Abstract
Brucella abortus 2308 derivatives with mini-Tn5 insertions in purE, purL, and purD display significant attenuation in the BALB/c mouse model, while isogenic mutants with mini-Tn5 insertions in pheA, trpB, and dagA display little or no attenuation in cultured murine macrophages or mice. These experimental findings confirm the importance of the purine biosynthesis pathways for the survival and replication of the brucellae in host macrophages. In contrast to previous reports, however, these results indicate that exogenous tryptophan and phenylalanine are available for use by the brucellae in the phagosomal compartment.
Brucella abortus is a facultative, gram-negative pathogen of humans and animals that inhabits macrophages (2). The capacity to withstand nutritional deprivation would be expected to be particularly important for the survival of B. abortus, since this organism does not escape from the phagosome into the nutrient-rich environment of the host cell cytoplasm (2). Indeed, previous studies suggest that the brucellae encounter a considerable degree of nutritional deprivation during their long-term residence in host macrophages. Brucella melitensis purE (5) and Brucella suis aroC (12) mutants, for example, are unable to maintain chronic infection in experimentally infected mice, and the attenuation of the B. melitensis purE mutant purE201 extends to the natural ruminant host (4) and nonhuman primates (M. Nikolich, personal communication). These observations are also consistent with those seen with auxotrophic mutants of Salmonella and Mycobacterium and indicate that the availability of certain nutrients is severely restricted in the phagosomal compartment of host macrophages (3, 11, 22).
In an effort to gain a better understanding of the metabolic versatility required for sustained intracellular residence by the brucellae in host macrophages, transposon mutagenesis and an in vitro screen were employed to identify B. abortus 2308 derivatives with mini-Tn5 insertions in genes required for resistance to nutrient deprivation. Transposon mutagenesis of this strain was performed by conjugative transfer of the mini-Tn5 derivative Km1 by employing pUT as the delivery vector and Escherichia coli S17-1λpir as the conjugal donor strain (6, 14, 28). Approximately 1,000 B. abortus mini-Tn5 mutants were patched with sterile toothpicks onto Schaedler agar supplemented with 5% defibrinated bovine blood (SBA), SBA supplemented with 45 μg of kanamycin/ml (SBAk), and Gerhardt's minimal medium (13) supplemented with 1.5% agar (GMMA). Plates were incubated at 37°C with 5% CO2 and examined for growth after 4, 7, and 10 days of incubation. Schaedler agar, the basal medium for SBA, is a complex culture medium containing enzymatic digests of casein, animal and plant tissues, yeast extract, glucose, cystine, and hemin (Difco manual, 10th ed., Difco Laboratories, Detroit, Mich.). In contrast, GMM, the base for GMMA, is a defined medium formulated during a study aimed at defining the minimal in vitro growth requirements of Brucella strains (13). GMM contains glycerol and lactate as carbon and energy sources. SBA and SBAk support luxuriant growth of B. abortus 2308 and 2308 carrying the plasmid pBBR1MCS2 (which confers kanamycin resistance) (19), respectively, with individual colonies being clearly visible after 48 h of incubation. The formation of visible colonies by 2308 on GMMA, on the other hand, requires 96 h or more of incubation. Thus, for the purposes of the study described in this report, SBA was considered to be a nutritionally complete growth medium and GMMA was considered a nutritionally restricted growth medium for the brucellae. Following incubation on GMMA and SBAk, 44 of the mini-Tn5 mutants displayed defective growth or no growth on GMMA but grew on SBAk as well as 2308(pBBR1MCS2). To eliminate the lack of standardization in inoculum size associated with patching the brucellae onto the growth medium with sterile toothpicks, the 44 B. abortus mini-Tn5 mutants that displayed defective growth on GMMA were inoculated into 1 ml of brucella broth supplemented with 45 μg of kanamycin/ml and incubated with shaking in a 37°C water bath. After 48 h of incubation, the bacterial cells were harvested by centrifugation, washed once with 1 ml of sterile phosphate-buffered saline, and resuspended to an optical density at 600 nm of 0.15 (approximately 109 CFU/ml). The resulting bacterial suspensions were diluted to a final cell concentration of 105 CFU/ml in sterile phosphate-buffered saline, 100 μl of each cell suspension was plated onto SBAk and GMMA supplemented with 45 μg of kanamycin/ml, and the plates were incubated at 37°C with 5% CO2. Following 4, 7, and 10 days of incubation, growth on these plates was examined and compared with the growth displayed by B. abortus 2308(pBBR1MCS2) on these two growth media. Following this second screen for defects in growth on the nutritionally deficient medium, 12 of the 44 mini-Tn5 mutants displayed defective or no growth on GMMA but growth comparable to that of the parental 2308 strain on SBA.
