Abstract
Children/adolescents with attention-deficit/hyperactivity disorder (ADHD) may have a poor or inadequate response to psychostimulants or be unable to tolerate their side-effects; furthermore, stimulants may be inappropriate because of co-existing conditions. Only one non-stimulant ADHD pharmacotherapy, the noradrenaline transporter inhibitor atomoxetine, is currently approved for use in Europe. We review recent advances in understanding of the pathophysiology of ADHD with a focus on the roles of catecholamine receptors in context of the α2A-adrenergic receptor agonist guanfacine extended release (GXR), a new non-stimulant treatment option in Europe. Neuroimaging studies of children/adolescents with ADHD show impaired brain maturation, and structural and functional anomalies in brain regions and networks. Neurobiological studies in ADHD and medication response patterns support involvement of monoaminergic neurotransmitters (primarily dopamine and noradrenaline). Guanfacine is a selective α2A-adrenergic receptor agonist that has been shown to improve prefrontal cortical cognitive function, including working memory. The hypothesized mode of action of guanfacine centres on direct stimulation of post-synaptic α2A-adrenergic receptors to enhance noradrenaline neurotransmission. Preclinical data suggest that guanfacine also influences dendritic spine growth and maturation. Clinical trials have demonstrated the efficacy of GXR in ADHD, and it is approved as monotherapy or adjunctive therapy to stimulants in Canada and the USA (for children and adolescents). GXR was approved recently in Europe for the treatment of ADHD in children and adolescents for whom stimulants are not suitable, not tolerated or have been shown to be ineffective. GXR may provide particular benefit for children/adolescents who have specific co-morbidities such as chronic tic disorders or oppositional defiant disorder (or oppositional symptoms) that have failed to respond to first-line treatment options.
Key Points
| Psychostimulants and the non-stimulant atomoxetine increase the extracellular availability of dopamine and noradrenaline at the synaptic cleft. Although methylphenidate is generally the first choice medication for children/adolescents with attention deficit/hyperactivity disorder (ADHD) in Europe, stimulants can be unsuitable for some patients. |
| Guanfacine is a selective α2A-adrenergic receptor agonist that acts directly on α2A-adrenergic receptors to enhance noradrenaline neurotransmission; preliminary evidence suggests that guanfacine also influences dendritic spine plasticity in the prefrontal cortex. |
| Guanfacine extended-release (GXR) is a new non-stimulant pharmacotherapy for ADHD in Europe for children and adolescents for whom stimulants are not suitable, not tolerated or have been shown to be ineffective. The selective mode of action of GXR may provide particular benefit for children/adolescents who have specific co-morbidities such as chronic tic disorders or oppositional defiant disorder (or oppositional symptoms) that have failed to respond to first-line treatment options. |
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a complex and multifactorial neurodevelopmental disorder [1] that is characterized by age-inappropriate and excessive levels of inattention, hyperactivity and impulsivity [2]. ADHD affects approximately 5 % of children and adolescents worldwide [3, 4] and symptoms persist into adulthood in most cases [5]. The disorder is associated with deficits in executive function [6], including working memory [7], and leads to impairment in a broad range of academic and social activities [2]. Emotional dysregulation is increasingly recognized as a common feature of ADHD, and may manifest as extreme irritability, frustration, reactive aggression and temper outbursts [8, 9]. Common ADHD co-morbidities include oppositional defiant disorder (ODD), conduct disorder and chronic tic disorders including Tourette’s syndrome [10, 11].
Although understanding of the pathophysiology of ADHD has improved greatly in the past decade, treatment options are still limited. The management of ADHD comprises non-pharmacological interventions, such as behavioural therapy, and pharmacotherapy [12–14]. The psychostimulants methylphenidate (MPH) and amphetamines were the only available ADHD medications for many years. Stimulants have a good effect size (0.8–1.5) [15, 16], and MPH is generally recommended in Europe as the first choice medication for children/adolescents with ADHD [2, 13]. However, approximately 30 % of children/adolescents with ADHD are unresponsive to a single stimulant medication and approximately 10 % fail to respond to any stimulants [17, 18]. Others are intolerant of the side effects of stimulants [19, 20]. Moreover, stimulants may not be the treatment of choice because of a personal or family history of medical conditions, risks of drug diversion or substance abuse, or parental preference [19, 21, 22].
One non-stimulant pharmacotherapy for ADHD, the noradrenaline transporter inhibitor atomoxetine (ATX), is currently approved for use in Europe. The efficacy of ATX in ADHD is well established [23], but potential clinical limitations include a delayed onset of action [24] and sympathomimetic cardiovascular side effects, which are similar to those of stimulants [25]. An alternative non-stimulant medication, particularly one without sympathomimetic cardiovascular side effects, could provide a useful therapeutic option for children/adolescents with ADHD. Guanfacine extended release (GXR; Intuniv) is approved for the treatment of ADHD as monotherapy or adjunctive therapy to stimulants in Canada and the USA (for children and adolescents, 6–17 years) [26, 27]. GXR was approved recently in Europe as part of a comprehensive programme for the treatment of ADHD in children and adolescents for whom stimulants are not suitable, not tolerated or have been shown to be ineffective [28].
Here we provide an overview of recent advances in our understanding of the pathophysiology of ADHD in the context of GXR. This article is intended to provide a clinical perspective on the neurobiology of ADHD and mode of action of pharmacotherapies. Given the broad scope of this article, a formal systematic literature review was not feasible. Rather, we used our combined clinical experience to develop an up-to-date and practical narrative review providing information on ADHD and pharmacological treatment options, with a focus on clinically relevant recent research findings.
Methods of Literature Search
Electronic literature searches via MEDLINE/PubMed were conducted for articles published in English (unlimited by date of publication). Combinations of keywords were used to identify relevant articles, including: ‘Attention deficit disorder with hyperactivity’, ‘ADHD’, ‘ADD’, ‘pathophysiology’, ‘neurobiology’, ‘neurochemistry’, ‘imaging’, ‘alpha 2A agonists’, ‘guanfacine’, ‘guanfacine extended release’, ‘GXR’, ‘mechanism’, ‘psychostimulants’, ‘methylphenidate’, ‘amphetamine’, ‘atomoxetine’, ‘contraindicated’, ‘adverse events’, ‘side effects’, ‘cardiovascular disease’, ‘hypertension’, ‘juvenile hypertension’, ‘tics’, ‘Tourette’s syndrome’, ‘conduct disorder’, ‘oppositional defiant disorder’ and ‘oppositional symptoms’. The last electronic searches were conducted on 15 July 2015. Meta-analyses, systematic and narrative reviews, and original research articles were included. Criteria for each search were adapted according to the initial number of hits. Formal literature searches were also supplemented with key publications, abstracts and posters that were known to the authors.
Pathophysiology of Attention-Deficit/Hyperactivity Disorder (ADHD)
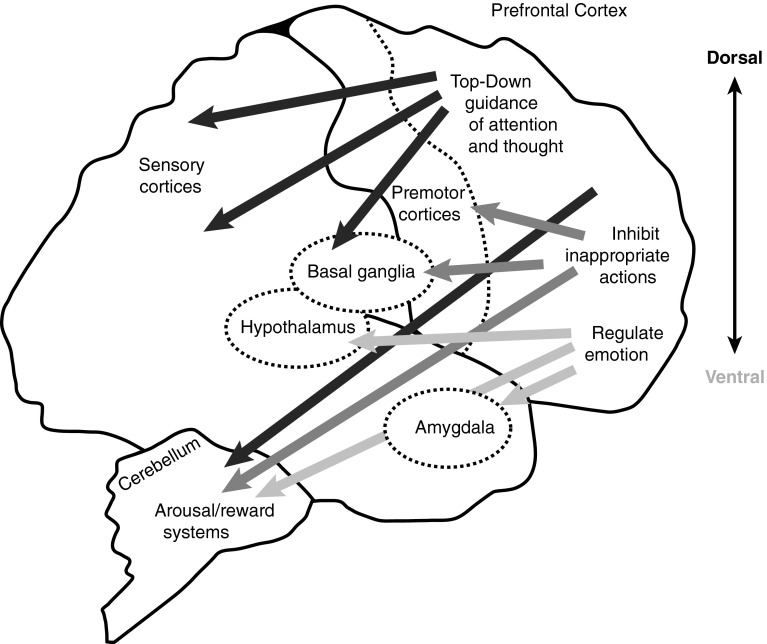
Although involvement of the prefrontal cortex (PFC) in ADHD has long been recognized, ADHD is now considered to be a neurodevelopmental disorder that affects the whole brain. The PFC regulates high-order cognitive functions, including attention, behaviour, planning and emotion through working memory [29]. These executive functions involve extensive neural network connections (Fig. 1) [29]. Recent evidence from systems neuroscience-based studies has demonstrated the importance of circuitry and connectivity involving large-scale neural networks in ADHD [30–32]. Accordingly, our understanding of the pathophysiology of ADHD has changed from a model based on specific regional differences to widespread altered connectivity in the whole brain [33].
Fig. 1.
Regulation of attention, behaviour and emotion through extensive network connections of the prefrontal cortex with other brain regions. Reproduced from Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–67, © (2012) with permission from Elsevier
Neuroimaging
Recent advances in neuroimaging have allowed the use of innovative high-resolution computational techniques to identify anatomical and functional anomalies in ADHD. Initial magnetic resonance imaging (MRI) studies in ADHD focused on the morphology of discrete brain regions, whereas more recent investigations have evaluated altered connectivity in large-scale neural networks [32, 34, 35].
Neuroanatomical differences between the brains of individuals with ADHD and healthy controls are well established [33]. Longitudinal and cross-sectional structural neuroimaging data indicate that ADHD is characterized by a global delay in brain maturation [30, 36, 37]. Cerebral, cerebellar, grey and white matter volumes are significantly smaller (2.5–4 %) among children/adolescents with ADHD compared with normal controls [30, 37, 38]. Peak cortical thickness is reached significantly later among children/adolescents with ADHD than normal controls (10.5 vs. 7.5 years; p < 1.0 × 10–20) and the delay is most profound in the lateral PFC (Fig. 2) [36]. A persistently smaller volume of the superior cerebellar vermis has also been reported in children/adolescents with ADHD versus normal controls, and the development trajectory of the cerebellum may be associated with clinical outcome [39]. Morphological and volumetric changes of subcortical structures, including the thalamus, basal ganglia and limbic system have also been reported [30, 40–42]. The functional significance of these differences in subcortical structures remains unclear but may represent disruption to neural network architecture in ADHD [41].
Fig. 2.
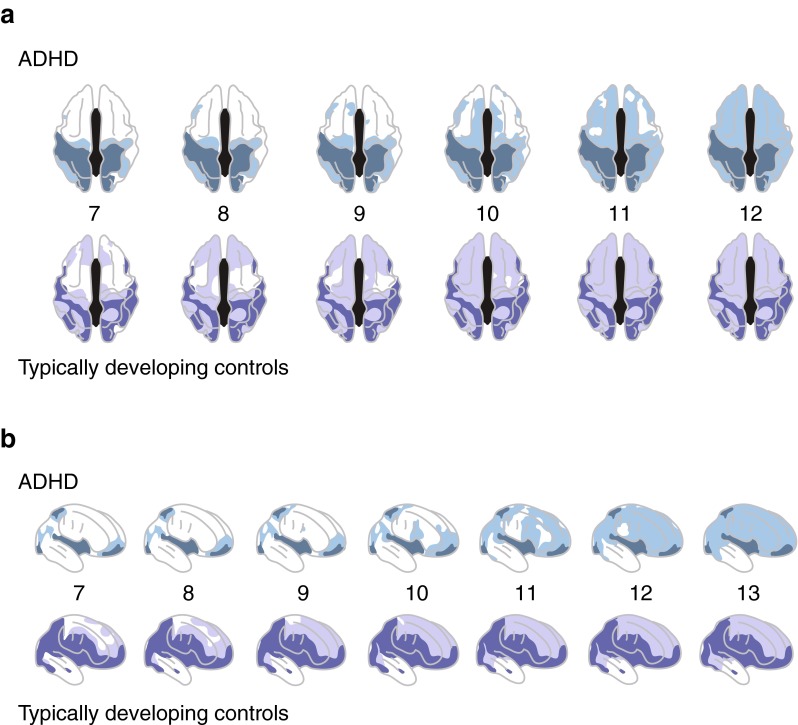
Attention-deficit/hyperactivity disorder (ADHD) is characterized by a delay in cortical maturation: a dorsal view and b right lateral view. View of the cortical regions where peak thickness was attained at each age (a 7–12 years, b 7–13 years) in ADHD (upper rows) and typically developing controls (lower rows). Darker colours indicate regions where a quadratic model was not appropriate (and thus a peak age could not be calculated), or the peak age was estimated to lie outside the age range covered. Both groups showed similar sequences of regions that attained peak thickness, but the ADHD group showed considerable delay in reaching this developmental marker. Reproduced with permission from Shaw et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–54. © (2007) National Academy of Sciences, USA
MRI studies using tractography 3D-modelling techniques have identified altered structural connectivity in white matter tracts among children with ADHD. Diffusion tensor imaging (DTI) provides quantitative data on the integrity of white matter tracts. Reduced volume and integrity of white matter tracts between the thalamus and striatum, hippocampus and PFC have been demonstrated in children with ADHD versus controls [42]. A meta-analysis of data from DTI studies (n = 15) suggests that the most consistent alterations in white matter integrity may be found in the right anterior corona radiata, right forceps minor, bilateral internal capsule and left cerebellum [43]. Abnormal connectivity and microstructural integrity in white matter linking the frontal lobe, striatum and cerebellum in children/adolescents with ADHD have also been demonstrated [44]. Moreover, in this study the altered connectivity in some fibre bundles correlated with attentional performance [44].
Network dysfunction in ADHD has been examined systematically using functional neuroimaging. Widespread differences in functional activation between patients with ADHD and normal controls have been described and suggest distinct domain-specific neurofunctional deficits [32, 38, 45]. Functional MRI (fMRI) provides a measure of the activity of neural regions involved at rest or during a particular task [33]. Meta-analyses of fMRI data have shown consistent right fronto-striatal dysfunction during inhibition- and attention-related tasks among patients with ADHD [46]. Attention tasks (data sets n = 13) involved the dorsolateral PFC, parietal and cerebellar areas. Inhibition tasks (data sets n = 21) involved the inferior frontal cortex, supplementary motor area and anterior cingulate cortex. A further meta-analysis of data from fMRI studies (n = 11) showed hypoactivation in the left fronto-parieto-cerebellar areas during timing tasks in ADHD [45].
