Abstract
The pathogenic mechanism of recurrent or chronic urinary tract infection is poorly understood. Escherichia coli cells bearing Dr fimbriae display unique tropism to the basement membrane (BM)-renal interstitium that enables the bacteria to cause chronic pyelonephritis in experimental mice. The renal receptors for Dr-fimbriated E. coli are type IV collagen and decay-accelerating factor (DAF). We hypothesized that type IV collagen receptor-mediated BM-interstitial tropism is essential for E. coli to cause chronic pyelonephritis. To test the role of the type IV collagen tropism of Dr-fimbriated E. coli in renal persistence, we constructed an isogenic mutant in the DraE adhesin subunit that was unable to bind type IV collagen but retained binding to DAF and examined its virulence in the mouse model. The collagen-binding mutant DrI113T was eliminated from the mouse renal tissues in 6 to 8 weeks, while the parent strain caused persistent renal infection that lasted at least 14 weeks (P ≤ 0.02). Transcomplementation with the intact Dr operon restored collagen-binding activity, BM-interstitial tropism, and the ability to cause persistent renal infection. We conclude that type IV collagen binding mediated by DraE adhesin is a critical step for the development of persistent renal infection in a murine model of E. coli pyelonephritis.
Escherichia coli is the most frequent etiologic agent of urinary tract infection (UTI) (35). Among the several virulence factors of uropathogenic E. coli, adhesins play an essential and critical role in the infectious process because they mediate the initial mucosal colonization of the host (12). These adhesins enable the bacterium to recognize specific cell surface receptors in the uroepithelium of the host. Such targeted receptor specificity may result in restricted tissue tropism, thereby associating specific adhesin subtypes with upper or lower UTI. Examples of such tissue tropism include recognition of monomannosyl residues on the bladder epithelium by type 1 fimbriae, resulting in cystitis (30), and binding of P antigen in the renal tubular epithelium by P fimbriae, leading to acute pyelonephritis (15). Although these adhesins are well described in the initiation of acute infections, traditional knowledge places emphasis on the host immune system and underestimates the roles of bacterial virulence factors in the subsequent events leading to chronic or recurrent renal pathology (27, 28). Few studies have proposed that the class III subtype of P fimbriae, type 1 fimbriae, and the Dr adhesins could play critical roles in the development of chronic pyelonephritis (7, 9, 13).
E. coli cells bearing Dr adhesins are associated with cystitis and recurrent UTI in young adults, protracted diarrhea in children, and pyelonephritis in pregnant women (1, 5, 6, 22). The family of Dr adhesins includes fimbrial and afimbrial adhesins of E. coli (Dr; Dr-II; F1845; Afa-I, -II, -III, -IV, -V, -VII, and -VIII; Nfa-I; and Aaf-I and Aaf-II) encoded by genetic clusters of similar organization and/or binding to the common apical epithelial cell receptor, decay-accelerating factor (DAF) (24). It has been shown that binding of these Dr adhesins to the DAF receptor leads to the internalization of Dr+ E. coli into nonfusogenic intracellular vacuoles, thereby providing a protected intracellular niche for the bacterium (29).
In a previous study, it was demonstrated that Dr fimbriae, members of the family of Dr adhesins, are important virulence factors in establishing persistent renal infection in an experimental mouse model of UTI (7). Transurethral infection of C3H/HeJ mice with a clinical isolate of Dr-fimbriated E. coli (Dr+ E. coli) IH11128 resulted in chronic tubulointerstitial nephritis and persistent colonization of renal interstitium for up to 1 year. The loss of Dr fimbriae resulted in the inability of E. coli to cause persistent renal infection. Renal histopathology of infected tissues demonstrated interstitial colonization by Dr+ E. coli with chronic inflammation, lymphocytic infiltration, fibrosis, and tubular atrophy. The unique tropism of Dr+ E. coli to the basement membrane (BM)-renal interstitium has been proposed to form the basis of chronicity (25). The BM-renal interstitium substructures in the kidney have abundant type IV collagen, a specialized form of extracellular matrix (ECM) that underlies all epithelia and compartmentalized tissues. Interestingly, among the family of Dr adhesins, the Dr fimbria is the only member with an unique ability to bind to type IV collagen, as well as DAF (36). Binding to type IV collagen is mediated by the major structural adhesin subunit DraE (32). Replacement of a single amino acid at position 113 of the DraE subunit results in loss of type IV collagen binding (2).
The pathogenic mechanism underlying the ability of Dr-fimbriated E. coli to cause chronic pyelonephritis by persistent colonization of the BM-renal interstitium is unclear. Our working hypothesis is that a bacterium causing chronic pyelonephritis should possess the following virulence properties: (i) bind specific receptors exposed on the uroepithelial cells; (ii) reach interstitial tissue, perhaps due to invasiveness; and (iii) bind specific receptors in the ECM.
Several microbial surface components have been demonstrated to bind specific ECM proteins (26). While the interactions of certain bacterial agents with ECM proteins have been demonstrated to form the basis of infection, the potential contribution of bacterial ECM tropism to chronic renal infection is not understood. In the present study, the contribution of Dr fimbrial type IV collagen binding to the pathogenesis of chronic pyelonephritis was investigated. This was accomplished by constructing a Dr fimbrial mutant unable to bind type IV collagen but retaining the ability to bind to DAF and evaluating the abilities of the wild type and mutant to bind renal tissue in vitro and to cause persistent renal colonization in an established model of chronic pyelonephritis. To fulfill the molecular Koch's postulate for pathogenesis, in vitro and in vivo studies were also performed with the collagen-binding mutants transcomplemented with plasmids carrying the wild-type or mutated Dr operon conferring collagen-hyperbinding capacity. Our findings demonstrate that the binding of DraE adhesin to type IV collagen is an essential step for establishing chronic renal infection.
