Abstract
Streptococcus pyogenes thiol proteinase, also known as streptococcal pyrogenic exotoxin B (SpeB), has been suggested to be a major virulence factor in S. pyogenes infection. SpeB was reported to induce apoptosis of host cells, but its mechanism of action is not yet fully understood. In this study, we examined the involvement of matrix metalloproteinases (MMPs) in SpeB-induced apoptosis. We first developed a large-scale preparation of recombinant SpeB and precursors of human MMP-9 and -2 (proMMPs) by using Escherichia coli Rosetta (DE3)pLysS and baculovirus-insect cell expression systems, respectively. Treatment with SpeB induced effective proteolytic activation of both proMMP-9 and -2. When RAW264 murine macrophages were incubated with SpeB-activated proMMP-9, the level of tumor necrosis factor alpha (TNF-α) in conditioned medium (CM), assessed by an enzyme immunoassay, was elevated. This increase was completely inhibited by addition of the MMP inhibitor SI-27 to the cell culture. The CM also produced marked induction of apoptosis of U937 human monocytic cells. Similarly, soluble Fas ligand (sFasL) was detected in CM of cultures of SW480 cells expressing FasL after treatment with SpeB-activated proMMPs; this CM also induced apoptosis in U937 cells. SpeB had a direct effect as well and caused the release of TNF-α and sFasL from the cells. SpeB-dependent production of MMP-9 and -2 and proapoptotic molecules (TNF-α and sFasL) was evident in a murine model of severe invasive S. pyogenes infection. These results suggest that SpeB or SpeB-activated MMPs contribute to tissue damage and streptococcal invasion in the host via extracellular release of TNF-α and sFasL.
Streptococcus pyogenes (group A Streptococcus [GAS]) causes a variety of human diseases ranging from pharyngitis and impetigo to severe invasive infections, including necrotizing fasciitis and toxic shock-like syndrome (TSLS), which are associated with high mortality (5). Many reports of severe invasive GAS infection have described the pathogenic roles of a number of virulent factors of GAS, including M protein and several streptococcal pyrogenic exotoxins (7). GAS species secrete an extracellular thiol proteinase also known as streptococcal pyrogenic exotoxin B (SpeB), which seems to have a critical function in the pathogenesis of severe invasive GAS infections (2, 17, 24, 26, 27). SpeB is secreted as a 40-kDa precursor that is autocatalytically converted to a 28-kDa active form. SpeB appears to contribute to GAS pathogenesis via cleavage and activation of host proteins (15, 21, 22, 55). SpeB was reported to induce apoptosis in macrophages and epithelial cells, possibly through activation of the caspase cascade (25, 52), although the extensive necrotic degeneration is the major pathological manifestation in TSLS. Details of the mechanism of GAS-induced apoptosis remain unclear, however.
Host cells undergo apoptosis via complicated host-pathogen interactions, which may have significant pathological consequences in hosts infected with various bacteria (54, 57). Much attention now focuses on the roles of two proapoptotic molecules, tumor necrosis factor alpha (TNF-α) and Fas ligand (FasL), in cell death. These ligand molecules bind to tumor necrosis factor receptor 1 and Fas, respectively, which are both members of the same family, so-called death receptors. After binding to ligands occurs, the intracellular domains of the death receptors interact with some adapter proteins that have been recruited and form the receptor-ligand complex. These complexes trigger activation of the caspase cascade, which leads to degradation of intracellular substrates and causes apoptosis (18, 39). TNF-α and FasL are known to be released from the cell surface by matrix metalloproteinases (MMPs) (3, 9, 23, 33, 34, 36, 37). Norrby-Teglund et al. reported that the number of cells producing proinflammatory cytokines, such as TNF-α, significantly increased in blood during severe invasive GAS infections (e.g., TSLS and necrotizing fasciitis) compared with noninvasive GAS infections (42). Soluble FasL (sFasL) was shown to be released as a biologically active, death-inducing mediator capable of inducing apoptosis of cells in patients with acute respiratory distress syndrome (31). These studies suggest that TNF-α and sFasL may contribute to apoptosis-related cell and tissue injury in severe GAS infections. Because disintegration of the extracellular matrix (ECM) is essential for bacterial invasion, and SpeB-induced MMP activation may cause ECM degradation, and apoptosis as well, it is important to examine the activation of precursors of human MMPs (proMMPs) by SpeB and its pathological consequences in GAS infections.
MMPs, which are a family of zinc-dependent neutral endopeptidases, participate in tissue degradation and remodeling under physiological and pathological conditions. It was previously reported that some bacterial proteinases activated proMMPs, via limited proteolysis, to generate the active forms of MMPs (45). Burns et al. also described SpeB activation of proMMP-2 (4). However, the activating potential of SpeB for various isoforms of proMMPs other than proMMP-2 and its pathological effect remain unclear.
In the present study, we investigated proteolytic activation of proMMP-2 and -9 by SpeB and the effect of this activation on the induction of apoptosis. We focused especially on the release of TNF-α and sFasL from the cell membrane as a function of the proteolytic activity of SpeB. We also analyzed MMP activation and generation of TNF-α and sFasL in a murine model of severe GAS infection, which was produced by combined infection with influenza virus and GAS. Our results suggest that SpeB has proapoptotic effects via activation of proMMPs as a result of the generation of the death ligands TNF-α and sFasL and thus contributes in a critical way to the pathogenesis of severe invasive GAS infections, such as TSLS and necrotizing fasciitis.
MATERIALS AND METHODS
Preparation of recombinant SpeB and its mutant protein.
pSK-SCP, with the cDNA sequence encoding the entire speB gene inserted, was obtained from H. Ohkuni, Nippon Medical School, Tokyo, Japan. To amplify the speB gene fragment, PCR was performed with Ex-Taq DNA polymerase (Takara Biomedicals, Kyoto, Japan) and the following primers: 5′-GTTCCATGGATCAAAACTTTGCTCGTAACG-3′ (sense) and 5′-TTGAATTCTTATCAATGATGATGATGATGATGAGGTTTGATGCCTACAACAGC-3′ (antisense). The amplified DNA fragment was purified with a PCR purification kit (Amersham International plc, Little Chalfont, Buckinghamshire, United Kingdom), and the purified DNA was then ligated to the pCRII TOPO cloning vector (Invitrogen Corp., Carlsbad, Calif.). Initial transformation was carried out with Escherichia coli DH5α, and the nucleotide sequences of the construct were verified by using a DNA sequencer (model 373A; Applied Biosystems, Tokyo, Japan). The plasmid clones expressing the speB gene were digested with NcoI and BamHI and then ligated into the expression vector pET3d (Stratagene, La Jolla, Calif.) to produce a C-terminal His-tagged protein in an E. coli Rosetta (DE3)pLysS strain (Novagen, Madison, Wis.). A Cys-192-Ser (C192S) mutant of SpeB (mSpeB; zymogen) was constructed by use of the QuiK Change site-directed mutagenesis kit (Stratagene). This mutant fragment was subcloned into pET3d (12, 13, 38). Expression of these His-tagged proteins was then induced with 1 mM isopropyl-β-d-thiogalactopyranoside, and the His-tagged wild-type and mSpeB proteins were purified by using the Ni2+-Hi-Trap column (Amersham). Recombinant SpeB proteins were treated with Detoxi-Gel endotoxin-removing gel (Pierce Biotechnology, Inc., Rockford, Ill.) to eliminate contaminant lipopolysaccharide (LPS). The amount of LPS that remained in the recombinant SpeB preparation after the Detoxi-Gel treatment was 2.8 ng of LPS/mg of SpeB protein.
