Abstract
Objective
Preoperative determinants of pain duration following surgery are poorly understood. We identified preoperative predictors of prolonged pain after surgery in a mixed surgical cohort.
Methods
We conducted a prospective longitudinal study of patients undergoing mastectomy, lumpectomy, thoracotomy, total knee replacement, or total hip replacement. We measured preoperative psychological distress and substance use, and then measured pain and opioid use after surgery until patients reported the cessation of both opioid consumption and pain. The primary endpoint was time to opioid cessation, and those results have been previously reported. Here we report preoperative determinants of time to pain resolution following surgery in Cox proportional hazards regression.
Results
Between January 2007 and April 2009 we enrolled 107 of 134 consecutively approached patients undergoing the aforementioned surgical procedures. In the final multivariate model, preoperative self-perceived risk of addiction predicted more prolonged pain. Unexpectedly, anxiety sensitivity predicted more rapid pain resolution after surgery. Each one-point increase (on a four point scale) of self-perceived risk of addiction was associated with a 38% (95% CI 3 - 61) reduction in the rate of pain resolution (p= 0.04). Furthermore, higher anxiety sensitivity was associated with an 89% (95% CI 23–190) increased rate of pain resolution (p=0.004).
Conclusions
Greater preoperative self-perceived risk of addiction, and lower anxiety sensitivity predicted a slower rate of pain resolution following surgery. Each of these factors was a better predictor of pain duration than preoperative depressive symptoms, PTSD symptoms, past substance use, fear of pain, gender, age, preoperative pain, or preoperative opioid use.
Introduction
Many studies have focused on immediate postsurgical pain intensity, and more recently there has been an increasing focus on chronic postsurgical pain.[1–9] In contrast, far fewer studies have described the intervening pain resolution that occurs between these immediate postoperative periods and periods associated with chronic pain. 10% of patients undergoing surgery do not experience resolution of postoperative pain and develop chronic pain. [10] The time course over which these patients experience pain resolution has received little attention. The factors controlling postoperative pain duration are important since they may be modifiable and targets for future treatments to expedite pain resolution. Also, there may be a link between factors causing continued post-surgical pain and factors leading to indefinite pain persistence and the development of chronic pain. This temporal aspect of acute pain (pain duration) has been minimally characterized in comparison to extensive research efforts aimed at other domains of pain such as reducing perioperative pain intensity or evaluating pain thresholds.
Previous studies of postoperative pain duration have been limited to hospitalized patients. In a cohort study of 88 postsurgical inpatients on a surgical ward, who underwent a variety of surgical procedures, only 30.7% of patients continued to suffer from pain 100 hours or more after surgery.[11] Consistent with these results, a study of patients after cardiac bypass surgery reported that five days postoperatively, 68% of patients were pain free at rest. [12] Without examining pain duration amongst patients discharged from the hospital, these studies introduced a time dependent selection bias examining the non-random subset of patients who remained hospitalized for more extensive time periods following surgery. In addition these studies did not explore the role of preoperative psychological distress on the duration of pain.
Predictors of post-surgical pain based on examination of intermittent time points after surgery include anxiety [2; 9; 13], state anxiety [7], pain catastrophizing [3; 13], preoperative pain both remote and at the surgical site, [7; 9] severity of acute postoperative pain [5; 7; 8], and pre-operative opioid use. [7] Depressive symptoms have been implicated in altered central pain processing, increased early postoperative pain intensity, [14] and an increased risk of developing chronic pain. [15]These findings contributed to our decision to look at similar domains as potential predictors of postoperative pain duration. However, we present a novel approach to characterizing postoperative pain duration through a Cox proportional hazards regression model of time to pain cessation, which has never been reported. In our prospective study, patient follow-up spanned 4 to 859 days after surgery. Furthermore, pain cessation was assessed via daily phone calls for the first three months, weekly phone calls up to 6 months after surgery, and then monthly calls thereafter. This novel model of postoperative pain cessation differs significantly from examining random intermittent intervals after surgery, which are more likely to capture pain intensity at a single point in time rather than the true waxing and waning nature of pain with eventual pain cessation.
We hypothesized that preoperative psychological distress would predict longer pain duration following surgery. To test our hypothesis, we conducted a prospective, longitudinal, cohort study of patients undergoing five different types of surgery to evaluate the effects of antecedent depressive symptoms and other measures of psychological distress on the duration of pain following surgery. Time to opioid cessation data from this study has previously been reported[16].
Materials and Methods
The Stanford University Institutional Review Board approved all aspects of the protocol, and all participants provided written informed consent.
Participants
We enrolled all patients in a consecutive sample scheduled to undergo five distinct surgical procedures: (1) thoracotomy, (2) primary total hip replacement, (3) primary total knee replacement, (4) radical mastectomy and (5) lumpectomy. To control for the confounding influences of the different emotional, physical and psychosocial contexts of these surgeries all subsequent analyses were stratified by surgery type except where stated. Patients were recruited between January 2007 and April 2009 at Stanford University Hospital. Final follow-up was November 2009. Inclusion criteria included patients undergoing one of the aforementioned surgeries at Stanford Hospital, ability to provide informed consent, ability to speak, read, and write English, and ability to fill out study questionnaires. Exclusion criteria included any inability to complete the study forms due to either mental incapacity or a language barrier. Patients were identified for recruitment by participating surgeons.
