106 cells/ml into six-well plates at 8 ml per well. Cells were cultured with the following stimuli and controls: PBS, live M. bovis, live M. avium subsp. paratuberculosis, M. tuberculosis subcellular cell wall extract, M. bovis CFP, M. tuberculosis ManLAM, and IPP. At 0, 2, 4, and 6 days following stimulation, cells were harvested with a cell scraper and washed with PBS plus 0.1% sodium azide. Cells were stained with anti-bovine CD2-phycoerythrin (BAQ95A) (VMRD, Pullman, Wash., and Chromaprobe, Mountain View, Calif.), anti-bovine δ TCR-fluorescein isothiocyanate (FITC) (GB21A) (VMRD-Chromaprobe), and appropriate isotype controls for 30 min at 4°C in the dark. The cells were washed twice in PBS plus 0.1% sodium azide, resuspended in 200 μl of PBS plus 0.1% sodium azide, and analyzed with a FACSCalibur flow cytometer. Lymphocytes were gated by forward and side scatter profiles. Percentages of total γδ T cells were determined by CELLQuest after setting quadrants based on staining of isotype controls.
Magnetic bead isolation of γδ T cells and monocytes.
PBMCs were resuspended in PBS at 2 × 106 cells/ml. Cells were stained with either murine anti-bovine δ TCR-FITC (VMRD-Chromaprobe), anti-bovine δ TCR-biotin (VMRD-Chromaprobe), murine anti-bovine monocyte (BAQ151A; VMRD), or murine anti-bovine monocyte-FITC (VMRD-Chromaprobe) for 30 min at 4°C in the dark. The cells were then washed twice in PBS plus 2 mM EDTA and incubated with anti-FITC, streptavidin, or anti-immunoglobulin G1 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 min at 4°C in the dark. Anti-immunoglobulin G1 peridinin chlorophyll-a protein (Becton Dickinson, San Diego, Calif.) was added to the cells that were stained with antimonocyte antibody and streptavidin RED670 (Gibco BRL) was added to the cells that were stained with anti-δ TCR-biotin antibody, and incubation was continued for an additional 10 min. The cells were washed twice with PBS plus 2 mM EDTA and resuspended to 500 μl/108 cells in PBS plus 2mM EDTA. The cell suspensions were then run over magnetic columns, and cells that were bound to the column were collected. Both purified monocytes and purified γδ T cells were analyzed for purity by flow cytometry.
Antigenic stimulation of purified γδ T cells.
PBMCs, purified monocytes, and purified γδ T cells were resuspended in complete RPMI 1640 and seeded into 96-well flat-bottom plates at a concentration of 106 cells/ml with 200 μl/well. For wells with both purified γδ T cells and purified monocytes 100 μl of each cell type was added at 2 × 106 cells/ml. Initially these cells were cultured with the following stimuli and controls: PBS, M. tuberculosis subcellular cell wall extract, M. bovis CFP, M. avium subsp. paratuberculosis CFP, M. tuberculosis ManLAM, IPP, live M. bovis, and live M. avium subsp. paratuberculosis. All cultures were incubated at 37°C with 5% CO2 for 48 h. Recombinant bovine IL-2 (a generous gift from Neil Wedlock) was added to the culture media (at 60 U/ml) in subsequent experiments. The stimuli listed above, in addition to noncapped lipoarabinomannan, M. bovis subcellular cell wall extract, proteolytically digested M. bovis crude cell wall extract, SDS-soluble M. bovis cell wall fraction, total lipid extract from M. tuberculosis, and mAGP from M. tuberculosis, were used along with IL-2 for stimulation in subsequent experiments.
IFN-γ ELISA.