MATERIALS AND METHODS
Bacterial strains.
A clinical uropathogenic E. coli strain, IH11128 (O75:K5:H−), expressing Dr fimbriae was isolated from a patient suffering from pyelonephritis (31). An isogenic mutant unable to bind type IV collagen (DrI113T) was constructed from a spontaneous nalidixic acid-resistant (Nalr) mutant of E. coli IH11128. Recombinant Dr+ E. coli cells expressing mutant forms of the DraE adhesins were constructed by site-directed mutagenesis (2). Bacteria were grown on plain Luria agar plates or plates supplemented with 100 μg of ampicillin/ml as appropriate. All strains were grown overnight in 5% CO2 at 37°C.
Construction of isogenic mutant DrI113T deficient in type IV collagen binding.
A strategy of allele exchange employing a conditional replicon (suicide vector) was applied to the construction of a collagen-binding mutant of E. coli IH11128. In this two-step procedure, a mutant allele of draE was constructed by replacing isoleucine at position 113 of the mature DraE protein with tyrosine by PCR mutagenesis, and the product was cloned into a suicide vector, pCVD442 (3). The resulting plasmid, pCC113, was introduced into E. coli IH11128 (Nalr) by filter mating with SM10 λpir (pCC113) and selection on minimal medium in the presence of ampicillin and nalidixic acid. Double recombinants that had the suicide vector sequences deleted were selected by screening for growth in the presence of 10% [scap]l[scap]-sucrose. Sucrose-resistant colonies were picked and tested for sensitivity to ampicillin. Among ampicillin-sensitive colonies, we selected colonies expressing chloramphenicol-resistant hemagglutination, indicative of the presence of the mutation I113T in the draE gene. The draE gene of the transconjugant was sequenced to confirm the mutation of codon ATT in position 113 to ACT.
Purification and in vitro binding of Dr fimbriae to renal tissue.
The recombinant E. coli DH5α strain carrying a plasmid-expressing Dr fimbria (pCC90) or the collagen-binding mutant (pCC90-I113T) was grown overnight on Luria-Bertani agar plates with ampicillin. The bacteria were suspended in phosphate-buffered saline (PBS), vortexed for 5 min, and centrifuged for 10 min at 10,000 × g in a Sorvall SS-34 rotor. The supernatants were filtered through a 0.22-μm-pore-size membrane. Fimbrial protein was purified from the filtrate by ammonium sulfate precipitation and size exclusion chromatography using Sepharose 4B. The column was connected to the Econo low-pressure liquid chromatography system (Bio-Rad, Hercules, Calif.). The eluted fimbriae were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% acrylamide).
Human kidney sections were fixed with cold acetone for 10 min and overlaid for 3 h with fimbrial preparation (200 μl) containing 50 μg of fimbrial protein/ml (for both the Dr and collagen mutants). A control section was incubated with PBS. The sections were washed in PBS and incubated for 1 h with rabbit anti-Dr antibody diluted 1:100 in PBS, followed by washing and 1 h of incubation with anti-rabbit antibody conjugated with Texas Red (for fimbrial antigen). A separate section was incubated for 1 h with anti-collagen antibody diluted 1:100, followed by washings and 1 h of incubation with anti-rabbit antibody conjugated with Cy2. Images were acquired with a CoolSnapcf digital camera (Roger Scientific Inc., Trenton, N.J.) hooked up to the Nikon Eclipse 600 microscope and processed with MetaMorph software version 5.7 (Universal Imaging Corp., Downingtown, Pa.). All procedures were done at room temperature (RT). Antibodies were used in 200-μl volumes to cover kidney sections. The magnification was ×100.
Fixed mouse kidney sections (4 μm thick) were incubated with bacterial suspensions adjusted to an optical density at 600 nm (OD600) of 1.0 for 3 h at RT. Following incubation, the slides were washed, and bound bacteria were detected by using antibodies to Dr fimbriae as described above. Controls for nonspecific staining were performed with preimmune rabbit immunoglobulin G and/or by replacing each step of staining by corresponding buffers.
Cryostat kidney sections of C3H/HeJ mice were fixed with cold acetone for 10 min and allowed to air dry. Next, the sections were incubated for 1 h at RT with 200 μl of bacterial suspensions made in PBS (2 ×109 CFU/ml) of Dr-positive recombinant E. coli pCC90 or with the collagen-negative mutant I113T. The sections were washed twice with PBS and incubated at RT for 45 min with 200 μl of anti-collagen antibody (NC1) diluted 1:250 in PBS. The sections were washed twice with PBS and subsequently incubated for 30 min with goat anti-rabbit antibody conjugated with Texas Red (Molecular Probes Inc., Eugene, Oreg.) diluted 1:50 in PBS. After two washings with PBS, the sections were incubated for 45 min with rabbit polyclonal anti-Dr antibody diluted 1:100, followed by washings and 30 min of incubation with goat anti-rabbit conjugated with Cy2 (Jackson Immuno Research Laboratories Inc., West Grove, Pa.) diluted 1:100. The sections were examined under a fluorescence microscope (Nikon Eclipse 600) equipped with a charge-coupled device CoolSnapcf camera driven by Metamorph software, version 5.0r2.
In vitro DAF and collagen-binding assay.