Procedures
We conducted an exploratory prospective, longitudinal observational study of patients’ pain and opioid use following surgery. Prior to surgery, patients completed several questionnaires to measure affective distress and previous substance use behaviors, using the series of patient self-report questionnaires described below. Completion of baseline measurements generally took between 30 and 60 minutes. Starting on post-operative day one and each day thereafter, we determined ongoing surgical pain using the Brief Pain Inventory (BPI).[17] We continued to collect daily BPI measurements by telephone until the patient reported five consecutive days of zero average pain and five consecutive days of zero prescription opioid use. Daily assessments generally lasted between 2 and 3 minutes per contact. After three months, the BPI was administered weekly, then after 6 months, the BPI was administered monthly. Major changes in patients’ health status were sought on a monthly basis (e.g., cancer recurrence, or chemotherapy) for evaluation as possible confounders. The primary endpoint was the time to pain resolution as defined by number of days between surgery and the first of five consecutive patient reports of zero average pain as assessed by the daily BPI.
Baseline Measures
Measures of pre-existing substance use disorders
Past substance use was measured with the Addiction Severity Index Drug and Alcohol Use Section,[18; 19] which assesses lifetime and past 30-day use of illicit substances and alcohol.
Measures of risk of developing problematic opioid use
To identify problematic preoperative opioid use, we administered The Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) [20]. In addition, patients were given an author-generated measure of Self-perceived Susceptibility to Addiction to quantify how patients’ baseline fears of opioids influence their subsequent use of opioids. Patients were asked, “How likely do you think it is that you will develop an addiction problem from pain medication you take after surgery?” and chose from one of four categorical answers: 1: “not at all”; 2: “unlikely”; 3: “somewhat likely”; or 4: “very likely.”
Measures of Mood, Anxiety-Sensitivity, PTSD and Fear Symptoms
We assessed depressive symptoms with the Beck Depression Inventory-II (BDI-II),[17] somatic fear and anxiety with The Anxiety-Sensitivity Index (ASI),[21] and fear of pain with the Fear of Pain Questionnaire (FOPQ).[22] Post-traumatic stress disorder symptoms were assessed with the brief Primary Care Post-Traumatic Stress Disorder (PTSD) Screen. [23]
Statistical Analysis
Time to pain resolution was analyzed using Kaplan Meier analysis and Cox proportional hazards regression. Different surgeries may have different underlying hazard rates due to differing injury, underlying disease, and coexisting emotional context (e.g., cancer surgery versus non-cancer surgery). To eliminate this confounding variable and to evaluate only factors that influenced the pain resolution rate across all surgery types, all Cox proportional hazard analyses were stratified by surgery type except where specifically noted. Statistical stratification ensures that comparisons between subjects were only made between subjects undergoing the same surgery (i.e., within the same strata) and eliminates confounding by surgery type.[24] Variables were first evaluated by univariate analysis of pain duration and included: age, gender, BDI-II score, ASI score, PTSD symptoms (answering yes to any question on the four question Primary Care PTSD screen [23]), SOAPP-R score, Self-Perceived Risk of Addiction, FOPQ, preoperative pain at the surgical site, preoperative pain anywhere over the entire body, legitimate preoperative opioid use, illicit preoperative opioid use, and any preoperative opioid use. Additionally recognized risk factors for addiction including a family history of substance abuse, and previous use of tobacco, alcohol, marijuana, or sedatives were also examined. For the multivariate model building process, preoperative factors that had an association with pain duration at a p-value <0.25 in univariate analysis, were assessed as candidates for the final multivariate model. Using this cutoff of a p-value <0.25 rather than more traditional levels such as 0.05 increases the identification of important variables such as confounders, which may not be significant in the final multivariate model. [25; 26] SAS version 9.3 (SAS Institute, Cary, N.C.) was used for all analyses. Inclusion in the final multivariate model was determined manually with use of the Akaike Information Criteria (AIC) to evaluate the effect of including variables until overall model fit was maximized as assessed by AIC. Sensitivity analyses were conducted to examine the potential influence of different model selection processes and also different patient subpopulations on our results. To examine the potential influence of specific multivariable model building processes, SAS’s automated forward, backward, and stepwise model building algorithms were used. Further sensitivity analysis involved reconstructing the multivariate model while omitting specific subpopulations including patients from each type of surgery and also opioid consuming patients.
Results
Patient characteristics are shown in Table 1. 134 patients undergoing surgery were approached for inclusion in this study, 107 unique individuals with individual time courses of post-operative pain resolution were examined. Duration of follow-up extended from time of study entry (between January 2007 and April 2009) to the resolution of the patients’ pain or censoring (for example, due to loss to follow-up, withdrawal by investigator, or additional surgery). At the time of the final follow-up in November 2009 pain duration ranged from 4 days to 859 days. Only one patient who agreed to participate provided no postoperative data. Cox proportional hazards regression not only allows for variable follow-up time across participants, but also retains data from censored patients for “partial credit.”[27] In more restrictive models, the data from censored participants would be wasted.[27] Of the remaining 106 patients, 85 (79%) met our predefined criteria for achieving pain resolution during the follow-up period and had a recorded time to pain resolution. Of these 85 patients, seven patients had pain that persisted beyond 200 days and then subsequently resolved, including 2 patients whose pain resolved after 437 days and 524 days respectively. Overall, median time to pain resolution was 52 days (95% CI 39–64) with the inter-quartile range between 27–116 days (95% CI 17–208). The number of patients undergoing each surgery, median and inter-quartile range of pain duration, and 95% confidence intervals are reported in Table 1.
Table 1.