All samples for IFN-γ enzyme-linked immunosorbent assays (ELISAs) were frozen at −80°C until they could be analyzed. A bovine IFN-γ EASIA kit (Biosource, Camarillo, Calif.) was used to qualitatively measure the IFN-γ in the supernatants. The kit protocol was followed. Briefly, 100 μl of tissue culture supernatant was added to anti-IFN-γ-coated wells. Positive and negative controls were supplied with the kit and added to anti-IFN-γ-coated wells. Fifty microliters of incubation buffer was added to each well, and the plates were incubated at room temperature on a horizontal shaker (700 rpm) for 1 h. The plates were then washed three times with 1× PBS-Tween. One hundred microliters of the working conjugate was added to each well and incubated at room temperature on a horizontal shaker (700 rpm) for 1 h. The plates were washed three times with 1× PBS-Tween. One hundred microliters of the chromogen (3,3′,5,5′-tetramethylbenzidine) was added to each well, and the plates were incubated at room temperature on a horizontal shaker (700 rpm) for 15 min. Two hundred microliters of stop solution was added to each well, and the plate was read at 450 nm against a 650-nm reference filter. Positive samples had optical densities (ODs) that were greater than the average OD of the negative control plus 0.15. All results are reported as the mean OD ± standard error of the mean. Interassay variability is a result of the inability to normalize the data due to the lack of a recombinant bovine IFN-γ standard.
Statistical analyses.
Statistical significance for the expansion study of bovine γδ T cells was determined by performing one-way analysis of variance (ANOVA) with least significant difference posttest. Statistical significance was determined by Student's t test for experiments comparing IFN-γ production induced by two stimuli and by one-way ANOVA with Tukey's posttest when comparing more than two stimuli.
RESULTS
Bovine γδ T-cell expansion.
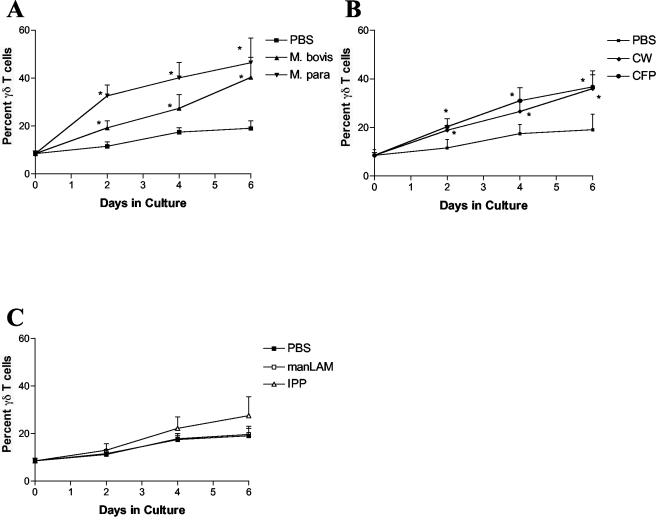
In order to determine if bovine γδ T cells recognized mycobacterial components, PBMCs from healthy cattle were cultured with live M. bovis, live M. avium subsp. paratuberculosis, M. tuberculosis cell wall, M. bovis CFP, M. tuberculosis ManLAM, IPP, and PBS. The percentage of γδ T cells of the total cultured cells were measured after 0, 2, 4, and 6 days in culture by flow cytometry. Bovine γδ T cells expanded significantly in response to stimulation with both live M. bovis and live M. avium subsp. paratuberculosis at all three time points (Fig. 1A). In addition, bovine γδ T cells expanded in response to stimulation with M. tuberculosis cell wall at days 2 and 4 and in response to M. bovis CFP at all three time points (Fig. 1B). There were, however, no significant responses to either IPP or M. tuberculosis ManLAM (Fig. 1C).
FIG. 1.
Expansion of bovine γδ T cells. In vitro expansion of bovine γδ T cells was measured following stimulation with live M. bovis and live M. avium subsp. paratuberculosis (A), mycobacterial cell wall (CW) and CFP (B), and ManLAM and IPP (C). Data are presented as the mean percent γδ T cells from four normal animals ± standard error of the mean. Statistical significance was determined by using ANOVA and least significant difference. *, P < 0.05.
Requirements for IFN-γ production by bovine γδ T cells.
