Abstract
Background
Tobramycin is a critical cystic fibrosis treatment however it causes ototoxicity. This study tested D-methionine protection from tobramycin-induced ototoxicity and potential antimicrobial interference.
Methods
Auditory brainstem responses (ABR) and outer hair cell (OHC) quantifications measured protection in guinea pigs treated with tobramycin and a range of D-methionine doses. In vitro antimicrobial interference studies tested inhibition and post antibiotic effect assays.
In vivo
antimicrobial interference studies tested normal and neutropenic E. coli murine survival and intraperitoneal lavage bacterial counts.
Results
D-methionine conferred significant ABR threshold shift reductions. OHC protection was less robust but significant at 20 kHz in the 420 mg/kg/day group.
In vitro
studies did not detect D-methionine-induced antimicrobial interference. In vivo studies did not detect D-methionine-induced interference in normal or neutropenic mice.
Conclusions
D-methionine protects from tobramycin-induced ototoxicity without antimicrobial interference. The study results suggest D-met as a potential otoprotectant from clinical tobramycin use in cystic fibrosis patients.
Keywords: D-methionine, aminoglycoside, otoprotection, ototoxicity, antimicrobial interference
1. Introduction
Since first introduced in 1942, aminoglycoside antibiotics have increased our ability to treat gram-negative bacterial infections that are not responsive to penicillin or other conventional antibiotics (Edson and Terrell 1999). However, aminoglycoside clinical use in the United States is limited by toxic side effects that include cochleo-, vestibulo-, and/or nephrotoxicities. Preferential damage to tissue types varies by the specific aminoglycoside employed (Begg et al. 1995; Schacht et al. 2012; Francis et al. 2013). Nonetheless, because of their low cost, high efficacy, and low incidence of resistance, aminoglycoside antibiotics are commonly used in many parts of the world today including South Africa and the United States (Forge et al. 2000; Bardien et al. 2009; Harris et al. 2012; Ababneh et al. 2012). Aminoglycosides have been commonly prescribed for sepsis, meningitis, complicated urinary tract and respiratory infections because they were highly effective against gram-negative infections (McCracken 1986).
The prevalence of aminoglycoside-induced ototoxicity has been documented to be high in populations where they are monitored for possible hearing loss. Studies have reported an incidence range of 6–41% (Brummet and Fox 1989; Matz 1993; Muhleran et al. 2001). This incidence does not appear to be decreasing. Fausti et al. (1999) monitored 370 patients receiving aminoglycoside antibiotics in the Veteran’s Administration system and found a 33% occurrence of aminoglycoside-induced ototoxicity. The incidence is higher in developing countries because audiologic monitoring for early ototoxicity detection is less common, dosing is less controlled than in the United States, and antibiotic options may be limited (Forge et al. 2000).
Aminoglycoside antibiotics exhibit in vitro activity against a variety of clinically significant gram-negative bacilli such as Escherichia coli, Salmonella spp., Shigella spp., Enterobacter spp., Citrobacter spp., Acinetobacter spp., Proteus spp., Klebsiella spp., Serratia spp., Morganella spp., and Pseudomonas spp. as well as gram positive Staphylococcus aureus and some streptococci. As with other antibiotic classes, significant differences in the spectrum exist among the various aminoglycosides (Edson and Terrell 1999).
Each aminoglycoside induces different overall and organ-specific side effects; with particular aminoglycoside vestibulo- and/or cochleotoxicities restricting clinical use (Lodhi et al. 1980; Aran 1995; Lima da Costa et al. 1998). Tobramycin generally causes equal cochlear and vestibular damage (Kahlmeter and Dahlager 1984; Matz 1986; Seligmann et al. 1996). Tobramycin first damages cochlear outer hair cells (OHCs) from base to apex of the organ of Corti, progressing to inner hair cells with continued use (Hawkins 1976), and causes irreversible hearing damage (Tange et al. 1982; Kusunoki et al. 2004).
In general, aminoglycosides, including tobramycin, induce reactive oxygen species (ROS) production as demonstrated in the cochlea (Hirose et al. 1997; Choung et al. 2009). These ROS can damage cochlear tissues and lead to apoptosis, loss of cochlear sensory cells, and resultant hearing loss. ROS production may be secondary to mitochondrial ribosome malfunction by causing protein mistranslation and impaired resistance to oxidative stress in the cochlea (Matt et al. 2012).
Several otoprotective agents have been proposed to prevent aminoglycoside-induced hearing loss with therapeutic targets at various steps of the proposed ototoxic pathway. However, none of the otoprotectants under development are currently FDA-approved for this purpose. Effective antioxidants, such as D-methionine (D-met), may be able to mitigate aminoglycoside-induced oxidative stress, prevent hair cell death, and reduce or alleviate subsequent hearing loss. If D-met reduces the toxicity profile of any, and hopefully all, aminoglycosides without compromising antimicrobial efficacy, a wider range of infections could be safely treated without the risk of inducing hearing loss.
D-met has effectively protected against gentamicin- and amikacin-induced ototoxicity (Sha and Schacht 2000; Campbell et al. 2007) by a presumptive free radical-detoxifying mechanism, prevention of hair cell death in the organ of Corti, and indirect cochlear mitochondrial glutathione level increases (Campbell et al. 2007; Xie et al. 2011) and may also advantageously be delivered orally (Campbell et al 2007). This translational study tested the antioxidant D-met as an otoprotective agent against tobramycin ototoxicity and tested whether D-met administration interferes with in vitro or in vivo aminoglycoside antimicrobial efficacy.
2. Materials and methods
2.1. D-methionine otoprotection studies
2.1.1. Subjects
Six groups of 10 male albino guinea pigs (250–350g; approximately 5–7 weeks old) were treated with daily injections of tobramycin sulfate (100 mg/kg/day subcutaneously) for 21 days. Group 1 served as a control and received tobramycin and an equivalent-volume saline intraperitoneal injection twice daily for 21 days. Groups 2–6 received subcutaneous tobramycin and two equal intraperitoneal D-met doses totaling 240, 300, 360, 420 or 480 mg/kg each day. The D-met or saline control doses were delivered 15 minutes before and 7 hours after the daily tobramycin injection. Campbell et al. (2003) demonstrated no functional or histological change of the inner ear in animals treated only with D-met. Thus, a D-met-only-treated group was not included in this study.
All animals were maintained on a regular diet and given free access to water. After arriving at the laboratory animal facilities, animals were allowed to acclimate one week before experiments began. Animal weight was recorded prior to each ABR assessment and once per week during drug administration. All animal protocols were reviewed and approved by Southern Illinois University School of Medicine Laboratory Animal Care and Use Committee.
2.1.2. Treatments
D-methionine powder (99+% pharmaceutical grade, Acros Organics; St. Louis, Mo) was diluted into sterile normal saline at 30 mg/mL.
A 40 mg/mL tobramycin injectable solution was purchased from APP Pharmaceuticals (Schaumberg, IL).
Animals were fully anesthetized throughout ABR procedures and at sacrifice with 86.21 mg/kg ketamine (Fort Dodge; Madison, NJ) and 2.76 mg/kg xylazine (Lloyd Laboratories; Shenandoah, IA), which was supplemented as needed with half doses.
2.1.3. Electrophysiology
All subjects underwent auditory brainstem response (ABR) testing prior to treatment and 2, 4, and 6 weeks after initiation of drug administration. ABR monitoring used an Intelligent Hearing System evoked potential unit in a double-walled IAC sound booth. ABR thresholds were measured in both ears in response to tone-bursts with 1 ms rise/fall and 0 ms plateau gated by a Blackman envelope and centered at the frequencies of 4, 8, 14, and 20 kHz presented at 30/s. Threshold was analyzed by readers blinded to condition and defined as the lowest intensity capable of eliciting a replicable, visually detectable response at the fourth ABR waveform. A total of 512 sweeps in replicate constituted each average, and the recording epoch was 15 ms following stimulus onset.
2.1.4. Histology
Anesthetized subjects were sacrificed by decapitation after the final ABR measurement was obtained. Cochleae were harvested for outer hair cell counts. Round window and stapes were removed and the round and oval window was punctured, a hole was hand-drilled into the first turn of the otic capsule with a sharpened pick and the perilymphatic space was perfused with 2.5% glutaraldehyde at 4°C in 0.1M Cacodylate (Cac) buffer (pH 7.4) within 5 minutes of sacrifice; allowing the fluid to exit through the opened round window.
After perfusion fixation, the cochleae were immersed in glutaraldehyde and stored at 4°C overnight. After overnight fixation, the cochleae were rinsed in 0.1 M Cac buffer and gently perfusion-rinsed with Cac through the perilymphatic spaces 3 times. After rinsing, the cochleae were perfusion-fixed with 4°C OsO4 in Cac buffer under a fume hood. Fixation continued by immersion and rotation in the same fixative for 15 minutes. The cochleae were then perfusion-rinsed three times with Cac and prepared for dissection.
Under the dissecting microscope, each cochlear bony capsule was carefully removed. The tissue was then serially dehydrated in 30%, 50%, 75%, 85%, 2 X 95%, and 3 X 100% EtOH. Each specimen was dried using hexamethyldisilazane (HMDS) and placed on a stub for sputter coating with 13 nm platinum. The tissue was viewed through a Hitachi S3000N Variable Pressure Scanning Electron Microscope at 3,000X–4,000X magnification.
2.1.5. Outer and inner hair cell (OHC and IHC) Quantification
OHC and IHC semiquantitative analyses were performed according to previously documented and published sampling techniques (Campbell et al. 1996; 2007, 2011; Claussen 2013). Briefly, a representative sample of 33 OHCs, or 11/row, and 11 IHCs were examined and counted for each frequency region (4, 8, 14, and 20 kHz) based off of previously published and documented frequency mapping (Eldridge et al. 1981) and the percentages of intact OHCs and IHCs were calculated. One cochlea per animal was assessed.
2.1.6. BUN/Creatinine Analysis
Serum samples were collected from anesthetized animals by a cardiac heart stick prior to sacrifice. Blood samples were centrifuged and serum collected for blood urea nitrogen (BUN) and creatinine analysis to identify potential permanent nephrotoxicity. BUN and creatinine levels were analyzed using an IDEX Vettest chemistry analyzer (IDEX Corporation).
