Abstract
Quorum sensing (QS) plays an important role in virulence of Pseudomonas aeruginosa, blocking of QS ability are viewed as viable antimicrobial chemotherapy and which may prove to be a safe anti-virulent drug. Bioactive components from Piper betle have been reported to possess antimicrobial ability. This study envisages on the anti-QS properties of ethanolic extract of P. betle leaf (PbLE) using P. aeruginosa PAO1 as a model organism. A marked reduction in swarming, swimming, and twitching ability of the bacteria is demonstrated in presence of PbLE. The biofilm and pyocyanin production also shows a marked reduction in presence of PbLE, though it does not affect the bacterial growth. Thus, the studies hint on the possible effect of the bioactive components of PbLE on reducing the virulent ability of the bacteria; identification of bioactive compounds should be investigated further.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-015-0348-8) contains supplementary material, which is available to authorized users.
Keywords: Anti Quorum Sensing properties, Piper betle leaf extract, Pseudomonas aeruginosa, Biofilm production, Pyocyanin assay, Mobility pattern
Introduction
Amongst proteobacteria, a widespread cell-to-cell communication (quorum sensing; QS) phenomenon is observed (Krishnan et al. 2012). QS is used to regulate the diverse bacterial function amongst which bioluminescence, biofilm formation, virulence, pigment production, motility and polysaccharide production have been widely studied (Fuqua and Greenberg 2002; Whitehead et al. 2001). Pseudomonas aeruginosa (belonging to Gamma Proteobacteria) is a prevalent opportunistic human pathogen and primarily infecting immune compromised patients (Govan and Deretic 1996). It causes serious eye (Zhu et al. 2004), ear (Tron et al. 2004), burn wounds (Friedstat et al. 2013), urinary tract (Packiavathy et al. 2014) and respiratory tract infection (cystic fibrosis) (Smith et al. 2013; Lyczak et al. 2002). Available antibiotic therapy does not respond to these infections; especially predisposed to infection with P. aeruginosa and hence the bacterium is developing new resistance, responsible for high rates of morbidity and mortality (Lanini et al. 2011). Alternative strategies to conventional antibiotic therapy are therefore required.
QS helps the bacteria to detect their population density by producing, releasing and perceiving the small autoinducer molecules and coordinate a common action such as releasing the virulence factors (Girard and Bloemberg 2008; Jimenez et al. 2012; Kumar et al. 2015). Thus, the virulence phenotypes of the bacteria can be quenched by blocking the QS. QS inhibitor (QSI) may inhibit the QS mechanism and be able to attenuate the virulence of the pathogen and are helpful to break the antibiotic resistance (Vattem et al. 2007; Adonizio et al. 2008). Recent studies have been demonstrated that QSI compound(s) can be found in higher plants such as vanilla (Choo et al. 2006), raspberry (Vattem et al. 2007), clove (Krishnann et al. 2012). In light of these findings, we look into Ayurveda, the oldest traditional medicine system of India, which reports large number of herbs possessing potential preventive and curative properties (Mukherjee and Wahile 2006). In Ayurveda, the use of betel leaves (Piper betle L.) in various ways, as carminative, stimulant, antiseptic, antifungal, antibacterial, anti-diabetic and anti-allergic agent have been mentioned (Guha 2006). The extract of betel leaves have been reported to possess many biological activities that are able to control the growth of many Gram positive and Gram negative microbes (Nair and Chanda 2008). No information however is available on betel leaf extracts to demonstrate its anti-QS activity.
In this study, we report of the anti-QS properties of ethanolic extract P. betle leaf (PbLE); effects of the mobility patterns (namely Swarming, Swimming and Twitching), reduction on biofilm and pyocyanin production in presence of different concentrations of PbLE. This in turn reflects on the virulence of model microorganism P. aeruginosa PAO1.