The ability of purified Dr fimbria and its collagen mutant to bind to DAF or type IV collagen was tested by the following assay. Ninety-six-well microtiter plates were coated with CHO DAF+ cells (20) or the 7S (tetramer) or noncollagenous (hexamer) domain of type IV collagen, and 10 μg of purified Dr fimbriae was added. After incubation for 1 h at RT, the plates were washed twice with PBS and fixed with 3% paraformaldehyde. Following fixation, rabbit polyclonal anti-Dr antibody at 1:100 dilution was added, and the plates were incubated for 1 h at RT and washed twice with PBS. The wells were then incubated with polyclonal goat anti-rabbit- horseradish peroxidase conjugate (Amersham Biosciences Co., Piscataway, N.J.) at 1:2,000 dilution for 1 h at RT. The reaction was developed with substrate and measured in an enzyme-linked immunosorbent assay plate reader at OD405.
Complementation of collagen-binding phenotype.
The isogenic collagen-binding mutant of IH1128 (Dr 113T) was transformed by electroporation with plasmid pCC90 carrying an insert of intact Dr operon or the plasmid pCC90-D54Y (2) carrying a mutation in the DraE adhesin gene coding for Dr fimbriae with collagen-hyperbinding capacity. The complemented strains were selected by resistance to ampicillin (50 μg/ml).
The 7S domain tetramer and the noncollagenous domain (hexamer) were isolated by collagenase digestion of bovine lens capsule BM, as previously described from glomerular BMs (17). Glass coverslips were coated with 10 μg of collagen type IV domains (noncollagenous domain) or with the 7S subunit, incubated for 18 h at 4°C, and washed twice with PBS. About 500 μl (each) of the Dr+ E. coli clinical strain IH11128; its isogenic mutant, Dr113T; and the complemented strains, including DrI113T(pCC90-D54Y) suspended in 2% α-methyl mannose PBS adjusted to a final OD600 of 0.4, was added to collagen-coated coverslips and incubated for 1 h at room temperature. The coverslips were washed three times with PBS, fixed with ice-cold methanol, and stained with a 1-in-20 dilution of Giemsa stain. The coverslips were examined for bound bacteria using a Nikon Eclipse 600 light microscope under ×100 magnification and photographed. The number of bound bacteria was reported as the number of bacteria per square centimeter of the photomicrograph.
Epithelial cell internalization assay.
The abilities of the Dr-fimbriated E. coli and its isogenic collagen-binding mutant to invade epithelial cells were studied by a previously described HeLa cell internalization assay (8).
Mouse model of experimental chronic pyelonephritis.
The Dr+ E. coli clinical strain IH11128 and its isogenic collagen-binding mutant, DrI113T, were tested for the ability to persist in an ascending UTI model (7). Female C3H/HeJ mice, 8 to 10 weeks old, were purchased from the Jackson Laboratory, Bar Harbor, Maine. Each mouse received two doses by intraperitoneal injection of 50 μl of streptomycin SO4 (28.5 mg/ml of saline) on consecutive days to clear possible UTIs. Two days after the last antibiotic treatment, the mice were anaesthetized by intraperitoneal injection of 200 μl of pentobarbital sodium (Nembutal; 5 mg/ml of saline). Following anesthesia, groups of mice were infected by urethral catheterization. About 50 μl of a bacterial suspension adjusted to an OD600 of 2.4 was instilled into the urinary bladder through a soft polyethylene catheter (outer diameter, 0.30 mm; Norton Performance Plastics, Akron, Ohio) adapted to a tuberculin syringe. The catheter was immediately withdrawn without further manipulation. The animals were subsequently allowed free access to food and water.
Quantitative tissue cultures.
Groups of mice were sacrificed by cervical dislocation on day 1 and at 1, 6, 8, and 14 weeks while under anesthesia. The kidneys were aseptically removed, weighed, and homogenized in 1 ml of PBS in a Teflon tissue grinder (Frank A. Thomas Co., Philadelphia, Pa.). About 20 μl of tissue homogenate was plated on Luria agar plates and MacConkey plates and incubated overnight at 37°C and 5% CO2 in the incubator. The following day, viable bacteria were counted, and the results were expressed as the number of CFU per gram of tissue. Statistical analysis was performed using the Student t test, and a P value of <0.05 was considered significant.
RESULTS
Isogenic mutant DrI113T produces adhesins which have lost collagen-binding but retain DAF-binding capacity.
E. coli Dr fimbriae mediate binding to DAF, a complement regulatory molecule expressed ubiquitously on epithelial cells and cells of the hematopoietic lineage, and type IV collagen, a BM protein (22, 36). The interactions of the Dr adhesin with its ligands have been described at the molecular level, indicating that Dr adhesin binds to the short consensus repeat 3 domain of DAF and the 7S domain (tetramer) of type IV collagen (20, 36). In order to characterize the DAF and type IV collagen-binding specificities of the recombinant constructs, we purified fimbriae from E. coli carrying the wild-type (pCC90) and mutated (pCC90-I113T) Dr operon and tested them in an in vitro binding assay. Fimbriae purified from the wild type bound to the 7S domain, whereas the mutant I113T did not (Fig. 1A). Also, the wild type did not bind the noncollagenous domain (hexamer) of type IV collagen, indicating that the binding of Dr adhesin is specific for the 7S domain, which connects four triple-helical molecules of type IV collagen at their N termini, forming supramolecular networks. Purified wild-type Dr fimbriae and the collagen-binding mutant I113T retained similar levels of binding to CHO DAF+ cells but did not bind to CHO DAF− cells fixed on microtiter plates (Fig. 1B). These results demonstrate that the isogenic mutant produced an intact and functional adhesin that lost its ability to bind only type IV collagen, but not DAF.
FIG. 1.