2.2. D-methionine/tobramycin in vitro antimicrobial efficacy studies
2.2.1. Isolates
Five isolates each of the following bacteria: Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Pseudomonas aeruginosa (P. aeruginosa) were obtained from St. John’s Hospital in Springfield, IL. Isolates were subcultured twice on nutrient agar or on selective medium (e.g. MacConkey’s agar for E. coli and Cetrimide agar for P. aeruginosa) for purification. Pure colonies were isolated and subcultured on nutrient agar. Bacteria were identified with API strips and Chromagar media. Fresh isolates were used to inoculate one milliliter skim milk medium and stored at −80°C. American Type Culture Collection (ATCC) E. coli 25922 (clinical isolate from FDA-1946), S. aureus 29213 (clinical isolate from Micro-Media Systems, Inc.) and P. aeruginosa 27853 (blood culture isolate from AA Medeiros) were used to determine minimal inhibitory concentrations (MIC).
2.2.2. Drugs
Tobramycin for in vitro studies and D-met for both in vitro and in vivo studies were obtained from Sigma Aldrich (St. Louis, MO). Injectable forms of aminoglycosides and cyclophosphamide were obtained for in vivo studies. Tobramycin sulfate and cyclophosphamide were obtained from Henry-Schein (Melville, NY). A ketamine (20.8 mg/kg) and xylazine (484 μg/kg) cocktail was used for anesthesia. Ketamine was obtained from Fort Dodge (Madison, NJ). Xylazine was purchased from Lloyd Laboratories (Shenandoah, IA).
2.2.3. Mean Inhibitory Concentration (MIC) Determinations
The MIC is the lowest antimicrobial concentration which inhibits microorganismal growth. Testing for MIC can provide important information about the susceptibility of organisms to antimicrobial drugs. Aminoglycoside and D-met MICs were determined by both broth dilutions and Bioscreen C analysis for E. coli, P. aeruginosa, and S. aureus. Antimicrobial susceptibility tests of each aminoglycoside and D-met were determined using the broth microdilution technique as described by the Clinical and Laboratory Standards Institute (M7-A6). Antimicrobial and D-met stock solutions were diluted into Cation Adjusted Mueller Hinton Broth (CAMHB), prepared at the highest tested concentration, and stored at −20°C. Tested serial dilutions ranged from 0.25–128 μg/ml for aminoglycosides and 125–6,400 μg/ml for D-met. Five microliters of inoculum (1×107 CFU/ml) in the same medium was added to each well of the prepared plates, resulting in a final inoculum of 5×105 CFU/ml. Each plate was incubated at 37°C for 18–24 hours. Following incubation, the plates were scored visually, and the MIC for each drug was determined.
2.2.4. Tobramycin and D-methionine combinations against bacteria in vitro
Briefly, 100 μl of a twofold serially-diluted tobramycin stock, diluted in CAMHB, was delivered to the first column of wells in a 96 well plate at twice the final concentration. D-met at twice the final concentrations (25–6,400 μg/ml) in CAMHB was delivered to plate rows 1 to 8 (checkerboard dilution method) (Odds 2003; Lewis et al. 2002; Rand et al. 1993). Five microliters of inoculum (1×107 CFU/ml) in CAMHB was added to each well, resulting in a final inoculum size of 5×105 CFU/ml. Plates were incubated at 37°C for 18–24 hours. Following incubation, the plates were scored visually. Checkerboard analyses were duplicated for each drug combination. Drug interaction was determined by calculation of the Fractional Inhibitory Concentration (ΣFIC) (Odds 2003). The interaction was defined as: Synergistic (FIC index ≤ 0.5); Indifferent (FIC > 0.5 and ≤ 4); and antagonistic (FIC > 4) (Odds 2003). Drug A referred to the aminoglycoside and Drug B referred to D-met.
2.2.5. Time Course Interference Assay
The Bioscreen C Automated Microbiology Growth Curve Analysis System (Growth Curves USA, Piscataway, NJ) directly measures microorganism growth with turbidity over time by generating an optical density (O.D.) curve (Medina et al. 2012; Cooper et al. 2011). Tobramycin MIC values against each isolate were established using a Bioscreen C prior to the time course interference assay. Tobramycin killing efficacy was tested in the presence of D-methionine (D-met) using the Bioscreen C. Tobramycin at 4x MIC were combined with serial dilution D-met concentrations (6,400 μg/ml-100 μg/ml) at twice the desired concentration and plated into a 100 well Bioscreen C honeycomb plate. Each combination was inoculated with 5 × 105 CFU/ml. Samples were diluted 5 fold and plated onto agar plates for quantitation. The Bioscreen C software was programmed to take a turbidometric measurement every 15 minutes at 580nm for 24 hours. The log phase measurements were completed after 12 hours. Time course interference assays were duplicated for each tobramycin/isolate combination. Results were exported to Microsoft Excel for graph production and analysis.
2.2.6. Post Antibiotic Effect (PAE) Studies
Post-antibiotic effect (PAE) is defined as the length of time bacterial growth is suppressed following brief exposure to an antibiotic. This pharmacodynamic parameter has long been studied as part of preclinical evaluations to determine dosing intervals. Aminoglycosides exhibit rapid concentration-dependent killing action; therefore, PAE is of particular interest due to short exposure time associated with persistent bacterial growth suppression (Craig 1991). Higher aminoglycoside dosages give greater post-antibiotic effects up to a certain maximal response. In vivo, the aminoglycosides post-antibiotic effect is lengthened by the synergistic effect of host leukocyte activity, with possible enhanced leukocyte phagocytic capabilities, following aminoglycoside exposure (Gonzalez and Spencer 1998). PAE was determined for the MIC, 2X MIC, 5X MIC, and 10X MIC of aminoglycosides for each isolate. Aminoglycosides at twice the desired concentration were combined with serial dilution D-met concentrations (6,400 μg/ml to 400 μg/ml) at 2x the desired concentration and placed into 125 mL Erlenmeyer flasks, to determine the effect of D-met on aminoglycoside PAE. Each flask was inoculated with the tested isolate at 5 X 105 CFU/ml and placed in a 37°C shaker for 2 hours. Each sample inoculum was diluted 5 fold and plated onto agar plates for quantitation. Flask contents were then transferred to centrifuge tubes, centrifuged at 500 x g, and washed three times with normal saline. Following the washing process, the remaining pellets were resuspended in CAMHB. Samples were then plated into a 100 well Bioscreen C honeycomb plate. An inoculum sample was diluted 5 fold and plated onto agar plates for quantitation. The Bioscreen C software was programmed to take a turbidometric measurement every 15 minutes at 580nm for 24 hours. PAE determinations were completed after six hours. Results were exported to Microsoft Excel for graph production and analysis.
2.3. D-methionine/tobramycin in vivo antimicrobial efficacy studies
2.3.1. Animals
White, male, specific pathogen free, Swiss-Webster mice weighing 18 to 20g (approx. 6–8 weeks old) were used in all in vivo antimicrobial studies. All animal care and use was approved by the Southern Illinois University School of Medicine Laboratory Animal Care and Use Committee and were performed under the supervision of the Southern Illinois University School of Medicine Division of Laboratory Animal Medicine.
We tested the possible D-met effect on aminoglycoside therapeutic efficacies in a lethal E. coli-infected murine model. Since antagonism may be clinically significant only in the host with diminished phagocytic function (Sande and Overton 1973), these studies were performed in both normal and neutropenic mice. This determination used a two-step process. Initially LD50 was determined in both healthy and neutropenic mice. These mice were used in the second phase of the study. The second phase determined if D-met interferes with tobramycin’s antimicrobial action in vivo. Result evaluations measured the length of animal survival as well as peritoneal cavity bacterial counts at the time of sacrifice.
2.3.2. Induction of Neutropenia
Cyclophosphamide, diluted in sterile saline immediately before use, was administered intraperitoneally (ip) in three equally divided doses (Days 0, 1, and 3) totaling 350 mg/kg. Animals were anesthetized using a ketamine (20.8 mg/kg) and xylazine (484 μg/kg) cocktail for sub-mandibular bleeding. Manual leukocyte and differential counts were performed on collected blood samples on days 2 through 4. Mice with neutrophil counts <500 neutrophils/μl were considered neutropenic (Han and Cutler 1997).
2.3.3. Inoculum preparation
E. coli was cultured overnight in CAMHB at 37°C. Cultures were diluted 1:10 and rotated on an orbital shaker for 1 hour in a 37°C water bath to reach log-phase growth. The suspension was standardized at 1 X 108 colony-forming units (CFU)/ml in CAMHB using a spectrophotometer and subsequently diluted in CAMHB to obtain the desired concentration. The final inoculum quantitation was established using bacterial plate counts.
2.3.4. Determination of Infective lethal dose (LD50)
Healthy and neutropenic mouse E. coli (ATCC 25922) LD50 levels were established. Neutropenic mice were used to model diminished phagocytic function in humans (Sande and Overton 1973). The LD50 determined an optimal challenge dose for our model prior to proceeding with the antimicrobial interference assays (Braun et al. 1981; Traub 1982; Lobry et al. 1992; Gottfredsson et al. 1995; Dominguez et al. 2001; Li and Tang 2004; Pliego-Castaneda et al. 2005).
By definition the LD50 establishes the number of microorganisms of a particular species that will kill 50% of a given animal population. We needed to first establish the LD50 in order to establish a challenge dose for our antimicrobial combination. The challenge dose was calculated such that a given concentration, based on the LD50, should kill all untreated mice. Soothill et al. (1992) established that values above the LD50 were appropriate for interference studies. If the challenge inoculum is too small, an infection will not be established. The antimicrobial efficacy was determined by the protection afforded the challenged mice. No E. coli ATCC 25922 LD50 data has previously been published in the Swiss-Webster strain of mice. ATCC was unaware of an LD50 in mice using this E. coli strain.
Seven groups of 3 healthy mice and seven groups of 3 neutropenic mice, for a total of 42 mice, were injected ip with 0.30 ml of 7 different E. coli concentrations escalating from 102 to 108 CFU/ml in 101 increments. In neutropenic mice, infection was induced on the 4th day of neutropenia induction. LD50 was defined as the bacterial concentration at which 2 out of 3 of the animals in any group died within 5 days following inoculation.