Recent data indicate that ADHD is associated with dysfunction in large-scale neural networks, including the default-mode network (DMN) [31, 32]. The DMN describes a distinctive set of reciprocally oscillating brain regions that, in normal controls, are active during the resting state and suppressed during goal-directed tasks [47]; in contrast, task-specific neural activity during goal-directed tasks is reduced in patients with ADHD [46]. Resting-state fMRI data have shown dysfunctional connectivity in the DMN in affected children/adolescents and adults versus controls, which supports the hypothesis that ADHD is related to delayed or disrupted maturation of the DMN [31, 32, 35, 48–50]. A comprehensive meta-analysis of fMRI studies (n = 55) showed evidence of dysfunction in the DMN and multiple other neuronal systems involved in high-level cognitive functions (executive function and attention) [31]. Moreover, recent data demonstrate altered connectivity in the DMN and other large-scale networks, and structural abnormalities in the dorsolateral PFC and anterior cingulate cortex [32]. Importantly, this modality-spanning study provides evidence of spatial correspondence in the pattern of structural and functional alterations in ADHD [32].
Neurochemistry
Dysregulated early brain network development (aberrant apoptosis, neurogenesis, neuritogenesis and synaptogenesis) may cause an imbalance in dopamine, noradrenaline and other neurotransmitter systems [51, 52]. Although animal models do not exactly match ADHD, they provide a useful method of studying the molecular pathophysiology of this complex neurodevelopmental disorder [53]. The spontaneously hypertensive rat (SHR) is considered to be the most valid available animal model for ADHD; others, including the Coloboma mouse and actin depolymerizing factor and n-cofilin double mutant mouse models, also provide good approximations. Recent advances in imaging modalities, such as positron emission tomography (PET) and magnetic resonance spectroscopy, have enabled investigation of the neurochemistry of ADHD in humans.
Dopamine and Noradrenaline
Dysregulation of monoaminergic neurotransmitter systems, primarily dopamine and noradrenaline, is hypothesized to play a central role in the pathophysiology of ADHD [33]. Involvement of the dopaminergic system is highlighted by the validity of neonatal 6-hydroxy-dopamine lesioned rats and dopamine transporter (DAT) knockout mice as ADHD models [54]. The SHR model has reduced levels of γ-aminobutyric acid (GABA), which interacts with both the dopaminergic and adrenergic systems in the hippocampus [55]. Alpha2A-adrenergic receptors in the dorsolateral PFC are involved in the regulation of locomotor hyperactivity in primates [56]. Dysregulated dopamine metabolism among treatment-naïve young adults with ADHD has been demonstrated in a PET study using a radiolabelled dopamine precursor [57]. Furthermore, PET imaging of treatment-naïve adult humans with ADHD has shown a reduction in dopamine (D2/D3) receptors in the caudate and limbic system, which correlates with symptoms of inattention [58].
Dopamine and adrenergic receptors are members of the G protein-coupled receptor superfamily of membrane proteins. Dopamine receptors may be classified as either D1 (D1 and D5 subtype) or D2 (D2, D3 and D4 subtype) receptors [59, 60]. Adrenergic receptors mediate physiological responses to adrenaline and noradrenaline. Alpha and β adrenergic receptors are recognized and each has several subtypes [61–63]. α1-Adrenergic receptors are primarily located in smooth muscle, whereas α2-adrenergic receptors are found predominantly in the central nervous system (CNS) and peripheral sympathetic nervous system. Three distinct subtypes of human α2-adrenergic receptors (α2A-, α2B- and α2C-) have been identified [61, 64, 65]. Alpha2B-adrenergic receptors are most prevalent in the thalamus, whereas α2A- and α2C-adrenergic receptors are widespread throughout the brain [66, 67]. The α2A-adrenergic receptor is the most common subtype found in the PFC [68].
In the PFC, α2A-adrenergic and D1 receptors are located predominantly on the dendritic spines of pyramidal neurons [69, 70]. Dendritic spines are tiny protrusions from a neuronal dendrite that interconnect via glutaminergic synapses with other pyramidal neurons in the PFC to form complex neural networks [29]. Noradrenaline and dopamine powerfully modulate network connectivity through their effects on α2A-adrenergic and D1 receptors on dendritic spines [29]. Imbalances in the fine-tuning of pyramidal neurons by noradrenaline and/or dopamine in the PFC affect information processing and are believed to underlie the symptoms of ADHD.
Stimulation of D1 receptors by dopamine is believed to increase the production of cyclic adenosine monophosphate (cAMP), thereby opening hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels near the synapse (Fig. 3) [71]. An open channel causes the signal to ‘leak out’, thus shunting inputs out of the dendritic spine [72] and weakening network connections [73]. Conversely, stimulation of α2A-adrenergic receptors by noradrenaline inhibits cAMP signalling, thereby closing HCN channels [72]. Closure of the channel allows the signal to be conducted down the pyramidal neuron. In theory, noradrenaline strengthens the appropriate ‘signal’ between neurons with shared inputs, whereas dopamine reduces ‘noise’ by dissipating inappropriate network connections [72]. Thus, dopamine and noradrenaline have contrasting but complementary actions to increase the ‘signal-to-noise ratio’ in the PFC [72].
Fig. 3.

Noradrenaline and dopamine modulate the strength of network connections at dendritic spines in the prefrontal cortex to influence information processing. Methylphenidate (MPH) primarily blocks the dopamine transporter; amphetamines (AMPs) block the dopamine and noradrenaline transporters, and increase output of dopamine; atomoxetine (ATX) blocks the noradrenaline transporter. Guanfacine (G) directly stimulates postsynaptic α2A-adrenergic receptors and thereby closes hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the post-synaptic neuron and enhances noradrenaline neurotransmission. Adapted from Wang M et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410 © (2007) with permission from Elsevier and Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–67, © (2012) with permission from Elsevier
Moderate stimulation of α2A-adrenergic receptors in the PFC improves working memory in animal models and humans [69, 74, 75]. Similarly, moderate stimulation of D1 receptors in the PFC enhances working memory performance [71]. Both noradrenaline and dopamine concentrations in the synaptic cleft have an ‘inverted U-shaped’ effect on working memory, in which too much or too little of either neurotransmitter impairs PFC function (Fig. 4) [73]. Excessive levels of noradrenaline at the synapse leads to binding of noradrenaline to other adrenergic receptor subtypes on distant synapses and impairs working memory [73]. Likewise, excessive levels of dopamine may result in the recruitment of other dopamine receptor subtypes [73].
Fig. 4.
Inverted U-shaped influence of noradrenaline (NA) and dopamine (DA) on the prefrontal cortex (PFC). Both NA and DA have an ‘inverted U-shaped’ influence on PFC physiology and cognitive performance; too little or too much of either neurotransmitter impairs PFC function. Adapted with permission from Macmillan Publishers Ltd from Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22
Other Neurotransmitters
ADHD probably results from interplay between several dysfunctional neurotransmitter systems. Another monoaminergic neurotransmitter, serotonin, is postulated to play a role in ADHD. Paediatric major depressive disorder and paediatric obsessive compulsive disorder share some neuropsychological deficits and affected neural circuits with ADHD [29]. Selective serotonin re-uptake inhibitors are an effective treatment for these two disorders and it has been suggested that they act to normalize serotonergic regulation of emotion and inhibitory control [29]. Moreover, 5HT1B knockout mice exhibit hyperactivity, and attention- and sensation-seeking behaviour [76]. Association studies have identified links between various serotonergic genes and ADHD and there may be a particular association between the serotonin receptor gene, HTR1B, and the inattentive subtype of ADHD [77].
Recent evidence suggests that dysfunction in the glutamatergic neurotransmitter system plays a role in the pathophysiology of ADHD [78]. Glutamate is the main excitatory neurotransmitter in the human brain, and a key neuronal growth factor [79]; the glutamate N-methyl-d-aspartate (NMDA) receptor NR2 subunit controls synaptic plasticity and memory function during brain development [80]. In vivo data indicate that the glutamatergic system is hyperfunctional in the PFC of the SHR [81]. In addition, increased glutamatergic tone in children with untreated ADHD versus controls [82] and effects on glutamatergic systems following pharmacotherapy have been detected in magnetic resonance spectroscopy studies [83, 84]. Recent data suggest that glutamine levels may be increased among children (but not adults) with ADHD versus controls [85].
Preliminary data suggest that histamine may also be associated with ADHD. Histaminergic pathways are associated with arousal, learning and memory [86–88]. Histamine H3 receptor antagonists improve the performance of the SHR in inhibitory avoidance tasks, thus suggesting a tentative role in the treatment of ADHD [89, 90]. In addition, MPH and ATX are reported to increase histamine release in the PFC of the SHR [89, 91, 92].
Genetics
The pathophysiology of ADHD has a strong genetic component [93]. Heritability of the disorder is estimated to range from 60 to 80 % [93]. Candidate gene studies have also shown a number of gene variants to be associated with ADHD, including those coding for synaptosomal-associated protein 25 (SNAP-25), dopamine receptors (DRD4) and the DAT (DAT1) [94]. Pooled molecular genetics data support the involvement in genes coding for regulators of synaptic plasticity, including CTNNA2 and KALRN, and cell adhesion molecules in ADHD [95]. However, each gene variant is likely to contribute only a small individual effect [33]. Complex ‘gene–gene’ and ‘gene–environment’ interactions may explain how individual genes modify the response to environmental or biological variables [96].
Pharmacological Treatment of ADHD
An understanding of the pathophysiology of ADHD and modes of action of pharmacotherapies may help clinicians select the medication with the most appropriate efficacy and safety profile to match the individual needs of each patient [33]. The complex modes of action of ADHD pharmacotherapies are not fully understood. Nonetheless, all empirically effective medications seem to enhance signalling of dopamine and/or noradrenaline in the CNS (Table 1) [72]. The stimulants (MPH and amphetamines) and ATX act indirectly to increase the availability of noradrenaline and dopamine in the synaptic cleft, whereas guanfacine has a direct effect on post-synaptic α2A-adrenergic receptors (Fig. 3).
Table 1.
Summary of the modes of action of stimulant and non-stimulant pharmacotherapies in attention-deficit/hyperactivity disorder (ADHD)
| Pharmacotherapy | Mechanism of action |
|---|---|
| Stimulants | |
| Amphetaminea | Blocks dopamine and noradrenaline transporters and increases output of dopamine |
| Methylphenidatea | Primarily acts by blocking the dopamine transporter to increase dopamine levels |
| Non-stimulants | |
| Atomoxetine | Blocks the noradrenaline transporter to moderately increase dopamine and noradrenaline levels |
| Clonidineb | Directly stimulates post-synaptic α2A-adrenergic receptors; also binds to other α2-adrenergic receptor subtypes |
| Guanfacinec | Directly stimulates post-synaptic α2A-adrenergic receptors to enhance noradrenaline neurotransmission |
aShort- and long-acting formulations are approved for the treatment of ADHD in Europe
bNot approved for the treatment of ADHD in Europe
cGuanfacine immediate release is not approved for the treatment of ADHD
α2-Adrenergic Receptor Agonists
Interest in the use of α2-adrenergic receptor agonists for the treatment of ADHD began 30 years ago [97]. Early studies focused on immediate-release clonidine and, later, guanfacine [97, 98]. Clonidine engages equally with all three α2-adrenergic receptor subtypes, and also binds to imidazoline I1 receptors [98–100]. In contrast, guanfacine has higher affinity for the α2A- than α2B- or α2C-adrenergic receptor subtypes [101, 102]. Indeed, receptor-binding studies have shown guanfacine to be the most selective α2A-adrenergic receptor agonist available [103] and is, thus, referred to as a ‘selective’ α2A-adrenergic receptor agonist.
Clinical use of immediate-release clonidine and guanfacine is limited by their pharmacokinetic (PK) profiles [98]. Rapid absorption of immediate-release α2-adrenergic receptor agonists leads to high peak plasma concentrations that are associated with side-effects such as sedation and a dry mouth; rapid clearance necessitates frequent dosing through the day [98, 104]. Extended-release formulations of guanfacine and clonidine have been developed to overcome these PK issues. Extended-release clonidine is approved for the treatment of ADHD as monotherapy or adjunctive therapy in children/adolescents in the USA [105]. GXR is approved for the treatment of ADHD as monotherapy or adjunctive therapy in Canada and the USA (for children and adolescents) [26, 27], and was approved recently in Europe as part of a comprehensive programme for the treatment of children and adolescents with ADHD for whom stimulants are not suitable, not tolerated or have been shown to be ineffective [28].
Guanfacine Extended Release (GXR)
Guanfacine directly stimulates postsynaptic α2A-adrenergic receptors to enhance noradrenaline neurotransmission (Fig. 3) [98, 106, 107]. The stimulation of α2A-adrenergic receptors in the PFC produces a beneficial effect on cognitive function. Improvement in attention, working memory and learning with guanfacine has been demonstrated in animal studies [74, 106–115]. A beneficial effect of guanfacine has also been shown on impulsive decision-making behaviour in rats and impulsive self-injurious behaviour in rhesus macaques [116–118]. Moreover, guanfacine (29 µg/kg single dose administered orally) improved spatial working memory and planning in a double-blind, placebo-controlled trial in healthy adults [75]. Guanfacine (1 mg single dose administered orally) has also been shown to modulate emotional biasing of cognitive control in healthy adults [119, 120].
fMRI data show that the effect of guanfacine on emotional biasing of cognitive control in healthy adults is associated with altered functional connectivity between the right and left amygdala and PFC [120]. Preliminary fMRI data suggest that clinical response to GXR (1 mg/day titrated to maximum 4 mg/day) in children/adolescents with ADHD is associated with reduced activation in the right midcingulate and left posterior cingulate cortices [121]. In vivo rat pharmacological MRI data support differing effects of guanfacine in specific areas of the brain [122]; functional blood oxygenation level dependent responses suggest that guanfacine increases neuronal activity in the frontal cortex but decreases activity in the basal ganglia (caudate, putamen and nucleus accumbens) and entorhinal cortex [122].
Three hypotheses, based on in vitro and in vivo animal model data, have been proposed to explain the underlying physiological mechanism of action of guanfacine: (1) the HCN channel and network hypothesis; (2) the excitatory postsynaptic current (EPSC) network hypothesis; and (3) the spinogenic hypothesis.