Patient Characteristics (n=107)
| Preoperative Characteristic (IQ Range)a | Thoracotomy | Total Knee Replacement | Total Hip Replacement | Mastectomy | Lumpectomy |
|---|---|---|---|---|---|
| Patients (n) | 27 | 19 | 25 | 25 | 11 |
| Age | 57(49–65) | 61(58–67) | 61(57–66) | 50.5(44.5–62) | 61(50–69) |
| Beck Depression Inventory-II Score | 6(3–11) | 7(6–13) | 7(5–15) | 10(5–14) | 4(2–5) |
| SOAPP-R Score | 10(8–12) | 15(8–18) | 14(9–19) | 13(9–18) | 10(7–13) |
| Anxiety Sensitivity Index Score | 10.5(5–16) | 14(6–27) | 17(9–26.5) | 18(7–23) | 12(4–23) |
| Fear of Pain Score | 56.5(44.0–73.0) | 74(42–87) | 78(69–90) | 76.5(64–88.5) | 82(68–92) |
| Baseline Pain at Surgical Site (0–10) | 0(0–0) | 3(2–6) | 5(4–7) | 0(0–0) | 0(0–1) |
| Baseline Pain Other Than Surgical Site (0–10) | 1(0–2) | 4(1–6) | 5(3–7) | 1(0–2) | 1(0–2) |
| Self-Perceived Risk of Addiction | 1(1–2) | 1(1–2) | 1(1–1) | 1(1–2) | 1(1–1) |
| Male Gender | 37% | 37% | 56% | 0% | 0% |
| Had PTSD Symptoms | 22% | 16% | 16% | 40% | 18% |
| Used Legitimate Preoperative Opioids | 15% | 32% | 42% | 4% | 0% |
| Used Illicit Preoperative Opioids | 11% | 21% | 17% | 12% | 0% |
| Used Any Preoperative Opioids | 19% | 42% | 40% | 16% | 0% |
| Used Preoperative Sedatives | 7% | 5% | 4% | 28% | 0% |
| Used Preoperative Marijuana | 4% | 0% | 5% | 13% | 0% |
| Used Tobacco Regularly (lifetime) | 33% | 26% | 28% | 24% | 18% |
| Median Time to Pain Resolution (days) and 95% CI | 58(35–92) | 81(49–146) | 81(43–118) | 27(16–35) | 7(0–27) |
Values presented are medians and interquartile range (IQ Range)
In univariate Cox regression analysis (Table 2) a number of factors were potentially associated (p-value <0.25) with prolonged pain duration including: self-perceived susceptibility to addiction, patient report of preoperative illicit opioid use, patient report of legitimate opioid use, patient report of any preoperative opioid use (both prescribed or illicit). An elevated Fear of Pain Score was associated with prolonged pain after surgery. However, an elevated Anxiety-sensitivity index score was associated with reduced pain duration after surgery. In contrast, a family history of substance abuse and preoperative sedative, marijuana, alcohol, or tobacco use were not associated with prolonged pain duration following surgery. Furthermore, pre-operative pain at the surgical site or anywhere over the entire body, and a number of other psychological measures were not associated with prolonged pain duration after surgery including presence of preoperative PTSD symptoms, SOAPP-R score, and BDI-II score. Each of the factors associated with pain duration at a p-value < 0.25 were evaluated for inclusion in the multivariate model in order to also evaluate for potential confounders. The proportional hazards assumption was met, and the linearity assumption of significant predictors was met.
Table 2.
Determinants of Pain Cessation Rate: Univariate Analysis
| Characteristic | Hazard Ratio | Hazard Ratio 95% Confidence Intervals | P-Value |
|---|---|---|---|
| Self-Perceived Risk of Addictiona | 0.65 | 0.42–0.99 | 0.05 |
| Preoperative Illicit Opioid Use | 0.56 | 0.27–1.16 | 0.12 |
| Fear of Pain Scorea | 0.75 | 0.52–1.08 | 0.12 |
| Anxiety Sensitivity Index Scorea | 1.25 | 0.92–1.70 | 0.16 |
| Any Preoperative Opioid Use | 0.68 | 0.38–1.21 | 0.19 |
| Legitimate Preoperative Opioid Use | 0.67 | 0.35–1.27 | 0.22 |
| Family History of Alcohol or Drug Abuse | 1.29 | 0.81–2.06 | 0.28 |
| Preoperative Sedative Use | 0.75 | 0.35–1.59 | 0.45 |
| Preoperative Marijuana Use | 0.68 | 0.21–2.23 | 0.52 |
| Preoperative Pain (Non-surgical Site)a | 1.06 | 0.64–1.76 | 0.82 |
| Positive PTSD Symptoms | 0.94 | 0.55–1.62 | 0.83 |
| SOAPP 24 Scorea | 0.98 | 0.77–1.25 | 0.85 |
| Beck Depression Inventory-II Scorea | 0.98 | 0.76–1.26 | 0.86 |
| Tobacco Use (lifetime) | 1.05 | 0.64–1.73 | 0.86 |
| Preoperative Alcohol Use | 0.97 | 0.61–1.53 | 0.89 |
| Male Gender | 0.98 | 0.57–1.67 | 0.94 |
| Preoperative Pain (Surgical Site)a | 1.02 | 0.63–1.65 | 0.94 |
| Agea | 1.00 | 0.73–1.36 | 1.00 |
Hazard Ratio between the 25th and 75th percentile.
The multivariate model was stratified by surgery type to control for confounding by the differing surgical injuries, emotional states, and psychological contexts associated with the various surgeries and underlying diagnoses. In multivariate analysis, a number of preoperative factors remained significantly associated with delayed pain resolution across surgery types (Table 3). ASI score and self-perceived addiction susceptibility were each associated with pain resolution after surgery. Unexpectedly, an elevated ASI score was associated with increased rates of pain resolution and reduced pain duration. In contrast, elevated self-perceived addiction susceptibility was associated with reduced rates of pain resolution and more prolonged pain after surgery. Fear of pain and patient-reported preoperative illicit opioid use were confounders of the relationship between ASI score and pain resolution.