IFN-γ is a key cytokine in the protective immune response to mycobacteria (10, 13). The ability of bovine γδ T cells to produce this key cytokine in response to mycobacterial components was assessed. Given the conflicting evidence for the requirement of antigen presentation for antigen recognition by γδ T cells (37, 38, 44), the ability of bovine γδ T cells to produce IFN-γ following stimulation with mycobacterial components was determined both with and without the presence of antigen-presenting cells. Bovine γδ T cells and monocytes were purified from peripheral blood of five healthy cattle by using magnetic beads and stimulated with several mycobacterial products and controls (M. bovis CFP, M. tuberculosis cell wall, M. tuberculosis ManLAM, IPP, live M. avium subsp. paratuberculosis, and live M. bovis). The purity of bovine γδ T-cell and monocyte populations was assessed by flow cytometry and found to be consistently greater than 90% for bovine γδ T cells and greater than 85% for bovine monocytes (data not shown). IFN-γ ELISAs were performed to detect production of IFN-γ by purified γδ T cells and by γδ T cells cultured with matched monocytes in response to stimulation with mycobacterial products. No detectable IFN-γ was produced by purified γδ T cells alone or in the presence of monocytes in response to stimulation with any of the mycobacterial products tested (data not shown).
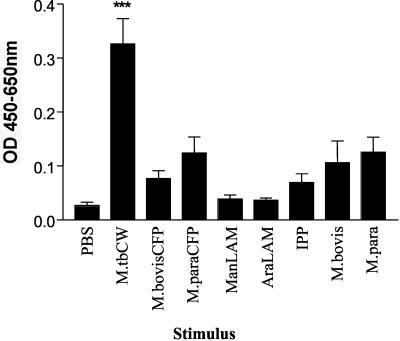
This result was not totally unexpected, as T cells need growth factors such as IL-2 for proliferation and cytokine secretion. The addition of IL-2 to cultures containing purified γδ T cells and purified monocytes resulted in the production of IFN-γ in response to mycobacterial cell wall (Fig. 2). In contrast, γδ T cells cultured with IL-2 and mycobacterial cell wall but no source of antigen-presenting cells did not produce IFN-γ (data not shown). This experiment has been repeated with cells from 10 additional normal blood donors, and we consistently observed that stimulation of purified γδ T cells cultured with purified monocytes, bovine IL-2, and M. tuberculosis cell wall resulted in significant IFN-γ secretion (data not shown).
FIG. 2.
Bovine γδ T cells produce significant amounts of IFN-γ in response to M. tuberculosis cell wall (M.tbCW) when cocultured with purified monocytes and IL-2. Monocytes and γδ T cells were isolated from the peripheral blood of four animals by using magnetic beads. Monocytes and γδ T cells from each individual animal were incubated with antigens or PBS for 48 h. The supernatants were then tested for the presence of IFN-γ. The data are presented as mean OD at 450 to 650 nm ± standard error for four animals. Significant IFN-γ production was determined by one-way ANOVA with the Tukey posttest. ***, P < 0.001.
IFN-γ production by bovine γδ T cells in response to proteolytically digested M. bovis cell wall.
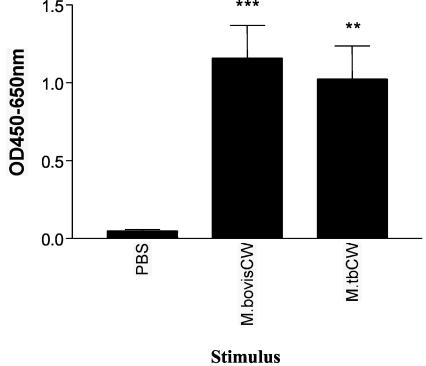
Since the production of IFN-γ by bovine γδ T cells in response to stimulation with M. tuberculosis cell wall was demonstrated, the subcellular cell wall fraction of a mycobacterial species that is relevant to cattle was harvested and tested for its ability to stimulate IFN-γ production by γδ T cells. γδ T cells and monocytes were purified from 10 healthy cattle and stimulated with the subcellular cell wall fraction from a virulent strain of M. bovis (PBS and M. tuberculosis cell wall were included as controls). Significant IFN-γ was detected in the culture supernatants of cells stimulated with M. bovis cell wall (Fig. 3). Culture supernatants from wells containing only monocytes were also tested to ensure that there were no contaminating T cells and that significant amounts of IFN-γ were not produced (data not shown).
FIG. 3.