(1) The HCN channel and network hypothesis provides a short-term mechanism by which α2A-adrenergic receptor agonists may regulate PFC networks. As previously described, stimulation of post-synaptic α2A-adrenergic receptors inhibits the production of cAMP, leading to closure of HCN channels and strengthening of the functional connectivity of PFC microcircuits. Blockade of HCN channels in superficial layers (II/III) of the rat PFC improves working memory performance [69] and guanfacine strengthens delay-related firing of PFC neurons during working memory tasks in electrophysiological primate studies [69].
(2) Stimulation of α2A-adrenergic receptors also suppresses excitatory synaptic inputs in pyramidal neurons [123, 124]. As described above, SHR model data suggest that the glutamatergic system is hyperfunctional in ADHD [81]. The EPSC network hypothesis is derived from in vitro and in vivo studies of rats and suggests that guanfacine may suppress glutamate transmission in deep layers (V–VI) of the medial PFC via complex intracellular signalling pathways [123, 124].
Further, it has been proposed that the intracellular pathways controlling HCN channels and the suppression of glutamate transmission may be linked [123, 124]. As such, α2A-adrenergic receptor activation may trigger both mechanisms, which work together to fine tune the synaptic inputs of pyramidal cells during different physiological conditions [123, 124]. It is hypothesized that HCN channel control (in layers II/III of the PFC) may predominate at low guanfacine doses, whereas the EPSC network (in layers V–VI) is principally affected at high doses [69, 123]. Indeed, neuronal firing in the PFC during working memory tasks is enhanced by low guanfacine doses and suppressed at high doses [69], whereas EPSC network effects can be demonstrated only at relatively high doses [123]. Thus, guanfacine seems to improve the signal-to-noise ratio in the PFC predominantly through an effect on HCN channels, but may also protect it during extreme stress (when noradrenaline is excessively released) through effects on the EPSC network [123].
(3) The spinogenic hypothesis describes a long-term effect of α2A-adrenergic receptor agonists mediated via dendritic spines. Dendritic spines can change rapidly in shape, volume and number [125, 126]. As previously described, dendritic spine plasticity in the PFC plays a key role in learning and memory [126]. α2A-adrenergic receptor agonists, such as guanfacine, increase the length and density of dendritic spines of cultured mouse cortical neurons [127, 128]. Guanfacine increases the expression of synaptic protein PSD95 and promotes the maturation of dendritic spines of cultured rat cortical neurons [129]. Indeed, recent data show that guanfacine prevents dendritic spine loss in the PFC and protects working memory performance in rats exposed to chronic stress [115].
α2A-Adrenergic receptor agonists also inhibit sympathetic nervous system activity. Guanfacine stimulates post-synaptic α2A-adrenergic receptors in the vasomotor centre of the brainstem to reduce peripheral sympathetic tone, and may also activate peripheral presynaptic α2A-adrenergic receptors [130–132]. Overall, guanfacine acts to decrease heart rate and vascular resistance [133]. Indeed, guanfacine was originally developed as an immediate-release formulation and clinically utilized as a centrally acting antihypertensive agent [134]. Guanfacine is associated with less potent hypotensive and sedative effects than is clonidine [135]. Data from a recent meta-analysis suggest that the relative risk of fatigue and somnolence is higher with extended-release clonidine than GXR as monotherapy for ADHD (10.86 [p = 0.02] and 3.21 [p = 0.0008], respectively, vs placebo) [136]. The selective affinity of guanfacine for α2A-adrenergic receptors is believed to underlie the differing physiological effects of these two α2A-adrenergic receptor agonists [98]. Detailed description of the pharmacodynamic and pharmacokinetic properties and posology of GXR are beyond the scope of this review, but information is available elsewhere for the interested reader [28, 137, 138].
Other ADHD Pharmacotherapies
Stimulants (various formulations of short- and long-acting MPH and amphetamines including lisdexamfetamine dimesylate) and ATX are approved for the treatment of ADHD in specific patient populations in many European countries and other regions. Stimulants and ATX act to block the re-uptake of dopamine and/or noradrenaline into the presynaptic neuron. Re-uptake by the presynaptic noradrenaline (norepinephrine) transporter (NET) is the main process by which the effects of noradrenaline are terminated in the CNS [139]. Similarly, dopamine is cleared from the synaptic cleft by re-uptake via the DAT; however, in the PFC, dopamine is also recognized by NETs [140].
Human and primate PET studies indicate that MPH binds predominantly to DATs in the striatum [141, 142]. Accordingly, the therapeutic effect of MPH in ADHD is believed to be primarily due to the blockade of DATs, which increases dopamine levels at the synapse (Fig. 3) [143, 144]. Preliminary data suggest that MPH may also downregulate dopamine turnover in children/adolescents with ADHD [57]. In addition, human PET data suggest that MPH also binds to NETs, but the clinical relevance of this is currently unclear [145]. Effects of MPH on neuronal remodelling in the nucleus accumbens, striatum and other brain regions have also been demonstrated [146–148]. Chronic exposure of mice to MPH increases dendritic spine density [146], and a sustained effect of MPH on synaptic gene and protein expression and synaptic and dendritic spine structure has been shown in rat models [147, 148].
Amphetamines inhibit the re-uptake of dopamine and noradrenaline from the synaptic cleft and induce an efflux of dopamine into the synapse in the PFC, striatum and nucleus accumbens (Fig. 3) [149, 150]. Amphetamines are a competitive substrate for the re-uptake transporters NET and DAT [151]. Inside neurons, amphetamines bind with the vesicular monoamine transporter 2 (VMAT2), thereby preventing the translocation of monoamines into intraneuronal storage vesicles [151]. Amphetamines also weakly inhibit monoamine oxidase (particularly monoamine oxidase A), which reduces intracellular catabolism of neurotransmitters [152]. As the concentration of neurotransmitters in the presynaptic neuron increases, the direction of action of the re-uptake transporter changes direction causing an efflux into the synapse [151].
The non-stimulant ATX inhibits NETs to moderately increase noradrenaline and dopamine levels in the PFC by indirect action (Fig. 3) [153]. ATX is reported to have no effect on dopamine efflux in the striatum or nucleus accumbens [153]. In vitro rodent data suggest that ATX may also block NMDA receptors on cortical and hippocampal neurons, thus affecting glutamatergic transmission [154]. A sustained effect of ATX on synaptic mRNA and proteins involved in neuronal plasticity has been demonstrated in rats [155], and a preliminary report of sustained maintenance of clinical response after discontinuation of long-term ATX warrants further investigation [156]. There seems to be some overlap in the neurophysiological effects of stimulants and ATX. Data from in vitro and in vivo animal studies suggest that both MPH and ATX may indirectly stimulate D1 and α2-adrenergic receptors on post-synaptic neurons in the PFC [157–159]. Human fMRI and arterial spin labelling MRI data also indicate common spatial patterns of brain activity after administration of MPH and ATX [160, 161].
Stimulants are the most widely prescribed class of pharmacotherapy for ADHD but may not be suitable for all children/adolescents [162, 163]. Patients may have a contraindication to MPH or amphetamines, or experience inadequate symptom control or side-effects [17–19, 21]. Common side-effects of stimulant treatment include loss of appetite, weight loss and sleep disturbance [19, 164, 165]. Long-acting formulations of MPH have been developed to allow once-daily dosing. However, long-acting MPH does not provide 24-h coverage [166], so some children/adolescents may experience troublesome symptoms of ADHD during the evening, night or early morning. In addition, recent work has shown that MPH causes chronic changes to pyramidal neuron function and synaptic plasticity in the juvenile rat PFC [167, 168], and the implication of these findings on human neurodevelopment remains unknown. Early PET evidence also suggests that long-term MPH therapy upregulates DATs in the striatum; this could reduce treatment efficacy and lead to ADHD symptom rebound on drug cessation, including during drug holidays [169]. As such, some clinicians may seek alternative treatment options for their patients.
Clinical Considerations on GXR
GXR directly stimulates α2A-adrenergic receptors and, according to the HCN channel and network hypotheses, thereby improves the ‘signal-to-noise’ ratio in the PFC. The selective effects of GXR may translate into clinical benefit for patients who require an alternative pharmacotherapy for ADHD. GXR is readily absorbed after oral ingestion and exhibits a linear PK profile in children and adults [137, 138]. Peak plasma concentration is achieved approximately 5 h after oral administration of a single dose of GXR [137, 170] and it has a long excretion half-life (of approximately 17 h) in adults [137]. Thus, the prolonged-release tablets provide a broad and flat GXR concentration profile [137, 138] that allows once-daily dosing [171]. GXR is bound moderately (70 %) to plasma proteins and metabolized primarily by cytochrome P450 (CYP) 3A4; plasma levels are affected by CYP3A4/5 inducers and inhibitors, such as ketoconazole [26].
Clinical Data on GXR in ADHD
Clinical trial data show that GXR is effective for the treatment of children and adolescents with ADHD as monotherapy or when administered as adjunctive therapy with stimulants [172–179]. Pivotal trial data on GXR are summarized in Table 2. Once-daily GXR significantly reduces ADHD core symptoms versus placebo among patients aged 6–17 years, and improvement is observed within 2 weeks of starting GXR treatment [173, 176, 177]. A significant improvement in functional outcomes (including Clinical Global Impressions of Improvement scale and Weiss Functional Impairment Rating Scale-Parent Report domains) with GXR was also observed in clinical trials [175, 177, 180]. The effect size of GXR monotherapy on ADHD Rating Scale version IV (ADHD-RS-IV) symptom scores in double-blind, placebo-controlled, pivotal trials ranges from 0.43 to 0.86 (Table 2). This is lower than the effect size of stimulants but similar to that reported for ATX (0.59–0.67) [15, 16, 23]. Head-to-head clinical direct comparison trial data are not available and to our knowledge not currently planned. Indirect comparisons, which must be interpreted cautiously in general, suggest that GXR may confer greater ADHD symptom control than does ATX [177, 181]. However, indirect comparisons should be replicated or supported by direct comparisons before being taken as scientific basis for clinical decision making.
Table 2.
Efficacy data from pivotal trials of GXR
| Study design | Primary efficacy endpoint | Key clinical findings (primary endpoint and effect size) |
|---|---|---|
| GXR as monotherapy | ||
| A randomized, multi-centre, double-blind, parallel-group, placebo-controlled, fixed-dose escalation study conducted in the USA [173] Children/adolescents (aged 6–17 years) with ADHD (N = 345) GXR dose: 2, 3 and 4 mg/day |
Change in ADHD-RS-IV total score from baseline to endpoint | Significant reduction in placebo-adjusted LS mean change from baseline in ADHD-RS-IV total scores in all GXR groups: −7.7 (p = 0.0002), −7.95 (p = 0.0001) and −10.39 (p < 0.0001) for the GXR 2, 3 and 4 mg/day groups, respectively GXR effect size: 0.64, 0.66 and 0.86 for the 2, 3 and 4 mg/day groups, respectively (post hoc analysis) |
| A randomized, double-blind, parallel-group, multi-centre, placebo-controlled, dose-ranging study conducted in the USA [176] Children/adolescents (aged 6–17 years) with ADHD (N = 324) GXR dose: 1–4 mg/day |
Change in ADHD-RS-IV total score from baseline to endpoint | Significant reduction in placebo-adjusted LS mean change in ADHD-RS-IV total scores in all GXR groups: −6.75 (p = 0.0041), −5.41 (p = 0.0176), −7.34 (p = 0.0016) and −7.88 (p = 0.0006) in the GXR 1a, 2, 3 and 4 mg/day groups, respectively GXR effect size: 0.53, 0.43, 0.58 and 0.62 for the GXR 1a, 2, 3 and 4 mg/day groups, respectively (post hoc analysis) |
| A double-blind, randomized, multi-centre, placebo-controlled, dose-optimization study conducted in the USA [179] Children (aged 6–12 years) with ADHD (N = 340) GXR dose: 1–4 mg/day (morning or evening dosing) |
Change in ADHD-RS-IV total score from baseline to Visit 10 (LOCF) | Significant reduction in LS mean change in ADHD-RS-IV total scores: −20.0, −19.8 and −20.1 for the GXR all active, GXR morning and GXR evening dosing groups, respectively (p < 0.001 for all groups vs. placebo) GXR effect size: 0.77, 0.75 and 0.78 for the GXR all active, GXR morning and GXR evening dosing groups, respectively |
| A study comprising an open-label optimization phase followed by a double-blind, placebo-controlled, multi-centre, randomized withdrawal phase, conducted in the EU, USA and Canada [231] Children/adolescents (aged 6–17 years) with ADHD (N = 528) [231] GXR dose: 1–7 mg/day |
Proportion of treatment failuresb [231] | Treatment failure occurred in 49 % of the GXR vs. 65 % of the placebo group (p = 0.006) [231] GXR effect sizec (at end of randomized withdrawal phase): 0.51d |
| A randomized, double-blind, multi-centre, parallel-group, placebo- and active-controlled (ATX reference arm), dose-optimization study conducted in the EU, USA and Canada [177] Children/adolescents (aged 6–17 years) with ADHD (N = 338) GXR dose: 0.05–0.12 mg/kg/day |
Change in ADHD-RS-IV total score from baseline to Visit 15 | Significant reduction in placebo-adjusted LS mean change from baseline in ADHD-RS-IV total score for GXR was −8.9 (p < 0.001) GXR effect size: 0.76 (ATX effect size: 0.32) |
| GXR as adjunctive therapy | ||
| A double-blind, randomized, multi-centre, placebo-controlled, dose-optimization study conducted in the USA [178, 180] Children/adolescents (aged 6–17 years) with ADHD and a suboptimal response to stimulants (N = 461) GXR dose: 1–4 mg/day (morning or evening dosing) plus current long-acting, oral stimulant (amphetamine or methylphenidate) |
Change in ADHD-RS-IV total score from baseline to endpoint | Significant reduction in placebo-adjusted LS mean change in ADHD-RS-IV total scores in both GXR groups: −4.5 (p = 0.002) and −5.3 (p < 0.001) GXR morning plus stimulant and GXR evening plus stimulant dosing groups GXR effect size: 0.38 and 0.45 for the GXR morning plus stimulant and GXR evening plus stimulant dosing groups, respectively |
ADHD attention-deficit/hyperactivity disorder, ADHD-RS-IV ADHD Rating Scale IV, ATX atomoxetine, GXR guanfacine extended release, LOCF last observation carried forward, LS least squares
a1 mg dose group weight-restricted to <110 lb
bTreatment failure defined as ≥50 % increase in ADHD-RS-IV total score and ≥2 point increase in Clinical Global Impression-Severity score at two consecutive visits vs double-blind randomized withdrawal phase baseline
cFor the change from baseline in LS mean ADHD-RS-IV total score (secondary endpoint)
dNewcorn et al. EPA-0666—long-term maintenance of efficacy of extended-release guanfacine hydrochloride (GXR) in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): double-blind, placebo-controlled, multicentre, phase 3 randomized withdrawal study. Poster presented at 22nd European Congress of Psychiatry, March 2014
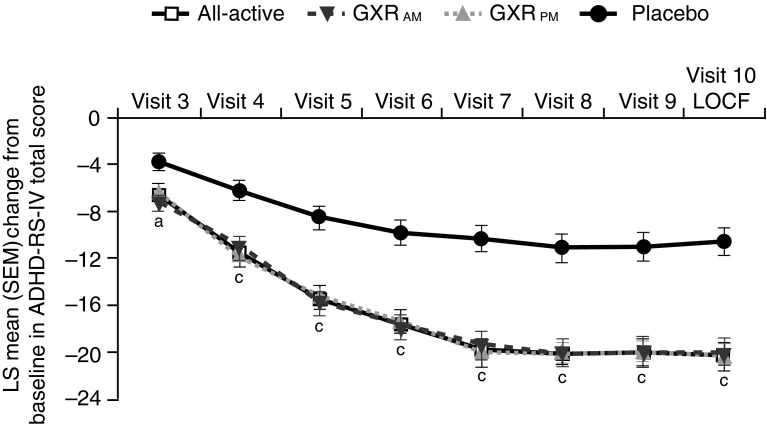
GXR has a broad, smooth and sustained concentration profile (that contrasts with the variable plasma levels associated with long-acting MPH) [137, 138, 166]. Administration of GXR in either the morning or evening is associated with significant and clinically meaningful improvements in ADHD symptoms (Fig. 5) [179]. At least 10–12 weeks of ATX treatment are required to achieve a full response [24]. Data suggest that GXR has a faster onset of efficacy than does ATX (significant placebo-adjusted improvement in ADHD-RS-IV total score observed at Week 1 and Week 3, respectively), although findings should be interpreted cautiously as this study was not designed or powered to compare active treatments [177].