Table 3.
Determinants of Pain Cessation Rate: Multivariate Analysis
| Characteristic | Hazard Ratio | Hazard Ratio 95% Confidence Intervals | P-Value |
|---|---|---|---|
| Anxiety Sensitivity Index Scorea | 1.89 | 1.23–2.90 | 0.004 |
| Self-Perceived Risk of Addictiona | 0.62 | 0.39–0.97 | 0.04 |
| Fear of Pain Scorea | 0.65 | 0.40–1.06 | 0.08 |
| Preoperative Illicit Opioid Use | 0.53 | 0.24–1.16 | 0.11 |
Hazard Ratio between the 25th and 75th percentile.
As noted in Table 3, the hazard ratio of continuous variables reflects the difference in pain cessation rates between patients in the 75th and 25th percentile. Thus, as ASI score increased from the 25th percentile value to the 75th percentile value in this cohort, the predicted rate of pain cessation increased by 89%. In contrast, self-perceived addiction-susceptibility significantly predicted the persistence of pain postoperatively in multivariate analysis. In response to the question “How likely do you think it is that you will develop an addiction problem from pain medication you take after surgery?” every one point increase (on a four point categorical scale) was associated with a 38% reduction in the rate of pain resolution (p-value=0.04) (Table 3). In addition, fear of pain and patient-reported preoperative illicit opioid use were statistical confounders of the relationship between ASI score and pain resolution. Removal of either of these confounders from the model changed the coefficient of the ASI score by greater than 10%. Both fear of pain and patient-reported preoperative illicit opioid use are quantitative negative confounders. [28] In other words, controlling for these confounders in the final model augments the association between ASI score and pain resolution resulting in a larger hazard ratio.
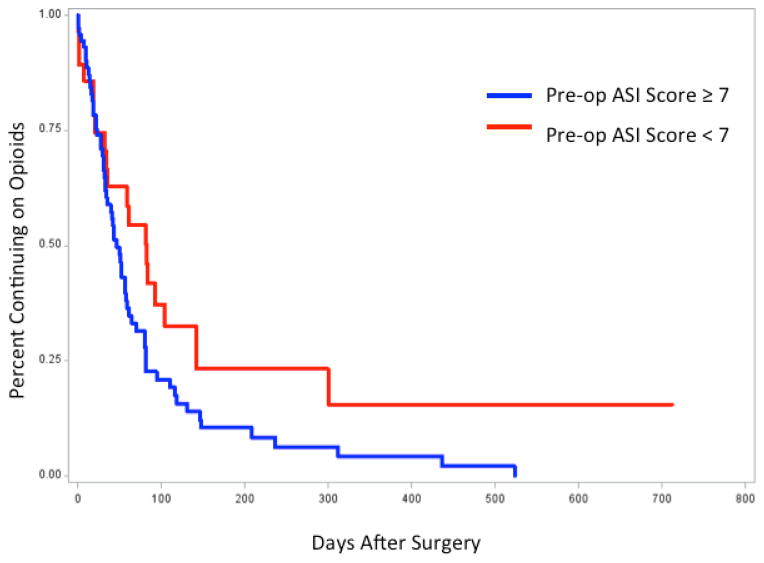
Figure 1 shows the Kaplan Meier survival curves of patients with continued pain after surgery divided into two groups by an ASI score of 7. 35% of the patients with an ASI score greater than or equal to 7 continued to report pain 61 days after surgery. However, 54% of the patients with an ASI score less than 7 continued to report pain 61 days after surgery.
Figure 1.
Preoperative Anxiety Sensitivity Index Score less than 7 predicts prolonged pain duration after surgery (Log rank p = 0.04)
Only the items retained in the final multivariate model optimized according to Akaike Information Criteria (AIC) are presented in Table 3. Examination of the resulting multivariate models from forward, backwards, and stepwise selection methods and their respective AIC values confirmed the final optimized model (Table 4). In addition, the addition of baseline pain severity at the future surgical site or baseline pain severity excluding the future surgical site resulted in greater AIC values (Table 4).
Table 4.
Sensitivity Analysis of Determinants of Pain Cessation Rate Multivariate Modelsa
| Multivariate Model Variation |
ASI Scorea | Fear of Pain Scorea |
Legitimate Preoperative Opioid Use |
Self-Perceived Risk of Addictiona |
Preoperative Illicit Opioid Use |
Preoperative Pain (Surgical Site)a |
Preoperative Pain (Non-Surgical Site)a |
AIC |
|---|---|---|---|---|---|---|---|---|
| HR (95%CI); P-Value |
HR (95%CI); P-Value |
HR (95%CI); P-Value |
HR (95%CI); P-Value |
HR (95%CI); P-Value |
HR (95%CI); P-Value |
HR (95%CI); P-Value |
||
| Full model | 329.654 | |||||||
| Alternative Model Building Algorithms (Full Patient Set) | ||||||||
| Backward Model Building Algorithm | 1.89(1.23–2.90); 0.004 | 0.65(0.40–1.06); 0.08 | 0.62(0.39–0.97); 0.04 | 0.53(0.24–1.16); 0.1 | 329.654 | |||
| Forward Model Building Algorithm | 1.95(1.27–3.01); 0.003 | 0.60(0.36–0.99); 0.05 | 0.61(0.29–1.31); 0.2 | 0.61(0.38–0.96); 0.03 | 0.67(0.29–1.55); 0.3 | 329.942 | ||
| Stepwise Model Building Algorithm | 1.89(1.23–2.90); 0.004 | 0.65(0.40–1.06); 0.08 | 0.62(0.39–0.97); 0.04 | 0.53(0.24–1.16); 0.1 | 329.654 | |||
| Final Model Plus Baseline Pain Severity at Surgical Site | 1.83(1.21–2.76); 0.004 | 0.64(0.40–1.03); 0.07 | 0.60(0.38–0.95); 0.03 | 1.36(0.79–2.35); 0.3 | 341.991 | |||
| Final Model Plus Baseline Pain Severity Other Than at Surgical Site | 1.87(1.23–2.83); 0.003 | 0.65(0.40–1.04); 0.07 | 0.58(0.37–0.94); 0.03 | 0.48(0.22–1.06); 0.07 | 1.51(0.89–2.57); 0.1 | 340.889 |
Hazard ratios presented as the difference in hazard between the 25th percentile and 75th percentile of that variable in the study population All models are stratified by surgery type; ASI= Anxiety Sensitivity Index.