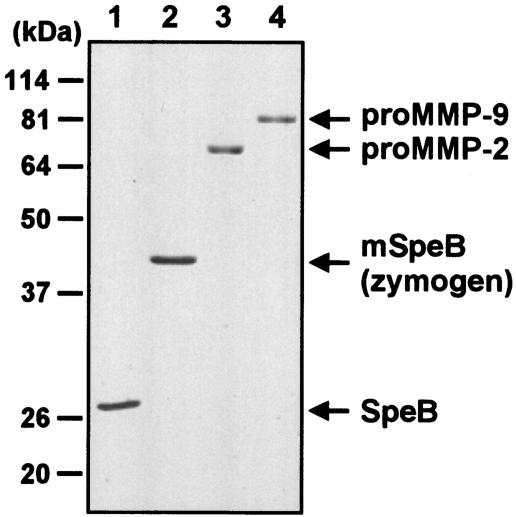
The yield of recombinant SpeB proteins in the culture of the Rosetta (DE3)pLysS system was ∼50 mg/liter. The recombinant SpeB proteins were >95% pure, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1). Although SpeB was expressed in precursor form (zymogen) in the E. coli system, the zymogen was autocatalytically processed during purification to form mature SpeB, which appeared as a 28-kDa homogeneous band on the gel.
FIG. 1.
SDS-PAGE results for recombinant SpeB and its C192S mutant protein and recombinant human proMMP-2 and -9 (5 μg each) under reducing conditions (12% acrylamide). Lanes: 1, SpeB; 2, C192S SpeB mutant (mSpeB; zymogen); 3, proMMP-2; 4, proMMP-9. The protein bands were stained with Coomassie brilliant blue. See the text for details.
Expression and purification of recombinant proMMP-9 and -2.
On the basis of a previous report that a baculovirus expression system provided an abundant source of recombinant human proMMP-9 (10), we also used a baculovirus system to produce recombinant human proMMP-9 and -2 in amounts and quality sufficient for investigations of the proteolytic activation of these zymogens. cDNA encoding the entire proMMP-9 gene inserted into pBluescript KS(+) (pBS-92-174) was kindly supplied by B. L. Marmer and G. I. Goldberg (Washington University, St. Louis, Mo.). preproMMP-9 cDNA was amplified by PCR with KOD DNA polymerase (Toyobo, Osaka, Japan) from a human MMP-9 pBS-92-174 vector as a template. The primers used were 5′-GTACCATATGAGCCTCTGGCAGCCCCTGGTCCTGG-3′ (sense) and 5′-GGTTACTAGTCCTCAGGGCACTGCAGGATGTCATA-3′ (antisense). The amplified DNA fragment was ligated into a cloning vector, pCR-Blunt II-TOPO (Invitrogen). pBacPAK9 (Clontech Laboratories, Inc., Palo Alto, Calif.) was used for subsequent subcloning and was ligated with the full-length gene for preproMMP-9 at an EcoRI site. The ligation mixture was used to transform the E. coli strain DH5α. One clone with a correct orientation of the preproMMP-9 gene was isolated and designated pBacPAK9-hMMP9. Sf21 cells were cotransfected with 0.5 μg of pBacPAK9-hMMP9 and BacPAK6 (Clontech) by using Bactofectin (Clontech), according to the manufacturer's instructions. Plaques of preproMMP9-positive viruses were isolated, and the recombinant proMMP-9 protein was produced according to the method described by George et al. (10), with purification by gelatin-Sepharose 4B column chromatography (Amersham). The yield of human recombinant proMMP-9 for the culture of Sf21 cells was ∼20 mg/liter. The purity of the recombinant proMMP-9 was assessed by SDS-PAGE as >95% (Fig. 1).
Recombinant proMMP-2 was also prepared by using a baculovirus expression system. First, full-length MMP-2 cDNA was cloned by reverse transcription-PCR on the basis of sequences reported earlier (19). The sense primer was 5′-GGCATATGGAGGCGCTAATGGCCCGG-3′, and the antisense primer was 5′-GGTTATCAGCAGCCTAGCCAGTCGGATTTG-3′. mRNA samples extracted from human fibrosarcoma HT-1080 cells were reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen) using an antisense primer. Aliquots of the reaction mixture were amplified by using Ex-Taq polymerase (Takara) and 30 rounds of PCR. The products were ligated into pCRII-TOPO TA cloning vector (Invitrogen), and the construct pCRII-TOPO-hMMP2 was obtained. The nucleotide sequences of the inserts were confirmed by DNA sequencing as described above. pCRII-TOPO-hMMP2 was digested with EcoRI, and a 2.0-kb insert was isolated and subcloned into pBacPAK9 to produce pBacPAK9-hMMP2. Sf21cells were cotransfected with pBacPAK9-MMP2 and BacPAK6 in the same manner as for pBacPAK9-hMMP9. Plaques formed by the putative recombinant baculovirus were isolated and screened for MMP activity by gelatin zymography, by using culture medium obtained 3 days after infection as described below (35). The isolated preproMMP-2-positive virus was designated vMMP2.
Mimic Sf9 cells (Invitrogen), which produce recombinant proteins glycosylated with terminal sialylated N-glycans similar to those synthesized by mammalian cells, were used for the culture of vMMP2 virus. Mimic Sf9 cells were grown in 500-ml spinner vessels (120 rpm; Bellco Glass, Vineland, N.J.) containing 100 ml of Grace's insect medium (Invitrogen) plus 10% heat-inactivated fetal calf serum (FCS; Sigma-Aldrich Fine Chemicals, St. Louis, Mo.), 0.1% Pluronic F-68, and 10 μg of gentamicin/ml to a cell density of ∼1.1 × 106/ml. The cells were infected by the addition of vMMP2 at a multiplicity of infection of 5 and were maintained at 28°C. The culture supernatant was harvested by centrifugation 72 h after infection, and proMMP-2 was purified by a method similar to that used for proMMP-9, as described above. The homogeneous band of the proMMP-2 protein, as shown by SDS-PAGE, appears in Fig. 1.