Fig. 5.
Guanfacine extended release (GXR) monotherapy administered in either the morning or evening improves attention-deficit hyperactivity disorder (ADHD) symptoms. Mean change from baseline in ADHD Rating Scale version IV (ADHD-RS-IV) total score among patients receiving GXR. p values based on type III sum of squares from an analysis of covariance model. a p < 0.05 vs. placebo based on change from baseline (Visit 2). c p < 0.001 vs. placebo based on change from baseline (Visit 2). Reproduced with permission from Newcorn et al. Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry. 2013;52:921–30. © (2013) with permission from Elsevier. LOCF last observation carried forward, LS least squares, SEM standard error of the mean
The safety and tolerability of GXR have been well established in clinical trials and post-marketing experience in Canada and the USA. Treatment with GXR seems to be generally well tolerated [173, 177]. The most frequently reported adverse events associated with GXR in an open-label extension study were somnolence (30.4 %), headache (26.3 %), fatigue (14.2 %) and sedation (13.3 %) [172]. Somnolence/sedation (6 %) and fatigue (2 %) were the most common adverse reactions leading to discontinuation of GXR monotherapy from two large USA studies [173, 176]. Indeed, dose-dependent sedative effects are well described but tend to be transient [172, 173, 175, 182].
GXR is associated with modest dose-dependent reductions in blood pressure and heart rate but hypotension and bradycardia may occur [26, 173, 175]. Cardiovascular effects are reported most frequently in the first month of treatment [172, 173]. Transient hypertension following abrupt discontinuation of GXR has also been reported so the dose should be tapered gradually in decrements of no more than 1 mg every 3–7 days [26, 27]. GXR is associated with QT/QTc prolongation; however, it has a stabilizing effect on cardiac restitution, in line with a lack of arrhythmia liability [183, 184].
GXR is associated with growth that is similar to normative data [26]. ATX carries a black box warning of suicidal ideation [185, 186] but, reassuringly, there are no reports to date of an increased risk of suicide-related behaviour with GXR. Additional information on contraindications, precautions and adverse reactions associated with GXR is available in the Summary of Product Characteristics as reviewed by the European Medicines Agency [28].
Practical Applications of GXR in ADHD Clinical Practice
Because of its distinct mode of action, GXR may offer a particularly useful alternative treatment option for specific patient populations. The prevalence of ADHD and some commonly co-existing conditions are shown in Table 3. The prevalence of most individual co-morbid conditions among patients with ADHD is relatively low but, when considered together, represents a sizable population with substantial impairment that may be poorly managed using stimulants alone. Clinical efficacy and safety data support the use of GXR among patients with a suboptimal response to stimulants, chronic tic disorders (including Tourette’s syndrome) and/or ODD (or oppositional symptoms; Table 4).
Table 3.
Prevalence of attention-deficit/hyperactivity disorder (ADHD) and some commonly co-existing conditions for which stimulants are not suitable, not tolerated or have been shown to be ineffective
| Range of prevalence of condition among patients with ADHD | Range of prevalence of ADHD among patients with condition | |
|---|---|---|
| Stimulants associated with a suboptimal response, contraindicated or potentially unsuitable | ||
| Suboptimal response to stimulant | 11–44 % [144, 232–236] | N/A |
| Intolerant of stimulant side-effects | 2–8 % [235, 237] | N/A |
| Chronic tic disorders (including Tourette’s syndrome) | 1–33 % [10, 238–246] | 11–73 % [241, 243, 244, 247–251] |
| Cardiovascular disorders (including hypertension and tachycardia) | No data found | 70 % in HLHS [252] |
| Problems relating to emotional regulation and impulse control | ||
| Emotional dysregulation | 11–66 % [253–258] | No data found |
| Oppositional defiant disorder | 12–67 % [10, 238–240, 242, 243, 245, 259–264] | 30 % [243] |
| Conduct disorder | 5–46 % [10, 238–240, 243, 245, 246, 260–264] | 40 % [243] |
| Addictive behaviour | ||
| Eating disorders | 1–16 % [239, 243, 245, 265, 266] | 10 % [243] |
| Substance abuse/dependence | 2–16 % [238, 239, 245, 262, 267] | 54 % (children) [268] 18–31 % (adolescents) [268, 269] |
HLHS hypoplastic left heart syndrome, N/A not applicable
Table 4.
Specific populations of patients with attention-deficit hyperactivity disorder (ADHD) for whom guanfacine extended release (GXR) or guanfacine immediate releasea has demonstrated clinical efficacy
| Study design | Key findings |
|---|---|
| Patients with a suboptimal response to stimulants | |
| An open-label, dose-optimization study of children/adolescents (aged 6–17 years) with a suboptimal response to MPH (n = 42) or amphetamine (n = 33) [187] | Adjunctive GXR was associated with a significant improvement in ADHD-RS-IV total scores (−16.1, p < 0.0001) |
| A randomized, placebo-controlled trial of children/adolescents (aged 6–17 years) with a suboptimal response to extended-release MPH or amphetamine, who received adjunctive GXR (morning dosing n = 150; evening dosing n = 152) or adjunctive placebo (n = 153) [178] | Adjunctive GXR significantly improved ADHD-RS-IV total scores (placebo-adjusted least squares mean reductions: −4.5 for GXR morning dosing [p = 0.002] and −5.3 [p < 0.001] for GXR evening dosing) |
| A pre-specified analysis of data from children/adolescents (aged 6–17 years) in an RCT and the open-label phase of a randomized withdrawal trial [188] | GXR monotherapy was associated with significant improvement in ADHD-RS-IV total scores in both prior-MPH-treated and stimulant-naïve patients (difference in least squares mean vs placebo: −9.8 [p < 0.001; n = 45] and −7.6 [p < 0.001; n = 60], respectively, for the RCT and −24.3 [n = 221] and −26.7 [n = 206], respectively, for the LTE trial [descriptive statistics only]) |
| Patients with chronic tic disorders (including Tourette’s syndrome) | |
| An open-label study of guanfacine immediate release among children (aged 8–16 years) with ADHD and Tourette’s syndrome (n = 10) [194] | Guanfacine immediate release was associated with improvement in the A task of the Continuous Performance Test (commission errors, p < 0.02; omission errors, p < 0.01) and the severity of motor and phonic tics (both p < 0.02) |
| A randomized, placebo-controlled trial of guanfacine immediate release among children (aged 7–14 years) with ADHD and tic disorders (n = 34) [195] | Guanfacine immediate release improved ADHD-RS total scores (37 vs. 8 %; p < 0.001) and Yale Global Tic Severity Scale scores (31 % vs. 0; p = 0.05) versus placebo |
| Patients with oppositional defiant disorder (or oppositional symptoms) | |
| A randomized, double-blind, placebo-controlled, dose-optimization study of children (aged 6–12 years) with ADHD and oppositional symptoms (GXR, n = 138 and placebo, n = 79) [174] | GXR significantly improved ADHD-RS-IV total scores and CPRS-R:L oppositional subscale scores compared with placebo (−23.8 vs. −11.5 [p < 0.001] and −10.9 vs. −6.8 [p < 0.001]). A post hoc analysis showed high correlation between improvement of oppositional and ADHD symptoms (r = 0.74) [174] |
| A matching-adjusted indirect comparison of randomized, double-blind, placebo-controlled trial data to assess the efficacy of GXR (n = 58) and ATX (n = 98) in reducing oppositional symptoms among children (mean age 10 years) with ADHD and ODD or oppositional/disruptive symptoms [200] | GXR was associated with a greater reduction in mean CPRS-R:S, oppositional subscale scores compared with ATX (−5.0 vs. −2.4; p = 0.01) |
| An assessment of the effect of GXR adjunctive to psychostimulant on oppositional symptoms using data from a multicentre, randomized, double-blind, placebo-controlled, dose-optimization study of children/adolescents (aged 6–17 years) with a suboptimal response to a stimulant alone [201]. Patients received GXR (1–4 mg/day) administered in the morning (n = 86) or evening (n = 93), or placebo (n = 95) | GXR adjunctive to a stimulant significantly improved CPRS–R:L oppositional subscale scores (placebo-adjusted least square mean change from baseline: GXR morning −3.6 [p = 0.001] and GXR evening −2.7 [p = 0.013]) in a predefined subgroup with oppositional symptoms. A post hoc analysis showed no significant improvement in CPRS–R:L oppositional subscale scores among children/adolescents with a diagnosis of ODD (n = 90; although the analysis may have been underpowered to detect a difference between groups) |
ADHD-RS-IV ADHD Rating Scale IV, CPRS-R:L Conners’ Parent Rating Scale-Revised: Long Form, CPRS-R:S Conners’ Parent Rating Scale-Revised: Short Form, LTE long-term efficacy, MPH methylphenidate, ODD oppositional defiant disorder, RCT randomized controlled trial
aGuanfacine immediate release is not approved for the treatment of ADHD
Suboptimal Response to Stimulants
GXR is approved for the treatment of ADHD as adjunctive therapy to stimulant medications in Canada and the USA (for children and adolescents). In an open-label trial of children/adolescents with a suboptimal response to long-acting MPH or amphetamine monotherapy, adjunctive GXR was associated with improvement in ADHD symptom scores from baseline (Table 4) [187]. These results were validated in a randomized controlled trial (RCT) of children/adolescents with suboptimal response to long-acting MPH or amphetamines, in which adjunctive GXR significantly improved ADHD symptom scores versus adjunctive placebo (Table 4) [178]. In a recent pre-specified subgroup analysis of phase III trial data, GXR monotherapy was associated with a significant improvement in ADHD-RS-IV total scores in both prior-MPH-treated and stimulant-naïve children/adolescents (Table 4), whereas ATX was associated with a significant improvement in ADHD-RS-IV total scores in stimulant-naïve patients only (RCT data only; difference in least squares mean vs. placebo: −5.0 [p = 0.022] and −1.8 [p = 0.452]) [188]. Appropriately designed randomized studies are needed to confirm the findings of these subgroup analyses and further support treatment sequencing decisions for individuals with ADHD.
Tics and Related Problems
Some patients with tic disorders benefit from stimulant medication, as improved concentration can suppress tics; however, others may find that tics are induced or exacerbated [189]. The benefit of α2-adrenergic receptor agonists on both ADHD and co-morbid tic disorders has been demonstrated in a number of studies [190–193]. Guanfacine was effective in reducing tics in two small studies of children with ADHD and co-morbid tic disorders (Table 4) [194, 195]. A systematic review and meta-analysis of data from RCTs (n = 6) demonstrated benefit of α2-adrenergic receptor agonists in treating tics among patients with ADHD [196]. At present, the use of GXR/guanfacine for tic disorders in children/adolescents is strongly recommended in Canadian, but not European, treatment guidelines [197, 198].
Emotional Dysregulation and Oppositional Defiant Disorder (ODD) Problems
Emotional dysregulation is a common feature of ADHD and is also present in other psychiatric disorders [8, 9]. Dysfunction of a neural network comprising the orbitofrontal cortex, ventral striatum and amygdala is implicated in emotional dysregulation [8]. Guanfacine modulates emotional responses [119] and has been shown to reduce feelings of frustration among children with ADHD [199]. GXR (vs. placebo) significantly reduced oppositional symptoms among children aged 6–12 years with ADHD and co-morbid oppositional symptoms [174], and was associated with significantly greater reduction in oppositional subscale scores than was ATX (p = 0.01; Table 4) [200]. Furthermore, adjunctive GXR significantly reduced oppositional symptoms among children/adolescents (aged 6–17 years) with ADHD and oppositional symptoms (Table 4) [201]. It has been postulated that GXR reduces symptoms of aggression by strengthening orbitofrontal cortical regulation of emotion [29]. GXR could offer a useful treatment option for patients with ADHD who experience severe impairment due to emotional dysregulation.
Intolerable Side Effects of Stimulants
GXR could hypothetically benefit other specific populations of patients with ADHD, as inferred by its distinct mode of action. This may include patients who experience intolerable side effects of stimulant medications. Weight loss is a commonly reported side effect of stimulants, and ADHD per se may also be associated with dysregulated growth [202, 203]. Clinicians may prefer to avoid using stimulants among patients with pre-existing low body mass index, as even relatively minor stimulant-related weight reduction may be associated with long-term changes in body composition [204]. In such cases, using, or switching to, a non-stimulant medication can provide a helpful alternative management strategy [205].