A sensitivity analysis was conducted to examine the potential influence on our results of different model selection processes and also different patient subpopulations. Importantly, higher ASI score predicted improved rates of pain resolution in all potential model subsets either with a p-value of 0.08 or lower (Table 5).
Table 5.
Sensitivity Analysis of Pre-Operative Determinants of Pain Cessation Rate Multivariate Modelsa
| Multivariate Model Variation | Anxiety Sensitivity Index Scorea | Self-Perceived Risk of Addictiona | Fear of Pain Scorea | Preoperative Illicit Opioid Use |
|---|---|---|---|---|
| HR (95%CI); P-Value | HR (95%CI); P-Value | HR (95%CI); P-Value | HR (95%CI); P-Value | |
| Full model | 1.89(1.23–2.90);0.004 | 0.62(0.39–0.97);0.04 | 0.65(0.40–1.06);0.08 | 0.53(0.24–1.16);0.11 |
| Models Using Specific Patient Subsets | ||||
| Thoracotomy Patients Omitted | 1.87(.120–2.92);0.006 | 0.67(0.43–1.10);0.12 | 0.57(0.33–0.97);0.04 | 0.64(0.29–1.43);0.28 |
| Total Knee Replacement Patients Omitted | 1.54(0.96–2.47);0.08 | 0.66(0.41–1.05);0.08 | 0.65(0.37–1.14);0.13 | 0.51(0.21–1.28);0.15 |
| Total Hip Replacement Patients Omitted | 1.85(1.07–3.18);0.03 | 0.58(0.34–0.98);0.04 | 0.64(0.37–1.13);0.12 | 0.55(0.21–1.41);0.21 |
| Mastectomy Patients Omitted | 1.64(1.03–2.61);0.04 | 0.49(0.24–1.01);0.05 | 0.91(0.52–1.57);0.72 | 0.48(0.18–1.28);0.14 |
| Lumpectomy Patients Omitted | 2.00(1.28–3.12);0.002 | 0.65(0.41–1.02);0.06 | 0.61(0.37–1.01);0.05 | 0.49(0.22–1.08);0.08 |
| Opioid Naïve Patients only | 1.73(0.98–3.04);0.06 | 0.54(0.310.94);0.03 | 0.59(0.31–0.94);0.08 | N/A |
Hazard ratios presented as the difference in hazard between 25th percentile and 75th percentile of that variable in the study population.
All models are stratified by surgery type.
Discussion
Our novel model of postoperative pain resolution using Cox proportional hazards regression more accurately characterizes the intervening period of pain resolution between immediate postoperative pain and the potential development of chronic postsurgical pain. No other perioperative study to date has characterized postoperative pain resolution through daily postoperative pain assessments that extend beyond hospital discharge well into the range of chronic post-surgical pain. Using this novel postoperative pain model, a higher ASI score was associated with increased rates of pain resolution. In contrast, elevated self-perceived addiction susceptibility was associated with reduced rates of pain resolution and more prolonged pain after surgery.
Overall, our results suggest postoperative pain persists much longer than the few previous reports specifically focused on pain duration following surgery. Melzack et al [11] reported that only 30.7% of patients continued to experience pain 100 hours or more after surgery in a cohort study of 88 postsurgical patients on a surgical ward. Similarly, Bachiocco et al. studied 120 inpatients following thoracotomy and reported the median duration of pain was 7.1 days.[29] Only about 10% of patients reported pain past 21 days. These reports of rapid pain resolution are somewhat at odds with the prevalence of chronic postsurgical pain described by studies that have focused on the occurrence of chronic postsurgical pain,[1–9] and are very different than the experience reported by patients in this study who reported much longer periods of time before pain resolution up to 859 days after surgery.
The duration of pain following surgery is important because overall suffering among those experiencing acute pain can be conceived of as the product of pain intensity and the time over which that pain intensity occurs.[30] Therefore, pain duration following surgery represents an independent, important, and clinically relevant endpoint for studies of postoperative pain. Additionally, following many surgeries, such as total joint replacement or herniorrhaphy the resolution of pain is nearly synonymous with recovery. However, to date few studies have examined pain duration as a primary endpoint following surgery. While significant progress has been made in the last fifty years to reduce overall suffering by expanding our options to reduce pain intensity at any given time, no general strategies (aside from reducing the scope of injury-- e.g. the development of laparoscopy) have been advanced to reduce suffering by reducing the duration of time over which patients feel this pain intensity.