Analysis for proteolytic activation of proMMPs by SpeB.
SDS-PAGE and gelatin zymography were used to evaluate the activation of proMMP-9 and -2, i.e., the proteolytic processing of proMMPs by SpeB. proMMP activation was also assessed by measuring the gelatinolytic activity of MMPs induced by SpeB treatment. SDS-PAGE of SpeB-treated proMMPs was performed to determine the change in the molecular sizes of proMMPs during activation. In this assay, 2 μM proMMP-9 was incubated with various concentrations of SpeB (0.4, 2, 10, and 20 μM) in 10 mM phosphate-buffered 0.15 M saline (PBS; pH 7.4) at 37°C for 30 min, after which 10-μl aliquots of the reaction mixture containing 2 μM proMMP-9 were subjected to SDS-10% PAGE. In addition, SDS-PAGE of the reaction mixture of proMMP-9 plus SpeB was used to investigate the time profile of generation of proteolytic fragments of proMMP-9. After 2 μM proMMP-9 was incubated with 2 μM SpeB in PBS (pH 7.4) at 37°C for various times, an aliquot containing 1.8 μg of proMMP-9 was subjected to SDS-PAGE as described above. proMMP-2, with or without SpeB treatment, was similarly analyzed.
The proteolytic activation of proMMPs was further examined by using gelatin zymography as described earlier (35). Briefly, reaction mixtures of proMMPs treated with SpeB were diluted 1:2 with sample buffer (125 mM Tris-HCl buffer, pH 6.8, containing 20% glycerol, 4% SDS, and 0.02% bromophenol blue). Each mixture was evaluated by the use of electrophoresis on SDS-10% PAGE containing 1 mg of gelatin (Sigma-Aldrich)/ml; 20-μl aliquots were applied per lane. After electrophoresis, the gels were rinsed in 2.5% Triton X-100 in 40 mM Tris-HCl buffer (pH 7.6) plus 0.01% Brij 35, 10 mM CaCl2, and 2 mM ZnCl2 for 3 h at room temperature to remove SDS. The gels were incubated overnight at 37°C and were then stained with Quick CBB (Wako Pure Chemicals, Osaka, Japan) so that protein bands with gelatinolytic activity could easily be identified as clear lytic bands.
To study the gelatinolytic activities of MMPs, human placenta type I collagen (Sigma-Aldrich) was heated at 65°C for 20 min to obtain denatured collagen (i.e., gelatin), which was used as a substrate for the activated MMPs. To obtain treated samples, after 2 μM proMMP-9 was incubated with 2 μM SpeB at 37°C for 30 min, samples of proMMP-9 treated with SpeB or untreated were incubated with gelatin (7 μg) at 37°C for 30 min, followed by analysis for gelatin hydrolysis by use of SDS-10% PAGE. In some assays, so that gelatinolysis caused solely by activated MMP-9 could be estimated, SpeB was treated with 10 μg of (28 μM) E64 [trans-epoxysuccinyl-l-leucylamido(4-guanidino)-butane] (Sigma-Aldrich)/ml, a cysteine proteinase inhibitor, at 37°C for 30 min before or after the reaction of SpeB and proMMP was started.
Detection of sFasL and TNF-α released from cultured cells treated with MMP-9 and SpeB.
The SpeB-induced release of sFasL was studied with human colon cancer SW480 cells in culture. Specifically, cells at a density of 107/dish were cultured overnight in a Falcon dish (diameter, 10 cm; Becton Dickinson Labware, Franklin Lakes, N.J.) in Dulbecco's modified Eagle medium (DMEM; Invitrogen) containing 10% heat-inactivated FCS (Sigma-Aldrich). After the cells were washed twice with PBS (pH 7.4), the medium was replaced with serum-free minimal essential medium (MEM) (4 ml; Invitrogen). The SW480 cells were then incubated for 1 h in the presence or absence of 0.5 μg of native proMMP-9 or proMMP-9/ml that had been treated with SpeB at a molar ratio of 1:1 in PBS (pH 7.4) at 37°C for 30 min. Supernatants of the culture medium were obtained by centrifugation at 5,000 × g and were concentrated 50-fold by using Centricon YM-10 filters (Amicon, Bedford, Mass.). sFasL released into the medium was analyzed by Western blotting: the concentrated culture supernatants were mixed with the sample buffer as described above and processed by electrophoresis on SDS-12% PAGE and immunoblotting with an anti-FasL monoclonal antibody (BD Biosciences Pharmingen, San Diego, Calif.) at a 1:250 dilution. The protein band was visualized by using the ECL system (Amersham) (35).
The effect of MMP treatment on the release of TNF-α from murine macrophage RAW264 cells was investigated via an enzyme-linked immunosorbent assay (ELISA). Cells were cultured at a density of 106/well (Falcon 24-well plate) in DMEM plus 10% FCS for 24 h. After the cells were washed twice with PBS (pH 7.4), they were incubated with SpeB-activated proMMP-9 (2 μg/ml), prepared as described above, in MEM for 1 h. The amount of TNF-α produced in the culture supernatant was quantified by use of an ELISA kit (BioSource International, Inc., Camarillo, Calif.). In some experiments, the MMP inhibitor SI-27 [l-N-(N-hydroxy-2-isobutylsuccinamoyl)-leucyl-isobutylamine; 50 μM; Banyu Pharmaceutical Co., Tsukuba, Japan] (56) was included in the culture medium when cells were incubated with SpeB-activated proMMP-9 to determine the direct involvement of MMPs in the production of TNF-α by the cells. SI-27 had no inhibitory activity for SpeB.
In addition, we examined the direct effect of SpeB thiol proteinase activity on SW480 and RAW264 cells. Specifically, SpeB (2.8 μg/ml; 10 nM) was untreated or treated with E64 (10 μg/ml; 28 μM) at 37°C for 30 min, diluted appropriately with serum-free MEM, and added to the cell culture for the analysis of sFasL and TNF-α release.
As described above, preparation of the recombinant SpeB had a very low level of LPS contamination. However, no appreciable sFasL and TNF-α releases were observed from SW480 and RAW264 cells, even when purified LPS was added to each cell culture at the same concentration as the level of LPS contamination in the reaction mixture of SpeB.
Identification of proapoptotic activity induced by SpeB-activated proMMP.