Hypertension or Other Cardiovascular Disorders
Juvenile hypertension is being increasingly recognized as an important public health issue [206–208]. Hypertension is estimated to affect up to 6 % of children and adolescents and up to 40 % of those who are overweight or obese [206, 209]. Stimulants and ATX have sympathomimetic effects on the cardiovascular system, including small but significant increases in blood pressure and heart rate [25]. Accordingly, stimulants and ATX are contraindicated for patients with underlying cardiovascular disorders that could be compromised by an increase in blood pressure or heart rate [186, 210]. Thus, a thorough history and examination are required prior to initiation of stimulant treatment to exclude a personal or family history of cardiovascular disorders [2, 186]. GXR does not cause hypertension or affect cardiac re-polarization but may reduce heart rate and blood pressure [131]. Thus, children/adolescents with ADHD and co-existing tachycardia, juvenile hypertension (or prehyper-tension) or other cardiovascular symptoms could potentially benefit from GXR [189].
Substance Use Disorder and Impulsivity Problems
Children, and adolescents in particular, who have ADHD are impulsive and novelty seeking, and therefore are inherently at higher risk of substance use disorder (SUD) [2, 211]. Unlike stimulants, GXR (and ATX) does not stimulate the nucleus accumbens, which is involved in the regulation of reward-related behaviour [122, 212]. By inference and despite a meta-analysis that demonstrated no association between stimulant use and increased SUD risks [213], GXR could provide a useful additional treatment option for patients who are at particularly high risk of SUD, unable to abstain from substance misuse despite stimulant treatment, or those with family members who are known to misuse drugs. Indeed, guanfacine has been shown to improve resistance to impulsive behaviour in a primate model and human studies [97, 214]. ADHD is a risk factor for children being obese or overweight [215], and dysregulated, impulsive over-eating behaviours are believed to contribute to weight gain [216]. Thus, GXR could theoretically offer a beneficial treatment option for patients with ADHD and associated obesity related to impulsive over-eating.
Circadian Cycle and/or Sleep Problems
The use of GXR to target specific periods of the circadian cycle may be a key application in clinical practice. The early morning routine can be a particularly challenging time of day for families of children with ADHD [217]. Our clinical experience suggests that executive functional deficit resulting in disorganized or oppositional behaviour before the morning dose of ADHD medication is a common and severely disruptive problem. Recent data suggest that GXR administered in the morning may result in a reduced total sleep time versus placebo among children with ADHD and pre-existing sleep problems (−57 vs. +31 min; p = 0.005) [218]. The author suggests a possible link between reduced sleep time with GXR and daytime somnolence [218]; however, as this study was terminated early and thus lacks sufficient statistical power, it should be considered as preliminary evidence that needs further investigation. Stimulants in particular may cause sleep disturbances if administered in the evening so should be administered at this time only very cautiously [19, 219], whereas GXR can be administered either in the morning or in the evening [179]. The mode of action of GXR infers that administration in the evening could help children with ADHD to sleep and wake up relatively symptom-free. Indeed, recent data show that morning or evening administration of GXR significantly improves ADHD-related symptoms in the morning and throughout the day (as assessed by modified Connors’ Parent Rating Scale–Revised: Short Form total scores; p < 0.001 vs. placebo at Week 8 regardless of the time of GXR administration) [220]. GXR administered in the morning or evening adjunctively with a psychostimulant is also associated with improvement in morning symptoms of ADHD [180].
Other Potential Clinical Applications of GXR
Previous studies also suggest that GXR, through its specific mode of action on the PFC, could offer an effective therapy for conditions other than ADHD. Post-traumatic stress disorder (PTSD) is associated with excessive noradrenaline release [221] and PFC dysfunction may contribute to the inadequate inhibition of traumatic memories [221]. Indeed, there is substantial symptom overlap and co-morbidity between ADHD and PTSD [222]. Rat studies have shown that guanfacine reverses stress-induced PFC deficits while not exacerbating conditioned fear [112, 223]. Moreover, GXR (1–4 mg administered once daily) was found to reduce the severity of traumatic stress symptoms among children/adolescents in an open-label pilot study [224].
α2A-Adrenergic receptor agonists, including guanfacine, have also been studied in other PFC-associated cognitive disorders. Early data suggest that guanfacine may have beneficial effects in the treatment of attention disorders associated with neurological conditions [225–227]. Improvement with guanfacine has been reported among selected patients who have mild traumatic brain injury, visual neglect following stroke, and deficits in arousal and sustained attention following acute disseminated encephalomyelitis [225–227]. In a rat study, guanfacine has been reported to prevent hypobaric hypoxia-induced spatial working memory deficits and neurodegeneration [228]. The results suggest a potential role for GXR in improving prefrontal cognitive function during high altitude exposure [228].
Future Perspectives
Further research into the pathophysiology and aetiological heterogeneity of ADHD and the modes of action of effective pharmacotherapies will increase the use of individualized patient management strategies [229]. The identification of patients with ADHD who are likely to respond to specific treatments and/or avoid side effects using baseline demographic or clinical characteristics (including co-morbidities and adverse reaction profiles) could improve outcomes [230]. Matching individual patients with the most appropriate treatment strategy would help to better address the needs of patients and their families and improve the cost-effectiveness of care [230]. Advances in the neurobiology of ADHD will also help to drive the research and development of new therapeutic options.
Conclusions
This article provides an overview of recent advances in neurobiology of ADHD and the mode of action of guanfacine. An understanding of the neurobiology of ADHD and available treatment options will help clinicians make informed decisions to optimize the care of patients who require an alternative pharmacotherapy. ADHD is a neurodevelopmental disorder that affects synaptic transmission and growth factors throughout the brain. Data from neurobiological studies and medication response patterns in clinical trials support a key role for dopamine and noradrenaline in ADHD. Psychostimulants, and the non-stimulant ATX, increase the extracellular availability of these catecholamines at the synaptic cleft. Although stimulants are the most widely prescribed treatment for ADHD, they can be unsuitable for a sizable proportion of patients.
The selective α2A-adrenergic receptor agonist, GXR, is an alternative non-stimulant ADHD pharmacotherapy. According to the HCN channel and network hypotheses, guanfacine acts directly on α2A-adrenergic receptors to enhance noradrenaline neurotransmission. Animal studies also provide preliminary evidence that guanfacine influences dendritic spine plasticity in the PFC. GXR is a new non-stimulant pharmacotherapy for ADHD in Europe for children and adolescents for whom stimulants are not suitable, not tolerated or have been shown to be ineffective. The selective mode of action of GXR may provide particular benefit for children/adolescents who have specific comorbidities such as chronic tic disorders or ODD (or oppositional symptoms) that have failed to respond to first-line treatment options.
Acknowledgments
This review was funded by Shire International GmbH. The authors determined the scope of the review. Under their direction, Hannah Wills, MBChB, CMPP, an employee of Caudex, Oxford, UK, provided writing assistance for development of the manuscript drafts, assisted with the collation of author comments, and performed exploratory literature searches. Debby Moss, PhD, and Joanna Wright, DPhil, also employees of Caudex, Oxford, UK, contributed to the initial literature searches and provided writing assistance for the manuscript outline. Editorial assistance in formatting, proofreading, copy-editing and fact checking was also provided by Caudex. Shire International GmbH provided funding to Caudex, Oxford, UK, for support in writing, editing, managing reviews, coordinating and collating comments for this manuscript. Antonia Panayi from Shire International GmbH also reviewed and edited the manuscript for scientific accuracy. Shire develops and manufactures treatments for psychiatric disorders including ADHD. The content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in Clinical Drug Investigation was made by the authors independently.
Michael Huss and Wai Chen would like to acknowledge the extraordinary commitment and contribution made by Andrea Ludolph to this article and her role as corresponding author until her sad and untimely death shortly before publication.
Compliance with Ethical Standards
Conflict of interest
MH has received unrestricted grants to conduct Investigator Initiated Trials (IIT) with products of Engelhard Arzneimittel and Medice. He has received speaker honoraria, travel support and served as an advisor for Lilly, Shire, Medice, Novartis, Engelhard Arzneimittel, Actelion, and Lundbeck. He also holds an international patent on Doppler radar to assess ADHD. WC has received research support from Shire, honoraria for lecturing and consultancy for Flynn Pharma, Janssen and Shire, and support for attending meetings from Janssen and Shire. WC was a member of advisory boards for Shire. AL has received unrestricted grants from Medice and Novartis to investigate modes of action of methylphenidate in vitro. She has received speaker honoraria from Shire and Lilly and served as an advisor for Shire.
Footnotes
Andrea G. Ludolph—Deceased September 2015.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, Fifth Edition (DSM-5) Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.NICE. Attention deficit hyperactivity disorder. Diagnosis and management of ADHD in children, young people and adults. National Clinical Practice Guideline Number 72. 2013. https://www.nice.org.uk/guidance/qs39. Accessed 3 Mar 2015.
- 3.Huss M, Holling H, Kurth BM, Schlack R. How often are German children and adolescents diagnosed with ADHD? Prevalence based on the judgment of health care professionals: results of the German health and examination survey (KiGGS) Eur Child Adolesc Psychiatry. 2008;17(Suppl 1):52–58. doi: 10.1007/s00787-008-1006-z. [DOI] [PubMed] [Google Scholar]
- 4.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Quiroga JA, Montoya A, Kutzelnigg A, Deberdt W, Sobanski E. Attention deficit hyperactivity disorder in the European adult population: prevalence, disease awareness, and treatment guidelines. Curr Med Res Opin. 2013;29:1093–1104. doi: 10.1185/03007995.2013.812961. [DOI] [PubMed] [Google Scholar]
- 6.Lambek R, Tannock R, Dalsgaard S, Trillingsgaard A, Damm D, Thomsen PH. Executive dysfunction in school-age children with ADHD. J Atten Disord. 2011;15:646–655. doi: 10.1177/1087054710370935. [DOI] [PubMed] [Google Scholar]
- 7.Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev. 2012;32:605–617. doi: 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry. 2014;171:276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asherson P, Manor I, Huss M. Attention-deficit/hyperactivity disorder in adults: update on clinical presentation and care. Neuropsychiatry. 2014;4:109–128. doi: 10.2217/npy.14.16. [DOI] [Google Scholar]
- 10.MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 11.Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Atten Defic Hyperact Disord. 2015;7:27–38. doi: 10.1007/s12402-014-0142-1. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson K, Hutchinson AD, Denson L. Nonpharmacological treatments for ADHD: a meta-analytic review. J Atten Disord. 2014;18:275–282. doi: 10.1177/1087054712444732. [DOI] [PubMed] [Google Scholar]
- 13.Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, et al. European clinical guidelines for hyperkinetic disorder—first upgrade. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):i7–i30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- 14.NICE. Attention deficit hyperactivity disorder. Diagnosis and management of ADHD in children, young people and adults. National Clinical Practice Guideline Number 72 (2008; includes 2013 updates). 2013. http://www.nice.org.uk/guidance/cg72/evidence/cg72-attention-deficit-hyperactivity-disorder-adhd-full-guideline-2. Accessed 13 Mar 2015.
- 15.Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- 16.Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. Pharm Ther. 2009;34:678–694. [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3:200–211. doi: 10.1177/108705470000300403. [DOI] [Google Scholar]
- 18.Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227–246. doi: 10.1111/jcpp.12036. [DOI] [PubMed] [Google Scholar]
- 20.Gajria K, Lu M, Sikirica V, Greven P, Zhong Y, Qin P, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder—a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543–1569. doi: 10.2147/NDT.S65721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matza LS, Secnik K, Rentz AM, Mannix S, Sallee FR, Gilbert D, et al. Assessment of health state utilities for attention-deficit/hyperactivity disorder in children using parent proxy report. Qual Life Res. 2005;14:735–747. doi: 10.1007/pl00022070. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz S, Correll CU. Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry. 2014;53:174–187. doi: 10.1016/j.jaac.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Bushe CJ, Savill NC. Systematic review of atomoxetine data in childhood and adolescent attention-deficit hyperactivity disorder 2009–2011: focus on clinical efficacy and safety. J Psychopharmacol. 2014;28:204–211. doi: 10.1177/0269881113478475. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27:15–30. doi: 10.1007/s40263-012-0019-9. [DOI] [PubMed] [Google Scholar]
- 26.Shire. Intuniv US prescribing information. 2014. http://pi.shirecontent.com/PI/PDFs/Intuniv_USA_ENG.pdf. Accessed 2 Feb 2015.
- 27.Shire Canada Inc. INTUNIV XR product monograph. 2015. http://www.shirecanada.com/en/documents/INTUNIV_XR_PM_EN.pdf. Accessed 20 Oct 2015.