2.3.5. Antimicrobial interference studies
Normal (non-neutropenic) and neutropenic mice were injected ip with inoculum at 10X the established LD50 levels or saline for control mice. Infected mice were divided into 4 treatment groups: saline; antibiotic only; D-met only; or antibiotic/D-met in combination. Normal and neutropenic mice were treated 5 times at 2, 12, 24, 36 and 48 hours after inoculum by ip injection with: saline (control group); D-met (200 mg/kg); tobramycin (15 mg/kg); or a D-met (200 mg/kg)/tobramycin (15 mg/kg) combination. Eight hours post-inoculation, three mice from each group were euthanized and peritoneal lavage bacteria counts were quantified. The remaining five mice in each group were observed for survival 5 days following inoculation.
2.4 Statistical Analysis
2.4.1. Electrophysiology
ABR threshold shift statistical analyses employed an Analysis of Variance (ANOVA) design in which D-met dosage was listed as a between subject variable and observation intervals (time) were within subject variables (repeated measure). Frequency was also a within subject variable nested within observation intervals. The major result of interest was the group (treatment) x time interaction. The between subjects factor (grouping variable) was the dose of D-met given with six levels (one control and 5 treatments) and the within subject variables were frequency (4 levels) and date of testing (3 levels).
For the ABR measurements, the left and right ear thresholds were first compared to check for a difference between ears. No significant differences were observed; thus, data from the each left ear only was used for histologic studies to eliminate concerns of using both right and left ears as independent data points. Baseline ABR thresholds were analyzed separately by a two factor ANOVA with frequency as a repeated measure with Dunnett’s post-hoc follow-up at p ≤ 0.05 and p ≤ 0.01 to compare D-met-treated groups to control groups. Tukey’s post-hoc follow-up also measured pairwise comparisons between D-met-treated groups at p ≤ 0.05 and p ≤ 0.01.
2.4.2. OHC quantification
OHC percentages were statistically analyzed by a two-way ANOVA with repeated measures (frequency) with Dunnett’s post-hoc follow-up at p ≤ 0.05. The between subjects factor as group with six groups (one control and five treatment groups) and within subjects factor as frequency region with four levels (4, 8, 14 and 20 kHz regions).
2.4.3. Antimicrobial efficacy
To compare different antimicrobial agent growth phase shifts in vitro, an optical density (OD) band ranging from 0.2 to 0.8 was used as an isolate linear growth phase filter. For every OD in that band, time was recorded to provide a mean and standard deviation for each agent. Thus, for every linear growth phase, an average time to the event comparison could be performed between the different combinations. This average time was then compared between the different combinations with an Analysis of Variance with Dunnett’s post-hoc follow-up at p ≤ 0.05.
In vivo antimicrobial efficacy statistical analysis comprised Fisher’s exact test for percent survival studies and an independent t-test for bacterial quantification. In vivo analysis compared D-met/Tobramycin combinations to mouse groups treated only with tobramycin.
Statistical significance for all tests was set at α-levels p ≤ 0.05 and p ≤ 0.01. Statistical analyses were performed with SAS 9.1 software.
3. Results
3.1. Electrophysiology
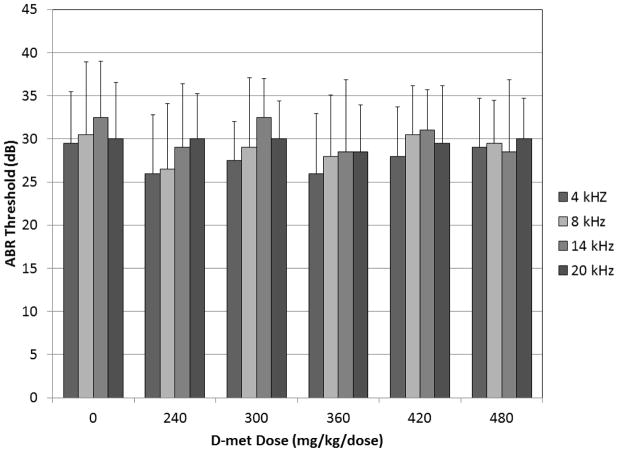
Overall ABR analysis revealed significant group effects (F = 9.98, df = 5, p ≤ 0.0001) and no interaction between group and frequency (F = 1.49, df = 15, p = 0.1145). Baseline thresholds are reported in Figure 1.
Figure 1.
Baseline ABR thresholds for 4, 8, 14, and 20 kHz tone-burst frequencies for all tobramycin-treated control and D-met dosing groups.
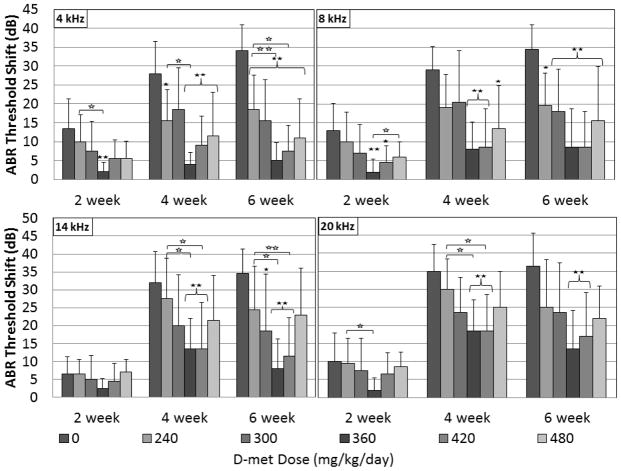
At 2 weeks, D-met ABR threshold shift protection was significant at the .01 level for the 360 mg/kg/day dosing group at 4 and 8 kHz (Figure 2). As expected, ABR threshold shifts in controls increased over time. At two weeks post-tobramycin initiation, mean control group ABR threshold shifts ranged from 6.5–13.5 dB; with the greatest threshold shifts at 4 and 8 kHz. Two week threshold shift for D-met dosing groups 240, 300 and 480 mg/kg/day tended to be less than controls but did not reach significance; possibly because of the minimal threshold shifts measured in the 2 week control group. D-met doses of 360 mg/kg/day resulted in threshold shift less than 5 dB at all frequencies, and 420 mg/kg/day doses resulted in threshold shift less than 5 dB at 8 and 14 kHz (Figure 2) at two weeks. The 360 mg/kg/day D-met group measured significant ABR threshold shift reductions compared to the 240 mg/kg/day at 4 and 20 kHz at the 0.05 level. The 360 mg/kg/day D-met group also measured significant ABR threshold shift reductions compared to the 480 mg/kg/day group at 8 kHz at the 0.05 level (Figure 2).
Figure 2.
ABR threshold shifts at 2, 4, and 6 weeks for 4, 8, 14 and 20 kHz tone-burst frequencies for all tobramycin-treated control and D-met dosing groups. Six guinea pig groups (n=10) were treated daily with 100 mg/kg/day tobramycin sulfate for 21 days. Five experimental groups received doses of 240, 300, 360, 420 or 480 mg/kg/day D-met 15 min prior to and 7 hours after each tobramycin administration. Significant results differing from the control group at an α-level of 0.05 or 0.01 as determined by a Dunnett’s post-hoc test are indicated by one or two closed stars, respectively. Significant results differing from D-met-treated groups at an α-level of 0.05 or 0.01 as determined by a Tukey’s post-hoc test are indicated by one or two open stars, respectively. Error bars represent +/− 1 SD.
D-met protection was more evident at four weeks because threshold shift was greater in the controls at that time point allowing greater contrast to protected groups. ABR threshold protection was significant at the .01 level for all frequencies for 360 and 420 mg/kg/day D-met dose groups and for the 480 mg/kg/day D-met group at 4 kHz. Additionally D-met protection occurred at the .05 level for 4 kHz at the 240 mg/kg/day dosing epoch and for 8 kHz at the 480 mg/kg/day D-met dose. At four weeks post-tobramycin initiation, mean control group ABR threshold shifts ranged from 28–35 dB; with the greatest shifts at 14 and 20 kHz. The most effective D-met doses of 360 and 420 mg/kg/day both resulted in ABR threshold shift less than 10 dB at 4 and 8 kHz (Figure 2) at four weeks. The 360 and 420 mg/kg/day D-met-treated group measured significant ABR threshold shift reductions compared to the 240 mg/kg/day group at 4, 14, and 20 kHz at the 0.05 level (Figure 2).
The greatest D-met protection of ABR thresholds related to controls was observed at 6 weeks. Mean control group ABR threshold shifts assessed six weeks post-tobramycin initiation (Figure 2) ranged from 34–36.5 dB. D-met doses of 360 and 420 mg/kg/day both resulted in threshold shift less than 10 dB at 4 and 8 kHz, and additionally at 14 kHz in the 360 mg/kg/day group. Threshold shift at 6-week ABR analyses of all D-met dosing groups averaged 11.5, 14, 16.9 and 20.2 dB for the tested frequencies of 4, 8, 14, and 20 kHz, respectively. Significant otoprotection occurred at the .01 level for all frequencies at the 360 and 420 mg/kg/day dosing levels. For the 300 mg/kg/day dosing level, protection was significant at 4 and 8 kHz at the .01 level and at 14 kHz at the .05 level. For the 240 mg/kg/day level, significant protection occurred at the .01 level for 4 kHz and at the .05 level for 8 kHz. For the 480 mg/kg/day D-met dosing level, significant protection occurred at the .01 level for 4 and 8 kHz but not for 14 or 20 kHz. The 360 and 420 mg/kg/day D-met-treated group measured significant ABR threshold shift reductions compared to the 240 mg/kg/day group at 4 and 14 20 kHz at the 0.05 level (Figure 2). The 360 mg/kg/day D-met-treated group also measured significant ABR threshold shift reductions compared to the 240 mg/kg/day group at 4 and 14 20 kHz at the 0.01 level (Figure 2).