IFN-γ production by γδ T cells in response to M. bovis cell wall (CW). Magnetic beads were used to isolate monocytes and γδ T cells from the peripheral blood of 10 animals. Monocytes and γδ T cells from each individual animal were incubated with M. bovis or M. tuberculosis cell wall or PBS for 48 h. The supernatants were tested for the presence of IFN-γ. Data are presented as mean OD at 450 to 650 nm ± standard error from two successive experiments with five animals per experiment. Significant IFN-γ production was determined by one-way ANOVA with the Tukey posttest. **, P < 0.01; ***, P < 0.001.
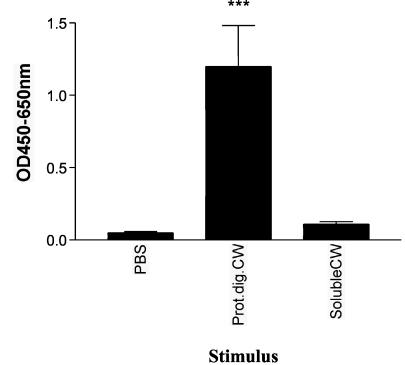
To determine if the IFN-γ-stimulatory component of the M. bovis subcellular cell wall fraction was a protein, both the SDS-soluble cell wall fraction and a proteolytically digested cell wall fraction were tested in the IFN-γ culture assay. Again, purified γδ T cells from 10 healthy cattle were cultured with matched monocytes, IL-2, and SDS-soluble subcellular cell wall fraction, proteolytically digested subcellular cell wall fraction, or PBS for 48 h. The proteolytically digested subcellular cell wall stimulated culture supernatants from all 10 animals, each of which had significant amounts of IFN-γ, while none of the soluble subcellular cell wall-stimulated culture supernatants from these animals contained any detectable amounts of IFN-γ (Fig. 4). Purified monocytes alone were also tested to ensure that there were no contaminating T cells and that significant amounts of IFN-γ were not produced (data not shown). These results indicated that the IFN-γ-stimulatory component of the M. bovis subcellular cell wall was unlikely to be a protein.
FIG. 4.
Bovine γδ T cells produce IFN-γ following culture with proteolytically digested mycobacterial cell wall (Prot.dig.CW). Monocytes and γδ T cells were isolated from the peripheral blood of 10 animals by using magnetic beads. Monocytes and γδ T cells from each individual animal were incubated with proteinase K-treated M. bovis cell wall, the SDS-soluble fraction of M. bovis cell wall, or PBS for 48 h. Supernatants were then tested for the presence of IFN-γ. Data are presented as mean OD at 450 to 650 nm ± standard error from two successive experiments with five animals per experiment. Significant IFN-γ production was determined by one-way ANOVA with the Tukey posttest. ***, P < 0.001.
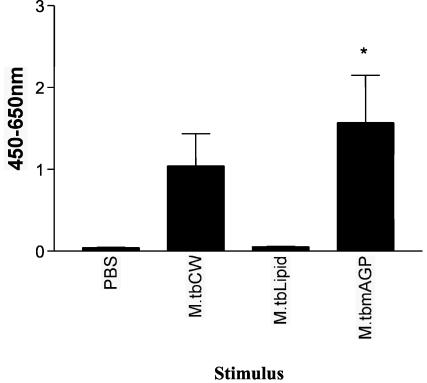
Bovine γδ T cells produce IFN-γ following stimulation with mAGP but not total lipid extract from M. tuberculosis.
Given that bovine γδ T cells produced significant IFN-γ following stimulation with proteolytically digested subcellular cell wall, the ability of total lipid extract and mAGP (both purified from M. tuberculosis) to stimulate the production of IFN-γ by γδ T cells was determined. γδ T cells and monocytes were purified from the peripheral blood of five healthy cattle and stimulated with IL-2 and M. tuberculosis total lipid extract or IL-2 and mAGP. γδ T cells and monocytes cultured with mAGP and IL-2 produced significant amounts of IFN-γ, whereas M. tuberculosis total lipid extract did not stimulate significant production of IFN-γ (Fig. 5). Purified monocytes alone were also tested to ensure that there were no contaminating T cells. Although in some of the mAGP-stimulated culture supernatants there were low levels of IFN-γ, the levels were not statistically significant (data not shown). These results further demonstrate the ability of bovine γδ T cells to produce significant amounts of IFN-γ when cultured with nonprotein components of the mycobacterial cell wall.