Based on the results of previous research, we hypothesized that preoperative psychological distress would predict longer pain duration following surgery. Using our model of postoperative pain duration, and in this mixed surgical cohort, increased self-perceived susceptibility to addiction (an author-generated measure) was an independent predictor of prolonged pain after surgery. It is possible that this self-reported measure is capturing an aspect of preoperative anxiety. Specifically, patients may have concerns regarding inability to control postoperative pain and the need to use high doses of pain medication after surgery with the development of addiction. In fact, we have previously reported results from this cohort that pre-operative self-perceived susceptibility to addiction was independently associated with a 53% (95% CI 23–71%) reduction in the rate of opioid cessation following surgery (p = 0.003)—i.e. people who thought they were at higher risk of addiction actually consumed opioids for longer periods of time after surgery--the opposite effect of our pre-study hypothesis.[16]
In addition, research has shown a connection between self-reported measures of opioid misuse and aspects of pain. In a cohort of 4,122 veterans, non-medical prescription opioid use was associated with pain interference. [31] In another cohort of veterans from primary care clinics, self-reported prescription drug abuse was significantly associated with chronic pain. [32] Similarly, in a nationally representative sample of U.S. adults, non-medical prescription analgesic use was significantly and independently associated with pain. [33] In our surgical cohort, patients reporting increased self-perceived susceptibility to addiction may have a history of non-medical pain medication use, which could directly translate to increased postoperative pain and pain interference with physical functioning. More work must be done to verify the reproducibility of these findings. Does higher self-perceived risk of addiction reflect a past experience of “opioid-liking”? If so, how might a tendency for “opioid liking” drive more persistent pain reports? Further research is necessary to characterize the association between this self-reported measure of addiction susceptibility and prolonged pain and opioid use after surgery.
In contrast, an elevated ASI score strongly predicted an increased rate of pain resolution after surgery in our surgical cohort. Also, fear of pain and patient-reported preoperative illicit opioid use were statistical confounders of the relationship between ASI score and pain resolution. Anxiety sensitivity is associated with fear of anxiety-related sensations and the belief that these sensations lead to harm. [21] Furthermore, anxiety sensitivity has been associated with enhanced pain intensity in both healthy subjects and patients with chronic pain. [34–36] However consistent with the potential protective effect suggested by our results, in a study of 82 patients presenting with new hand fractures at a fracture clinic located at a general hospital, higher levels of anxiety sensitivity as measured by the ASI score were related to lower levels of disability. [37] Keogh et al. postulated that heightened awareness of bodily sensations may result in protective behaviors, which may prevent further injury or re-injury. [37] In fact, patients with high physical anxiety sensitivity (a previously identified factor of the ASI) show selective attention to physically threatening material rather than socially threatening or positive material. [38] Similarly, ASI score may have resulted in a counter-intuitive protective effect in our surgical population as patients with elevated ASI scores may have engaged in more protective behaviors after surgery leading to earlier pain resolution. As can be seen from Table 5 this potential protective effect is also robust to reanalyzing the data while omitting various subgroups of patients. In other words, no matter how we reanalyze the data this effect seems to persist. Furthermore, the median ASI score of 14 in our population was similar to the mean ASI scores in Keogh’s study of 10.3 and 10.26 in males and females respectively, suggesting comparability.
Other studies directly examining anxiety sensitivity in the perioperative setting have not found an association with postoperative pain intensity. In a prospective study of 160 patients undergoing third molar removal, preoperative ASI scores were not predictive of postoperative anxiety and pain. [39] Also, although anxiety sensitivity was associated with pre-operative elevated anxiety, depression, and stress prior to surgery in patients undergoing implantable cardioverter defibrillator implantation, anxiety sensitivity was not associated with anxiety or depression up to 6 months after surgery.[40] Unexpectedly, patients with higher levels of preoperative anxiety sensitivity showed greater reductions in stress after surgery. [40] Lemon et al. postulated that preoperative anxiety sensitivity was measuring realistic concerns regarding threats to survival in these patients at-risk for sudden cardiac death rather than unrealistic concerns in physically healthy respondents. Furthermore, the authors postulated that once a threatening event (surgery) has passed, and harm has been reduced, patients with preoperative elevated anxiety sensitivity might experience enhanced psychological adjustment. This theory may also apply to our surgical cohort, as patients with preoperative elevated ASI scores had a higher rate of pain resolution. However, given the construct of anxiety sensitivity, it is more likely that those patients with elevated preoperative ASI scores are exhibiting unrealistic concerns regarding impending surgery, and they may experience greater psychological adjustment after surgery.
In contrast to hypotheses pointing to the potential protective effect of elevated preoperative ASI scores. It is possible that those participants with elevated ASI scores were able to discriminate between pain at their surgical site and other areas of pain over their entire body. In other words, participants with elevated ASI scores were specifically able to isolate pain associated with their surgery, and the association between elevated ASI scores and an increased rate of pain resolution may be an artifact of how the BPI was administered to our participants. Another possible hypothesis is that participants with elevated ASI scores were more likely to remain in the study and not be censored resulting in a responder bias. However, the association between preoperative ASI score and non-censored status in logistic regression was non-significant. Nonetheless, this counter-intuitive finding will need to be examined in future perioperative studies specifically to see if the association between ASI and pain duration is reproduced.
Our unique model of postoperative pain duration resulted in a set of predictors that has not been identified through past research examining intermittent time points after surgery. Preoperative measures of psychological distress other than anxiety sensitivity and fear of pain were not associated with pain duration after surgery. Additionally, depressive symptoms as measured by the BDI-II were not associated with pain duration after surgery in our population. The fact that elevated preoperative BDI-II score was associated strongly with duration of opioid use in this cohort [16] suggests that the BDI-II as employed here was sensitive to small but meaningful differences in depressive symptoms. In this context, the failure to detect any association between preoperative depressive symptoms and more prolonged pain suggests strongly that if any such relationship exists it is unlikely to be large enough to be clinically relevant. Future research is necessary to examine postoperative pain duration as an outcome separate from pain intensity or severity after a multitude of surgeries to determine the reproducibility of our results. For example, further characterization of pain sensitivity in patients with elevated self-perceived susceptibility to addiction may establish a vulnerable patient phenotype. In addition, risk assessment for prolonged postoperative pain may be achievable through patient-reported outcomes. With additional research using our novel model of postoperative pain duration, new interventions could be developed to reduce the duration of suffering related to postoperative pain by modifying established preoperative risk factors.