3.2. Histology
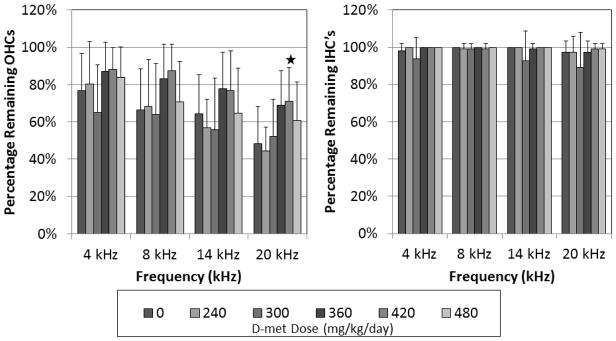
Overall OHC counts revealed significant group effect (F = 2.53, df = 5, p = 0.0394) and no interaction between group and frequency (F = 1.42, df = 15, p = 0.1430) (Figure 3). Dunnett’s follow-up revealed a significant difference between the 420 mg/kg/day D-met dosing group at 20 kHz and treated control at p ≤ 0.05. The mean percentage of remaining OHC present in all tested frequencies for the tobramycin-treated control group ranged from 48–77%, in comparison to groups treated with tobramycin and 360 and 420 mg/kg/day D-met; which ranged from 69–87% and 71–88%, respectively. Thus, D-met-treated animals demonstrated increased remaining OHC percentages compared to controls (Figures 3, 4).
Figure 3.
Remaining outer and inner hair cell percentages at sacrifice for treatment groups at 4, 8, 14 and 20 kHz tone-burst frequencies. Three weeks after a 21 day treatment course, cochleae were harvested and prepared for OHC viewing and analysis. Significant results differing from the control group at an α-level of 0.05 using Dunnett’s test are indicated with one star. Error bars represent +/− 1 SD.
Figure 4.
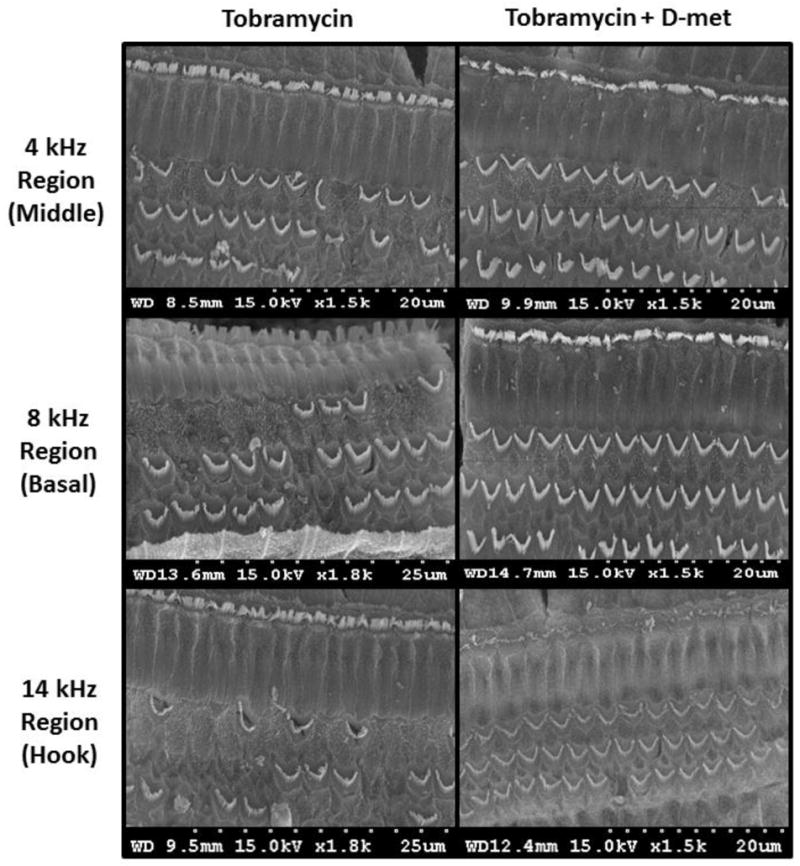
Cochlear outer hair cell SEM micrographs of animals treated with tobramycin and saline (left) or tobramycin and D-met (420 mg/kg/dose); right) in the 4 kHz (middle), 8 kHz (basal), and 14 kHz (hook) cochlear regions. Three weeks after a 21 day treatment course, cochleae were harvested and prepared for OHC viewing and analysis. D-met treatment significantly increased remaining OHC counts only in the 20 kHz region (p ≤ 0.05). However, increased OHC counts were observed throughout the cochlea. Scale for each image ranges from 20–25 μm.
3.3. BUN/Creatinine
No differences in BUN and creatinine occurred between groups. BUN concentrations ranged from 18.1–20.8 across all groups. Creatinine concentrations ranged from 0.33–0.37 across all groups. Even in the tobramycin-treated controls, BUN and creatinine values were within normal limits suggesting that this tobramycin ototoxicity animal model does not induce nephrotoxicity. D-met did not influence BUN or creatinine measurements; suggesting that D-met also does not induce nephrotoxicity. Thus, we could not test for D-met protection from nephrotoxicity because the tobramycin-treated controls did not exhibit nephrotoxicity.
3.4. Minimal Inhibitory Concentration (MIC)
The MICs against E. coli isolates ranged from: 1–64 μg/ml (tobramycin); and ≥6,400 μg/ml (D-met). The MICs for Staphylococcus aureus isolates ranged from: 0.5–4 μg/ml (tobramycin); and ≥6,400 μg/ml (D-met). The MICs against Pseudomonas aeruginosa ranged from: 0.5–128 μg/ml (tobramycin); and ≥6,400 μg/ml (D-met). All ATCC control strains measured aminoglycoside MICs within expected ranges.
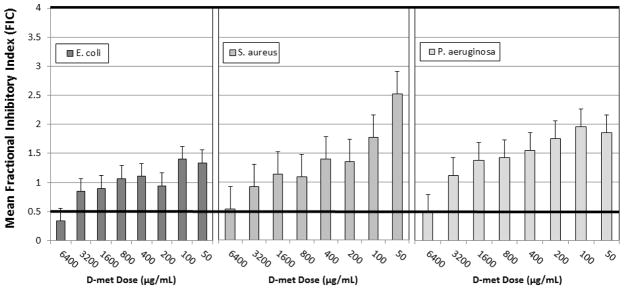
3.5. Mean Fractional Inhibitory Concentration Index (FIC)
FIC shows tobramycin indifference to all tested D-methionine (D-met) concentrations. At high D-met concentrations, tobramycin/D-met (6,400 μg/ml) combinations showed synergy in 42% of tested isolates. After repeated trials, one P. aeruginosa isolate was identified as an extreme outlier and removed from analysis because it measured non-replicable variability that was significantly greater than the average. Thus, these results do not demonstrate antagonism at high D-met concentrations for any of the isolates tested (Figure 5).
Figure 5.
Mean Fractional Inhibitory Index (FIC) for tobramycin and D-met concentrations. A checkerboard assay was used to determine MIC values of tobramycin (128–0.25 μg/ml) in combination with a serial dilution of D-met (6400–100 μg/ml). FIC values were calculated using previously determined MIC results for tobramycin and D-met and E. coli, S. aureus, or P. aeruginosa (n = 6 for E. coli and S. aureus; n = 5 for P. aeruginosa).
3.6. Time course inhibition assays
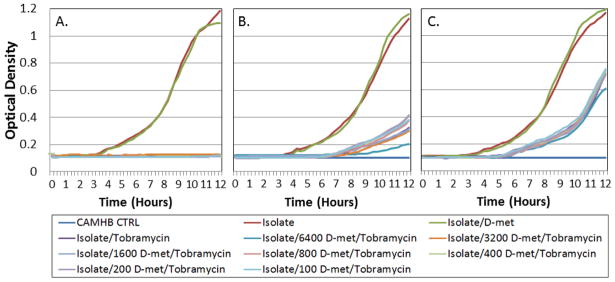
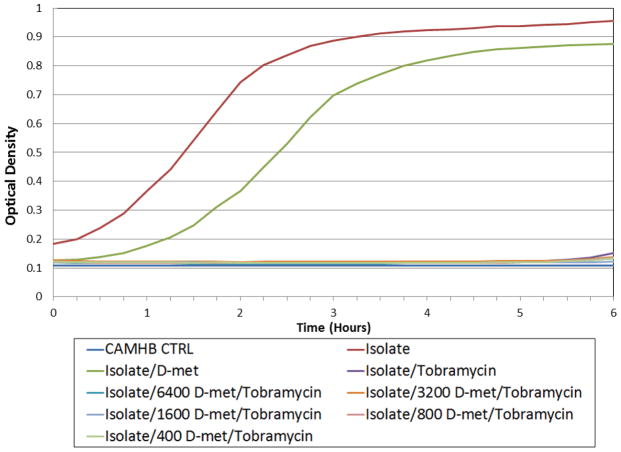
Tobramycin microbial inhibitory efficacy in combination with D-met was tested using time course microbial inhibition assays. The Bioscreen C instrument was used to establish inhibitory curves (Cooper et al. 2011). Bioscreen C-determined MIC’s were coordinated with each time course inhibition to optimize the Bioscreen C system to each isolate. Growth during the logarithmic phase was used to analyze whether D-met affects the aminoglycoside inhibitory efficacy over time. The time period during the log growth phase was used to determine whether D-met had an inhibitory antimicrobial effect on tobramycin. D-met alone at 6,400 μg/ml produced growth curves which paralleled bacterial growth rates without antimicrobials, which indicates that D-met did not have an inhibitory effect on bacterial growth. All generated tobramycin/D-met combination growth curves, when compared to the media control, showed no inhibitory effects (Figure 6). Effect comparisons on tobramycin tested against typical clinical isolates indicated no D-met-induced antimicrobial interference.
Figure 6.
Clinical isolate time course inhibition assays for tobramycin/D-met combinations. Graphs show optical density plotted over time during bacterial log growth phase. Tobramycin (at MIC) was combined with serial D-met dilutions (6400–100 μg/ml) and compared to clinical isolate growth. Studies were performed in triplicate. D-met did not significantly inhibit antimicrobial efficacy (p ≤ 0.05). Clinical isolates tested were: A) E. coli; B) S. aureus; and C) P. aeruginosa.
3.7. Post Antibiotic Effect (PAE)
Experimentation was performed using the E. coli model system to further test for tobramycin/D-met PAE interactions. PAE studies confirmed growth inhibition assay results. No tobramycin/D-met combinations produced growth curves with a shorter lag growth phase than either the E. coli alone or D-met only control. Optical density (OD580) measurements after D-met and aminoglycoside removal revealed that a 4–5 hour PAE was produced in each of the original antimicrobial combinations (Figure 7). Microbial growth resumption began approximately 5–6 hours after tobramycin removal. PAE results were not affected when E. coli was treated with D-met/tobramycin combinations. These results were consistent with all tobramycin MIC’s tested and at all D-met concentrations. PAE studies further confirmed results found in the time course inhibition assays. Across all antibiotics, D-met and tobramycin combinations did not grow before D-met and isolate controls. Therefore, PAE experiments did not detect D-met-induced antimicrobial inhibition.