FIG. 5.
IFN-γ production by γδ T cells following stimulation with mycobacterial mAGP but not with mycobacterial lipids. Monocytes and γδ T cells were isolated from the peripheral blood of five animals by using magnetic beads. Monocytes and γδ T cells were incubated with M. tuberculosis cell wall (M.tbCW), lipid extract from M. tuberculosis, M. tuberculosis mAGP, or PBS for 48 h. Supernatants were frozen and then tested for the presence of IFN-γ. Data are presented as mean OD at 450 to 650 nm ± standard error for five animals. Significant IFN-γ production was determined by one-way ANOVA with the Tukey posttest. *, P < 0.05.
DISCUSSION
This study provided new information regarding the antigen recognition and function of bovine γδ T cells. Live mycobacteria, mycobacterial cell wall, and M. bovis CFP significantly stimulated the in vitro expansion of bovine γδ T cells. In addition, the mAGP fraction of the mycobacterial cell wall was identified as a potent stimulus for the production of IFN-γ by γδ T cells.
The in vitro expansion of T-cell populations in response to antigen is a useful way to identify potential antigens that are recognized by T cells. This study demonstrated that both live mycobacteria and subcellular fractions of mycobacteria, namely, cell wall and CFP, were capable of inducing the in vitro expansion of bovine γδ T cells. This indicates that mycobacteria and mycobacterial components are potential antigens for bovine γδ T cells. Given that the readout for this experiment was percent γδ T cells, it is possible that the in vitro expansion of γδ T cells seen could also be attributed to the in vitro death of another cell population. Interestingly, there was no significant expansion following stimulation with either ManLAM or IPP. Although IPP has been identified as a human γδ T-cell antigen (27, 40), there did not appear to be recognition by bovine γδ T cells, which is consistent with a recent study of M. bovis-infected cattle (33).
The ability to produce IFN-γ during a mycobacterial infection is essential for an effective host response (10, 13). This study demonstrated that γδ T cells, isolated from the peripheral blood of healthy cattle, were able to produce significant amounts of IFN-γ following stimulation with mycobacterial cell wall (from both M. tuberculosis and M. bovis). Interestingly, proteolytic digestion of the mycobacterial cell wall did not affect the production of IFN-γ by bovine γδ T cells. To confirm that the immunostimulatory component of the cell wall was not a protein, bovine γδ T cells were stimulated with an SDS-soluble cell wall extract, and no IFN-γ was produced. This suggests that a nonprotein component of the cell wall induced the IFN-γ production. We cannot, however, rule out a protein that is tightly associated and protected by the cell wall as the immunostimulatory component. Such a protein would likely be resistant to proteolytic digestion and may not be released from the cell wall following SDS extraction. In addition to SDS-soluble cell wall proteins, this fraction would also contain other mycobacterial cell wall components such as lipoarabinomannan, lipomannan, and phosphatidylinositol mannosides. The absence of a significant IFN-γ response to this fraction also eliminates these components as the stimulatory material.
Since a protein component of the cell wall could not easily be identified as the immunostimulatory component, the total lipid extract and mAGP from M. tuberculosis were also tested for the ability to induce IFN-γ production by bovine γδ T cells. No significant amount of IFN-γ was produced following stimulation with the total lipid extract, but the mAGP fraction did induce IFN-γ production. Although the individual components of mAGP (mycolic acids, arabinogalactan, and peptidoglycan) (2) were not tested, peptidoglycan is the likely component that induced IFN-γ production by bovine γδ T cells. Mycolic acids would have been present in the total lipid extract of M. tuberculosis, and since this fraction did not induce IFN-γ production, they are not likely the IFN-γ inducing component. Arabinogalactan has been demonstrated to be immunosuppressive and is thus not likely to induce IFN-γ production by bovine γδ T cells (22, 26, 29). Peptidoglycan and/or mAGP has been shown to induce the production of inflammatory cytokines (24, 28, 42, 47). Moreover, mAGP isolated from M. tuberculosis was shown to induce the production of TNF-α, and this was dependent upon Toll-like receptor-2 expression (43). It is therefore likely that the peptidoglycan component of mAGP induces the production and secretion of TNF-α, IL-1, and IL-12 by bovine monocytes, which then act on the bovine γδ T cells to induce the production of early IFN-γ. Further studies testing each individual component of mAGP would be necessary to confirm this hypothesis.