Binding of purified Dr fimbriae to type IV collagen and DAF. Dr fimbriae purified from E. coli Dr(pCC90) (DAF+ COL+) or E. coli Dr(pCC90-I113T) (DAF+ COL−) were incubated with microtiter plates coated with the 7S domain (tetramer) or noncollagenous (NC1) domain (hexamer) of type IV collagen purified from the bovine lens capsule BM (A) or recombinant CHO cells transfected with cDNA for human DAF (CHO− DAF+) or vector only (CHO− DAF−) (B). The Dr fimbriae (DAF+ COL+) bound to the 7S domain of type IV collagen and human DAF, while its isogenic collagen-binding mutant lost binding to the 7S domain but retained binding activity to human DAF. Each bar represents the mean plus the standard error of the mean from three experiments done in quadruplicate.
Loss of collagen-binding phenotype of Dr fimbriae results in loss of tubular BM binding.
The Dr receptors, DAF and type IV collagen, are present in the urinary tract and facilitate colonization by Dr+ E. coli (21, 25). Previous studies using in vitro binding to human tissue and in vivo mouse models have characterized tissue-specific binding of Dr fimbriae to the transitional epithelium in the urethra, bladder, ureter, and renal pelvis, with intense binding confined to Bowman's capsule and tubular BMs of the nephron (25). In order to understand the contributions of individual Dr receptors to ascending renal infection by Dr+ E. coli, fimbriae purified from recombinant E. coli expressing wild-type Dr and collagen-binding mutant I113T fimbriae were incubated with cryostat sections of human kidney. Intense staining of Dr fimbriae was noticed for Bowman's capsule and the tubular BM (Fig. 2C), a region rich in type IV collagen networks. In sharp contrast, the collagen-binding mutant I113T fimbriae lost binding to the tubular BM and Bowman's capsule (Fig. 2D).
FIG. 2.
Binding of purified Dr fimbriae to glomerulus and renal BM of human kidney. Cryostat sections of human kidney were incubated with Dr fimbriae purified from E. coli Dr(pCC90) (DAF+ COL+) or E. coli Dr(pCC90-I113T) (DAF+ COL−) to detect tissue tropism to glomerular and renal interstitial structures. (A) Hematoxylin and eosin staining of kidney. (B) Distribution of type IV collagen in human kidney. (C) Dr fimbriae showed intense binding to Bowman's capsule and BMs of the renal tubules. (D) DrI113T fimbriae from collagen-binding mutant completely lost binding to Bowman's capsule and BMs of the renal tubules. All micrographs were taken at magnification ×1,000. The glomeruli are indicated by arrows, and the tubules are indicated by arrowheads.
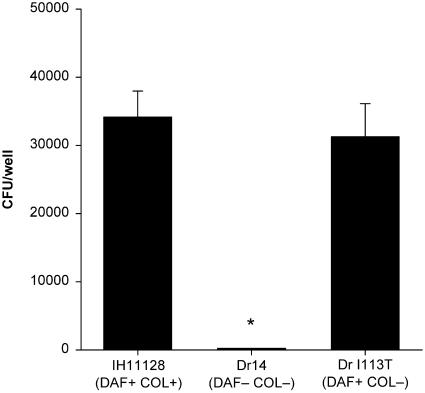
Live clinical strains of Dr+ E. coli IH11128 bound in appreciable numbers to coverslips coated with the 7S domain of type IV collagen (average, 800 cells per microscope field at ×600), while the isogenic collagen-binding mutant DrI113T lost the ability to bind (36 cells/field) (Fig. 3). Carnoy and Moseley demonstrated that replacement of aspartic acid at position 54 of DraE with tyrosine resulted in an increased capacity of Dr fimbriae to bind the 7S domain (2). A plasmid construct carrying this substitution (pCC90-D54Y), when transformed into the isogenic collagen-binding mutant, not only restored the binding capacity but resulted in increased binding to the 7S domain (Fig. 3) (2,060 cells/field).
FIG. 3.
Adherence of Dr+ E. coli to coverslips coated with 7S domain of type IV collagen. Bacterial strains were incubated with coverslips coated with collagen, and bacterial binding was detected by Giemsa staining. (A) Clinical Dr+ strain IH11128 (DAF+ COL+). (B) Isogenic collagen-binding mutant DrI113T (DAF+ COL−). (C) Strain transcomplemented with collagen-binding phenotype DrI113T(pCC90-D54Y) (DAF+ COL++). All micrographs were taken at magnification ×600.
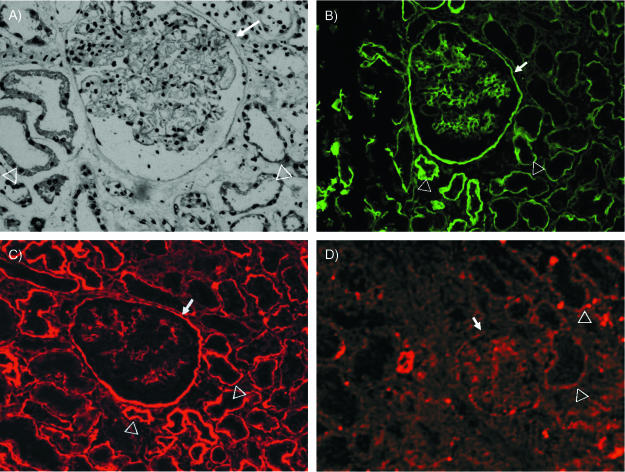
To test if mouse kidneys expressed Dr receptors, live Dr+ E. coli IH11128, its isogenic collagen-binding mutant DrI113T, and the transcomplemented strain DrI113T-D54Y exhibiting collagen-hyperbinding capacity were incubated with cryostat sections of mouse kidneys. The Dr+ E. coli bound to mouse kidney, while the collagen nonbinder displayed markedly decreased binding capacity (Fig. 4A and B). The transcomplemented strain, DrI113T-54Y, bound in high numbers to the mouse kidney section (Fig. 4C). Double immunofluorescence staining was performed to better visualize E. coli binding to type IV collagen-rich elements. Mouse renal tissue was stained with rabbit antibody to type IV collagen and secondary antibody conjugated with fluorochrome. The attached bacteria were visualized with anti-Dr antibody and secondary antibody conjugated with a different color fluorochrome. The micrographs shown in Fig. 4D visualize Dr+ E. coli clearly associated with BM-stained type IV collagen, while the collagen-negative mutant does not bind (Fig. 4E). The binding pattern was similar to the binding of purified fimbriae to the human kidney. These results indicate that the increased binding of Dr-fimbriated E. coli to renal tissues was mediated by binding to the type IV collagen of BMs.