Figure 7.
Post Antibiotic Effect (PAE) assays of an E. coli clinical isolate with tobramycin (2 μg/ml). E. coli was exposed to tobramycin alone and in combination with serially diluted D-met (6400–400 μg/ml) for 2 hours at 37°C. Tobramycin/D-met combinations were compared to isolate and D-met (6400 μg/ml D-met and media) controls. Studies were performed in triplicate. All tobramycin/D-met growth curves were significantly inhibited (p ≤ 0.05) when compared with isolate controls and D-met controls.
3.8. Antimicrobial efficacy In vivo studies
D-met did not significantly affect in vivo tobramycin efficacy in either normal or neutropenic mice challenged with 10 LD50 of E. coli (1×105 CFU). Peritoneal lavage counts performed 8 hours post-antibiotic treatment showed no significant bacterial increases for tobramycin-/D-met- treated mice over tobramycin-treated mice. No significant bacterial increases in either normal (p = 0.110) or neutropenic (p = 0.414) mice were seen in any of the tested tobramycin treatment groups (Table 1).
Table 1.
Antimicrobial interference study in normal (non-neutropenic) and neutropenic mice treated with tobramycin (n = 5/survival group; 3/bacterial recovery group) with and without D-met. The addition of D-met to tobramycin treatment did not significantly influence survival (p = 0.444) or mean bacterial recovery (p = 0.197).
| Swiss Webster mouse in vivo model | ||||
|---|---|---|---|---|
| Normal Mice | Neutropenic Mice | |||
| Treatment | % Survival | Mean Bacterial Recovery | % Survival | Mean Bacterial Recovery |
| Saline Only | 100 | 0 | 100 | 0.5 × 101 |
| Isolate Only | 0 | 8.33 × 104 | 80 | 1.35 × 105 |
| Tobramycin1 + Isolate | 60 | 0.33 × 101 | 40 | 1.17 × 101 |
| D-met2 + Isolate | 0 | 5.17 × 104 | 0 | 8.47 × 104 |
| Tobramycin + D-met3+ Isolate | 100 | 1.75 × 102 | 100 | 4.8 × 101 |
Tobramycin dosage: tobramycin (15 mg/kg)
D-met dosage: (200 mg/kg)
Dosage combinations: tobramycin (15 mg/kg) + D-met (200 mg/kg)
D-met combined with tobramycin treatments did not significantly influence mouse survival rates for normal (p = 0.444) or neutropenic (p = 0.166) mice compared to groups treated only with tobramycin. However, D-met/tobramycin combination groups measured increased survival compared to tobramycin-alone groups that did not achieve statistical significance (Table 1).
4. Discussion
4.1. D-met confers dose-dependent protection
This study demonstrates D-met’s ability to significantly decrease tobramycin-induced ototoxicity via reducing ABR permanent threshold shifts and decreasing OHC loss. To our knowledge, we are the first to report dose-dependent D-met otoprotection against tobramycin. D-met doses at 360 and 420 mg/kg/day administered 15 minutes prior to and 7 hours after tobramycin resulted in a highly significant ototoxicity reduction at all ABR-tested frequencies. Doses below 360 and above 420 mg/kg/day showed a lesser degree of protection and suggest an optimal bi-phasic therapeutic D-met dosing range exists in tobramycin combinations. While significant otoprotection at some frequencies was detected in all dosing groups, 360 and 420 mg/kg/day D-met doses showed the most robust significance across all frequencies tested. This pattern was detected histologically by OHC analysis in which the 420 mg/kg/day dosing group showed significant otoprotection at 20 kHz using a Dunnett’s Test. From a descriptive standpoint, the average percent remaining OHCs is higher in the 360 and 420 dosing categories than in the controls for all frequency regions which is consistent with the same dosing levels and frequency for ABR measures. However the variability and multiple measures, while correcting for Bonferroni effect for multiple comparisons, diluted the analyses and precluded us from reaching statistical significance at all the 420 dosing level at 20 kHz. Mechanistically, some of the protection reflected in the ABRs may also have been contributed by protection of the spiral ganglion cells or another area however this hypothesis requires further investigation.
Clinical D-met dosing ranges for protection from tobramycin are not yet determined. However, clinical tobramycin dosing, ranging from 8–15 mg/kg/day for cystic fibrosis patients (Massie and Cranswick 2006; Flume et al 2009), is a fraction of the high dose (100 mg/kg/day) used to rapidly induce ototoxicity in the animal model. Although a clinical D-met dose is not yet determined to protect from tobramycin, D-met has demonstrated significant protection from noise-induced hearing loss in animal models with low doses (Clifford et al. 2011). Therefore, although somewhat preliminary, the dose-dependent protection measured in the current study and D-met’s previous low-dose protection from noise suggests that optimal D-met protection from clinically-prescribed tobramycin may also require only a fraction of the currently tested D-met doses. Further clinical D-met dosing for tobramycin ototoxicity is warranted.
In this study, we also measured body weight, BUN, and creatinine. Comparisons showed no significant difference between treated controls and D-met experimental groups for these measures; although the saline included with intraperitoneal injections may have eliminated any nephrotoxicities or protection by flushing the kidneys throughout the experiment. Animals may have recovered from acute nephrotoxicity 6 weeks post-treatment. However, unchanged BUN and creatinine measures suggest that D-met may not induce permanent nephrotoxicity.
4.2. D-met does not interfere with tobramycin antimicrobial efficacy
Based on limited published reports, D-met does not interfere with aminoglycoside antimicrobial action (Herr et al. 2001) and does not alter serum gentamicin levels (Sha and Schacht 2000). Therefore, we postulated treatment efficacy should be retained even with D-met protection from ototoxicity. Although little information is available regarding D-met’s antimicrobial activity, some evidence indicates that D-met may itself have antimicrobial activity and enhance the antimicrobial activity of other antibiotics. D-met, at high concentration, had lethal effects against bacteria when used alone due to its incorporation into the peptidoglycan layer of the cell wall (Caparros et al. 1992). This report was not supported by our data; indicating that at 6,400 μg/ml D-met concentrations, no significant logarithmic growth reduction was observed.
We studied E. coli, P. aeruginosa and S. aureus clinical isolates for their growth response in the presence of tobramycin, D-met, and D-met/tobramycin combinations. We further studied the effect of these combinations in an in vivo murine model using E. coli infections. These data support the observations of Herr et al. (2001) who reported that D-met does not interfere with aminoglycoside antimicrobial activities. Time course assays using tobramycin against E. coli, P. aeruginosa and S. aureus clinical strains indicated no D-met interference with tobramycin antimicrobial efficacy. D-met alone did not have an inhibitory effect on the microorganismal growth rate. Further, the use of D- met in combination with tobramycin had no effect on their post antibiotic effect in a murine model.
A murine model of lethal E. coli infection did not report significant antagonism with D-met/tobramycin combinations. Antagonism, if it exists, may be clinically significant only in a host with diminished phagocytic function (Sande and Overton 1973); thus, we performed experiments in both normal and neutropenic mice. Tobramycin in vivo antimicrobial activity was not affected by D-met in either neutropenic or non-neutropenic mice. In fact, D-met combined with tobramycin therapy may have increased survival in the murine model compared to isolate only and tobramycin only controls in normal and neutropenic mice (Table 1); possibly through its incorporation into the peptidoglycan layer of the cell wall (Caparros et al. 1992). Thus, D-met administration may protect from multiple tobramycin-induced adverse drug reactions.
Another sulfhydryl antioxidant compound, N-acetyl cysteine (NAC), has also been tested to prevent aminoglycoside-induced hearing loss. However, when tested for protection from kanamycin-induced ototoxicity, NAC actually exacerbated hearing loss (Bock et al. 1983). Further, NAC demonstrated antagonistic effects against gentamicin and tobramycin antimicrobial efficacy (Parry and Neu 1977) and demonstrated increased but insignificant gram-negative bacterial incidence in clinical trials (Feldman et al. 2007). D-met provided protection from aminoglycoside-induced ototoxicity (Sha and Schacht 2000; Campbell 2003; current study) and has now demonstrated a lack of interference with aminoglycoside antimicrobial efficacy. Thus, although D-met and NAC are both antioxidant compounds, they may protect by different mechanisms.
Aminoglycoside ototoxicity, aminoglycoside bactericidal efficacy and D-met otoprotection act by different mechanisms. Aminoglycosides induce ototoxicity by inducing iNOS pathways and creating hydroxyl radicals via the Fenton Reaction, which induces lipid peroxidation and activates cell death via apoptotic and necrotic pathways (Sha and Schacht 1999). Aminoglycoside bactericidal efficacy, however, is dependent on 30s ribosomal inhibition, protein synthesis inhibition, and resultant cell death. Although D-met otoprotective mechanisms have not been fully elucidated, it is known to be a direct and indirect antioxidant and has not demonstrated interaction with 30s ribosomal inhibition. To the best of our knowledge, this study is the first in vitro and in vivo antimicrobial interference study testing D-met and aminoglycoside combinations. Our results also confirm previous studies: D-met does not alter the in vivo or in vitro aminoglycoside antimicrobial activity against either gram positive or gram negative bacteria.
4.3. Clinical implications and impact
Currently, no FDA-approved agent exists for protection from tobramycin-induced ototoxicity. Our current findings suggest D-met affords highly significant (p ≤ 0.01) otoprotection from tobramycin and shows promise for improving quality of life outcomes for patients receiving high dose or repeated tobramycin treatments.
Tobramycin- and aminoglycoside-induced ototoxicity reports range from nearly absent to approximately 35% of cystic fibrosis patients (Mulheran et al. 2001, 2006; Piltcher et al. 2003; Cheng et al. 2009; Guy et al. 2010; Martins et al. 2010; Scheenstra et al. 2010; Al-Malky et al. 2011; Hennig et al. 2014) with an increased risk of aminoglycoside-induced hearing loss with repeated and higher cumulative doses. The majority of adolescent and adult cystic fibrosis patients are colonized with Pseudomonas aeruginosa (P. aeruginosa) (Cystic Fibrosis Foundation Registry 2011). These patients require long-term intravenous and inhaled tobramycin dosing regimens due to chronic and acute Pseudomonas aeruginosa infections (Treggiari 2009; Prayle and Smyth 2010). Thus, a large cystic fibrosis patient population may benefit from D-met otoprotection.