Southern blot analysis (27) with probes specific for the kanamycin resistance gene in the mini-Tn5 derivative Km1 and the ampicillin resistance gene in pUT was used to confirm that the transposon had inserted into a single genetic locus in each of these mutants. To clone the mini-Tn5-disrupted genes, genomic DNA from the mutants was digested with either EcoRI or NcoI, yielding fragments containing the mini-Tn5 and flanking B. abortus genomic sequences and cloned into either the EcoRI site of pBluescript II KS(+) or the NcoI site of pGEM5Zf+. The resulting recombinant plasmids were used to transform E. coli DH5α cells, and transformants containing plasmids carrying the mini-Tn5-disrupted genes were selected by plating on Luria-Bertani medium supplemented with kanamycin and ampicillin. The nucleotide sequences of the mini-Tn5-disrupted genes cloned in these plasmids were determined using the dideoxy-based methods described by Sanger et al. (30). Brucella-specific DNA sequences identified in this fashion were compared against those in the B. melitensis 16M genome sequence (7) by using the tblastx algorithm. The strain designations for these mutants along with the identity of the mini-Tn5-disrupted loci in these strains are presented in Table 1.
TABLE 1.
Identity of the mini-Tn5-disrupted loci in B. abortus mutants displaying nutritional defects in vitro
| Mutant | mini-Tn5- disrupted gene | Proposed function of disrupted gene product | Accession no.a |
|---|---|---|---|
| AR54 | purL | Purine biosynthesis | BMEI1124 |
| AR86 | rplS | 50S ribosomal subunit L19 | BMEI0156 |
| AR93 | trpB | Tryptophan biosynthesis | BMEI2018 |
| AR408 | pheA | Phenylalanine biosynthesis | BMEI1905 |
| AR416 | fatD | Ferric siderophore transport | BMEII0606 |
| AR423 | bvrS | Two-component response regulator | BMEI2034 |
| AR425 | eryC | Erythritol catabolism | BMEII0428 |
| AR536 | dagA | Glycine, alanine, and serine transport | BMEII0783 |
| AR609 | purSL | Purine biosynthesis | BMEI1123 |
| AR875 | purH | Purine biosynthesis | BMEI0233 |
| AR943 | ilvD | Branched-chain amino acid biosynthesis | BMEI1848 |
| AR975 | purE | Purine biosynthesis | BMEI0296 |
Open reading frame designation in the B. melitensis genome.
Four of the B. abortus mutants identified in this genetic screen had mini-Tn5 insertions in genetic loci involved in purine metabolism (36) and, as expected, B. abortus AR54 (purL::miniTn5) and AR975 (purE::miniTn5) both displayed purine auxotrophy in vitro. Neither strain would grow in GMM or on GMMA unless the medium was supplemented with 5 mM adenine and 0.3 mM guanine. All three of the B. abortus derivatives with mini-Tn5 insertions in purine biosynthesis genes also displayed significant attenuation in cultured murine macrophages (Fig. 1) and in experimentally infected mice (Fig. 2). A previously described directed reversion strategy (21) was used to replace the mini-Tn5-disrupted purL and purE loci in AR54 and AR975, respectively, with the corresponding wild-type purL and purE genes. The resulting revertants, AR54R and AR975R, grew as well as 2308 on GMMA without adenine or guanine supplementation and also displayed wild-type virulence in cultured murine macrophages (Fig. 1 and data not shown) and in experimentally infected BALB/c mice (Fig. 2). Thus, the results of the study presented here support and extend those reported previously for B. melitensis (5, 8) and B. suis (17) and indicate that the brucellae require intact purine biosynthesis pathways in order to maintain long-term survival in the phagosome. These experimental findings also agree with those observed with other intracellular bacteria that remain confined to the phagosomal compartment, such as Salmonella enterica serovar Typhimurium (20, 32) and the Mycobacterium spp. (16), and suggest that exogenous purine pools are limiting in this intracellular environment.