- 28.Shire Pharmaceuticals Ireland Limited. Intuniv summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003759/WC500195130.pdf. Accessed 20 Oct 2015
- 29.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Greven CU, Bralten J, Mennes M, O’Dwyer L, van Hulzen KJ, Rommelse N, et al. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry. 2015;72:490–499. doi: 10.1001/jamapsychiatry.2014.3162. [DOI] [PubMed] [Google Scholar]
- 31.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessler D, Angstadt M, Welsh RC, Sripada C. Modality-spanning deficits in attention-deficit/hyperactivity disorder in functional networks, gray matter, and white matter. J Neurosci. 2014;34:16555–16566. doi: 10.1523/JNEUROSCI.3156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortese S. The neurobiology and genetics of attention-deficit/hyperactivity disorder (ADHD): what every clinician should know. Eur J Paediatr Neurol. 2012;16:422–433. doi: 10.1016/j.ejpn.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, et al. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J Neurosci. 2012;32:17753–17761. doi: 10.1523/JNEUROSCI.3272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sripada C, Kessler D, Fang Y, Welsh RC, Prem KK, Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2014;35:4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 38.Vaidya CJ. Neurodevelopmental abnormalities in ADHD. Curr Top Behav Neurosci. 2012;9:49–66. doi: 10.1007/7854_2011_138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, III, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- 40.Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, et al. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia S, Li X, Kimball AE, Kelly MS, Lesser I, Branch C. Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;204:161–167. doi: 10.1016/j.pscychresns.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Hong SB, Zalesky A, Fornito A, Park S, Yang YH, Park MH, et al. Connectomic disturbances in attention-deficit/hyperactivity disorder: a whole-brain tractography analysis. Biol Psychiatry. 2014;76:656–663. doi: 10.1016/j.biopsych.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2012;36:2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 47.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- 50.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fredriksson A, Archer T. Neurobehavioural deficits associated with apoptotic neurodegeneration and vulnerability for ADHD. Neurotox Res. 2004;6:435–456. doi: 10.1007/BF03033280. [DOI] [PubMed] [Google Scholar]
- 52.Lou HC, Rosa P, Pryds O, Karrebaek H, Lunding J, Cumming P, et al. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev Med Child Neurol. 2004;46:179–183. doi: 10.1111/j.1469-8749.2004.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 53.Purper-Ouakil D, Ramoz N, Lepagnol-Bestel AM, Gorwood P, Simonneau M. Neurobiology of attention deficit/hyperactivity disorder. Pediatr Res. 2011;69:69R–76R. doi: 10.1203/PDR.0b013e318212b40f. [DOI] [PubMed] [Google Scholar]
- 54.van der Kooij MA, Glennon JC. Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2007;31:597–618. doi: 10.1016/j.neubiorev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Sterley TL, Howells FM, Russell VA. Evidence for reduced tonic levels of GABA in the hippocampus of an animal model of ADHD, the spontaneously hypertensive rat. Brain Res. 2013;1541:52–60. doi: 10.1016/j.brainres.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 56.Ma CL, Arnsten AF, Li BM. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Biol Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK, et al. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage. 2008;41:718–727. doi: 10.1016/j.neuroimage.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 58.Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 59.Dearry A, Gingrich JA, Falardeau P, Fremeau RT, Jr, Bates MD, Caron MG. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990;347:72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- 60.Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Frothingham L, et al. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci USA. 1989;86:9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lomasney JW, Lorenz W, Allen LF, King K, Regan JW, Yang-Feng TL, et al. Expansion of the alpha 2-adrenergic receptor family: cloning and characterization of a human alpha 2-adrenergic receptor subtype, the gene for which is located on chromosome 2. Proc Natl Acad Sci USA. 1990;87:5094–5098. doi: 10.1073/pnas.87.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinberg DH, Trivedi P, Tan CP, Mitra S, Perkins-Barrow A, Borkowski D, et al. Cloning, expression and characterization of human alpha adrenergic receptors alpha 1a, alpha 1b and alpha 1c. Biochem Biophys Res Commun. 1994;201:1296–1304. doi: 10.1006/bbrc.1994.1845. [DOI] [PubMed] [Google Scholar]
- 63.Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, et al. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- 64.Kobilka BK, Matsui H, Kobilka TS, Yang-Feng TL, Francke U, Caron MG, et al. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987;238:650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- 65.Regan JW, Kobilka TS, Yang-Feng TL, Caron MG, Lefkowitz RJ, Kobilka BK. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc Natl Acad Sci USA. 1988;85:6301–6305. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, et al. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328X(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 67.Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of alpha 2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 68.Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- 69.Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 70.Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 72.Arnsten AF. The emerging neurobiology of attention deficit hyperactivity disorder: the key role of the prefrontal association cortex. J Pediatr. 2009;154:I-S43. doi: 10.1016/j.jpeds.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramos BP, Stark D, Verduzco L, van Dyck CH, Arnsten AF. Alpha2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 76.Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037/0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- 77.Smoller JW, Biederman J, Arbeitman L, Doyle AE, Fagerness J, Perlis RH, et al. Association between the 5HT1B receptor gene (HTR1B) and the inattentive subtype of ADHD. Biol Psychiatry. 2006;59:460–467. doi: 10.1016/j.biopsych.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 78.Carrey NJ, MacMaster FP, Gaudet L, Schmidt MH. Striatal creatine and glutamate/glutamine in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:11–17. doi: 10.1089/cap.2006.0008. [DOI] [PubMed] [Google Scholar]
- 79.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 80.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci USA. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller EM, Pomerleau F, Huettl P, Gerhardt GA, Glaser PE. Aberrant glutamate signaling in the prefrontal cortex and striatum of the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder. Psychopharmacology. 2014;231:3019–3029. doi: 10.1007/s00213-014-3479-4. [DOI] [PubMed] [Google Scholar]
- 82.MacMaster FP, Carrey N, Sparkes S, Kusumakar V. Proton spectroscopy in medication-free pediatric attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;53:184–187. doi: 10.1016/S0006-3223(02)01401-4. [DOI] [PubMed] [Google Scholar]
- 83.Carrey N, MacMaster FP, Fogel J, Sparkes S, Waschbusch D, Sullivan S, et al. Metabolite changes resulting from treatment in children with ADHD: a 1H-MRS study. Clin Neuropharmacol. 2003;26:218–221. doi: 10.1097/00002826-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 84.Wiguna T, Guerrero AP, Wibisono S, Sastroasmoro S. Effect of 12-week administration of 20-mg long-acting methylphenidate on Glu/Cr, NAA/Cr, Cho/Cr, and mI/Cr ratios in the prefrontal cortices of school-age children in Indonesia: a study using 1H magnetic resonance spectroscopy (MRS) Clin Neuropharmacol. 2012;35:81–85. doi: 10.1097/WNF.0b013e3182452572. [DOI] [PubMed] [Google Scholar]
- 85.Bollmann S, Ghisleni C, Poil SS, Martin E, Ball J, Eich-Hochli D, et al. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl Psychiatry. 2015;5:e589. doi: 10.1038/tp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A, et al. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res. 2007;57:306–313. doi: 10.1016/j.neures.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 87.He C, Luo F, Chen X, Chen F, Li C, Ren S, et al. Superficial layer-specific histaminergic modulation of medial entorhinal cortex required for spatial learning. Cereb Cortex. 2015 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 88.Williams RH, Chee MJ, Kroeger D, Ferrari LL, Maratos-Flier E, Scammell TE, et al. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci. 2014;34:6023–6029. doi: 10.1523/JNEUROSCI.4838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fox GB, Pan JB, Esbenshade TA, Bennani YL, Black LA, Faghih R, et al. Effects of histamine H(3) receptor ligands GT-2331 and ciproxifan in a repeated acquisition avoidance response in the spontaneously hypertensive rat pup. Behav Brain Res. 2002;131:151–161. doi: 10.1016/S0166-4328(01)00379-5. [DOI] [PubMed] [Google Scholar]
- 90.da Silva WC, Bonini JS, Bevilaqua LR, Izquierdo I, Cammarota M. Histamine enhances inhibitory avoidance memory consolidation through a H2 receptor-dependent mechanism. Neurobiol Learn Mem. 2006;86:100–106. doi: 10.1016/j.nlm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Liu LL, Yang J, Lei GF, Wang GJ, Wang YW, Sun RP. Atomoxetine increases histamine release and improves learning deficits in an animal model of attention-deficit hyperactivity disorder: the spontaneously hypertensive rat. Basic Clin Pharmacol Toxicol. 2008;102:527–532. doi: 10.1111/j.1742-7843.2008.00230.x. [DOI] [PubMed] [Google Scholar]
- 92.Horner WE, Johnson DE, Schmidt AW, Rollema H. Methylphenidate and atomoxetine increase histamine release in rat prefrontal cortex. Eur J Pharmacol. 2007;558:96–97. doi: 10.1016/j.ejphar.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 93.Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2011;13:333–344. doi: 10.1007/s11920-011-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 95.Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Roser C, Nguyen TT, et al. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115:1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- 96.Nikolas M, Klump KL, Burt SA. Youth appraisals of inter-parental conflict and genetic and environmental contributions to attention-deficit hyperactivity disorder: examination of GxE effects in a twin sample. J Abnorm Child Psychol. 2012;40:543–554. doi: 10.1007/s10802-011-9583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arnsten AF, Jin LE. Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. Yale J Biol Med. 2012;85:45–58. [PMC free article] [PubMed] [Google Scholar]
- 98.Arnsten AF, Scahill L, Findling RL. Alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol. 2007;17:393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- 99.Uhlen S, Porter AC, Neubig RR. The novel alpha-2 adrenergic radioligand [3H]-MK912 is alpha-2C selective among human alpha-2A, alpha-2B and alpha-2C adrenoceptors. J Pharmacol Exp Ther. 1994;271:1558–1565. [PubMed] [Google Scholar]
- 100.Ernsberger P, Meeley MP, Mann JJ, Reis DJ. Clonidine binds to imidazole binding sites as well as alpha 2-adrenoceptors in the ventrolateral medulla. Eur J Pharmacol. 1987;134:1–13. doi: 10.1016/0014-2999(87)90125-7. [DOI] [PubMed] [Google Scholar]
- 101.Uhlen S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202:235–243. doi: 10.1016/0014-2999(91)90299-6. [DOI] [PubMed] [Google Scholar]
- 102.Uhlen S, Muceniece R, Rangel N, Tiger G, Wikberg JE. Comparison of the binding activities of some drugs on alpha 2A, alpha 2B and alpha 2C-adrenoceptors and non-adrenergic imidazoline sites in the guinea pig. Pharmacol Toxicol. 1995;76:353–364. doi: 10.1111/j.1600-0773.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 103.Uhlen S, Xia Y, Chhajlani V, Felder CC, Wikberg JE. [3H]-MK 912 binding delineates two alpha 2-adrenoceptor subtypes in rat CNS one of which is identical with the cloned pA2d alpha 2-adrenoceptor. Br J Pharmacol. 1992;106:986–995. doi: 10.1111/j.1476-5381.1992.tb14446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122:78–87. doi: 10.3810/pgm.2010.09.2204. [DOI] [PubMed] [Google Scholar]
- 105.Concordia Pharmaceuticals Inc. Kapvay (clonidine hydrochloride) extended-release tablets. 2014. http://www.kapvay.com/pdf/kapvay-conc-v1-USPI.pdf. Accessed 28 Jan 2015.
- 106.Kawaura K, Karasawa J, Chaki S, Hikichi H. Stimulation of postsynapse adrenergic alpha2A receptor improves attention/cognition performance in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2014;270:349–356. doi: 10.1016/j.bbr.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 107.Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav. 1996;55:415–422. doi: 10.1016/S0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 109.Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 110.Decamp E, Clark K, Schneider JS. Effects of the alpha-2 adrenoceptor agonist guanfacine on attention and working memory in aged non-human primates. Eur J Neurosci. 2011;34:1018–1022. doi: 10.1111/j.1460-9568.2011.07815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang M, Tang ZX, Li BM. Enhanced visuomotor associative learning following stimulation of alpha 2A-adrenoceptors in the ventral prefrontal cortex in monkeys. Brain Res. 2004;1024:176–182. doi: 10.1016/j.brainres.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 112.Jin XC, Ma CL, Li BM. The alpha(2A)-adrenoceptor agonist guanfacine improves spatial learning but not fear conditioning in rats. Sheng Li Xue Bao. 2007;59:739–744. [PubMed] [Google Scholar]
- 113.Sagvolden T. The alpha-2A adrenoceptor agonist guanfacine improves sustained attention and reduces overactivity and impulsiveness in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD) Behav Brain Funct. 2006;2:41. doi: 10.1186/1744-9081-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hains AB, Yabe Y, Arnsten AF. Chronic stimulation of alpha-2A-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Neurobiol Stress. 2015;2:1–9. doi: 10.1016/j.ynstr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, et al. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology. 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abela AR, Chudasama Y. Noradrenergic alpha-receptor stimulation in the ventral hippocampus reduces impulsive decision-making. Psychopharmacology. 2014;231:521–531. doi: 10.1007/s00213-013-3262-y. [DOI] [PubMed] [Google Scholar]
- 118.Freeman ZT, Rice KA, Soto PL, Pate KA, Weed MR, Ator NA, et al. Neurocognitive dysfunction and pharmacological intervention using guanfacine in a rhesus macaque model of self-injurious behavior. Transl Psychiatry. 2015;5:e567. doi: 10.1038/tp.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulz KP, Clerkin SM, Fan J, Halperin JM, Newcorn JH. Guanfacine modulates the influence of emotional cues on prefrontal cortex activation for cognitive control. Psychopharmacology. 2013;226:261–271. doi: 10.1007/s00213-012-2893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schulz KP, Clerkin SM, Newcorn JH, Halperin JM, Fan J. Guanfacine modulates the emotional biasing of amygdala-prefrontal connectivity for cognitive control. Eur Neuropsychopharmacol. 2014;24:1444–1453. doi: 10.1016/j.euroneuro.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bedard AC, Schulz KP, Krone B, Pedraza J, Duhoux S, Halperin JM, et al. Neural mechanisms underlying the therapeutic actions of guanfacine treatment in youth with ADHD: a pilot fMRI study. Psychiatry Res. 2015;231:353–6. [DOI] [PubMed]