Given the importance of IFN-γ production to controlling mycobacterial infections and the high percentage of circulating γδ T cells in cattle, identifying mycobacterial components that induce IFN-γ production by γδ T cells may provide new information towards developing efficacious bovine tuberculosis vaccines. This study demonstrates that bovine γδ T cells are capable of producing large quantities of innate IFN-γ when stimulated by the mAGP fraction of the mycobacterial cell wall.
Acknowledgments
This work was supported by a USDA training grant to Colorado State University.
We thank John Belisle for many of the antigens used in this study, Neil Wedlock for the recombinant bovine IL-2, XY Inc. for the generous use of their cattle, and Brian Fraley for excellent technical assistance.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Asarnow, D., W. Kuziel, M. Bonyhadi, R. Tigelaar, P. Tucker, and J. Allison. 1988. Limited diversity of γ/δ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell 55:837-847. [DOI] [PubMed] [Google Scholar]
- 2.Besra, G. S. 1998. Preparation of cell-wall fractions from mycobacteria. Methods Mol. Biol. 101:91-107. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone, J., R. Cron, T. Barrett, B. Houlden, A. Sperling, A. Dent, S. Hedrick, B. Rellahan, and L. Matis. 1991. Repertoire development and ligand specificity of murine TCR γδ T cells. Immunol. Rev. 129:5-33. [DOI] [PubMed] [Google Scholar]
- 4.Boom, W. H., K. N. Balaji, R. Nayak, K. Tsukaguchi, and K. A. Chervenak. 1994. Characterization of a 10- to 14-kilodalton protease-sensitive Mycobacterium tuberculosis H37Ra antigen that stimulates human gamma delta T cells. Infect. Immun. 62:5511-5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Born, W., L. Hall, A. Dallas, J. Boymel, T. Shinnick, D. Young, P. Brennan, and R. O'Brien. 1990. Recognition of peptide antigen by heat shock-reactive γδ T lymphocytes. Science 249:67-69. [DOI] [PubMed] [Google Scholar]
- 6.Brenner, M. B., J. McLean, D. P. Dialynas, J. L. Strominger, J. A. Smith, F. L. Owen, J. G. Seidman, S. Ip, F. Rosen, and M. S. Krangel. 1986. Identification of a putative second T-cell receptor. Nature 322:145-149. [DOI] [PubMed] [Google Scholar]
- 7.Brown, W. C., W. C. Davis, S. H. Choi, D. A. Dobbelaere, and G. A. Splitter. 1994. Functional and phenotypic characterization of WC1+ gamma/delta T cells isolated from Babesia bovis-stimulated T cell lines. Cell. Immunol. 153:9-27. [DOI] [PubMed] [Google Scholar]
- 8.Collins, R. A., P. Sopp, K. I. Gelder, W. I. Morrison, and C. J. Howard. 1996. Bovine gamma/delta TcR+ T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. Scand. J. Immunol. 44:444-452. [DOI] [PubMed] [Google Scholar]
- 9.Constant, P., Y. Poquet, M. A. Peyrat, F. Davodeau, M. Bonneville, and J. J. Fournie. 1995. The antituberculous Mycobacterium bovis BCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human gamma delta T cells. Infect. Immun. 63:4628-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty, M. L., H. F. Bassett, P. J. Quinn, W. C. Davis, A. P. Kelly, and M. L. Monaghan. 1996. A sequential study of the bovine tuberculin reaction. Immunology 87:9-14. [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza, C. D., A. M. Cooper, A. A. Frank, R. J. Mazzaccaro, B. R. Bloom, and I. M. Orme. 1997. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J. Immunol. 158:1217-1221. [PubMed] [Google Scholar]
- 13.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fournie, J. J., and M. Bonneville. 1996. Stimulation of gamma delta T cells by phosphoantigens. Res. Immunol. 147:338-347. [DOI] [PubMed] [Google Scholar]