FIG. 1.
Intracellular survival and replication of B. abortus strains 2308 (•), AR54 (2308 purL::miniTn5) (▪), AR875 (2308 purH::miniTn5) (▴), and AR975 (2308 purE::miniTn5) (×) (A) and of B. abortus 2308, AR975 (2308 purE::miniTn5), and AR975R (AR975 purE::miniTn5→purE+) (B) in cultured resident peritoneal macrophages from BALB/c mice. Macrophages were isolated and plated at a density of 5 × 105 cells per well in 96-well microtiter plates and infected with brucellae opsonized with hyperimmune murine serum at a multiplicity of infection of 100:1, using the methods described by Elzer et al. (10). Results are expressed as percent survival, which was calculated by dividing the number of intracellular brucellae present at a particular sampling time by the number of intracellular brucellae present immediately after phagocytosis (t = 0). *, P < 0.05; **, P < 0.01 for comparisons of 2308 versus AR54, AR875, or AR975. †, P < 0.05 for comparisons of AR975R versus AR975 by using the Student t test (26).
FIG. 2.
Spleen colonization profiles of B. abortus strains 2308 (•), AR54 (2308 purL::miniTn5) (□), AR875 (2308 purH::miniTn5) (×), and AR975 (2308 purE::miniTn5) (▴) (A) and B. abortus 2308 (•), AR54 (2308 purL::miniTn5) (▴), AR54R (AR54 purL::miniTn5→purL) (⧫), AR975 (2308 purE::miniTn5) (▪), and AR975R (AR975 purE::miniTn5→purE) (▪) (B) in experimentally infected BALB/c mice. Brucella strains were grown on SBA or SBAk, and infection doses were prepared as described elsewhere (9). Seven- to 9-week-old female BALB/c mice were infected with 5 × 104 (A) or 2 × 104 (B) CFU of brucellae via the intraperitoneal route. At 1, 4, 8, and 12 weeks postinfection, three to five mice from each experimental challenge group were sacrificed by isoflurane overdose, their spleens were removed and homogenized, and the total number of brucellae per spleen was determined by serial dilution and plating onto SBA and/or SBAk. Results are expressed as total CFU per spleen ± the standard deviation. *, P < 0.05; **, P < 0.01 for comparisons of 2308 versus AR54, AR875, or AR975. †, P < 0.05 for comparisons of AR54R versus AR54; ‡‡, P < 0.01 for comparisons of AR975R versus AR975 (Student's t test [26]).
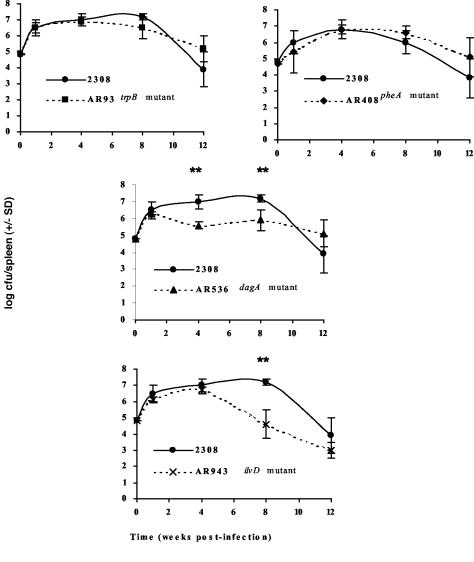
Of those B. abortus mutants with mini-Tn5 insertions in genes predicted to be involved in amino acid biosynthesis and transport, only the ilvD mutant, AR943, displayed attenuation in both macrophages (Fig. 3) and mice (Fig. 4). The remaining amino acid biosynthesis mutants [AR93 (trpB::miniTn5) and AR408 (pheA::miniTn5)] displayed wild-type virulence in mice. The ilvD gene product, dihydroxy acid dehydratase, participates in the biosynthesis of all three of the branched chain amino acids, leucine, isoleucine, and valine (34) and, as expected, the B. abortus ilvD mutant AR943 displays auxotrophy for all three of these amino acids when cultured on GMMA. In contrast, the products of the trpB and pheA genes are specifically dedicated to the biosynthesis of tryptophan and phenylalanine, respectively (24), and the B. abortus trpB and pheA mutants display auxotrophy only for the corresponding single amino acid when cultivated on GMMA. Consequently, our experimental findings with the B. abortus ilvD, trpB, and pheA mutants suggest that tryptophan and phenylalanine are available to the brucellae in their intracellular niche but that other amino acids (e.g., leucine, isoleucine, or valine) are not. The E. coli dagA gene (also known as cycA) encodes a glycine, alanine, and serine transporter (25). The slight attenuation displayed by the B. abortus dagA mutant AR536 in experimentally infected mice suggests that the transport of these particular amino acids by the brucellae may be important at some stage during the infectious process. The modest and transient nature of the attenuation displayed by AR536 in mice, however, coupled with the failure of this mutant to display a phenotype in cultured murine macrophages, makes it impossible to make definitive conclusions regarding the availability of glycine, alanine, or serine in the phagosomal compartment. Indeed, an analysis of the virulence profiles of isogenic B. abortus mutants with genetic lesions specifically affecting the biosynthesis of each of these individual amino acids would be more informative in this regard.