Materials and methods
Bacterial strains and subculture conditions
Pseudomonas aeruginosa PAO1 (MTCC-3541) was obtained from microbial type culture collection and Gene Bank, Chandigarh, India. The stock culture of the test organism was maintained in medium containing 30 % glycerol in cryogenic vials were kept at −70 °C. Working cultures were kept at 4 °C on nutrient agar slants and were periodically transferred to fresh slants. A loop full of culture from the slants were transferred to nutrient broth and grown overnight at 37 °C. The overnight grown culture was used for the subsequent study.
Preparation of ethanolic extract of betel leaves (PbLE)
Freshly cut betel leaves (Piper betle L. ver. Kali Bangla; landrace of Paschim Medinipur, West Bengal, India) were dried in hot air oven (40 ± 1 °C) for 48 h, crushed in mortar and pestle and the leaf powder were stored at −20 °C. Leaf powder was Soxhlet extracted using 80 % ethanol for 20 h. The crude extract was concentrated and dried in rotary vacuum evaporator below 50 °C, 100 mg dried extract was dissolved in 500 µl of dimethylsulfoxide (DMSO) and then diluted to 10 mg/ml working PbLE stock by adding triple distilled water (Maity et al. 2014).
Mobility patterns assays
Swarming assay
Tan et al. (2013) was followed with minor modifications for the preparation of the swarm plate assay of P. aeruginosa PAO1. Petri plates were prepared using 0.5 g bacto agar, 0.5 g peptone, 0.2 g yeast extract and 1.0 g glucose per 100 ml of distilled water. A set containing 25, 50, 75, 100 µg/ml of PbLE was seeded with 5 ml of media and poured immediately on a 10 ml of pre-warmed agar plate as an overlay. Overnight culture of the P. aeruginosa PAO1 culture was inoculated at the centre of the agar surface and the plate was incubated for 24 h at 37 °C.
With minor modifications, swimming and twitching assay was done following Inoue et al. (2008).
Swimming assay
Like the swarming plate, swimming plate assay media contained 1 % nutrient broth, glucose 0.5 %, agar 0.3 %. The agar media was air dried for 5–10 min and the bacterial cells were gently inoculated using a tooth pick at the centre of the agar surface. Then the plate was incubated at 37 °C for 24–48 h.
Twitching assay
The media for twitching assay contained 1 % tryptone, 0.5 % yeast extract, 0.5 % NaCl, 1 % agar. In twitching assay, bacterial cells were stabbed into the bottom of a petri dish containing the said agar medium using a toothpick and incubated at 37 °C for 20 h. The movement of the colony on the interface between the agar medium and the petri dish was observed.
Pyocyanin assay
Pyocyanin was extracted from overnight grown P. aeruginosa PAO1 culture supernatant. 25 µg/ml up to 200 µg/ml (25, 50, 75, 100, 125, 150, 175 and 200 µg/ml) of PbLE were mixed with freshly prepared P. aeruginosa culture (2 ml, OD600 = 0.1) and incubated overnight at 37 °C. After 24 h, 2 ml of chloroform was added to the culture supernatant and mixed vigorously. The chloroform layer was mixed with 1 ml of HCl (0.2 M). After centrifugation (8000 rpm for 10 min at 28 °C) the relative concentration of Pyocyanin was measured as OD of the HCl layer at 520 nm against 0.2 M HCl as blank (Chong et al. 2011).
Biofilm formation and quantification
An overnight culture of P. aeruginosa PAO1 was diluted to 1:100 into fresh medium and 100 µl was added to each well of microtiter plate and incubated for 28–30 h at 37 °C. After incubation, cells were dumped out by turning the plate over and shaking out the liquid. The plates were submerged in small tub of water, and shake the water out. The process was repeated. 125 µl of a 0.1 % aqueous solution of a crystal violet was added and the plates are incubated at room temperature for 10–15 min. Plates were rinsed 3–4 times in water and blotted on a stack of paper towels by turning the plates upside down and dried for a few hours or overnight. To quantitate, 200 µl of 30 % acetic acid was added to each well. Incubated for 10–15 min and 125 µl was transferred to a new, flat-bottomed microtiter dish. OD at A540–A600 was read on a plate reader (Siddiqui et al. 2012).