To investigate whether cultured cells treated with SpeB-activated proMMP have proapoptotic activity, culture supernatants of RAW264 and SW480 cells were analyzed for the induction of apoptosis in a human monocyte-like cell line, U937 cells, in culture. After RAW264 or SW480 cells were incubated for 3 h with SpeB-treated proMMP-9 as described above, a 100-μl aliquot of culture supernatant (concentrated 15 times) was added to a suspension culture (100 μl; DMEM) of U937 cells used at a density of 106/well (Falcon 96-well dish). After 12 h of incubation, the U937 cells were harvested and analyzed for apoptosis by flow cytometry (FACSCalibur; BD Biosciences Immunocytometry Systems, San Jose, Calif.) using an Annexin V-FITC apoptosis detection kit (BD Biosciences Pharmingen) according to the manufacturer's instructions.
To examine whether apoptosis induction is attributable solely to sFasL or TNF-α, U937 cells were incubated with the culture supernatant of SW480 or RAW264 cells in the presence of various concentrations of neutralizing antibodies for sFasL and TNF-α (anti-FasL monoclonal immunoglobulin G [IgG] antibody [Medical and Biological Laboratories, Nagoya, Japan]; anti-TNF-α IgG antibody [R&D Systems, Inc., Minneapolis, Minn.]) or in the presence of nonimmune control IgG.
Induction of severe GAS infection in mice.
An animal model of severe GAS infection was produced in ddY mice (Sea:ddY males, 3 weeks old; Seac Yoshitomi, Ltd., Fukuoka, Japan) as reported by Okamoto et al. (43) and Akaike et al. (1), with modifications. The mice received the influenza virus A/Aichi/2/68 (H3N2) strain by inhalation of 7 × 104 PFU/ml of viral suspension (1). At 36 h after inhalation, a superinfection with GAS strain SSI-1, a clinical isolate from a TSLS patient and a gracious gift from S. Murai (Toho University, Tokyo, Japan), was produced by intranasal injection of 105 CFU of the bacteria in 20 μl of PBS; this technique resulted in severe disease due to fulminating GAS pneumonia and septicemia.
Similarly, severe GAS infection was produced by inoculating a GAS clinical isolate SSI-9 strain and its SpeB-deficient isogenic mutant into the virus-infected mice. An SpeB-deficient isogenic mutant was generated from strain SSI-9 (a gift from S. Murai) via inactivation of the speB gene of the bacteria by allelic replacement. Specifically, chromosomal DNA derived from GAS strain SSI-9 was purified and used as a template for PCR amplification of the speB gene. The primers used were 5′-CAGCCATCAATTCTGAAATCGCC-3′ (sense) and 5′-CTCACCAGCGGATCATTTGACGC-3′ (antisense). The PCR fragment was ligated into pSF152 (51). The resulting plasmid, pTM16, was used for chromosomal inactivation of the speB gene, as described previously (51). The inactivated mutant strain TR11 (speB::aad9 Spr) was then selected by using spectinomysin-containing agar plates. The lack of SpeB production by the TR-11 strain was confirmed by Western blotting with a specific anti-SpeB antibody that was purified from rabbit antiserum raised after SpeB immunization. The Western blotting was performed with the supernatant of the bacterial culture (37°C for 24 h in Todd-Hewitt broth) in the same manner as for the sFasL analysis. To produce severe GAS infection, 36 h after mice were inoculated with influenza virus as described above, GAS strain SSI-9 or TR11 (107 CFU of each bacterium in 20 μl of PBS) was intranasally administered to the animals.
All animal experiments were carried out with the approval of the Ethical Committee at the Center for Animal Resources and Development, Kumamoto University.
Detection of apoptotic changes and induction of MMP and proapoptotic molecules during severe GAS infection in mice.
Apoptotic changes in mouse lungs occurring during severe GAS infection were investigated in situ by means of the terminal deoxynucleotide transferase (TdT)-mediated dUTP-biotin nick end-labeling (TUNEL) assay as reported earlier (41). Briefly, lung tissues were fixed in 2% periodate-lysine-paraformaldehyde, embedded in Tissue-Tek OCT compound (Miles, Elkhart, Ind.), and frozen in dry ice-acetone. For the TUNEL assay, 6-μm-thick sections were TdT-mediated biotin nick-end labeled, followed by use of a streptavidin-labeled peroxidase reaction to obtain the blue color. The sections that were used for the TUNEL assay were also stained with hematoxylin-eosin (HE) for histopathological examination.
At various times after the GAS superinfection, blood was collected via a heparin-containing syringe from the superior mesenteric veins of infected mice under ether anesthesia. Plasma obtained by centrifugation was used for the assay of TNF-α and sFasL. Simultaneously with blood collection, bronchoalveolar lavage (BAL) was performed by using a cannula inserted into the trachea, with a 1-ml syringe filled with 1 ml of PBS (pH 7.4) containing 2.6 mM EDTA, as described previously (1). BAL fluid (BALF) thus recovered (usually >90% recovery) was centrifuged (600 × g for 10 min at 4°C), and the supernatant was subjected to gelatin zymography for MMP detection and to ELISA for determination of sFasL and TNF-α, as described earlier in the text. Aliquots of BALF (10 μl each) were used for gelatin zymography. Levels of TNF-α and sFasL in plasma from GAS-infected mice were also measured by using the TNF-α ELISA, as detailed earlier for the cultured cells, and a mouse FasL ELISA kit (R&D Systems), respectively.
Statistical analysis.
Statistical differences were determined by the unpaired t test or by the Mann-Whitney U test. Fisher's exact probability test was performed to analyze statistical differences in survival rates.
RESULTS
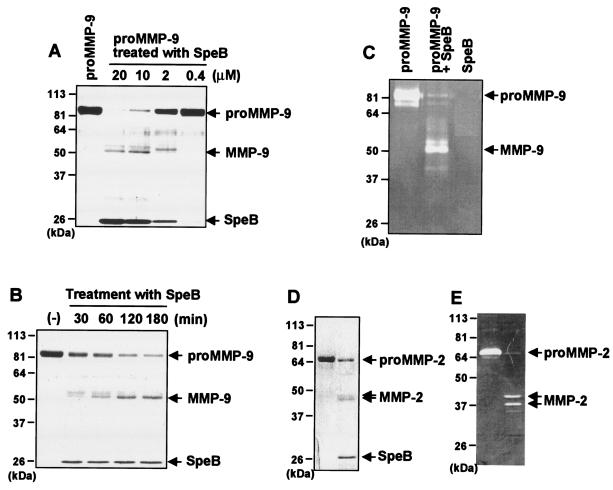
Proteolytic processing and activation of proMMP-9 by SpeB. The processing and activation of proMM-2 and -9 by SpeB were evaluated by SDS-PAGE. As shown in Fig. 2A, SDS-PAGE of SpeB-treated proMMP-9 (92 kDa) revealed that conversion of proMMP-9 to a 51-kDa fragment depended on the concentration of SpeB. In addition, with the reaction mixture of proMMP-9 and SpeB at a 1:1 molar ratio, conversion of proMMP-9 to the major 51-kDa fragment was time dependent (Fig. 2B). Gelatin zymography further showed that this 51-kDa fragment was functionally active and produced a gelatinolytic band, which indicates that SpeB effectively processes proMMP-9 to its active form, MMP-9 (Fig. 2C). In a similar fashion, 72-kDa proMMP-2 was proteolytically converted to active forms of MMP-2, as illustrated in Fig. 2D and E; two major active fragments (44 and 46 kDa) were found.