Acknowledgments
Funding:
Dr. Carroll gratefully acknowledges funding from the Foundation for Anesthesia Education and Research (FAER) in the form of a mentored research training grant. Subsequent funding was provided by K23 grant 1K23DA025152 from the National Institute on Drug Abuse.
Dr. Hah was supported by K23 grant 1K23DA035302 from the National Institute on Drug Abuse.
Dr. Humphreys was supported by a senior career scientist award from the Veterans Affairs Health Services Research and Development Service.
Dr. Mackey was supported by NIH K24 DA029262 and the Chris Redlich Pain Research Fund.
Any views expressed here are not necessarily those of the U.S. Government.
Initial results of this study were presented as a poster at the American Pain Society 2008 Annual Scientific Meeting in Tampa, FL.
The authors would like to thank Richard I. Whyte, MD, MBA (Department of Cardiothoracic Surgery), Jessica S. Donington (Department of Cardiothoracic Surgery), and Walter B. Cannon, MD (Department of Cardiothoracic Surgery) at Stanford University for aiding in patient recruitment.
Footnotes
Conflicts of Interest:
None
Contributor Information
Ian R. Carroll, Stanford University Department of Anesthesiology, Perioperative, and Pain Medicine, Division of Pain Medicine, Palo Alto, CA.
Jennifer M. Hah, Stanford University Department of Anesthesiology, Perioperative, and Pain Medicine, Division of Pain Medicine, Palo Alto, California.
Peter L. Barelka, (Affiliated), Anesthesiology Service Department of Veterans Affairs, VA, Palo Alto Health Care System, Palo Alto, CA and Stanford University School of Medicine, Department of Anesthesiology, Perioperative, and Pain Medicine, Palo Alto, CA.
Charlie KM. Wang, Stanford Systems Neuroscience and Pain Lab (SNAPL), Stanford School of Medicine, Department of Anesthesia, Palo Alto, CA.
Bing M. Wang, Stanford Systems Neuroscience and Pain Lab (SNAPL), Stanford School of Medicine, Department of Anesthesia, Palo Alto, CA.
Matthew J. Gillespie, Stanford Systems Neuroscience and Pain Lab (SNAPL), Stanford School of Medicine, Department of Anesthesia, Palo Alto, CA.
Rebecca McCue, Stanford Systems Neuroscience and Pain Lab (SNAPL), Stanford School of Medicine, Department of Anesthesia, Palo Alto, CA.
Jarred W. Younger, Stanford Department of Anesthesiology, Perioperative, and Pain Medicine, Division of Pain Medicine, Palo Alto, California.
Jodie Trafton, Psychiatry & Behavioral Sciences, Stanford University, Palo Alto, CA.
Keith Humphreys, VA, Palo Alto Health Care System, Palo Alto, CA and Stanford University, Palo Alto, CA.
Stuart B. Goodman, Department of Orthopaedic Surgery, Stanford University, Palo Alto, CA.
Fredrick M. Dirbas, Assistant Professor, Department of Surgery, Stanford University, Palo Alto, CA.
Sean C. Mackey, Stanford University Department of Anesthesiology, Perioperative, and Pain Medicine, Palo Alto, CA.
References
- 1.Lavand'homme PM, Grosu I, France MN, Thienpont E. Pain Trajectories Identify Patients at Risk of Persistent Pain After Knee Arthroplasty: An Observational Study. Clin Orthop Relat Res. 2013 doi: 10.1007/s11999-013-3389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masselin-Dubois A, Attal N, Fletcher D, et al. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain. 2013;14:854–64. doi: 10.1016/j.jpain.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araujo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13:1045–57. doi: 10.1016/j.jpain.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Archer KR, Seebach CL, Mathis SL, Riley LH, 3rd, Wegener ST. Early postoperative fear of movement predicts pain, disability, and physical health 6 months after spinal surgery for degenerative conditions. Spine J. 2013 doi: 10.1016/j.spinee.2013.06.087. [DOI] [PubMed] [Google Scholar]
- 5.Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155:232–43. doi: 10.1016/j.pain.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Martinez V, Ben Ammar S, Judet T, Bouhassira D, Chauvin M, Fletcher D. Risk factors predictive of chronic postsurgical neuropathic pain: the value of the iliac crest bone harvest model. Pain. 2012;153:1478–83. doi: 10.1016/j.pain.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Vandenkerkhof EG, Hopman WM, Goldstein DH, et al. Impact of Perioperative Pain Intensity, Pain Qualities, and Opioid Use on Chronic Pain After Surgery: A Prospective Cohort Study. Reg Anesth Pain Med. 2011 doi: 10.1097/AAP.0b013e318237516e. [DOI] [PubMed] [Google Scholar]
- 8.Hickey OT, Burke SM, Hafeez P, Mudrakouski AL, Hayes ID, Shorten GD. Severity of acute pain after breast surgery is associated with the likelihood of subsequently developing persistent pain. Clin J Pain. 2010;26:556–60. doi: 10.1097/AJP.0b013e3181dee988. [DOI] [PubMed] [Google Scholar]
- 9.Gerbershagen HJ, Dagtekin O, Rothe T, et al. Risk factors for acute and chronic postoperative pain in patients with benign and malignant renal disease after nephrectomy. Eur J Pain. 2009;13:853–60. doi: 10.1016/j.ejpain.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 11.Melzack R, Abbott FV, Zackon W, Mulder DS, Davis MW. Pain on a surgical ward: a survey of the duration and intensity of pain and the effectiveness of medication. Pain. 1987;29:67–72. doi: 10.1016/0304-3959(87)90179-5. [DOI] [PubMed] [Google Scholar]