Assessment of bacterial growth
Actively growing culture of P. aeruginosa PAO1 (1 ml, OD600 = 0.1) was incubated in Luria Broth with different concentrations of PbLE (50, 100, 150 µg/ml). OD600 nm was observed at every 2 h interval for consecutively 40 h to evaluate the relative growth in different experimental conditions.
Statistical analysis
All the above experiments were carried out in triplicates. The data were statistically analysed by conducting student’s t test and ANOVA. Statistical analysis and the graphs were constructed using MS excel.
Results and discussion
Mobility patterns: swarming, swimming and twitching
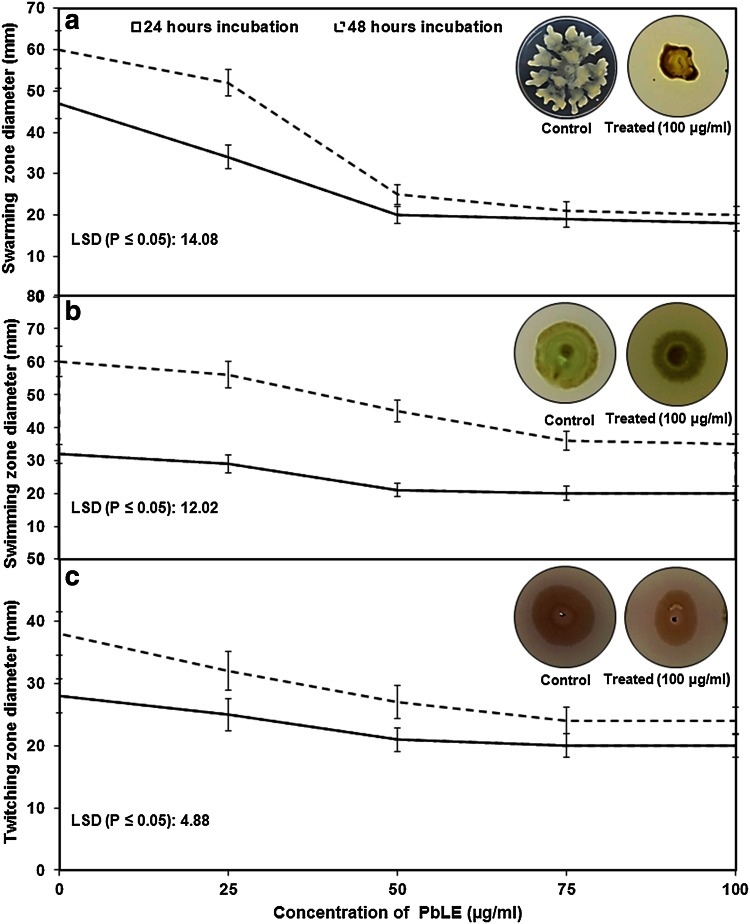
The graph (Fig. 1a) shows that a concentration beyond 25 μg/ml PbLE inhibits the swarming activity drastically. A PbLE concentration of 50 μg/ml was seen to be inhibitory for swarming activity of the bacteria wherein more than 50 % inhibition was observed irrespective of the hours of incubation. The slope between 25 and 50 μg/ml (m = 0.56) after 24 h while the slope at the same concentration after 48 h (m = −1.08) indicates significantly that the rate of inhibition drastically increases after 24 h.
Fig. 1.