FIG. 4.
(A to C) Adherence of Dr+ E. coli to the mouse kidney. Cryostat sections of mouse kidneys were incubated with bacterial strains to detect tissue tropism. The clinical Dr+ strain IH11128 (DAF+ COL+) bound in high numbers to Bowman's capsule (A), while the collagen-binding mutant, DrI113T (DAF+ COL−), displayed reduced binding to these structures (B). The transcomplementation of the collagen-binding phenotype in strain DrI113T(pCC90-D54Y) (DAF+ COL++) restored binding to Bowman's capsule (C). Bacteria are indicated by arrows. g, glomerulus. All micrographs were taken at magnification ×600. (D and E) Acetone-fixed cryostat kidney sections of C3H/HeJ mice were incubated with bacterial suspensions of Dr+ E. coli IH11128 (D) or the collagen-negative mutant I113T (E). Extracellular collagen (red) was visualized by staining sections with polyclonal rabbit anti-collagen (NC1) antibody, followed by staining with goat anti-rabbit antibody conjugated with Texas Red. Fimbrial antigen (green) was visualized by staining with polyclonal rabbit anti-Dr antibody, followed by staining with goat anti-rabbit conjugated with Cy2. Dr+ E. coli showed intense binding, forming clusters of bacteria (arrows) along the collagen fibers (D), while the collagen-negative mutant lost the ability to adhere to collagen IV-containing substructures (E).
Inability to bind collagen does not affect internalization capacity.
While the binding of Dr+ E. coli to DAF has been demonstrated to mediate internalization of the bacterium into tissue culture cells, the role of the collagen-binding phenotype in the internalization process of Dr+ E. coli is unknown (8). However, ECM-binding phenotypes of OpaA+ gonococci and Staphylococcus aureus have been shown to mediate internalization into eukaryotic cells (4, 34). Hence, to test the effect of the collagen-binding mutation on the internalization process, the clinical Dr+ E. coli strain IH11128 and its isogenic collagen-binding mutant DrI113T were tested for the ability to internalize into HeLa epithelial cells expressing abundant DAF. The internalization rate of the collagen-binding mutant was comparable to that of the wild-type strain (Fig. 5), suggesting that the loss of the collagen-binding phenotype did not affect the internalization capacity of the constructed mutant.
FIG. 5.
Internalization of Dr+ E. coli, or its isogenic non-collagen-binding mutant, into HeLa epithelial cells. The internalization capacities of Dr+ E. coli and the collagen mutant were evaluated in a gentamicin protection assay. The internalization rate of Dr+ E. coli IH11128 (DAF+ COL+) and its isogenic non-collagen-binding mutant DrI113T (DAF+ COL−) were comparable. The bars represent means plus standard errors of the mean from three independent experiments done in duplicate. *, P = 0.004 by Student's t test.
Elimination of collagen-binding ability abolished chronic renal colonization in the experimental mouse model.
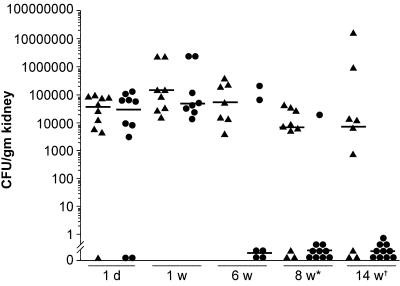
To test the in vivo contribution of the collagen-binding phenotype of Dr adhesin to chronic renal colonization, the virulences of the collagen-binding mutant and the parent clinical strain were evaluated in an experimental model of chronic pyelonephritis developed in our laboratory (7). The virulences of these strains were evaluated by analyzing their capabilities to persist in the renal tissues of infected mice. Two groups of female C3H/HeJ mice, 8 to 10 weeks old, were infected with the Dr+ E. coli clinical strain IH11128 (n = 45) or its isogenic collagen-binding mutant, DrI113T (n = 44). Kidney cultures from the group of mice sacrificed on day 1 (10 animals in each group) showed efficient colonization by the Dr+ E. coli IH11128 and its collagen-binding mutant, DrI113T (P = 0.762). At 1-week postinfection, both groups of mice (n = 8) had comparable rates of bacterial colonization in the renal tissues (P = 0.328). At the 6-week time point, four out of six mice tested in the collagen-binding mutant group eliminated infection (66% elimination), while all the mice (n = 7) in the Dr+ E. coli group displayed positive cultures (0% elimination) (P = 0.10). Ten mice from each group were sacrificed 8 weeks following infection, and 9 out of 10 mice eliminated infection in the collagen-binding mutant group (90% elimination), in contrast to the Dr+ E. coli group, in which 3 out of 10 mice eliminated infection (30% elimination), and the colonization rates in both groups differed significantly (P = 0.02) (Fig. 6). The last time point tested was 14 weeks following infection. While all of the mice (n = 10) in the collagen-binding mutant group eliminated infection (100% elimination), only three out of nine mice eliminated infection in the Dr+ E. coli group (33% elimination). One mouse in the Dr+ E. coli group died at this time point. The colonization rate in the Dr+ E. coli group ranged from 103 to 107 CFU/g of tissue, in contrast to the collagen-binding mutant group, in which all the mice eliminated infection (P = 0.015). The results suggested a gradual elimination of the collagen-binding mutant from the renal tissues of the infected mice.