- 12.Nay PG, Elliott SM, Harrop-Griffiths AW. Postoperative pain: expectation and experience after coronary artery bypass grafting. Anaesthesia. 1996;51:741–743. doi: 10.1111/j.1365-2044.1996.tb07887.x. [DOI] [PubMed] [Google Scholar]
- 13.Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain. 2012;28:819–41. doi: 10.1097/AJP.0b013e31824549d6. [DOI] [PubMed] [Google Scholar]
- 14.Rudin A, Wolner-Hanssen P, Hellbom M, Werner MU. Prediction of post-operative pain after a laparoscopic tubal ligation procedure. Acta Anaesthesiol Scand. 2008;52:938–45. doi: 10.1111/j.1399-6576.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 15.Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003:27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 16.Carroll I, Barelka P, Wang CK, et al. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg. 2012;115:694–702. doi: 10.1213/ANE.0b013e31825c049f. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 18.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Makela K. Studies of the reliability and validity of the Addiction Severity Index. Addiction. 2004;99:398–410. doi: 10.1111/j.1360-0443.2003.00665.x. discussion 411–8. [DOI] [PubMed] [Google Scholar]
- 20.Butler S, Budman S, Fernandez K, Jamison R. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 22.McNeil DW, Rainwater AJ., 3rd Development of the Fear of Pain Questionnaire--III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 23.Kimerling R, Ouimette P, Prins A, et al. Brief report: Utility of a short screening scale for DSM-IV PTSD in primary care. Journal of general internal medicine. 2006;21:65–67. doi: 10.1111/j.1525-1497.2005.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker GA. Common Statistical Methods for Clinical Research with SAS Examples. 2. Cary, NC: SAS Institute Inc; 2002. p. 464. [Google Scholar]
- 25.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 27.Vittinghoff E. Statistics for biology and health. New York: Springer; 2005. Regression methods in biostatistics : linear, logistic, survival, and repeated measures models; p. xv.p. 340. [Google Scholar]
- 28.Rao CR, Miller JP, Rao DC. Handbook of statistics. 1. xviii. Amsterdam ; Boston: Elsevier; 2008. Epidemiology and medical statistics; p. 852. [Google Scholar]
- 29.Bachiocco V, Morselli-Labate AM, Rusticali AG, Bragaglia R, Mastrorilli M, Carli G. Intensity, latency and duration of post-thoracotomy pain: relationship to personality traits. Functional neurology. 1990;5:321–332. [PubMed] [Google Scholar]
- 30.Lacroix R, Barbaree HE. Pain-elicited responses and their role in predicting future pain duration and severity. Behaviour Research and Therapy. 1992;30:471–478. doi: 10.1016/0005-7967(92)90031-b. [DOI] [PubMed] [Google Scholar]
- 31.Barry DT, Goulet JL, Kerns RK, et al. Nonmedical use of prescription opioids and pain in veterans with and without HIV. Pain. 2011;152:1133–8. doi: 10.1016/j.pain.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker WC, Fiellin DA, Gallagher RM, Barth KS, Ross JT, Oslin DW. The association between chronic pain and prescription drug abuse in Veterans. Pain Med. 2009;10:531–6. doi: 10.1111/j.1526-4637.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 33.Novak SP, Herman-Stahl M, Flannery B, Zimmerman M. Physical pain, common psychiatric and substance use disorders, and the non-medical use of prescription analgesics in the United States. Drug Alcohol Depend. 2009;100:63–70. doi: 10.1016/j.drugalcdep.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keogh E, Cochrane M. Anxiety sensitivity, cognitive biases, and the experience of pain. J Pain. 2002;3:320–9. doi: 10.1054/jpai.2002.125182. [DOI] [PubMed] [Google Scholar]
- 35.Terry EL, Kerr KL, DelVentura JL, Rhudy JL. Anxiety sensitivity does not enhance pain signaling at the spinal level. Clin J Pain. 2012;28:505–10. doi: 10.1097/AJP.0b013e31823984f9. [DOI] [PubMed] [Google Scholar]
- 36.Asmundson GJ, Norton GR. Anxiety sensitivity in patients with physically unexplained chronic back pain: a preliminary report. Behav Res Ther. 1995;33:771–7. doi: 10.1016/0005-7967(95)00012-m. [DOI] [PubMed] [Google Scholar]
- 37.Keogh E, Book K, Thomas J, Giddins G, Eccleston C. Predicting pain and disability in patients with hand fractures: comparing pain anxiety, anxiety sensitivity and pain catastrophizing. Eur J Pain. 2010;14:446–51. doi: 10.1016/j.ejpain.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Keogh E, Dillon C, Georgiou G, Hunt C. Selective attentional biases for physical threat in physical anxiety sensitivity. J Anxiety Disord. 2001;15:299–315. doi: 10.1016/s0887-6185(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 39.van Wijk AJ, de Jongh A, Lindeboom JA. Anxiety sensitivity as a predictor of anxiety and pain related to third molar removal. J Oral Maxillofac Surg. 2010;68:2723–9. doi: 10.1016/j.joms.2010.06.174. [DOI] [PubMed] [Google Scholar]
- 40.Lemon J, Edelman S. Psychological adaptation to ICDs and the influence of anxiety sensitivity. Psychol Health Med. 2007;12:163–71. doi: 10.1080/13548500500448478. [DOI] [PubMed] [Google Scholar]