Effect of piper betle ethanolic leaf extract (PbLE) on the mobility pattern of Pseudomonas aeruginosa PAO1. a Effect of swarming activity, b swimming activity and c twitching activity of Pseudomonas aeruginosa PAO1 with increasing concentrations (25, 50, 75 and 100 μg/ml) of PbLE (inset showing the colony morphology of the bacteria)
After 24 h of incubation at 25 μg/ml with respect to control a difference of 13 mm in the zone of inhibition are observed. At the same period of incubation at 50 μg/ml swimming activity of P. aeruginosa PAO1 in presence of PbLE (Fig. 1b) diminished with increase in concentration (at 24 h) but after 48 h of exposure, the rate of inhibition is almost 7.40 %. Though, beyond 50 μg/ml the inhibition rate comes to a constant irrespective of the hours of incubation.
Twitching activity in presence of PbLE (Fig. 1c) was seen to be diminishing but is not much of significance when compared to the control; a mere 10.71 % decrease is seen after 24 h of incubation at 25 μg/ml. Thereafter the rate of decrease remains constant.
Form the above observations, it could be said that PbLE has a destructive effect on the QS mechanisms of P. aeruginosa PAO1. The above inhibition activities were compared simultaneously with certain antibiotics like Ciprofloxacin, Gentamicin. The antibiotic Ciprofloxacin inhibited the growth of P. aeruginosa PAO1 but was found to be resistant to the antibiotic Gentamicin (Data not shown). Of all three QS motility mechanisms observed it has the most devastating effect on swarming activity which correlates with the findings of Tan et al. (2013).
Biofilm and pyocyanin assay; inhibition biology of biofilm formation and resistance
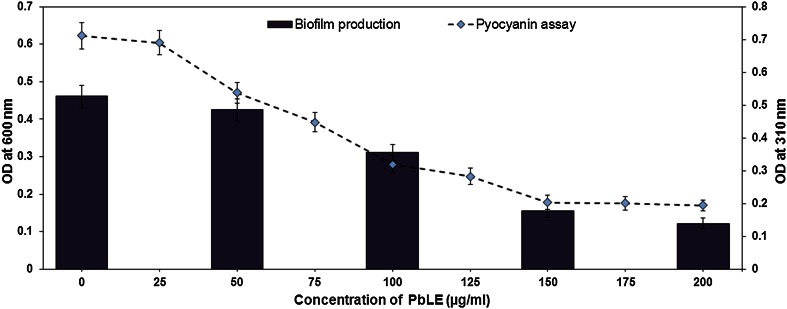
In our studies, a reduction is observed in biofilm production (Fig. 2) at concentrations beyond 50 μg/ml. When compared to control sets the inhibition percentage could be said as 50 μg/ml (7.81 %), 100 μg/ml (32.54 %), 150 μg/ml (66.16 %), 200 μg/ml (75.35 %) i.e. almost approximately fourfolds of decrease per 50 μg/ml of increase in PbLE concentration. Because biofilm resistance depends on aggregation of bacteria in multicellular communities, one strategy might be to develop therapies that disrupt the multicellular structure of the biofilm. If the multi-cellularity of the biofilm is defeated, the host defences might be able to resolve the infection, and the efficacy of antibiotics might be restored. Potential therapies include enzymes that dissolve the matrix polymers of the biofilm, chemical reactions that block biofilm matrix synthesis, and analogues of microbial signalling molecules that interfere with cell-to-cell communication, required for normal biofilm formation (Stewart and Costerton 2001).
Fig. 2.
Inhibition of Pseudomonas aeruginosa PAO1 biofilm and pyocyanin production in presence of different concentrations of PbLE. Bar chart represents the mean results from triplet cultures of three independent experiments, with error bar representing standard deviation
This study lays a foundation that a simultaneous administration of PbLE along with antibiotics (reported to be resistant to P. aeruginosa) may help in bringing back the sensitivity to effect in antibiotic therapy.
Pyocyanin contributes to the persistence of P. aeruginosa infection by causing detrimental effects toward lung (Lyczak et al. 2002) epithelial cells and by deregulating inflammatory response initiated by the host. Pyocyanin synthesis is regulated by a complex synchrony of lasR-lasI, rhlR-rhlI and mvfR-haq QS-system whereby mutations in these systems lead to the deficiency of pyocyanin synthesis (Priya et al. 2013).