FIG. 3.
Intracellular survival and replication of B. abortus strains 2308 (•), AR93 (2308 trpB::miniTn5) (▪), AR408 (2308 pheA::miniTn5) (⧫), AR536 (2308 dagA::miniTn5) (▴), and AR943 (2308 ilvD::miniTn5) (×) in cultured murine resident peritoneal macrophages. Macrophages were isolated and plated at a density of 5 × 105 cells per well in 96-well microtiter plates and infected with brucellae opsonized with hyperimmune murine serum at a multiplicity of infection of 100:1 using the methods described by Elzer et al. (10). Results are expressed as percent survival, which was calculated by dividing the number of intracellular brucellae present at a particular sampling time by the number of intracellular brucellae present immediately after phagocytosis (t = 0). **, P < 0.01 for comparisons of 2308 versus AR943 by using the Student t test (26).
FIG. 4.
Spleen colonization profiles of B. abortus strains 2308 (•), AR93 (2308 trpB::miniTn5) (▪), AR408 (2308 pheA::miniTn5) (⧫), AR536 (2308 dagA::miniTn5) (▴), and AR943 (2308 ilvD::miniTn5) (×) in experimentally infected BALB/c mice. Brucella strains were grown on SBA or SBAk, and infection doses were prepared as described elsewhere (11). Seven- to 9-week-old female BALB/c mice were infected with 5 × 104 CFU brucellae via the intraperitoneal route. At 1, 4, 8, and 12 weeks postinfection, three to five mice from each experimental challenge group were sacrificed by isoflurane overdose, their spleens were removed and homogenized, and the total number of brucellae per spleen was determined by serial dilution and plating onto SBA and/or SBAk. Results are expressed as total CFU per spleen ± the standard deviation. **, P < 0.01 for comparisons of 2308 versus AR943 or AR536 by using the Student t test (26).
The results of earlier studies with a B. suis aroC mutant, which cannot produce a wide variety of aromatic compounds, including the amino acids tryptophan and phenylalanine (12), and B. suis mutants with mini-Tn5 insertions in a variety of amino acid biosynthesis loci (17) suggested that a variety of amino acids, including leucine, glycine, and serine, are not available to B. suis 1330 in its intracellular niche. It is important to note in this regard, however, that the basis for the attenuation of the B. suis aroC mutant in the mouse model in these earlier studies could well have been its inability to produce other aromatic compounds rather than its auxotrophy for tryptophan and phenylalanine. In this same vein, the B. suis mutants with mini-Tn5 insertions in amino acid biosynthesis genes were identified in these earlier studies based on their attenuation in the human monocytic cell line THP-1, but these strains were not evaluated in the mouse model. Whether the differential behavior of the B. abortus and B. suis amino acid biosynthesis mutants in cultured phagocytes reflects differences between the bacterial host strains being examined or differences in nutrient availability between the phagosomal compartments of murine and human phagocytes is presently unknown. Nevertheless, the disparate nature of these findings indicates that a more thorough investigation of the in vivo phenotypes of Brucella amino acid auxotrophs will be necessary before we have an accurate picture of the availability of these compounds to the brucellae during their long-term residence in the phagosomal compartment of host macrophages.