FIG. 2.
Proteolytic processing of proMMP-9 (A to C) and proMMP-2 (D and E) by SpeB. (A) proMMP-9 (2 μM) was incubated with various concentrations of SpeB (0.4, 2, 10, and 20 μM) in PBS (pH 7.4) at 37°C for 30 min, after which aliquots of the reaction mixtures containing 1.8 μg of proMMP-9 were subjected to SDS-10% PAGE. (B) Time profile of generation of proteolytic fragments from MMP-9 in the reaction mixture of proMMP-9 plus SpeB. After 2 μM proMMP-9 was incubated with 2 μM SpeB in PBS (pH 7.4) at 37°C for various times, aliquots containing 1.8 μg of proMMP-9 were subjected to SDS-10% PAGE. Similar analysis was performed with proMMP-2 with or without SpeB treatment. (C) Proteolytic activation of proMMP-9 was assessed by using gelatin zymography. Reaction mixtures of proMMP-9 treated with SpeB underwent electrophoresis on SDS-10% PAGE containing 1 mg of gelatin/ml. After electrophoresis, the gels were incubated overnight at 37°C and were stained with Coomassie brilliant blue. (D and E) SDS-PAGE and gelatin zymography for proMMP-2 treated with SpeB. proMMP-2 (2 μM) was incubated with 2 μM SpeB in PBS (pH 7.4) at 37°C for 15 min, followed by SDS-PAGE (D) and gelatin zymography (E), as described for proMMP-9. See the text for details.
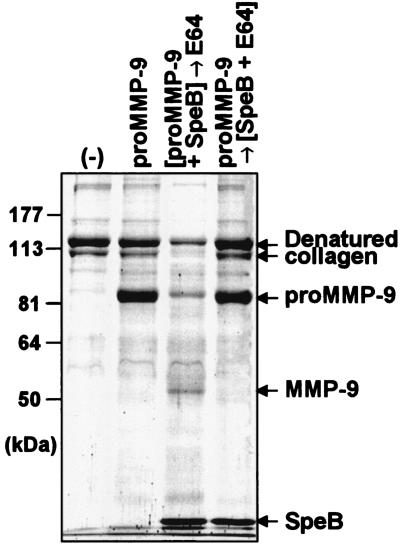
Proteolytic activation of proMMP-9 by SpeB was also evaluated by means of a gelatinolytic assay. SpeB-treated proMMP-9 degraded denatured type I collagen, whereas proMMP-9 alone had no measurable effect (Fig. 3). However, because SpeB alone, without proMMP-9, showed gelatinolytic activity (data not shown), further experiments were carried out to determine whether gelatin degradation observed with the reaction mixture of proMMP-9 plus SpeB depended on activated proMMP-9. For this purpose, the cysteine protease inhibitor E64 was added to the reaction mixture after treatment of proMMP-9 with SpeB, which completely nullified the proteolytic activity of SpeB. The result showed that SpeB-treated proMMP-9, even in the presence of E64, produced marked gelatin degradation, as seen in Fig. 3. However, when SpeB was reacted with E64 before being incubated with proMMP-9, gelatin digestion was completely abrogated (Fig. 3). SpeB digested denatured type I collagen; this reaction was also completely inhibited by E64. Similar results were obtained for proMMP-2 after treatment with SpeB (data not shown).
FIG. 3.
Proteolytic activation of proMMP-9 by SpeB as assessed by gelatinolytic activity. The gelatinolytic activity of MMP-9 produced after SpeB treatment was measured by using denatured type I collagen (gelatin) as a substrate for activated MMP-9. After 2 μM proMMP-9 was incubated with 2 μM SpeB, proMMP-9 treated with SpeB or untreated was incubated with gelatin (7 μg), followed by analysis for gelatin hydrolysis by use of SDS-10% PAGE. In some assays, E64 was added to the reaction mixture of proMMP with SpeB to inhibit SpeB proteinase activity. Lanes: (−), gelatin alone; proMMP-9, gelatin incubated with proMMP-9 alone; [proMMP-9+SpeB]→E64, gelatin incubated with SpeB-treated proMMP-9 after E64 treatment; and proMMP-9→[SpeB+E64], gelatin incubated with proMMP-9 treated with SpeB inactivated by E64. See the text for details.
These results thus indicate that SpeB is a potent activator of proMMP-9 and -2, with the effect being produced via specific, limited proteolysis of these proMMPs by SpeB.
FasL and TNF-α processing via SpeB-activated proMMP-9.
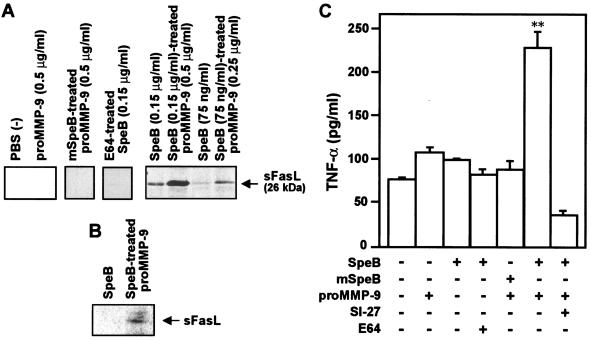
FasL is a type II transmembrane protein that induces apoptosis after binding to its receptor, Fas (39). FasL is mainly expressed on natural killer cells and activated T cells (40). MMP-7 was reported to cleave the 40-kDa membrane-bound FasL to generate a 26-kDa soluble form, sFasL (47). Human colon cancer SW480 cells expressed FasL more strongly than other cell lines (49), whereas no apparent production of MMPs, including MMP-2 and MMP-9, was observed with SW480 cells used in the present study by gelatin zymography, which is consistent with a recent report describing a lack of proMMP-2 and proMMP-9 expression (32). In the present study, SW480 cells were incubated with SpeB-treated proMMP-9, and sFasL released by the cells into the culture medium was analyzed by Western blotting. As shown in Fig. 4A, sFasL was not detected in the culture media of cells incubated with proMMP-9 alone, but it was identified in the culture supernatants of cells treated with various concentrations of SpeB-activated proMMP-9. However, when proMMP-9 preincubated with mSpeB, which has a C192S mutation at the active center of the thiol proteinase and thus lacks proteinase activity, was used to treat the cells, no measurable immunoreactive band was observed. SpeB added alone to the SW480 cell culture also induced extracellular release of sFasL, which was completely eliminated by E64, although its potential was weak compared with that of SpeB-activated proMMP-9. This result indicates that proteolytic activation of proMMP-9 was essential for triggering the extracellular release of sFasL.