Our studies depict Pyocyanin production to be having a steady decrease with increasing concentrations of PbLE i.e. beyond 150 μg/ml the rate of decrease comes to a constant (Fig. 2).
From our studies and available literature, it could be postulated that the PbLE may have inhibited the QS-systems at its gene expression level or acts as an antagonist to decrease pyocyanin production.
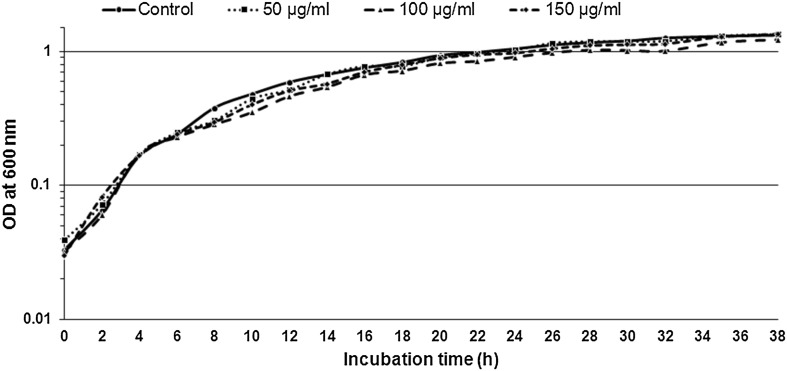
Comparing the above observations (Fig. 3) with the growth curve of P. aeruginosa PAO1 in presence different concentrations of PbLE; it does not show any variation or decrease in the growth pattern as compared to PbLE free medium. Thus, it makes it evident that PbLE irrespective of its concentration has no effect on the cell division of P. aeruginosa PAO1 but the biofilm assay and pyocyanin production does indicate that PbLE has a marked effect on the anabolism of biofilm and pyocyanin production. Hence, this effect also has an effect on the QS abilities of the bacteria in presence of PbLE. This indicates on the possible reducing virulence of P. aeruginosa.
Fig. 3.
Growth curve patterns of Pseudomonas aeruginosa PAO1 in presence of different concentrations of PbLE (0, 50, 100 and 150 μg/ml)
Conclusion
Based on the results obtained from this study, it is proven that the ethanolic extract of P. betle demonstrates anti-QS capabilities. It may have also been able to attenuate QS-regulated virulence determinants of P. aeruginosa PAO1. Newer insights may be provided by the bioactive compounds in the extract leading towards discovering potential anti-pathogenic drugs to combat emerging multidrug resistant pathogens. Future work should direct to the isolation and characterization of active molecule that is responsible for the anti-QS properties of the ethanolic extract of P. betle leaf.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work is supported in part by Science and Engineering Research Board (Grant No. SR/FT/LS-102/2010), Government of India.