- 122.Easton N, Shah YB, Marshall FH, Fone KC, Marsden CA. Guanfacine produces differential effects in frontal cortex compared with striatum: assessed by phMRI BOLD contrast. Psychopharmacology. 2006;189:369–385. doi: 10.1007/s00213-006-0558-1. [DOI] [PubMed] [Google Scholar]
- 123.Yi F, Liu SS, Luo F, Zhang XH, Li BM. Signaling mechanism underlying alpha -adrenergic suppression of excitatory synaptic transmission in the medial prefrontal cortex of rats. Eur J Neurosci. 2013;38:2364–2373. doi: 10.1111/ejn.12257. [DOI] [PubMed] [Google Scholar]
- 124.Ji XH, Ji JZ, Zhang H, Li BM. Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–2271. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- 125.Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006;21:38–47. doi: 10.1152/physiol.00042.2005. [DOI] [PubMed] [Google Scholar]
- 126.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 127.Hu J, Vidovic M, Chen MM, Lu QY, Song ZM. Activation of alpha 2A adrenoceptors alters dendritic spine development and the expression of spinophilin in cultured cortical neurones. Brain Res. 2008;1199:37–45. doi: 10.1016/j.brainres.2007.12.073. [DOI] [PubMed] [Google Scholar]
- 128.Song ZM, Abou-Zeid O, Fang YY. Alpha2a adrenoceptors regulate phosphorylation of microtubule-associated protein-2 in cultured cortical neurons. Neuroscience. 2004;123:405–418. doi: 10.1016/j.neuroscience.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 129.Ren WW, Liu Y, Li BM. Stimulation of α2A-adrenoceptors promotes the maturation of dendritic spines in cultured neurons of the medial prefrontal cortex. Mol Cell Neurosci. 2012;49:205–216. doi: 10.1016/j.mcn.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 130.Barber ND, Reid JL. Comparison of the actions of centrally and peripherally administered clonidine and guanfacine in the rabbit: investigation of the differences. Br J Pharmacol. 1982;77:641–647. doi: 10.1111/j.1476-5381.1982.tb09342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sorkin EM, Heel RC. Guanfacine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31:301–336. doi: 10.2165/00003495-198631040-00003. [DOI] [PubMed] [Google Scholar]
- 132.van Zwieten PA, Chalmers JP. Different types of centrally acting antihypertensives and their targets in the central nervous system. Cardiovasc Drugs Ther. 1994;8:787–799. doi: 10.1007/BF00877397. [DOI] [PubMed] [Google Scholar]
- 133.Feldstein CA, Cohen AA, Sabaris RP, Burucua JE. Hemodynamic effects of guanfacine in essential hypertension. Clin Ther. 1984;6:325–334. [PubMed] [Google Scholar]
- 134.Saameli K, Jerie P, Scholtysik G. Guanfacine and other centrally acting drugs in antihypertensive therapy; pharmacological and clinical aspects. Clin Exp Hypertens A. 1982;4:209–219. doi: 10.3109/10641968209061586. [DOI] [PubMed] [Google Scholar]
- 135.Kugler J, Seus R, Krauskopf R, Brecht HM, Raschig A. Differences in psychic performance with guanfacine and clonidine in normotensive subjects. Br J Clin Pharmacol. 1980;10(Suppl 1):71S–80S. doi: 10.1111/j.1365-2125.1980.tb04909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014;53:153–173. doi: 10.1016/j.jaac.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 137.Swearingen D, Pennick M, Shojaei A, Lyne A, Fiske K. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29:617–625. doi: 10.1016/j.clinthera.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 138.Boellner SW, Pennick M, Fiske K, Lyne A, Shojaei A. Pharmacokinetics of a guanfacine extended-release formulation in children and adolescents with attention-deficit-hyperactivity disorder. Pharmacotherapy. 2007;27:1253–1262. doi: 10.1592/phco.27.9.1253. [DOI] [PubMed] [Google Scholar]
- 139.Seneca N, Gulyas B, Varrone A, Schou M, Airaksinen A, Tauscher J, et al. Atomoxetine occupies the norepinephrine transporter in a dose-dependent fashion: a PET study in nonhuman primate brain using (S, S)-[18F]FMeNER-D2. Psychopharmacology. 2006;188:119–127. doi: 10.1007/s00213-006-0483-3. [DOI] [PubMed] [Google Scholar]
- 140.Carboni E, Silvagni A. Experimental investigations on dopamine transmission can provide clues on the mechanism of the therapeutic effect of amphetamine and methylphenidate in ADHD. Neural Plast. 2004;11:77–95. doi: 10.1155/NP.2004.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- 142.Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/S0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- 143.Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 144.Wilens TE, Spencer TJ. The stimulants revisited. Child Adolesc Psychiatr Clin N Am. 2000;9:573–603. [PubMed] [Google Scholar]
- 145.Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH, et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68:854–860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci USA. 2009;106:2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cavaliere C, Cirillo G, Bianco MR, Adriani W, De Simone A, Leo D, et al. Methylphenidate administration determines enduring changes in neuroglial network in rats. Eur Neuropsychopharmacol. 2012;22:53–63. doi: 10.1016/j.euroneuro.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 148.Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, et al. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31:1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- 149.Nielsen JA, Chapin DS, Moore KE. Differential effects of d-amphetamine, beta-phenylethylamine, cocaine and methylphenidate on the rate of dopamine synthesis in terminals of nigrostriatal and mesolimbic neurons and on the efflux of dopamine metabolites into cerebroventricular perfusates of rats. Life Sci. 1983;33:1899–1907. doi: 10.1016/0024-3205(83)90674-4. [DOI] [PubMed] [Google Scholar]
- 150.Guix T, Hurd YL, Ungerstedt U. Amphetamine enhances extracellular concentrations of dopamine and acetylcholine in dorsolateral striatum and nucleus accumbens of freely moving rats. Neurosci Lett. 1992;138:137–140. doi: 10.1016/0304-3940(92)90490-X. [DOI] [PubMed] [Google Scholar]
- 151.Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27:479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mantle TJ, Tipton KF, Garrett NJ. Inhibition of monoamine oxidase by amphetamine and related compounds. Biochem Pharmacol. 1976;25:2073–2077. doi: 10.1016/0006-2952(76)90432-9. [DOI] [PubMed] [Google Scholar]
- 153.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 154.Ludolph AG, Udvardi PT, Schaz U, Henes C, Adolph O, Weigt HU, et al. Atomoxetine acts as an NMDA receptor blocker in clinically relevant concentrations. Br J Pharmacol. 2010;160:283–291. doi: 10.1111/j.1476-5381.2010.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Udvardi PT, Fohr KJ, Henes C, Liebau S, Dreyhaupt J, Boeckers TM, et al. Atomoxetine affects transcription/translation of the NMDA receptor and the norepinephrine transporter in the rat brain—an in vivo study. Drug Des Devel Ther. 2013;7:1433–1446. doi: 10.2147/DDDT.S50448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Buitelaar J, Asherson P, Soutullo C, Colla M, Adams DH, Tanaka Y, et al. Differences in maintenance of response upon discontinuation across medication treatments in attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2015. [DOI] [PubMed]
- 157.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marquand AF, O’Daly OG, De Simoni S, Alsop DC, Maguire RP, Williams SC, et al. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: a multi-class pattern recognition approach. Neuroimage. 2012;60:1015–1024. doi: 10.1016/j.neuroimage.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schulz KP, Fan J, Bedard AC, Clerkin SM, Ivanov I, Tang CY, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69:952–961. doi: 10.1001/archgenpsychiatry.2011.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27:1–10. doi: 10.1097/00004703-200602000-00001. [DOI] [PubMed] [Google Scholar]
- 163.Spencer TJ, Biederman J, Harding M, O’Donnell D, Faraone SV, Wilens TE. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry. 1996;35:1460–1469. doi: 10.1097/00004583-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 164.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]
- 165.Sonuga-Barke EJ, Coghill D, Wigal T, DeBacker M, Swanson J. Adverse reactions to methylphenidate treatment for attention-deficit/hyperactivity disorder: structure and associations with clinical characteristics and symptom control. J Child Adolesc Psychopharmacol. 2009;19:683–690. doi: 10.1089/cap.2009.0024. [DOI] [PubMed] [Google Scholar]
- 166.Maldonado R. Comparison of the pharmacokinetics and clinical efficacy of new extended-release formulations of methylphenidate. Expert Opin Drug Metab Toxicol. 2013;9:1001–1014. doi: 10.1517/17425255.2013.786041. [DOI] [PubMed] [Google Scholar]
- 167.Urban KR, Waterhouse BD, Gao WJ. Distinct age-dependent effects of methylphenidate on developing and adult prefrontal neurons. Biol Psychiatry. 2012;72:880–888. doi: 10.1016/j.biopsych.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Urban KR, Li YC, Gao WJ. Treatment with a clinically-relevant dose of methylphenidate alters NMDA receptor composition and synaptic plasticity in the juvenile rat prefrontal cortex. Neurobiol Learn Mem. 2013;101:65–74. doi: 10.1016/j.nlm.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang GJ, Volkow ND, Wigal T, Kollins SH, Newcorn JH, Telang F, et al. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One. 2013;8:e63023. doi: 10.1371/journal.pone.0063023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bukstein OG, Head J. Guanfacine ER for the treatment of adolescent attention-deficit/hyperactivity disorder. Expert Opin Pharmacother. 2012;13:2207–2213. doi: 10.1517/14656566.2012.721778. [DOI] [PubMed] [Google Scholar]
- 171.Faraone SV, McBurnett K, Sallee FR, Steeber J, Lopez FA. Guanfacine extended release: a novel treatment for attention-deficit/hyperactivity disorder in children and adolescents. Clin Ther. 2013;35:1778–1793. doi: 10.1016/j.clinthera.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 172.Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr. 2008;13:1047–1055. doi: 10.1017/s1092852900017107. [DOI] [PubMed] [Google Scholar]
- 173.Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 174.Connor DF, Findling RL, Kollins SH, Sallee F, Lopez FA, Lyne A, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6–12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24:755–768. doi: 10.2165/11537790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 175.Sallee FR, Lyne A, Wigal T, McGough JJ. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:215–226. doi: 10.1089/cap.2008.0080. [DOI] [PubMed] [Google Scholar]
- 176.Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 177.Hervas A, Huss M, Johnson M, McNicholas F, van Stralen J, Sreckovic S, et al. Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, Phase III trial. Eur Neuropsychopharmacol. 2014;24:1861–1872. doi: 10.1016/j.euroneuro.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 178.Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:74–85. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 179.Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, et al. Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry. 2013;52:921–930. doi: 10.1016/j.jaac.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 180.Wilens TE, McBurnett K, Turnbow J, Rugino T, White C, Youcha S. Morning and evening effects of guanfacine extended release adjunctive to psychostimulants in pediatric ADHD: results from a Phase III multicenter trial. J Atten Disord. 2013 (Epub ahead of print). [DOI] [PubMed]
- 181.Sikirica V, Findling RL, Signorovitch J, Erder MH, Dammerman R, Hodgkins P, et al. Comparative efficacy of guanfacine extended release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in children and adolescents: applying matching-adjusted indirect comparison methodology. CNS Drugs. 2013;27:943–953. doi: 10.1007/s40263-013-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ruggiero S, Clavenna A, Reale L, Capuano A, Rossi F, Bonati M. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24:1578–1590. doi: 10.1016/j.euroneuro.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 183.Martin P, Satin L, Kahn RS, Robinson A, Corcoran M, Purkayastha J, et al. A thorough QT study of guanfacine. Int J Clin Pharmacol Ther. 2015;53:301–316. doi: 10.5414/CP202065. [DOI] [PubMed] [Google Scholar]
- 184.Fossa AA, Zhou M, Robinson A, Purkayastha J, Martin P. Use of ECG restitution (beat-to-beat QT-TQ interval analysis) to assess arrhythmogenic risk of QTc prolongation with guanfacine. Ann Noninvasive Electrocardiol. 2014;19:582–594. doi: 10.1111/anec.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bangs ME, Tauscher-Wisniewski S, Polzer J, Zhang S, Acharya N, Desaiah D, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47:209–218. doi: 10.1097/chi.0b013e31815d88b2. [DOI] [PubMed] [Google Scholar]
- 186.Eli Lilly and Co. Strattera PI. 2014. http://pi.lilly.com/us/strattera-pi.pdf. Accessed 13 Nov 2014.
- 187.Spencer TJ, Greenbaum M, Ginsberg LD, Murphy WR. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:501–510. doi: 10.1089/cap.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Huss M, Hervas A, Newcorn J, Harpin V, Lyne A, Robertson B, et al. Guanfacine extended release for attention-deficit/hyperactivity disorder following inadequate response to prior methylphenidate (P.7.d.013) Eur Neuropsychopharmacol. 2014;24:S727–S728. doi: 10.1016/S0924-977X(14)71172-1. [DOI] [Google Scholar]
- 189.Warikoo N, Faraone SV. Background, clinical features and treatment of attention deficit hyperactivity disorder in children. Expert Opin Pharmacother. 2013;14:1885–1906. doi: 10.1517/14656566.2013.818977. [DOI] [PubMed] [Google Scholar]
- 190.Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:884–893. doi: 10.1097/CHI.0b013e3181b26e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cohen DJ, Detlor J, Young JG, Shaywitz BA. Clonidine ameliorates Gilles de la Tourette syndrome. Arch Gen Psychiatry. 1980;37:1350–1357. doi: 10.1001/archpsyc.1980.01780250036004. [DOI] [PubMed] [Google Scholar]
- 192.Tourette’s Syndrome Study Group Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/WNL.58.4.527. [DOI] [PubMed] [Google Scholar]
- 193.Rizzo R, Gulisano M, Cali PV, Curatolo P. Tourette syndrome and comorbid ADHD: current pharmacological treatment options. Eur J Paediatr Neurol. 2013;17:421–428. doi: 10.1016/j.ejpn.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 194.Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, Arnsten A, et al. Guanfacine treatment of comorbid attention-deficit hyperactivity disorder and Tourette’s syndrome: preliminary clinical experience. J Am Acad Child Adolesc Psychiatry. 1995;34:1140–1146. doi: 10.1097/00004583-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 195.Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 196.Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders—efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev. 2013;37:1162–1171. doi: 10.1016/j.neubiorev.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. 2012;57:133–143. doi: 10.1177/070674371205700302. [DOI] [PubMed] [Google Scholar]
- 198.Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. 2011;20:173–196. doi: 10.1007/s00787-011-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hunt RD, Arnsten AF, Asbell MD. An open trial of guanfacine in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 200.Signorovitch J, Erder MH, Xie J, Sikirica V, Lu M, Hodgkins PS, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):130–137. doi: 10.1002/pds.3246. [DOI] [PubMed] [Google Scholar]
- 201.Findling RL, McBurnett K, White C, Youcha S. Guanfacine extended release adjunctive to a psychostimulant in the treatment of comorbid oppositional symptoms in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2014;24:245–252. doi: 10.1089/cap.2013.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Spencer T, Biederman J, Wilens T. Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics. 1998;102:501–506. [PubMed] [Google Scholar]