The remaining B. abortus mutants isolated in our genetic screen had mini-Tn5 insertions in genes predicted to be involved in translation (rplS [23]), erythritol catabolism (eryC [29]), iron acquisition (fatD [18]), and the maintenance of cell envelope integrity (bvrS [33]). Since the phenotypes of B. abortus ery and bvrS mutants have been described previously and an evaluation of the contribution of the fat locus to iron acquisition in B. abortus is the subject of a separate study in our laboratory, the B. abortus mini-Tn5 mutants AR425 (eryC), AR423 (bvrS), and AR416 (fatD) were not further characterized during the course of this study. Similarly, the B. abortus mini-Tn5 mutant AR86 was excluded from this study because the rplS mutation would be expected to have a detrimental effect on ribosome assembly (23) and the resulting defect in translational efficiency would make the results of in vivo studies with this mutant difficult, if not impossible, to interpret.
The experimental findings presented in this report provide us with a better understanding of the nutritional environment within which the brucellae reside in the phagosomal compartment of host macrophages and the ways in which they adapt to this niche. They may also have important implications with regard to vaccine development and chemotherapy. Although no safe and effective vaccine for human brucellosis currently exists, an attenuated B. melitensis purE mutant has been extensively studied in a variety of experimental hosts (4, 5, 15), including nonhuman primates (M. Nikolich, personal communication) and appears to be an excellent candidate in this regard. If B. melitensis purH and purL mutants show similar differences in their attenuation in nonhuman primates as their B. abortus counterparts do in the mouse model, it may be possible to exploit this relationship to improve upon the B. melitensis purE-based vaccine candidate. This will be particularly important if the B. melitensis purE mutant fails to display the balance of attenuation and immunogenicity required of human vaccines. Purine analogs have also been evaluated as potential chemotherapeutic agents against Mycobacterium tuberculosis (1, 31). Based on the critical nature of de novo purine biosynthesis for the successful survival of the brucellae in their intracellular niche, these purine analogs may be effective as brucellacidal agents in vivo. Such a finding would be particularly important with regard to the treatment of human brucellosis, since this disease is notoriously difficult to cure with the currently used chemotherapeutic regimens (35).
Acknowledgments
We thank John Baumgartner for his technical assistance.
This work was supported by a contract (DAMD17-98-C-8045) from the U.S. Army Medical Research and Materiel Command and a grant (AI-48499) from the National Institute of Allergy and Infectious Diseases.
Editor: V. J. DiRita
REFERENCES
- 1.Bakkestuen, A. K., L.-L. Gundersen, G. Langli, F. Liu, and J. M. J. Nolsøe. 2000. 9-Benzylpurines with inhibitory activity against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 10:1207-1210. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, C. L., and R. M. Roop II. 1999. Brucella infections and immunity, p. 255-279. In L. J. Paradise, H. Friedman, and M. Bendinelli (ed.), Opportunistic intracellular bacteria and immunity. Plenum Press, New York, N.Y.
- 3.Bange, F.-C., A. M. Brown, and W. R. Jacobs, Jr. 1996. Leucine auxotrophy restricts growth of Mycobacterium bovis BCG in macrophages. Infect. Immun. 64:1794-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheville, N. F., S. C. Olsen, A. E. Jensen, M. G. Stevens, A. M. Florance, H. H. Houng, E. S. Drazek, R. L. Warren, T. L. Hadfield, and D. L. Hoover. 1996. Bacterial persistence and immunity in goats vaccinated with a purE deletion mutant or the parental 16M strain of Brucella melitensis. Infect. Immun. 64:2431-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford, R. M., L. Van deVerg, L. Yuan, T. L. Hadfield, R. L. Warren, E. S. Drazek, H. H. Houng, C. Hammock, K. Sasala, T. Polsinelli, J. Thompson, and D. L. Hoover. 1996. Deletion of purE attenuates Brucella melitensis infection in mice. Infect. Immun. 64:2188-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLorenzo, V., M. Herrero, U. Jakubzik, and K. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DelVecchio, V. G., V. Kapatral, R. R. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J.-J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drazek, E. S., H. H. Houng, R. M. Crawford, T. L. Hadfield, D. L. Hoover, and R. L. Warren. 1995. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect. Immun. 