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong YM, Yin WF, Ho CY, Mustafa MR, Hadi AHA, Awang K, Narrima P, Koh CL, Appleton DR, Chan KG. Malabaricone C from Myristica cinnamomea exhibits anti-quorum sensing activity. J Nat Prod. 2011;74:2261–2264. doi: 10.1021/np100872k. [DOI] [PubMed] [Google Scholar]
- Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42:637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- Friedstat JS, Moore ME, Weber JM, Fagan SP, Goverman J. Selection of appropriate empiric gram-negative coverage in a multinational pediatric burn hospital. J Burn Care Res. 2013;34:203–210. doi: 10.1097/BCR.0b013e3182781829. [DOI] [PubMed] [Google Scholar]
- Fuqua C, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- Girard G, Bloemberg GV. Central role of quorum sensing in regulating the production of pathogenicity factors in Pseudomonas aeruginosa. Future Microbiol. 2008;3:97–106. doi: 10.2217/17460913.3.1.97. [DOI] [PubMed] [Google Scholar]
- Govan JR, Deretic V. Microbiol pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P. Betel leaf: the neglected green gold of India. J Hum Ecol. 2006;19:87–93. [Google Scholar]
- Inoue T, Shingaki R, Fukui K. Inhibition of swarming motility of Pseudomonas aeruginosa by branched-chain fatty acids. FEMS Microbiol Lett. 2008;281:81–86. doi: 10.1111/j.1574-6968.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnann T, Yin WF, Chan KG. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors. 2012;12:4016–4030. doi: 10.3390/s120404016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L, Chhibber S, Kumar R, Kumar M, Harjai K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia. 2015;102:84–95. doi: 10.1016/j.fitote.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Lanini S, D’Arezzo S, Puro V, Martini L, Imperi F, Piselli P, Montanaro M, Paoletti S, Visca P, Ippolito G. Molecular epidemiology of a Pseudomonas aeruginosa hospital outbreak driven by a contaminated disinfectant-soap dispenser. PLoS ONE. 2011 doi: 10.1371/journal.pone.0017064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity TR, Samanta A, Jana D, Saha B, Datta S. Effect of Piper betle leaf extract on post-harvest physiology and vascular blockage in relation to vase life and keeping quality of cut spike of tuberose (Polianthes tuberosa L. cv. single) Ind J Plant Physiol. 2014;19:250–256. doi: 10.1007/s40502-014-0110-y. [DOI] [Google Scholar]
- Mukherjee PK, Wahile A. Integrated approaches towards drug development from ayurveda and other Indian system of medicines. J Ethnopharmacol. 2006;103:25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Nair R, Chanda S. Antimicrobial activity of Terminalia catappa, Manilkara zapota, and Piper betle leaf. Indian J Pharm Sci. 2008;70:390–393. doi: 10.4103/0250-474X.43012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packiavathy IASV, Priya S, Pandian SK, Ravi AV. Inhibition of biofilm development of uropathogens by curcumin—an anti-quorum sensing agent from Curcuma longa. Food Chem. 2014;148:453–460. doi: 10.1016/j.foodchem.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Priya K, Yin WF, Chan KG. Anti-quorum sensing activity of the traditional Chinese herb, Phyllanthus amarus. Sensors. 2013;13:14558–14569. doi: 10.3390/s131114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MF, Sakinah M, Ismail AF, Matsuura T, Zularisam AW. The anti-biofouling effect of Piper betle extract against Pseudomonas aeruginosa and bacterial consortium. Desalination. 2012;288:24–30. doi: 10.1016/j.desal.2011.11.060. [DOI] [Google Scholar]
- Smith DJ, Lamont IL, Anderson GJ, Reid DW. Targeting iron uptake to control Pseudomonas aeruginosa infections in cystic fibrosis. Eur Respir J. 2013;42:1723–1736. doi: 10.1183/09031936.00124012. [DOI] [PubMed] [Google Scholar]
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Tan LY, Yin WF, Chan KG. Piper nigrum, Piper betle and Gnetum gnemon—natural food sources with anti-quorum sensing properties. Sensors. 2013;13:3975–3985. doi: 10.3390/s130303975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron EAM, Wilke HL, Petermann SR, Rust L. Pseudomonas aeruginosa from canine otitis externa exhibit a quorum sensing deficiency. Vet Microbiol. 2004;99:121–129. doi: 10.1016/j.vetmic.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Vattem DA, Mihalik K, Crixell SH, McLean RJC. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007;78:302–310. doi: 10.1016/j.fitote.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum sensing in gram-negative bacteria. FEMS Microbiol Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Bandara R, Conibear TCR, Thuruthyil SJ, Rice SA, Kjelleberg S. Pseudomonas aeruginosa with LasI quorum-sensing deficiency during corneal infection. Invest Ophthalmol Vis Sci. 2004;45:1897–1903. doi: 10.1167/iovs.03-0980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.