FIG. 4.
Release of sFasL and TNF-α from cultured SW480 cells after treatment with MMP-9 and SpeB. (A) SW480 cells were incubated for 1 h in the presence or absence of various concentrations of native proMMP-9 or proMMP-9 that had been treated with SpeB at a molar ratio of 1:1 in PBS (pH 7.4) at 37°C for 30 min. In some experiments, the cells were treated with SpeB alone (with or without E64 treatment) or mSpeB-treated proMMP-9. The culture supernatant was subjected to Western blotting for sFasL. (B) SpeB was eliminated from the reaction mixture of SpeB alone or SpeB plus proMMP-9 before incubation with SW480 cells by using a nickel-chelated column. The culture supernatant was analyzed by Western blotting in the same manner as for panel A. (C) Effect of MMP treatment on TNF-α release from RAW264 cells as assessed by ELISA. RAW264 cells were incubated with SpeB (0.6 μg/ml)-activated proMMP-9 (2 μg/ml) for 1 h, and the amount of TNF-α produced in the culture supernatant was quantified. The cells were also incubated with 2 μg of proMMP-9/ml alone, 0.6 μg of SpeB treated with E64/ml or untreated, or mSpeB (0.6 μg/ml)-treated proMMP-9 (2 μg/ml). In some experiments, SI-27 was added to the culture medium when cells were incubated with SpeB (0.6 μg/ml)-activated proMMP-9 (2 μg/ml). The data are means plus standard errors (n = 3). **, P < 0.01 by the unpaired t test versus all other groups. See the text for details.
To clarify the contribution of SpeB-activated proMMP-9 to FasL processing by SW480 cells, SpeB was eliminated from the reaction mixture of SpeB alone or SpeB plus proMMP-9 before incubation with the cells by using a nickel-chelated column. The nickel selectively binds to the recombinant SpeB via a His tag added to the protein as described earlier. As demonstrated in Fig. 4B, SpeB-treated proMMP-9, even after nickel-chelated column chromatography, generated appreciable sFasL release from the cells, whereas SpeB alone after the same treatment produced no immunoreactive band. The minimum concentration of SpeB required for direct sFasL release from SW480 cells was 0.075 μg/ml, as assessed by the Western blotting shown in Fig. 4A. This value is much higher than the concentration of SpeB remaining after treatment of the SpeB and proMMP-9 reaction mixture after nickel-chelated column chromatography, which was <0.01 μg/ml as judged by a semiquantitative analysis with Western blotting for SpeB (data not shown). These results indicate that proMMP-9 activated by SpeB is a potent inducer of extracellular release of sFasL.
We also examined whether another important proapoptotic factor, TNF-α, is released extracellularly from cells after stimulation with SpeB or proMMP-9 activated by SpeB. The amount of TNF-α generated in the culture medium of RAW264 cells with or without proteinase treatment was quantified by use of ELISA. Similar to results for sFasL release, the greatest release of TNF-α into the culture supernatant occurred after the treatment of cells with SpeB-activated proMMP-9 (Fig. 4C). Treatment with SpeB alone or proMMP-9 alone resulted in weak TNF-α release. E64-treated SpeB and mSpeB-treated proMMP-9 showed only marginal TNF-α release. This indicates that SpeB had a direct TNF-α-processing potential for the cells, although it was weak compared with that of SpeB-activated proMMP-9. Also, the low level of TNF-α release after proMMP-9 treatment may be due to autoactivation of proMMP-9 during incubation with RAW264 cells. Increased TNF-α production by all these proteinases was, however, strongly inhibited by the MMP inhibitor SI-27. This finding suggests that extracellular release of TNF-α was caused mostly via MMP-dependent proteolytic processing of a precursor of TNF-α rather than transcriptional up-regulation of TNF-α.
Proapoptotic effects induced by culture media of cells treated with SpeB-activated proMMP.
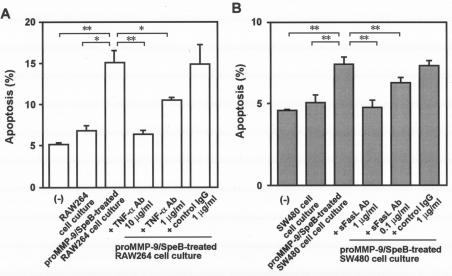
After RAW264 cells were incubated with or without SpeB-treated proMMP-9, samples of the conditioned media were collected and analyzed for apoptosis-inducing potential. To assess this potential, supernatants of these samples were added to U937 cells in culture. Culture medium from RAW264 cells treated with SpeB-activated proMMP-9 produced marked apoptotic changes in U937 cells, whereas culture medium from untreated RAW264 cells increased apoptosis of U937 cells only slightly (Fig. 5A). Thus, the apoptosis-inducing potential of the culture supernatant from treated RAW264 cells correlated well with the level of TNF-α induction shown in Fig. 4C. Similarly, apoptosis was induced with culture medium from SW480 cells treated with proMMP-9 activated by SpeB, although to a more moderate degree than with culture medium from treated RAW264 cells (Fig. 5B). This less efficient induction of apoptosis by culture medium of SW480 cells treated with MMP-9 may be due to an insufficient amount of sFasL generated from the SW480 cells to cause apoptosis of U937 cells, even though proteolytic processing of FasL induced by MMP-9 in SW480 cells did occur.
FIG. 5.
Proapoptotic activity induced in cells treated with proMMP activated by SpeB. Culture supernatants of RAW264 cells (A) and SW480 cells (B) were analyzed for induction of apoptosis in U937 cells in culture. After the RAW264 or SW480 cells were incubated for 3 h with SpeB-treated proMMP-9, samples of culture supernatants were added to cultured U937 cells, followed by analysis for apoptosis by flow cytometry using an Annexin V-FITC apoptosis detection method. In some assays, U937 cells were incubated with the culture supernatant of SW480 or RAW264 cells in the presence of various concentrations of the neutralizing antibody (Ab) for sFasL and TNF-α or in the presence of nonimmune control IgG. The data are means plus standard errors (n = 3). *, P < 0.05, and **, P < 0.01 by the unpaired t test. See the text for details.
proMMP or SpeB alone did not show appreciable apoptosis induction for U937 cells when it was added to cell culture at the same concentration used for the reaction mixture of SpeB and proMMP-9 that showed potent apoptosis-inducing activity (data not shown). More important, the apoptotic effects, which were caused by the supernatants from the cell culture with the SpeB-treated proMMP-9, were almost completely nullified by the treatment of the culture supernatant with a neutralizing antibody for sFasL or TNF-α (Fig. 5).