- 15.Fu, Y. X., R. Cranfill, M. Vollmer, R. Van Der Zee, R. L. O'Brien, and W. Born. 1993. In vivo response of murine gamma delta T cells to a heat shock protein-derived peptide. Proc. Natl. Acad. Sci. USA 90:322-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, V. E., P. A. Sieling, J. Gong, P. F. Barnes, K. Uyemura, Y. Tanaka, B. R. Bloom, C. T. Morita, and R. L. Modlin. 1997. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J. Immunol. 159:1328-1335. [PubMed] [Google Scholar]
- 17.Griffin, J. P., K. V. Harshan, W. K. Born, and I. M. Orme. 1991. Kinetics of accumulation of gamma delta receptor-bearing T lymphocytes in mice infected with live mycobacteria. Infect. Immun. 59:4263-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haregewoin, A., G. Soman, R. C. Hom, and R. W. Finberg. 1989. Human γδ+ T cells respond to mycobacterial heat-shock protein. Nature 340:309-312. [DOI] [PubMed] [Google Scholar]
- 19.Hein, W. R., and C. R. Mackay. 1991. Prominence of gamma delta T cells in the ruminant immune system. Immunol. Today 12:30-34. [DOI] [PubMed] [Google Scholar]
- 20.Itohara, S., A. Farry, J. Lafaille, M. Bonneville, Y. Takagaki, W. Haas, and S. Tonegawa. 1990. Homing of a γ/δ thymocyte subset with homogeneous T-cell receptors to the mucosal epithelial. Nature 343:754-757. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy, H. E., M. D. Welsh, D. G. Bryson, J. P. Cassidy, F. I. Forster, C. J. Howard, R. A. Collins, and J. M. Pollock. 2002. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+ γδ T cells. Infect. Immun. 70:1488-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinhenz, M. E., J. J. Ellner, P. J. Spagnuolo, and T. M. Daniel. 1981. Suppression of lymphocyte responses by tuberculous plasma and mycobacterial arabinogalactan. Monocyte dependence and indomethacin reversibility. J. Clin. Investig. 68:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matis, L., A. Fry, R. Cron, M. Cotterman, R. Dick, and J. Bluestone. 1989. Structure and specificity of a class II MHC alloreactive γδ T cell receptor. Science 245:746-749. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson, E., L. Verhage, J. Rollof, A. Fleer, J. Verhoef, and H. van Dijk. 1993. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol. Med. Microbiol. 7:281-287. [DOI] [PubMed] [Google Scholar]
- 25.Modlin, R. L., C. Pirmez, F. M. Hofman, V. Torigian, K. Uyemura, T. H. Rea, B. R. Bloom, and M. B. Brenner. 1989. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature 339:544-548. [DOI] [PubMed] [Google Scholar]
- 26.Moreno, C., A. Mehlert, and J. Lamb. 1988. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin. Exp. Immunol. 74:206-210. [PMC free article] [PubMed] [Google Scholar]
- 27.Morita, C. T., E. M. Beckman, J. F. Bukowski, Y. Tanaka, H. Band, B. R. Bloom, D. E. Golan, and M. B. Brenner. 1995. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity 3:495-507. [DOI] [PubMed] [Google Scholar]
- 28.Nagao, S., Y. Kawabata, M. Kitano, K. Suzuki, T. Nishikawa, and H. Takada. 1995. Modification of delayed-type hypersensitivity reactions by muramyldipeptide in guinea pigs. FEMS Immunol. Med. Microbiol. 11:231-245. [DOI] [PubMed] [Google Scholar]
- 29.Niinaka, T., S. Kishimoto, T. Aoki, H. Ikegami, and F. Ito. 1975. Skin reaction, inhibition of macrophage migration, and lymphocyte transformation with tuberculin active peptide (TAP) and arabinogalactan obtained from tubercle bacilli. Int. Arch. Allergy Appl. Immunol. 49:585-596. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer, K., B. Schoel, H. Gulle, S. H. Kaufmann, and H. Wagner. 1991. Human gamma/delta T cells responding to mycobacteria. Behring Inst. Mitteilung. 1991:36-42. [PubMed] [Google Scholar]