P. aeruginosa is also a common cause of respiratory infections in intensive care unit and ventilator-dependent patients (American Thoracic Society 2005) that require tobramycin treatment. Many patients with these respiratory infections have multiple co-morbid health conditions that are typically more serious with advanced age (American Thoracic Society 2005). Aging is associated with decreased glutathione levels, reduced natural antioxidant defenses and cellular damage, mechanisms similar to ototoxic aminoglycosides (Lautermann et al. 1997). D-met’s antioxidant characteristics may be particularly helpful in these tobramycin-treated patient populations.
Internationally, aminoglycosides are frequently utilized for infections because of low cost, infrequent drug-induced allergy, and accessibility. However, in developing countries, aminoglycosides are more likely to result in ototoxic damage secondary to widespread use without physician monitoring and, infrequent audiologic evaluation (Oghan et al. 2011). Social factors such as stress and poor nutritional status may also predispose patients to aminoglycoside ototoxicity and increase drug-induced hearing damage particularly in developing nations (Forge and Schacht 2000). Thus, D-met therapy combined with aminoglycosides may prevent ototoxicity internationally without compromising aminoglycoside antimicrobial efficacy.
4.4. Future studies
This study provides evidence supporting D-met as an otoprotective therapeutic agent, particularly for an aminoglycoside with varied cochlear and vestibular toxicities, without exacerbating antimicrobial efficacy. D-met combined with tobramycin may allow for longer dosing intervals with decreased concern for hearing loss. Future human clinical trials are now needed to assess D-met’s safety and efficacy with tobramycin to confirm these in vivo and in vitro studies.
The required preclinical functional measures for FDA submission are protection from auditory threshold shift which we measure with auditory brainstem response (ABR). For clinical ototoxicity monitoring, the standards are based on auditory threshold measures, both in the conventional and high frequency audiometric ranges (ASHA 1994, AAA 2009). Otoacoustic measures are not a part of the required clinical ototoxicity standards. Thus, distortion product otoacoustic emissions (DP-OAEs) and spiral ganglion cell assessment did not meet the current study’s translational bench to bedside focus. However, they would be of great interest in future studies of specific OHC function, protection, and lesion sites; particularly to address the protection differences measured between auditory threshold and OHC quantification analysis.
Highlights.
D-met protects from tobramycin-induced ototoxicity as early as 2 weeks after administration.
D-met protection is dose-dependent and increases over time.
D-met does not interfere with aminoglycoside antimicrobial efficacy.
D-met protection may help cystic fibrosis or other vulnerable patient populations.
Acknowledgments
This work was funded by the National Institutes of HealthR01 DC008412-01A1 NIH/NIDCD “Developing D-methionine as an Aminoglycoside Otoprotectant’.R01. PI Kathleen Campbell, PhD.
We wish to thank Scott Bergman, PharmD, BCPS, and the Department of Pharmacy Practice at Southern Illinois University Edwardsville for their financial contribution toward the Bioscreen C purchase.
Abbreviations
- ABR
auditory brainstem response
- AG
aminoglycosides
- ANOVA
analysis of variance
- ATCC
American Type Culture Collection
- BUN
blood urea nitrogen
- CAMHB
cation-adjusted Mueller-Hinton broth
- CFU
colony forming unit
- D-met
D-methionine
- DP-OAEs
distortion product otoacoustic emissions
- HMDS
hexamethyldisilazane
- IHC
inner hair cell
- IP
intraperitoneal
- MIC
minimum inhibitory concentration
- FIC
fractional inhibitory concentration
- OHC
outer hair cell
- PAE
post-antibiotic effect
- ROS
reactive oxygen species
Footnotes
Declaration of Interest: Dr. Kathleen Campbell is the sole inventor on the patents for D-methionine as a protective agent. All of her patents are owned by her employer, Southern Illinois University School of Medicine. All other authors do not have personal relationships with organizations that could potentially be perceived as influencing the described research. All authors have read the journal’s disclosure policy of potential conflicts of interest.
Data presented at the Association for Research in Otolaryngology Midwinter Meeting; San Diego, California February 22, 2014. PS-028.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (AAA) American Academy of Audiology. American Academy of Audiology Position Statement and Clinical Practice Guidelines. 2009 Audiology.org.
- Ababneh M, Harpe S, Oinonen M, Polk R. Trends in Aminoglycoside Use and Gentamicin-Resistant Gram-Negative Clinical Isolates in US Academic Medical Centers: Implications for Antimicrobial Stewardship. Infect Control Hosp Epidemiol. 2012;33(6):594–601. doi: 10.1086/665724. [DOI] [PubMed] [Google Scholar]
- Al-Malky G, Suri R, Dawson S, Sirimanna T, Kemp D. Aminoglycoside antibiotics cochleotoxicity in pediatric cystic fibrosis (CF) patients: A student using extended high-frequency audiometry and distortion product otoacoustic emissions. Intl J Audiol. 2011;50:112–122. doi: 10.3109/14992027.2010.524253. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- Aran JM. Current perspectives on inner ear toxicity. Otolaryngol Head Neck Surg. 1995;1:133–44. doi: 10.1016/S0194-59989570313-6. [DOI] [PubMed] [Google Scholar]
- (ASHA) American Speech-Language-Hearing Association. Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy. 1994 [Guidelines]. Available from www.asha.org/policy.
- Avent M, Rogers B, Cheng A, Paterson D. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J. 2011;41:441–449. doi: 10.1111/j.1445-5994.2011.02452.x. [DOI] [PubMed] [Google Scholar]
- Bardien S, de Jong G, Schaaf H, Harris T, Fagan J, Peterson L. Aminoglycoside- induced hearing loss: South Africans at risk. SAMJ. 2009;99(6):440–441. [PubMed] [Google Scholar]
- Begg EJ, Barclay ML. Aminoglycosides-50 years on. Br J Clin Pharmac. 1995;39:597–603. [PMC free article] [PubMed] [Google Scholar]
- Begg E, Peddie B, Chambers S, Boswell D. Comparison of gentamicin dosing regimens using an in-vitro model. J Antimicrob Chemother. 1992;29:427–433. doi: 10.1093/jac/29.4.427. [DOI] [PubMed] [Google Scholar]
- Bock G, Yates G, Miller J, Moorjani P. Effects of N -acetylcysteine on kanamycin ototoxicity in the guinea pig. Hear Res. 1983;9:255–262. doi: 10.1016/0378-5955(83)90030-8. [DOI] [PubMed] [Google Scholar]
- Braun R, Fitzi K, Gutzwiller F, Hohl H, van der Linde F, Gelzer J. Frequency of Escherichia coli resistance in Switzerland. Schweiz Med Wochenschr. 1981;111:1048–1057. [PubMed] [Google Scholar]
- Brummett RE, Fox KE. Aminoglycoside-induced hearing loss in humans. Antimicrob Agents Chemother. 1989;33:797–800. doi: 10.1128/aac.33.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundtzen R, Gerber A, Cohn D, Craig W. Post-antibiotic suppression of bacterial growth. Res Infect Dis. 1981;3:28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- Campbell KCM, Fox DJ, Roberts MH, Yanik S, Meech RP, Hargrove TL, Verhulst S, Rybak L, Cooper M. D-methionine Reduces Tobramycin-Induced Ototoxicity Without Antimicrobial Interference. PS-028. Presented at The Association for Research in Otolaryngology Midwinter Meeting; San Diego, California. February 22, 2014. [Google Scholar]
- Campbell K, Claussen A, Meech R, Verhulst S, Fox D, Hughes L. D-Methionine (D-met) Significantly Rescues Noise Induced Hearing Loss: Timing Studies. Hear Res. 2011;282:138–144. doi: 10.1016/j.heares.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Klemens JJ. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226(1–2):92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Rybak LP, Hughes LF. The Effect of D-Methionine on Cochlear Oxidative State with and without Cisplatin Administration: Mechanisms of Otoprotection. J Am Acad Audiol: Special Edition on Ototoxicity. 2003;14(3):144–156. [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Rybak LP, Hughes LF. D-Methionine protects against cisplatin damage to the stria vascularis. Hear Res. 1999;138:13–28. doi: 10.1016/s0378-5955(99)00142-2. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Rybak LP, Meech RP, Hughes LF. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Caparros MG, Pisabarro AG, De Pedro MA. Effect of D-amino acids on structure and Synthesis of peptidoglycan in E. coli. J Bact. 1992;147:5549–5559. doi: 10.1128/jb.174.17.5549-5559.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Johnston P, Luz J, Uluer A, Filgor B, Licameli G, Kenna M, Jones D. Sensorineural hearing loss in patients with cystic fibrosis. Otolaryng Head Neck. 2009;141:86–90. doi: 10.1016/j.otohns.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Cheng P, Liu S, Young Y, Lin-Shiau S. D-Methionine attenuated cisplatin-induced vestibulotoxicity through altering ATPase activities and oxidative stress in guinea pigs. Toxicol Appl Pharmacol. 2006;5(2):228–36. doi: 10.1016/j.taap.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Cheng P, Liu S, Hsu C, Lin-Shiau S. Correlation of increased activities of Na+K+-ATPase and Ca2÷ ATPase with reversal of cisplatin ototoxicity induced by D-methionine in guinea pigs. Hear Res. 2005;205(1–2):102–9. doi: 10.1016/j.heares.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng P, Liu S, Young Y, Hsu C, Lin-Shiau S. Protection from noise-induced temporary threshold shift by D-methionine is associated with preservation of ATPase activities. Ear Hear. 2008;29(1):65–75. doi: 10.1097/AUD.0b013e31815d635b. [DOI] [PubMed] [Google Scholar]