Induction of apoptosis, MMPs, and proapoptotic molecules during severe GAS infection in mice.
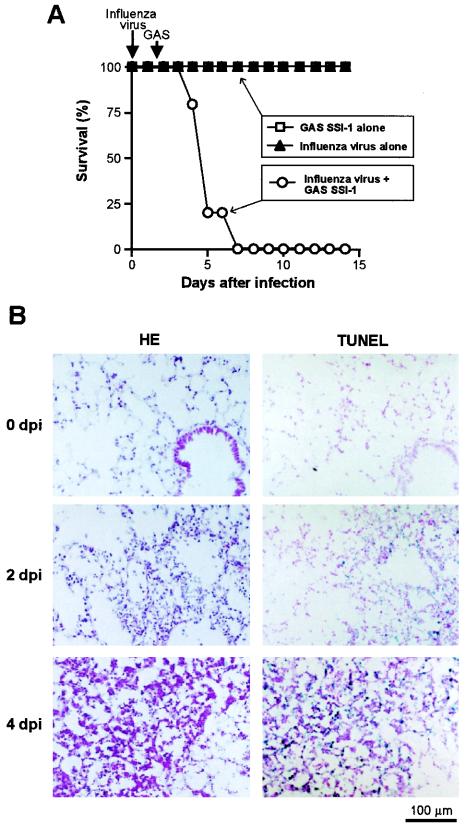
A murine model of severe GAS infection was used for analysis of proMMPs and apoptosis induction in vivo. In this model, a nonlethal dose of influenza virus plus superinfection with GAS caused severe GAS pneumonia combined with septicemia, with high mortality (Fig. 6A). Histopathological examination by means of HE staining of lung tissues revealed thickening of alveolar septa and moderate infiltration of inflammatory cells on day 2 after GAS infection (4 days after influenza virus infection) (Fig. 6B). On day 4 after GAS infection (6 days after influenza virus infection), the alveolar architecture of the lung was destroyed, and extensive infiltration of inflammatory cells, such as neutrophils and macrophages, was observed. Figure 6B also demonstrates time-related pathological changes and apoptosis in the lungs of mice assessed in situ by use of the TUNEL assay after GAS superinfection. The TUNEL reaction was evident not only in inflammatory cells but also in epithelial cells in the lung on day 2 after GAS infection, with a greater reaction seen on day 4 after GAS infection (Fig. 6B). When mice were infected with influenza virus alone and with GAS strain SSI-1 alone at the same dose as that used for virus-GAS complex infection, appreciable pathological changes were not observed in the lung as assessed by HE staining and TUNEL analysis (data not shown).
FIG. 6.
Pathological changes during severe GAS infections in mice. (A) Time profile of the survival rates of mice infected with either influenza virus alone or GAS strain SSI-1 alone and of mice infected with both influenza virus and GAS strain SSI-1. The model of severe GAS infection was produced in ddY mice, which received the influenza virus A/Aichi/2/68 (H3N2) strain by inhalation of 7 × 104 PFU/ml of viral suspension. Then, at 36 h after inhalation of the influenza virus, superinfection with GAS strain SSI-1, a clinical isolate from a TSLS patient, was produced by intranasal injection of 105 CFU of the bacteria in 20 μl of PBS. Severe diseases caused by fulminating GAS pneumonia and septicemia resulted. n = 5 for each group. P < 0.01 for groups infected with GAS SSI-1 and virus alone versus the GAS superinfection group by Fisher's exact probability test. (B) Pathological and apoptotic changes in mouse lungs occurring during severe GAS infection were studied histologically by HE staining and in situ by TUNEL assay of serial sections. 0 dpi (day postinfection), uninfected mice; 2 and 4 dpi, 2 and 4 days after GAS infection, respectively. See the text for details.
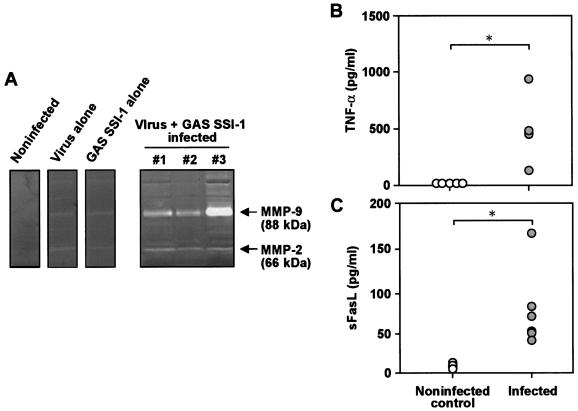
Induction of MMP was also studied by the use of gelatin zymography of BALF obtained from infected animals. Two major gelatinolytic bands corresponding to the 88- and 66-kDa active forms of murine MMP-2 and -9 were found 4 days after GAS infection in BALF from mice infected with influenza virus plus GAS, but not in uninfected control mice (Fig. 7A). Precursor and active forms of murine MMP-9 and -2 are reported to be larger than human isoforms (6, 29). MMP production was scarcely observed with the BALF from mice infected with GAS and virus alone (Fig. 7A).
FIG. 7.
Induction of MMP (A) and proapoptotic molecules (B and C) in severe GAS infection in mice. (A) BALF was obtained on day 4 after GAS superinfection of influenza virus-infected mice. The BALF supernatant was subjected to gelatin zymography for MMP detection. Representative results are shown for control groups (uninfected and infected with virus and GAS alone), and data for three different animals are shown for the combined-infection group. (B and C) Plasma from these mice was used for assay of TNF-α (B; 2 days postinfection) and sFasL (C; 4 days postinfection) by ELISA. n = 4 to 7 for each group. *, P < 0.05 by the Mann-Whitney U test. See the text for details.
More important, the levels of TNF-α and sFasL were significantly elevated in plasma from mice with superinfection compared with levels in uninfected mice (Fig. 7B and C). Therefore, induction of MMP and apoptotic changes in GAS-infected lungs seem to be closely related to the production of proapoptotic molecules, such as TNF-α and sFasL, in vivo.