- 31.Pfeffer, K., B. Schoel, H. Gulle, S. H. Kaufmann, and H. Wagner. 1990. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur. J. Immunol. 20:1175-1179. [DOI] [PubMed] [Google Scholar]
- 32.Porcelli, S., M. Brenner, J. Greenstein, S. Balk, C. Terhorst, and P. Bleicher. 1989. Recognition of cluster differentiation I antigens by CD4-CD8-cytolytic T lymphocytes. Nature 341:447-449. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes, S. G., R. G. Hewinson, and H. M. Vordermeier. 2001. Antigen recognition and immunomodulation by gamma delta T cells in bovine tuberculosis. J. Immunol. 166:5604-5610. [DOI] [PubMed] [Google Scholar]
- 34.Rivas, A., J. Koide, M. Cleary, and E. Engleman. 1989. Evidence for the involvement of the γδ T cell antigen receptor in cytotoxicity mediated by human alloantigen-specific T cell clones. J. Immunol. 142:1840-1847. [PubMed] [Google Scholar]
- 35.Saito, H., D. Kranz, Y. Takagaki, A. Hayday, H. Eisen, and S. Tonegawa. 1984. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature 309:757-762. [DOI] [PubMed] [Google Scholar]
- 36.Salvetti, M., C. Buttinelli, G. Ristori, M. Carbonari, M. Cherchi, M. Fiorelli, M. G. Grasso, L. Toma, and C. Pozzilli. 1992. T-lymphocyte reactivity to the recombinant mycobacterial 65- and 70-kDa heat shock proteins in multiple sclerosis. J. Autoimmun. 5:691-702. [DOI] [PubMed] [Google Scholar]
- 37.Schild, H., N. Mavaddat, C. Litzenberger, E. W. Ehrich, M. M. Davis, J. A. Bluestone, L. Matis, R. K. Draper, and Y. H. Chien. 1994. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell 76:29-37. [DOI] [PubMed] [Google Scholar]
- 38.Sciammas, R., R. Johnson, A. Sperling, W. Brady, P. Linsley, P. Spear, F. Fitch, and J. Bluestone. 1994. Unique antigen recognition by a herpesvirus-specific TCR-γ-δ cell. J. Immunol. 152:5392-5397. [PubMed] [Google Scholar]
- 39.Smith, R. A., J. M. Kreeger, A. J. Alvarez, J. C. Goin, W. C. Davis, D. L. Whipple, and D. M. Estes. 1999. Role of CD8+ and WC1− gamma/delta T cells in resistance to Mycobacterium bovis infection in the SCID-bo mouse. J. Leukoc. Biol. 65:28-34. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, Y., C. T. Morita, Y. Tanaka, E. Nieves, M. B. Brenner, and B. R. Bloom. 1995. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375:155-158. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, Y., S. Sano, E. Nieves, G. De Libero, D. Rosa, R. L. Modlin, M. B. Brenner, B. R. Bloom, and C. T. Morita. 1994. Nonpeptide ligands for human gamma delta T cells. Proc. Natl. Acad. Sci. USA 91:8175-8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of Toll-like receptors. Infect. Immun. 68:6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weintraub, B., M. Jackson, and S. Hedrick. 1994. γ-δ T cells can recognize non-classical MHC in the absence of conventional antigenic peptides. J. Immunol. 153:3051-3058. [PubMed] [Google Scholar]
- 45.Welsh, M. D., H. E. Kennedy, A. J. Smyth, R. M. Girvin, P. Andersen, and J. M. Pollock. 2002. Responses of bovine WC1+ γδ T cells to protein and nonprotein antigens of Mycobacterium bovis. Infect. Immun. 70:6114-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson, E., M. K. Aydintug, and M. A. Jutila. 1999. A circulating bovine gamma delta T cell subset, which is found in large numbers in the spleen, accumulates inefficiently in an artificial site of inflammation: correlation with lack of expression of E-selectin ligands and L-selectin. J. Immunol. 162:4914-4919. [PubMed] [Google Scholar]
- 47.Yang, S., R. Tamai, S. Akashi, O. Takeuchi, S. Akira, S. Sugawara, and H. Takada. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 69:2045-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]