- 203.Biederman J, Spencer TJ, Monuteaux MC, Faraone SV. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157(635–40):e1. doi: 10.1016/j.jpeds.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Poulton A, Briody J, McCorquodale T, Melzer E, Herrmann M, Baur LA, et al. Weight loss on stimulant medication: how does it affect body composition and bone metabolism? A prospective longitudinal study. Int J Pediatr Endocrinol. 2012;2012:30. doi: 10.1186/1687-9856-2012-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hasnain M, Vieweg WV. Weight considerations in psychotropic drug prescribing and switching. Postgrad Med. 2013;125:117–129. doi: 10.3810/pgm.2013.09.2706. [DOI] [PubMed] [Google Scholar]
- 206.Bassareo PP, Mercuro G. Pediatric hypertension: an update on a burning problem. World J Cardiol. 2014;6:253–259. doi: 10.4330/wjc.v6.i5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lurbe E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invitti C, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–1742. doi: 10.1097/HJH.0b013e32832f4f6b. [DOI] [PubMed] [Google Scholar]
- 208.George MG, Tong X, Wigington C, Gillespie C, Hong Y. Hypertension screening in children and adolescents—National Ambulatory Medical Care Survey, National Hospital Ambulatory Medical Care Survey, and Medical Expenditure Panel Survey, United States, 2007–2010. Morb Mortal Wkly Rep. 2014;63:47–53. [PubMed] [Google Scholar]
- 209.Martin L, Oepen J, Reinehr T, Wabitsch M, Claussnitzer G, Waldeck E, et al. Ethnicity and cardiovascular risk factors: evaluation of 40,921 normal-weight, overweight or obese children and adolescents living in Central Europe. Int J Obes (Lond) 2015;39:45–51. doi: 10.1038/ijo.2014.167. [DOI] [PubMed] [Google Scholar]
- 210.Wigal SB. Efficacy and safety limitations of attention-deficit hyperactivity disorder pharmacotherapy in children and adults. CNS Drugs. 2009;23(Suppl 1):21–31. doi: 10.2165/00023210-200923000-00004. [DOI] [PubMed] [Google Scholar]
- 211.Wood AC, Rijsdijk F, Asherson P, Kuntsi J. Inferring causation from cross-sectional data: examination of the causal relationship between hyperactivity-impulsivity and novelty seeking. Front Genet. 2011;2:6. doi: 10.3389/fgene.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 213.Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: a meta-analysis. JAMA Psychiatry. 2013;70:740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim S, Bobeica I, Gamo NJ, Arnsten AF, Lee D. Effects of alpha-2A adrenergic receptor agonist on time and risk preference in primates. Psychopharmacology. 2012;219:363–375. doi: 10.1007/s00213-011-2520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fliers EA, Buitelaar JK, Maras A, Bul K, Hohle E, Faraone SV, et al. ADHD is a risk factor for overweight and obesity in children. J Dev Behav Pediatr. 2013;34:566–574. doi: 10.1097/DBP.0b013e3182a50a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cortese S, Comencini E, Vincenzi B, Speranza M, Angriman M. Attention-deficit/hyperactivity disorder and impairment in executive functions: a barrier to weight loss in individuals with obesity? BMC Psychiatry. 2013;13:286. doi: 10.1186/1471-244X-13-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Whalen CK, Henker B, Ishikawa SS, Jamner LD, Floro JN, Johnston JA, et al. An electronic diary study of contextual triggers and ADHD: get ready, get set, get mad. J Am Acad Child Adolesc Psychiatry. 2006;45:166–174. doi: 10.1097/01.chi.0000189057.67902.10. [DOI] [PubMed] [Google Scholar]
- 218.Rugino TA. Effect on primary sleep disorders when children with ADHD are administered guanfacine extended release. J Atten Disord. 2014 (Epub ahead of print). [DOI] [PubMed]
- 219.Corkum P, Moldofsky H, Hogg-Johnson S, Humphries T, Tannock R. Sleep problems in children with attention-deficit/hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adolesc Psychiatry. 1999;38:1285–1293. doi: 10.1097/00004583-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 220.Young J, Rugino T, Dammerman R, Lyne A, Newcorn JH. Efficacy of guanfacine extended release assessed during the morning, afternoon, and evening using a modified conners’ parent rating scale-revised: short form. J Child Adolesc Psychopharmacol. 2014;24:435–441. doi: 10.1089/cap.2013.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–i15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 222.Biederman J, Petty CR, Spencer TJ, Woodworth KY, Bhide P, Zhu J, et al. Examining the nature of the comorbidity between pediatric attention deficit/hyperactivity disorder and post-traumatic stress disorder. Acta Psychiatr Scand. 2013;128:78–87. doi: 10.1111/acps.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Birnbaum SG, Podell DM, Arnsten AF. Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharmacol Biochem Behav. 2000;67:397–403. doi: 10.1016/S0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 224.Connor DF, Grasso DJ, Slivinsky MD, Pearson GS, Banga A. An open-label study of guanfacine extended release for traumatic stress related symptoms in children and adolescents. J Child Adolesc Psychopharmacol. 2013;23:244–251. doi: 10.1089/cap.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Malhotra PA, Parton AD, Greenwood R, Husain M. Noradrenergic modulation of space exploration in visual neglect. Ann Neurol. 2006;59:186–190. doi: 10.1002/ana.20701. [DOI] [PubMed] [Google Scholar]
- 226.Singh-Curry V, Malhotra P, Farmer SF, Husain M. Attention deficits following ADEM ameliorated by guanfacine. J Neurol Neurosurg Psychiatry. 2011;82:688–690. doi: 10.1136/jnnp.2009.195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.McAllister TW, McDonald BC, Flashman LA, Ferrell RB, Tosteson TD, Yanofsky NN, et al. Alpha-2 adrenergic challenge with guanfacine one month after mild traumatic brain injury: altered working memory and BOLD response. Int J Psychophysiol. 2011;82:107–114. doi: 10.1016/j.ijpsycho.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kauser H, Sahu S, Kumar S, Panjwani U. Guanfacine ameliorates hypobaric hypoxia induced spatial working memory deficits. Physiol Behav. 2014;123:187–192. doi: 10.1016/j.physbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 229.Bruxel EM, Akutagava-Martins GC, Salatino-Oliveira A, Contini V, Kieling C, Hutz MH, et al. ADHD pharmacogenetics across the life cycle: new findings and perspectives. Am J Med Genet B Neuropsychiatr Genet. 2014;165:263–282. doi: 10.1002/ajmg.b.32240. [DOI] [PubMed] [Google Scholar]
- 230.Hodgkins P, Dittmann RW, Sorooshian S, Banaschewski T. Individual treatment response in attention-deficit/hyperactivity disorder: broadening perspectives and improving assessments. Exp Rev Neurother. 2013;13:425–433. doi: 10.1586/ern.13.31. [DOI] [PubMed] [Google Scholar]
- 231.clinicaltrials.gov. NCT01081145. 2014. http://clinicaltrials.gov/ct2/show/study/NCT01081145?sect=X01256. Accessed 4 Aug 2014.
- 232.Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 233.Ahmann PA, Theye FW, Berg R, Linquist AJ, Van Erem AJ, Campbell LR. Placebo-controlled evaluation of amphetamine mixture-dextroamphetamine salts and amphetamine salts (Adderall): efficacy rate and side effects. Pediatrics. 2001;107:E10. doi: 10.1542/peds.107.1.e10. [DOI] [PubMed] [Google Scholar]
- 234.Wang Y, Zheng Y, Du Y, Song DH, Shin YJ, Cho SC, et al. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry. 2007;41:222–230. doi: 10.1080/00048670601057767. [DOI] [PubMed] [Google Scholar]
- 235.Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165:721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- 236.Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40:180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 237.Wilens T, McBurnett K, Stein M, Lerner M, Spencer T, Wolraich M. ADHD treatment with once-daily OROS methylphenidate: final results from a long-term open-label study. J Am Acad Child Adolesc Psychiatry. 2005;44:1015–1023. doi: 10.1097/01.chi.0000173291.28688.e7. [DOI] [PubMed] [Google Scholar]
- 238.Smalley SL, McGough JJ, Moilanen IK, Loo SK, Taanila A, Ebeling H, et al. Prevalence and psychiatric comorbidity of attention-deficit/hyperactivity disorder in an adolescent Finnish population. J Am Acad Child Adolesc Psychiatry. 2007;46:1575–1583. doi: 10.1097/chi.0b013e3181573137. [DOI] [PubMed] [Google Scholar]
- 239.Gau SS, Ni HC, Shang CY, Soong WT, Wu YY, Lin LY, et al. Psychiatric comorbidity among children and adolescents with and without persistent attention-deficit hyperactivity disorder. Aust N Z J Psychiatry. 2010;44:135–143. doi: 10.3109/00048670903282733. [DOI] [PubMed] [Google Scholar]
- 240.Steinhausen HC, Novik TS, Baldursson G, Curatolo P, Lorenzo MJ, Rodrigues PR, et al. Co-existing psychiatric problems in ADHD in the ADORE cohort. Eur Child Adolesc Psychiatry. 2006;15(Suppl 1):I25–I29. doi: 10.1007/s00787-006-1004-y. [DOI] [PubMed] [Google Scholar]
- 241.Schlander M, Schwarz O, Rothenberger A, Roessner V. Tic disorders: administrative prevalence and co-occurrence with attention-deficit/hyperactivity disorder in a German community sample. Eur Psychiatry. 2011;26:370–374. doi: 10.1016/j.eurpsy.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 242.Zavadenko NN, Suvorinova NI. Disorders comorbid to attention deficit hyperactivity syndrome in children. Zh Nevrol Psikhiatr Im S S Korsakova. 2007;107:30–35. [PubMed] [Google Scholar]
- 243.Anckarsater H, Lundstrom S, Kollberg L, Kerekes N, Palm C, Carlstrom E, et al. The child and adolescent twin study in Sweden (CATSS) Twin Res Hum Genet. 2011;14:495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- 244.Amiri S, Fakhari A, Golmirzaei J, Mohammadpoorasl A, Abdi S. Tourette’s syndrome, chronic tics, and comorbid attention deficit/hyperactivity disorder in elementary students. Arch Iran Med. 2012;15:76–78. [PubMed] [Google Scholar]
- 245.Biederman J, Faraone SV. The Massachusetts General Hospital studies of gender influences on attention-deficit/hyperactivity disorder in youth and relatives. Psychiatr Clin N Am. 2004;27:225–232. doi: 10.1016/j.psc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 246.Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127:462–470. doi: 10.1542/peds.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H, et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2012;21:451–457. doi: 10.1007/s00787-012-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Freeman RD. Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):15–23. doi: 10.1007/s00787-007-1003-7. [DOI] [PubMed] [Google Scholar]
- 249.Khalifa N, von Knorring AL. Psychopathology in a Swedish population of school children with tic disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1346–1353. doi: 10.1097/01.chi.0000251210.98749.83. [DOI] [PubMed] [Google Scholar]
- 250.Kadesjo B, Gillberg C. Tourette’s disorder: epidemiology and comorbidity in primary school children. J Am Acad Child Adolesc Psychiatry. 2000;39:548–555. doi: 10.1097/00004583-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 251.Ghanizadeh A, Mosallaei S. Psychiatric disorders and behavioral problems in children and adolescents with Tourette syndrome. Brain Dev. 2009;31:15–19. doi: 10.1016/j.braindev.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 252.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105:1082–1089. doi: 10.1542/peds.105.5.1082. [DOI] [PubMed] [Google Scholar]
- 253.Strine TW, Lesesne CA, Okoro CA, McGuire LC, Chapman DP, Balluz LS, et al. Emotional and behavioral difficulties and impairments in everyday functioning among children with a history of attention-deficit/hyperactivity disorder. Prev Chronic Dis. 2006;3:A52. [PMC free article] [PubMed] [Google Scholar]
- 254.Spencer TJ, Faraone SV, Surman CB, Petty C, Clarke A, Batchelder H, et al. Toward defining deficient emotional self-regulation in children with attention-deficit/hyperactivity disorder using the Child Behavior Checklist: a controlled study. Postgrad Med. 2011;123:50–59. doi: 10.3810/pgm.2011.09.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sjowall D, Roth L, Lindqvist S, Thorell LB. Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. J Child Psychol Psychiatry. 2013;54:619–627. doi: 10.1111/jcpp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Anastopoulos AD, Smith TF, Garrett ME, Morrissey-Kane E, Schatz NK, Sommer JL, et al. Self-regulation of emotion, functional impairment, and comorbidity among cildren with AD/HD. J Atten Disord. 2011;15:583–592. doi: 10.1177/1087054710370567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Stringaris A, Goodman R. Mood lability and psychopathology in youth. Psychol Med. 2009;39:1237–1245. doi: 10.1017/S0033291708004662. [DOI] [PubMed] [Google Scholar]
- 258.Sobanski E, Banaschewski T, Asherson P, Buitelaar J, Chen W, Franke B, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51:915–923. doi: 10.1111/j.1469-7610.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- 259.Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol. 2007;22:538–542. doi: 10.1177/0883073807303221. [DOI] [PubMed] [Google Scholar]
- 260.Ter-Stepanian M, Grizenko N, Zappitelli M, Joober R. Clinical response to methylphenidate in children diagnosed with attention-deficit hyperactivity disorder and comorbid psychiatric disorders. Can J Psychiatry. 2010;55:305–312. doi: 10.1177/070674371005500506. [DOI] [PubMed] [Google Scholar]
- 261.Harty SC, Miller CJ, Newcorn JH, Halperin JM. Adolescents with childhood ADHD and comorbid disruptive behavior disorders: aggression, anger, and hostility. Child Psychiatry Hum Dev. 2009;40:85–97. doi: 10.1007/s10578-008-0110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hurtig T, Ebeling H, Taanila A, Miettunen J, Smalley S, McGough J, et al. ADHD and comorbid disorders in relation to family environment and symptom severity. Eur Child Adolesc Psychiatry. 2007;16:362–369. doi: 10.1007/s00787-007-0607-2. [DOI] [PubMed] [Google Scholar]
- 263.Souza I, Pinheiro MA, Denardin D, Mattos P, Rohde LA. Attention-deficit/hyperactivity disorder and comorbidity in Brazil: comparisons between two referred samples. Eur Child Adolesc Psychiatry. 2004;13:243–248. doi: 10.1007/s00787-004-0402-2. [DOI] [PubMed] [Google Scholar]
- 264.August GJ, Realmuto GM, MacDonald AW, III, Nugent SM, Crosby R. Prevalence of ADHD and comorbid disorders among elementary school children screened for disruptive behavior. J Abnorm Child Psychol. 1996;24:571–595. doi: 10.1007/BF01670101. [DOI] [PubMed] [Google Scholar]
- 265.Biederman J, Ball SW, Monuteaux MC, Surman CB, Johnson JL, Zeitlin S. Are girls with ADHD at risk for eating disorders? Results from a controlled, five-year prospective study. J Dev Behav Pediatr. 2007;28:302–307. doi: 10.1097/DBP.0b013e3180327917. [DOI] [PubMed] [Google Scholar]
- 266.Rastam M, Taljemark J, Tajnia A, Lundstrom S, Gustafsson P, Lichtenstein P, et al. Eating problems and overlap with ADHD and autism spectrum disorders in a nationwide twin study of 9- and 12-year-old children. Sci World J. 2013;2013:315429. doi: 10.1155/2013/315429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Madsen AG, Dalsgaard S. Prevalence of smoking, alcohol and substance use among adolescents with attention-deficit/hyperactivity disorder in Denmark compared with the general population. Nord J Psychiatry. 2014;68:53–59. doi: 10.3109/08039488.2013.768293. [DOI] [PubMed] [Google Scholar]
- 268.Wu LT, Gersing K, Burchett B, Woody GE, Blazer DG. Substance use disorders and comorbid Axis I and II psychiatric disorders among young psychiatric patients: findings from a large electronic health records database. J Psychiatr Res. 2011;45:1453–1462. doi: 10.1016/j.jpsychires.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hovens JG, Cantwell DP, Kiriakos R. Psychiatric comorbidity in hospitalized adolescent substance abusers. J Am Acad Child Adolesc Psychiatry. 1994;33:476–483. doi: 10.1097/00004583-199405000-00005. [DOI] [PubMed] [Google Scholar]