63:3297-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elzer, P. H., R. W. Phillips, M. E. Kovach, K. M. Peterson, and R. M. Roop II. 1994. Characterization and genetic complementation of a Brucella abortus high-temperature-requirement A (htrA) deletion mutant. Infect. Immun. 62:4135-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elzer, P. H., R. W. Phillips, G. T. Robertson, and R. M. Roop II. 1996. The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect. Immun. 64:4838-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foulongne, V., K. Walravens, G. Bourg, M. L. Boschiroli, J. Godfroid, M. Ramuz, and D. O'Callaghan. 2001. Aromatic compound-dependent Brucella suis is attenuated in both cultured cells and mouse models. Infect. Immun. 69:547-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhardt, P., and J. B. Wilson. 1948. The nutrition of brucellae: growth in simple chemically defined media. J. Bacteriol. 56:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrero, M., V. DeLorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoover, D. L., R. M. Crawford, L. L. Van de Verg, M. J. Izadjoo, A. K. Bhattacharjee, C. M. Paranavitana, R. L. Warren, M. P. Nikolich, and T. L. Hadfield. 1999. Protection of mice against brucellosis by vaccination with Brucella melitensis WR201 (16MΔpurEK). Infect. Immun. 67:5877-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köhler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J. P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köster, W. L., L. A. Actis, L. S. Waldbeser, M. E. Tomalsky, and J. H. Crosa. 1991. Molecular characterization of the iron transport system mediated by the pJM1 plasmid in Vibrio anguillarum 775. J. Biol. Chem. 266:23829-23833. [PubMed] [Google Scholar]
- 19.Kovach, M. E., R. W. Phillips, P. H. Elzer, and R. M. Roop II. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800. [PubMed] [Google Scholar]
- 20.Leung, K. Y., and B. B. Finlay. 1991. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 88:11470-11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeVier, K., R. W. Phillips, V. K. Grippe, R. M. Roop II, and G. C. Walker. 2000. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 287:2492-2493. [DOI] [PubMed] [Google Scholar]
- 22.O'Callaghan, D., D. Maskell, F. Y. Liew, C. S. F. Easmon, and G. Dougan. 1988. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attenuation, persistence, and ability to induce protective immunity in BALB/c mice. Infect. Immun. 56:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson, B. C., G. O. Bylund, D. E. Berg, and P. M. Wikstrom. 1995. Functional analysis of the ffh-trmD region of the Escherichia coli chromosome by using reverse genetics. J. Bacteriol. 177:5554-5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittard, A. J. 1996. Biosynthesis of the aromatic amino acids, p. 458-484. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Maganasik W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 25.Robbins, J. C., and D. L. Oxender. 1973. Transport systems for alanine, serine, and glycine in Escherichia coli K-12. J. Bacteriol. 116:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner, B. 2000. Fundamentals of biostatistics, 5th ed. Duxbury, Pacific Grove, Calif.
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Sangari, F. J., and J. Agüero. 1991. Mutagenesis of Brucella abortus: comparative efficiency of three transposon delivery systems. Microb. Pathog. 11:443-446. [DOI] [PubMed] [Google Scholar]
- 29.Sangari, F. J., J. Agüero, and J. M. García-Lobo. 2000. The genes for erythritol catabolism are organized as an inducible operon in Brucella abortus. Microbiology 146:487-495. [DOI] [PubMed] [Google Scholar]
- 30.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scozzafava, A., A. Mastrolorenzo, and C. T. Supuran. 2001. Antimycobacterial activity of 9-sulfonylated/sulfenylated-6-mercaptopurine derivatives. Bioorg. Med. Chem. Lett. 11:1675-1678. [DOI] [PubMed] [Google Scholar]
- 32.Sigwart, D. F., B. A. D. Stocker, and J. D. Clements. 1989. Effect of a purA mutation on efficacy of Salmonella live-vaccine vectors. Infect. Immun. 57:1858-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sola-Landa, A., J. Pizzaro-Cerdá, M.-J. Grilló, E. Moreno, I. Moriyón, J.-M. Blasco, J.-P. Gorvel, and I. López-Goñi. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29:125-138. [DOI] [PubMed] [Google Scholar]
- 34.Umbarger, H. E. 1996. Biosynthesis of the branched-chain amino acids, p. 442-457. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Maganasik W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 35.Young, E. J. 2000. Brucella species, p. 2386-2393. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 36.Zalkin, H., and P. Nygaard. 1996. Biosynthesis of purine nucleotides, p. 561-579. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Maganasik W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.