- Chuong Y, Taura A, Pak K, Choi S, Masuda M, Ryan A. Generation of highly- reactive oxygen species is closely related to rat organ of Corti treated with gentamicin. Neuroscience. 2009;16 (161):214–26. doi: 10.1016/j.neuroscience.2009.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen A, Fox D, Yu X, Meech R, Verhulst S, Hargrove T, Campbell K. D-methionine pre-loading reduces noise-induced permanent threshold shift and outer hair cell loss. Int J Aud Dec. 2013;52 (12):801–807. doi: 10.3109/14992027.2013.840933. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield D. Direct detection of ototoxicant- induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Clifford R, Coleman J, Balough B, Liu J, Kopke R, Jackson R. Low-dose D-methionine and N-acetyl-L-cysteine for protection from permanent noise-induced hearing loss in chinchillas. Otolaryngol Head Neck Surg. 2011 Dec;145(6):999–1006. doi: 10.1177/0194599811414496. [DOI] [PubMed] [Google Scholar]
- Coleman J, Liu J, Jackson R, Kopke R. Post-noise administration of methionine attenuates noise-induced hearing loss in the chinchilla. Abstracts of the Association for Research in Otolaryngology. 2002a;25:226. [Google Scholar]
- Coleman JKM, Liu J, Wood K, Kopke R. Low dose methionine with N-acetyl-L-cysteine reduces noise-induced threshold shift in the chinchilla. Abstracts of the Association for Research in Otolaryngology. 2002b;25:226. [Google Scholar]
- Cooper CJ, Denyer SP, Maillard JY. Rapid and quantitative automated measurement of bacteriophage activity against cystic fibrosis isolates of Pseudomonas aeruginosa. J Appl Microbiol. 2011;110:631–640. doi: 10.111/j.1365-2672.2010.04928.x. [DOI] [PubMed] [Google Scholar]
- Conrad D, Stenbit A, Zettner E, Wick I, Eckhardt C, Hardiman G. Frequency of mitochondrial 12S ribosomal RNA variants in an adult cystic fibrosis population. Pharmacogenet Genom. 2008;8(12):1095–1102. doi: 10.1097/FPC.0b013e328312b072. [DOI] [PubMed] [Google Scholar]
- Craig WA, Gudmundsson S. Antibiotics in laboratory medicine. 4. Baltimore, MD: 1996. [Google Scholar]
- Craig WA, Gundmundsson S. Antibiotics in laboratory medicine. 3. Baltimore, MD: 1991. Postantibiotic effect. [Google Scholar]
- Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis. 1990;74:63–70. [PubMed] [Google Scholar]
- Craig WA, Vogelman B. The postantibiotic effect. Ann Intern Med. 1987;106:900–902. doi: 10.7326/0003-4819-106-6-900. [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation. [Accessed 12/16/12];Annual Patient Registry. 2011 ( http://tobramycin.cff.org/UploadedFiles/research/ClinicalResearch/2011-Patient-Registry)
- Dominguez MC, de La Rosa M, Borobio MV. Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. J Antimicrob Chemother. 2001;47:391–398. doi: 10.1093/jac/47.4.391. [DOI] [PubMed] [Google Scholar]
- Drusano GL. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemothe. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson R, Terrell C. Symposium on Antimicrobial Agents-Part VIII - The Aminoglycosides. Mayo Clin Proc. 1999;74:519–528. doi: 10.4065/74.5.519. [DOI] [PubMed] [Google Scholar]
- Fantin B, Ebert S, Leggett J, Vogelman B, Craig W. Factors affecting duration of in-vivo postantibiotic effect for aminoglycosides against gram-negative bacilli. J Antimicrob Chemother. 1991;27:829–836. doi: 10.1093/jac/27.6.829. [DOI] [PubMed] [Google Scholar]
- Fausti S, Henry J, Helt W, Phillips D, Frey R, Noffsinger D, Larson V, Fowler C. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Fausti S, Henry J, Schaffer H, Olson D, Frey R, McDonald W. High-Frequency Audiometric Monitoring for Early Detection of Aminoglycoside Ototoxicity. J Infect Dis. 1992;165:1026–1032. doi: 10.1093/infdis/165.6.1026. [DOI] [PubMed] [Google Scholar]
- Feldman L, Efrati S, Eviatar E, Abramsohn R, Yarovoy I, Gersch E, Averbukh Z, Weissgarten J. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007 Aug;72(3):359–63. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa J, Kapolowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: Importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- Flume P, Mogayzel P, Robinson K, Goss C, Rosenblatt R, Kuhn R, Marshall B. Cystic Fibrosis Pulmonary Guidelines, Treatment of Pulmonary Exacerbations. American J Respir Crit Care Medicine. 2009;180:802–809. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- Forge A, Schacht J. Aminoglycoside Antibiotics. Audiol Neuro-Otol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- Fourmy D, Recht M, Blanchard S, Puglisi J. Structure of the A Site of Escherichia coli 16S Ribosomal RNA Complexed with an Aminoglycoside Antibiotic. Science. 1996 Nov 22;274(5291):1367–71. doi: 10.1126/science.274.5291.1367. [DOI] [PubMed] [Google Scholar]
- Francis S, Katz J, Fanning K, Harris K, Nicholas B, Lacy M, Pagana J, Agris P, Shin J. A Novel Role of Cytosolic Protein Synthesis Inhibition in Aminoglycoside Ototoxicity. J Neurosci. 2013;33(7):3379–3093. doi: 10.1523/JNEUROSCI.3430-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick PJ. Toxicity of methionine in humans. J Nutr. 2006;136(6 Suppl):1722S–1725S. doi: 10.1093/jn/136.6.1722S. [DOI] [PubMed] [Google Scholar]
- Gatell J, Ferran F, Araujo V, Bonet M, Soriano E, Traserra J, SanMiguel J. Univariate and Multivariate Analyses of Risk Factors Predisposing to Auditory Toxicity in Patients Receiving Aminoglycosides. Antimicrob Agents CH. 1987;31(9):1383–1387. doi: 10.1128/aac.31.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibelli L, Fanelli C, Rotilio G, Lafavia E, Coppola S, Colussi C, Civitareale P, Ciriolo M. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- Gonzalez LS, 3rd, Spencer JP. Aminoglycosides: a practical review. Am Fam Physician. 1998;58(8):1811–20. [PubMed] [Google Scholar]
- Gottfredsson M, Erlendsdottir H, Gunmundsson A, Gudmundsson S. Different patterns of bacterial DNA synthesis during postantibiotic effect. Antimicrob Agents Chemother. 1995;39:1313–1319. doi: 10.1128/aac.39.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin Y, Cotanche DA, Manneberg O, Molina R, et al. Pulmonary delivery of D-methionine is associated with an increase in ALCAR and glutathione in cochlear fluids. Hear Res. 2013 Jan 4; doi: 10.1016/j.heares.2012.12.011. E-pub ahead of print: pii: S0378-5955(12)00306-1. [DOI] [PubMed] [Google Scholar]
- Guan MX, Fischel-Ghodisan N, Attardi G. A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum Mol Genet. 2000;9(12):1787–1793. doi: 10.1093/hmg/9.12.1787. [DOI] [PubMed] [Google Scholar]
- Guy E, Bosomworth M, Denton M, Conway S, Brownlee K, Lee T. Serum tobramycin levels following delivery of tobramycin (Tobi ®) via eFLow® advanced nebulizer in children with cystic fibrosis. J Cyst Fibros. 2010;9:292–295. doi: 10.1016/j.jcf.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Hamstra D, Eisbruch A, Naidu M, Ramana G, Sunkara P, Campbell K, Ross B, Rehemtulla A. Pharmacokinetic analysis and phase 1 study of MRX-1024 in patients treated with radiation therapy with or without cisplatinum for head and neck cancer. Clin Cancer Res. 2010;16 (9):2666–76. doi: 10.1158/1078-0432.CCR-09-3318. [DOI] [PubMed] [Google Scholar]
- Han Y, Cutler J. Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J Infect Dis. 1997;175:1169–1175. doi: 10.1086/516455. [DOI] [PubMed] [Google Scholar]
- Harris T, Bardien S, Schaaf H, Petersen L, De Jong G, Fagan J. Aminoglycoside-induced hearing loss in HIV-positive and HIV-negative multidrug-resistant tuberculosis patients. S Afr Med J. 2012;102:363–366. doi: 10.7196/samj.4964. [DOI] [PubMed] [Google Scholar]
- Hawkins J, Keidel W, Neff W. Drug Ototoxicity Handbook of Sensory Physiology. part III. V. Berlin: Springer; 1976. pp. 707–728. [Google Scholar]
- Hennig S, McKay K, Vidmar S, O’Brien K, Stacey S, Cheney J, Wainwright C. Safety of inhaled (Tobi) and intravenous tobramycin in young children with cystic fibrosis. J Cyst Fibros. 2014 Jul;13(4):428–34. doi: 10.1016/j.jcf.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Herr L, Koirala J, Campbell K, Starks N. D-methionine Does Not Interfere With Antimicrobial Effectiveness. Proceedings of Infectious Disease Society of America. 2001:457. [Google Scholar]
- Hessen MT, Pitsakis PG, Levison ME. Postantibiotic effect of penicillin plus gentamicin versus Enterococcus faecalis in vitro and in vivo. Antimicrob Agents Chemother. 1989;33:608–611. doi: 10.1128/aac.33.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res. 1997;104:1–14. doi: 10.1016/s0378-5955(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Hyde GE, Rubel EW. Mitochondrial role in hair cell survival after injury. Otolaryngol- Head Neck Surg. 1995;113(5):530–540. doi: 10.1177/019459989511300503. [DOI] [PubMed] [Google Scholar]
- Kahlmeter G, Dahlager JI. Aminoglycoside toxicity - a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984;13(Suppl A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Steyger PS. Intracellular mechanisms of aminoglycoside-induced ototoxicity. Integr Biol. 2011;3(9):879–86. doi: 10.1039/c1ib00034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kies C, Fox H, Aprahamian S. Comparative value of L-, and D-methionine supplementation of an oat-based diet for humans. J Nutr. 1975;105(7):809–14. doi: 10.1093/jn/105.7.809. [DOI] [PubMed] [Google Scholar]
- Klemens J, Meech R, Hughes L, Somani S, Campbell K. Antioxidant levels inversely covary with hearing loss after amikacin treatment. J Am Acad Audiol: Special Edition on Ototoxicity. 2003;14(3):134–143. [PubMed] [Google Scholar]