FIG. 6.
Renal colonization rates in mice infected with Dr+ E. coli and its isogenic collagen-binding mutant strain. Each symbol represents the quantitative bacterial count from the kidneys of individual mice sacrificed at a particular time point. The earliest statistical significances in the renal colonization rates in IH11128 (DAF+ COL+), represented by triangles, and DrI113T (DAF+ COL−), represented by circles, occurred at 8 (P = 0.02) and 14 (P = 0.01) weeks. Statistical analysis was performed by the Mann-Whitney rank sum test.
To test if complementation of the collagen-binding phenotype would restore the bacterial capacity to persist in the mouse renal tissue, we constructed and tested two transcomplemented strains of the isogenic collagen-binding mutants. The first complemented strain was transfected with plasmid pCC90 carrying inserts of the wild-type Dr operon, and the second complemented strain was transfected with the plasmid pCC90-D54Y carrying a mutated Dr operon, which encodes a collagen-hyperbinding ability. In vitro tests showed a restored collagen-binding capacity for both transconjugants. Four groups of mice were infected with the wild type (Dr+ wt), the isogenic collagen-binding mutant (Dr Col−), and two versions of complemented strains (Dr Col+ and Dr Col++) to test for restoration of virulence. Comparable infection rates were achieved in all four groups, as evidenced by renal cultures on day 1 following infection (Table 1). Six weeks following infection, the infection rates for the three phenotypic groups were as follows: Dr+ wt, 75%; Dr Col−, 16%; and Dr Col++, 50%, suggesting a gradual loss of infection in the Col− group. Fifteen weeks following infection, the renal-culture rates were as follows: Dr+ wt, 50%; Dr Col−, 0%; Dr Col+, 50%; and Dr Col++, 100%. These results suggest that the complementation of the collagen-binding phenotype restored renal persistence. Interestingly, the ability to bind collagen avidly (Dr Col++) seemed to be a more successful and advantageous phenotype, leading to a higher rate of renal persistence at 15 weeks than the wild type.
TABLE 1.
Complementation restores persistence of renal infection
| Strain used | Colonization (%)
|
Pa | ||
|---|---|---|---|---|
| 1 day | 6 wk | 15 wk | ||
| Dr11128 wt | 12/14 (85) | 9/12 (75) | 3/6 (50) | < 0.1 |
| DrI113T Col− | 13/14 (92) | 2/12 (16) | 0/6 (0)b | < 0.0001 |
| DrI113T/Dr Col+ | 13/14 (92) | NTc | 3/6 (50) | < 0.06 |
| DrI113T/54Y Col++ | 12/14 (85) | 6/12 (50) | 6/6 (100) | < 0.4 |
Fisher exact probability test was used to evaluate statistical significance.
Statistically significant reduction in colonization rates at week 15 compared to day 1 of infection. The remaining P values showed a lack of significance.
NT, not tested.
DISCUSSION
This study demonstrated that the type IV collagen-binding phenotype of Dr-fimbriated E. coli was essential for the establishment of persistent renal infection in an experimental model. A point mutation in the DraE adhesin abolished binding to type IV collagen, prevented attachment to the renal BM in human and mouse kidney sections, and protected animals from chronic infection. The transcomplementation of the collagen-binding mutant with a collagen-binding or collagen-hyperbinding phenotype restored the E. coli capacity to cause persistent renal infection in the mouse model.
Several lines of evidence suggest that the C3H/HeJ mouse model is appropriate for studying the role of type IV collagen-Dr fimbrial interaction in chronic or recurrent kidney infection. The receptors recognized by the Dr fimbriae of uropathogenic E. coli, DAF, and type IV collagen appear to have similar renal distributions in the mouse and human kidneys. The amino acid sequences of the type IV collagen in mouse and human show significant homology, suggesting that the Dr+ E. coli binding to type IV collagen may be similar in the two species. The use of C3H/HeJ mice, which are lipopolysaccharide (LPS) nonresponders, allows us to study the role of Dr fimbriae without the influence of LPS-mediated pathology and suggests that the Dr fimbriae are a virulence factor necessary to establish chronic tubulointerstitial nephritis. Finally, the relevance of the C3H/HeJ model is further supported by our findings that LPS hyporesponsiveness occurs during pregnancy (unpublished data) and that Dr+ E. coli is found at high frequency in gestational pyelonephritis in pregnant women (10, 11, 23).
The present results demonstrate that, despite the loss of type IV collagen binding by the Dr fimbriae, good colonization persisted for 4 to 5 weeks. However, the gradual elimination of a collagen-binding mutant, DrI113T, from mouse kidneys began at 6 to 8 weeks, which was significantly earlier than for the Dr+ E. coli that recognizes collagen. This finding indicates that the interaction between Dr fimbriae and type IV collagen may play a crucial role in sustaining bacterial persistence in the renal tissue. A study of the in vivo affinities of Dr fimbriae of E. coli and type 3 fimbriae of Klebsiella pneumoniae to type IV and type V collagens, respectively, have demonstrated that following intravenous injection, purified Dr fimbriae, but not type 3 fimbriae, formed mesangial deposits in the rat kidney that persisted for several months (18). This study further validates our current observations supporting the unique capability of Dr+ E. coli to cause collagen-dependent persistent colonization.