- Kopke R, Coleman K, Liu J, Campbell K, Riffenburgh R. Enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002;112:1515–1532. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- Korver K, Rybak L, Whitworth C, Campbell K. Round Window Application of D-Methionine Provides Complete Cisplatin Otoprotection. Otolaryngol Head Neck Surg. 2002;126:683–689. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- Kusunoki T, Cureoglu S, Schachern P, Sampaio A, Fukushima H, Oktay M, Paparella M. Effects of aminoglycoside administration on cochlear elements in human temporal bones. Auris Nasus Larynx. 2004;31(4):383–8. doi: 10.1016/j.anl.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Lautermann J, Crann S, McLaren J, Schacht J. Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hearing Res. 1997;114:75–82. doi: 10.1016/s0378-5955(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Lewis R, Diekema D, Messer S, Pfaller M, Klepser M. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J Antimicrob Chemother. 2002;49(2):345–351. doi: 10.1093/jac/49.2.345. [DOI] [PubMed] [Google Scholar]
- Li H, Steyger PS. Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci Rep. 2011;1:159. doi: 10.1038/srep00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Tang MC. Post-antibiotic effect induced by antibiotic combination: influence of mode, sequence and interval of exposure. J Antimicrob Chemother. 2004;54(5):904–908. doi: 10.1093/jac/dkh435. [DOI] [PubMed] [Google Scholar]
- Lima da Costa D, Erre J, Pehourq F, Aran J. Aminoglycoside ototoxicity and the medial efferent system: II. Comparison of acute effects of different antibiotics. Audiology. 1998;37(3):162–73. doi: 10.3109/00206099809072970. [DOI] [PubMed] [Google Scholar]
- Lobry JR, Carret G, Flandrois JP. Maintenance requirements of Escherichia coli ATCC 25922 in the presence of sub-inhibitory concentrations of various antibiotics. J Antimicrob Chemother. 1992;29:121–127. doi: 10.1093/jac/29.2.121. [DOI] [PubMed] [Google Scholar]
- Lodhi S, Weiner N, Mechigian I, Schacht J. Ototoxicity of aminoglycosides correlated with their action on monomolecular films of polyphosphoinositides. Biochem Pharmacol. 1980;29(4):597–601. doi: 10.1016/0006-2952(80)90382-2. [DOI] [PubMed] [Google Scholar]
- Lu S. Regulation of glutathione synthesis. Semin in Liver Dis. 1998;18:331–334. doi: 10.1055/s-2007-1007168. [DOI] [PubMed] [Google Scholar]
- Martins L, Camargos P, Becker H, Becker C, Guimaraes R. Hearing loss in Cystic Fibrosis. Int J Pediatr Otorhi. 2010;74:469–473. doi: 10.1016/j.ijporl.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Massie J, Cranswick N. Pharmacokinetic profile of once daily intravenous tobramycin in children with cystic fibrosis. Journal of Paediatrics and Public Health. 2006;42:601–605. doi: 10.1111/j.1440-1754.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- Matt T, Ng C, Lang K, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka S, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Bottger E. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. PNAS. 2012;109:10984–10989. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz GJ. Aminoglycoside Ototoxicity. Am J Otolaryngol. 1986;7:117–119. doi: 10.1016/s0196-0709(86)80040-0. [DOI] [PubMed] [Google Scholar]
- McCracken GH. Aminoglycoside toxicity in infants and children. Am J Med. 1986;80(Suppl):172–178. doi: 10.1016/0002-9343(86)90497-3. [DOI] [PubMed] [Google Scholar]
- Medina A, Lambert R, Magan N. Rapid throughput analysis of filamentous fungal growth using turbidometric measurements with the Bioscreen C: a tool for screening antifungal compounds. Fungal Bio. 2012;116:161–169. doi: 10.1016/j.funbio.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Mulheran M, Degg C, Burr S, Morgan D, Stableforth D. Occurrence and risk of cochleotoxicity in cystic fibrosis patients receiving repeated high-dose aminoglycoside therapy. Antimicrob Agents. 2001;Ch. 45(9):2502–2509. doi: 10.1128/AAC.45.9.2502-2509.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulheran M, Hyman-Taylor P, Tan K, Lewis S, Stableforth D, Knox A, Smyth A. Absence of cochelotoxicity measured by standard and high-frequency pure tone audiometry in a trial of once- versus three-times-daily tobramycin in cystic fibrosis patients. Antimicrob Agents Ch. 2006;50(7):2293–2299. doi: 10.1128/AAC.00995-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Bodmer M, Brors D, Bodmer D. Early gene expression in the organ of Corti exposed to gentamicin. Hear Res. 2004;195(1–2):1–8. doi: 10.1016/j.heares.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- O’Donnell EP. Risk factors for aminoglycoside ototoxicity in adult cystic fibrosis patients. Int J Antimicrob Agents. 2010;36:94–95. doi: 10.1016/j.ijantimicag.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Oghan F, Apuhan T, Yilmaz F. Ototoxicity caused by topical administration of gentamicin versus tobramycin in rabbits. Int J Pediatr Otorhino. 2011;75:915–918. doi: 10.1016/j.ijporl.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Parry MF, Neu HC. Effect of N-acetycysteine on antibiotic activity and bacterial growth in vitro. J Clin Microbiol. 1977;5(1):58–61. doi: 10.1128/jcm.5.1.58-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliego-Castaneda QF, Yanez-Viguri JA, Lopez-Valle T. Multiresistant pseudomonas spp. In vitro susceptibility to a combination of two antibiotics. Cir. 2005;73:465–470. [PubMed] [Google Scholar]
- Piltcher O, Teixeria V, de Oliveira M, Scattolin I, Piltcher S. The prevalence of neurosensorial hearing loss among cystic fibrosis patients from Hospital de Clinicas de Porto Alegre. Int J Pediatr Otorhino. 2003;67:939–941. doi: 10.1016/s0165-5876(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Prayle A, Smyth AR. Aminoglycoside use in cystic fibrosis: therapeutic strategies and toxicity. Curr Opin Pulm Med. 2010;16:604–610. doi: 10.1097/MCP.0b013e32833eebfd. [DOI] [PubMed] [Google Scholar]
- Rand K, Houck H, Brown P, Bennett D. Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob Agents Chemother. 1993;37(3):613–615. doi: 10.1128/aac.37.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reser D, Rho M, Dewan D, Herbst L, Li G, Stupak H, Zur K, Romaine J, Frenz D, Goldbloom L, Kopke R, Arezzo J, Van De Water T. L- and D- methionine provide equivalent long term protection against CDDP-induced ototoxicity in vivo, with partial in vitro and in vivo retention of antineoplastic activity. Neurotoxicology. 1999;20 (5):731–748. [PubMed] [Google Scholar]
- Ryan G, Jahnke N, Remmington T. Inhaled antibiotics for pulmonary exacerbations in cystic fibrosis. Cochrane Database Syst Rev. 2012;12(12) doi: 10.1002/14651858.CD008319.pub2. [DOI] [PubMed] [Google Scholar]
- Sande MA, Overton JW. In vivo antagonism between gentamicin and chloramphenicol in neutropenic mice. J Infect Dis. 1973;128(2):247–50. doi: 10.1093/infdis/128.2.247. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken) 2012;295(11):1837–50. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheenstra R, Heijerman H, Zuur C, Touw D, Rijntjes E. No hearing loss after repeated courses of tobramycin in cystic fibrosis patients. Acta Oto-Laryngol. 2010;130:253–258. doi: 10.3109/00016480903015150. [DOI] [PubMed] [Google Scholar]
- Seligmann H, Podoshin L, Ben-David J, Fradis M, Goldsher M. Drug-induced tinnitus and other hearing disorders. Drug Saf. 1996;14(3):198–212. doi: 10.2165/00002018-199614030-00006. [DOI] [PubMed] [Google Scholar]
- Sha S, Schacht J. Stimulation of free radical formation by aminoglycoside antibiotics. Hear Res 1999. 1999;128:112–118. doi: 10.1016/s0378-5955(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free-radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear Res. 2000;142:34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Soothill JS, Morton DB, Ahmad A. The HID50 (hypothermia-inducing dose 50): an alternative to the LD50 for measurement of bacterial virulence. Int J Exp Pathol. 1992 Feb;73(1):95–8. [PMC free article] [PubMed] [Google Scholar]
- Takumida M, Anniko M. Nitric oxide in guinea pig vestibular sensory cells following gentamicin exposure in vitro. Acta Oto-Laryngol. 2001;121:346–350. doi: 10.1080/000164801300102734. [DOI] [PubMed] [Google Scholar]
- Takumida M, Anniko M. Simultaneous detection of both nitric oxide and reactive oxygen species in guinea pig vestibular sensory cells. J Othorhinolaryngol Relat Spec. 2002;64(2):143–147. doi: 10.1159/000057794. [DOI] [PubMed] [Google Scholar]
- Tange R, Conijn E, van Zeijl L, Huizing E. Pattern of gentamicin-induced cochlear degeneration in the guinea pig. A morphological and electrophysiological study. Arch Otorhinolaryngol. 1982;236(2):173–84. doi: 10.1007/BF00454037. [DOI] [PubMed] [Google Scholar]
- Traub WH. Interactions of antimicrobial drugs and combined phagocytic/serum bactericidal activity of defibrinated human blood against Serratia marcescens. Chemotherapy. 1982;28:434–443. doi: 10.1159/000238135. [DOI] [PubMed] [Google Scholar]
- Treggiari M, Rosenfeld M, Mayer-Hamblett M, et al. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study. Contemp Clin Trials. 2009;30(3):256–268. doi: 10.1016/j.cct.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt W. Oxidation of methionyl residues in protein: tools, targets and reversal. Free Radic Biol Med. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- Williams SE, Zenner HP, Schacht J. Three molecular steps of aminoglycoside ototoxicity demonstrated in outer hair cells. Hear Res. 1987;30:11–8. doi: 10.1016/0378-5955(87)90177-8. [DOI] [PubMed] [Google Scholar]
- Wimmer C, Mees K, Stumpf P, Welsch U, Reichel O, Suckfull M. Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otol Neurotol. 2004;25:33–40. doi: 10.1097/00129492-200401000-00007. [DOI] [PubMed] [Google Scholar]
- Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011;281(1–2):28–37. doi: 10.1016/j.heares.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanel GG, Hoban DJ, Harding GK. The postantibiotic effect: a review of in vitro and in vivo data. Ann Pharmacother. 1991;25:153–163. doi: 10.1177/106002809102500210. [DOI] [PubMed] [Google Scholar]