The type IV collagen-binding phenotype did not affect adherence and internalization into epithelial cells mediated by the DAF receptor-Dr fimbrial interaction. The purified wild-type Dr fimbriae and the collagen-binding mutant DrI113T fimbriae demonstrated similar levels of binding to DAF-expressing cell lines. Moreover, internalization of the non-collagen-binding mutant did not differ from that of the parent strain. The contribution of DAF binding to establishment of chronic kidney infection in the mouse model needs further investigation. DraE adhesin mutants which have lost DAF binding but retain collagen type IV attachment have recently been constructed and will be useful in further defining the roles of the two binding activities of Dr fimbriae in the murine model of chronic pyelonephritis (33).
Overall, these findings suggest that chronic kidney infection due to uropathogenic Dr+ E. coli is at least a two-phase process supported by dual receptor specificity of the DraE adhesin. Binding to DAF receptor expressed on epithelial cells may promote the first step of kidney infection, followed by internalization and translocation of bacteria to the renal parenchyma. This concept is reinforced by our observation that human proximal tubule cell lines shows positive staining for DAF receptor and internalize Dr+ but not Dr− E. coli (unpublished data). Alternatively or in parallel, bacteria may disseminate through the renal microvascular system to reach basolateral sites rich in type IV collagen. A preliminary experiment supporting this mechanism showed invasion of endothelial cells of the mouse kidney (unpublished data). As proposed by other investigators, processes such as apoptosis and sloughing off of the epithelia also could expose deeper layers of tissue and contribute to the invasion of the renal parenchyma (16, 19).
Type IV collagen binding may be required in the second step of infection for establishing persistent colonization. Type IV collagen and other ECM proteins, such as laminin, fibronectin, fibrinogen, and vitronectin, are recognized by several bacterial proteins collectively referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMM) (26). The clinical significance of MSCRAMM interaction with the ECM and its impact on the virulence of the microbial pathogen is beginning to be understood. Recent studies have shown that binding to ECM may mediate bacterial internalization. The fibronectin binding proteins of S. aureus mediate the internalization of the bacteria into eukaryotic cells (4). Further studies are needed to explore whether the entry of Dr+ E. coli into intracellular compartments from the basolateral site can be mediated by interaction with collagen.
Collagen binding does not fully explain why Dr+ E. coli can persist in the kidney despite evidence of an interstitial inflammatory response in renal tissue triggered by bacterial infection. A vigorous interleukin-8 response was found in HeLa cells and in human proximal tubule cell lines infected with Dr+ (but not with Dr−) E. coli (Goluszko et al., unpublished). This finding may imply that host phagocytes recruited to the site of infection are unable to perform effective digestion and intracellular killing or that Dr+ E. coli can resist phagocytosis. Interestingly, the latter notion is supported by the finding of Johnson et al. that Dr-fimbriated E. coli can survive adherence to human neutrophils, a feature that could contribute to chronic or recurrent infection (14). Finally, there is some evidence that the impaired LPS responsiveness observed in C3H/HeJ mice takes place in humans. A clinical study recently completed by our group demonstrates that 95% of pregnant women downregulate TLR-4 (on the surfaces of monocytes/macrophages), resembling LPS functional hyporesponsiveness (unpublished data). This is a critically important clinical issue, as it was previously reported that pregnant women have high incidences of pyelonephritis caused by Dr+ E. coli in the third trimester (10, 11, 23).
Our study provides evidence that the type IV collagen-binding phenotype of Dr fimbriae is essential for the development of experimental chronic pyelonephritis. This observation is reinforced by the complementation study, which restored both collagen binding and renal persistence. It is noteworthy that other members of the Dr family of adhesins that do not bind type IV collagen (afimbrial adhesins I and III) are still associated with UTI, resulting in acute infections of the urinary bladder (cystitis) (24). Hence, the kidney tropism may be partially explained on the basis of the renal distribution of type IV collagen and the functional ability of E. coli to bind this ECM protein. Despite the uncertainties about the contributions of various host and microbial factors to establishing chronic or recurrent UTI, it is conceivable that the collagen- and/or ECM-binding property of uropathogenic E. coli adhesins is an important factor involved in establishing chronic or recurrent UTI processes.
Acknowledgments
This project was funded in part by a predoctoral fellowship from the McLaughlin endowment to R. Selvarangan, grants from the National Institutes of Health (DK-42029 to B. Nowicki, DG-49862 to S. Moseley, and DK-18381 to B. Hudson), and a grant from the Centre Hospitalier Regional et Universitaire de Lille to C. Carnoy.
Editor: V. J. DiRita
REFERENCES
- 1.Arthur, M., C. E. Johnson, R. H. Rubin, R. D. Arbeit, C. Campanelli, C. Kim, S. Steinbach, M. Agarwal, R. Wilkinson, and R. Goldstein. 1989. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect. Immun. 57:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnoy, C., and S. L. Moseley. 1997. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol. Microbiol. 23:365-379. [DOI] [PubMed] [Google Scholar]
- 3.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Germani, Y., E. Begaud, P. Duval, and C. Le Bouguenec. 1996. Prevalence of enteropathogenic, enteroaggregative, and diffusely adherent Escherichia coli among isolates from children with diarrhea in New Caledonia. J. Infect. Dis. 174:1124-1126. [DOI] [PubMed] [Google Scholar]
- 6.Giron, J. A., T. Jones, F. Millan-Velasco, E. Castro-Munoz, L. Zarate, J. Fry, G. Frankel, S. L. Moseley, B. Baudry, and J. B. Kaper. 1991. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J. Infect. Dis. 163:507-513. [DOI] [PubMed] [Google Scholar]
- 7.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goluszko, P., V. Popov, R. Selvarangan, S. Nowicki, T. Pham, and B. J. Nowicki. 1997. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J. Infect. Dis. 176:158-167. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, R., N. K. Ganguly, V. Ahuja, K. Joshi, and S. Sharma. 1995. An ascending non-obstructive model for chronic pyelonephritis in BALB/c mice. J. Med. Microbiol. 43:33-36. [DOI] [PubMed] [Google Scholar]
- 10.Hart, A., B. J. Nowicki, B. Reisner, E. Pawelczyk, P. Goluszko, P. Urvil, G. Anderson, and S. Nowicki. 2001. Ampicillin-resistant Escherichia coli in gestational pyelonephritis: increased occurrence and association with the colonization factor Dr adhesin. J. Infect. Dis. 183:1526-1529. [DOI] [PubMed] [Google Scholar]
- 11.Hart, A., T. Pham, S. Nowicki, E. B. Whorton, Jr., M. G. Martens, G. D. Anderson, and B. J. Nowicki. 1996. Gestational pyelonephritis-associated Escherichia coli isolates represent a nonrandom, closely related population. Am. J. Obstet. Gynecol. 174:983-989. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, J. R., C. E. Johnson, and J. N. Maslow. 1999. Clinical and bacteriologic correlates of the papG alleles among Escherichia coli strains from children with acute cystitis. Pediatr. Infect. Dis. J. 18:446-451. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, J. R., K. M. Skubitz, B. J. Nowicki, K. Jacques-Palaz, and R. M. Rakita. 1995. Nonlethal adherence to human neutrophils mediated by Dr antigen-specific adhesins of Escherichia coli. Infect. Immun. 63:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallenius, G., S. Svenson, R. Mollby, B. Cedergren, H. Hultberg, and J. Winberg. 1981. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet ii:604-606. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 102:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langeveld, J. P., M. E. Noelken, K. Hard, P. Todd, J. F. Vliegenthart, J. Rouse, and B. G. Hudson. 1991. Bovine glomerular basement membrane. Location and structure of the asparagine-linked oligosaccharide units and their potential role in the assembly of the 7 S collagen IV tetramer. J. Biol. Chem. 266:2622-2631. [PubMed] [Google Scholar]
- 18.Miettinen, A., B. Westerlund, A. M. Tarkkanen, T. Tornroth, P. Ljungberg, O. V. Renkonen, and T. K. Korhonen. 1993. Binding of bacterial adhesins to rat glomerular mesangium in vivo. Kidney Int. 43:592-600. [DOI] [PubMed] [Google Scholar]
- 19.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 20.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowicki, B., H. Holthofer, T. Saraneva, M. Rhen, V. Vaisanen-Rhen, and T. K. Korhonen. 1986. Location of adhesion sites for P-fimbriated and for 075X-positive Escherichia coli in the human kidney. Microb. Pathog. 1:169-180. [DOI] [PubMed] [Google Scholar]
- 22.Nowicki, B., A. Labigne, S. Moseley, R. Hull, S. Hull, and J. Moulds. 1990. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect. Immun. 58:279-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowicki, B., M. Martens, A. Hart, and S. Nowicki. 1994. Gestational age-dependent distribution of Escherichia coli fimbriae in pregnant patients with pyelonephritis. Ann. N. Y. Acad. Sci. 730:290-291. [DOI] [PubMed] [Google Scholar]
- 24.Nowicki, B., R. Selvarangan, and S. Nowicki. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl. 1):S24-S27. [DOI] [PubMed] [Google Scholar]
- 25.Nowicki, B., L. Truong, J. Moulds, and R. Hull. 1988. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am. J. Pathol. 133:1-4. [PMC free article] [PubMed] [Google Scholar]
- 26.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 27.Risdon, R. A. 1994. Pyelonephritis and reflux nephropathy, p. 777-808. In C. C. Tisher and B. M. Brenner (ed.), Renal pathology with clinical and functional correlations. J. B. Lippincott Co., Philadelphia, Pa.
- 28.Roberts, J. A. 1991. Etiology and pathophysiology of pyelonephritis. Am. J. Kidney Dis. 17:1-9. [DOI] [PubMed] [Google Scholar]
- 29.Selvarangan, R., P. Goluszko, V. Popov, J. Singhal, T. Pham, D. M. Lublin, S. Nowicki, and B. Nowicki. 2000. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect. Immun. 68:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokurenko, E. V., V. Chesnokova, R. J. Doyle, and D. L. Hasty. 1997. Diversity of the Escherichia coli type 1 fimbrial lectin. Differential binding to mannosides and uroepithelial cells. J. Biol. Chem. 272:17880-17886. [DOI] [PubMed] [Google Scholar]
- 31.Vaisanen-Rhen, V. 1984. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect. Immun. 46:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Loy, C. P., E. V. Sokurenko, and S. L. Moseley. 2002. The major structural subunits of Dr and F1845 fimbriae are adhesins. Infect. Immun. 70:1694-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Loy, C. P., E. V. Sokurenko, R. Samudrala, and S. L. Moseley. 2002. Identification of amino acids in the Dr adhesin required for binding to decay-accelerating factor. Mol. Microbiol. 45:439-452. [DOI] [PubMed] [Google Scholar]
- 34.van Putten, J. P., T. D. Duensing, and R. L. Cole. 1998. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol. Microbiol. 29:369-379. [DOI] [PubMed] [Google Scholar]
- 35.Warren, J. W. 1996. Clinical presentation and epidemiology of urinary tract infections, p. 3-27. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections. ASM Press, Washington, D.C.
- 36.Westerlund, B., P. Kuusela, J. Risteli, L. Risteli, T. Vartio, H. Rauvala, R. Virkola, and T. K. Korhonen. 1989. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol. Microbiol. 3:329-337. [DOI] [PubMed] [Google Scholar]