The levels of these proapoptotic molecules in BALF from mice with superinfection were below the detection limit, however. This may be due to effects of dilution of the original alveolar lining fluid, which might contain biologically functioning sFasL and TNF-α, occurring during the lung lavage. Another possible explanation is that the GAS pneumonia was accompanied by septicemia with GAS, which may induce systemic response to elevate levels of sFasL and TNF-α in plasma via a mechanism involving SpeB and MMPs. It is also possible that activated MMPs generated in the lung and recovered in the blood circulation may cause systemic induction of sFasL and TNF-α release.
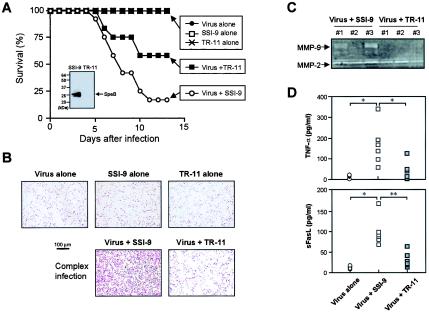
We have performed several in vivo studies using a SpeB-deficient isogenic mutant of S. pyogenes that illustrated a clear cause-and-effect relationship with regard to the pathogenesis of S. pyogenes infection involving SpeB (Fig. 8). Specifically, the mouse model of severe GAS infection was produced in the same manner as the infection with virus plus GAS strain SSI-1, except that GAS strain SSI-9 and its SpeB-deficient isogenic mutant (strain TR-11) were used instead of strain SSI-1. Mice infected with virus alone and with GAS strain SSI-I or TR-11 alone did not show significant pathological changes as assessed by the mortality of the infected animals (Fig. 8A) and by means of HE staining (data not shown) and TUNEL analysis of the mouse lung (Fig. 8B). In contrast, the combined infections with influenza virus and GAS strain SSI-1 produced a severe disease similar to the superinfection with GAS strain SSI-9. It was also found that the pathogenicity of strain SSI-9 was significantly attenuated by deletion of SpeB production as assessed by the mortality and the lung TUNEL reaction (apoptotic change) of infected animals (Fig. 8A and B). Of considerable importance is the finding that induction of sFasL and TNF-α and the activation of proMMP-9 and -2 were markedly reduced in the superinfection with strain TR-11 compared with those in superinfection with strain SSI-9 (Fig. 8C and D). These results indicate that activation of MMP-9 and -2 and generation of proapoptotic molecules, such as TNF-α and sFasL, occurred in a manner that depended on SpeB produced by GAS during infection.
FIG. 8.
Survival rate (A), apoptotic changes of the lung (B), and induction of MMPs (C) and proapoptotic molecules (D) during severe GAS infections in mice. Mice were infected with influenza virus and GAS in the same manner as for Fig. 6, except that GAS strain SSI-9 and its SpeB-deficient isogenic mutant (strain TR-11) were used to produce infections instead of GAS strain SSI-1. (A) Time profile of survival rates of mice infected with either influenza virus alone or GAS SSI-9 or TR-11 alone and of mice infected with both influenza virus and GAS strain SSI-9 or TR-11. n = 12 for each group. P < 0.01 for infections with GAS (SSI-1 and TR-11) and virus alone versus SSI-1 superinfection; P < 0.05 for SSI-1 superinfection versus TR-11 superinfection; P < 0.05 for infections with GAS (SSI-1 and TR-11) and virus alone versus TR-11 superinfection by Fisher's exact probability test. The inset shows the Western blot for SpeB production from each GAS strain. (B) Apoptotic changes in mouse lungs observed on day 5 after GAS superinfection were analyzed in situ by the TUNEL assay. (C) BALF was obtained on day 3 after GAS superinfection of influenza virus-infected mice. The BALF supernatant was subjected to gelatin zymography for MMP detection. Results for three different animals are shown for each group. (D) Plasma from these mice was used for assay of TNF-α (B; 3 days postinfection) and sFasL (C; 5 days postinfection) by ELISA. n = 3 to 6 for each group. *, P < 0.05, and **, P < 0.01 by the Mann-Whitney U test. See the text for details.
DISCUSSION
SpeB may contribute to the pathogenesis of GAS infection, particularly TSLS, in several ways, depending on the modulation of various host proteins. For example, as shown in earlier studies, this enzyme degrades human fibronectin and vitronectin (22) and cleaves interleukin-1β precursor to form the active interleukin-1β (21). It also processes the urokinase receptor of monocytic cells (55) and directly releases kinins from kininogen (15).
In the present work, we confirmed that SpeB proteolytically activated MMP-9 and -2 (Fig. 2 and 3), which then processed the release of TNF-α and sFasL from the cell membrane (Fig. 4). Furthermore, SpeB itself had gelatinase-like activity and also showed weak TNF-α- and sFasL-releasing potentials (Fig. 4). SpeB-released TNF-α and sFasL indeed caused apoptosis of cultured cells in vitro (Fig. 5). We further demonstrated, by using a murine model of severe GAS infection, that extensive apoptosis occurred in the lung (the primary infectious focus of GAS), accompanied by up-regulation of MMP-9 and -2 expression and by increased systemic TNF-α and sFasL production (Fig. 6 to 8). We thus suggest that extensive tissue damage in severe invasive GAS infection may be mediated by SpeB-activated MMPs, with SpeB and SpeB-activated proMMPs causing induction of proapoptotic molecules, such as TNF-α and sFasL.
The mechanisms of bacterially induced apoptosis have been well defined, especially for infections caused by Shigella flexneri, Salmonella enterica serovar Typhimurium, Yersinia enterocolitica, Yersinia pestis, and Pseudomonas aeruginosa. For example, SipB (Salmonella invasion protein B) of serovar Typhimurium and IpaB (invasive plasmid antigen B) of S. flexneri activate caspase 1 in host cells via type III secretion systems (14, 16). Yersinia species express two proteins, YopP (Yersinia outer protein) and YopJ, which are transferred to host cells via the type III secretion system. YopP and YopJ have been shown to block the stimulation of NF-κB, mitogen-activated protein kinases, and mitogen-activated protein kinase kinases, which are key molecules in cell survival pathways. Moreover, YopP and YopJ cleave procaspases to translocate Bid to mitochondria. Mitochondrial Bid induces the release of cytochrome c, followed by activation of caspases 3, 9, and 7, to finally result in apoptosis (8, 46). P. aeruginosa induces apoptosis in host cells because of up-regulation of CD95/CD95 ligand (Fas/FasL) on the cell surface (11). All these apoptosis induction mechanisms may help bacterial invasion of the host tissue and impair host defense against the bacteria.
Although Kuo et al. (25) and Tsai et al. (52) reported that SpeB directly induced apoptosis of human monocytes and epithelial cells via activation of the caspase pathway and that SpeB reduced phagocytic activity of monocytic cells, the detailed mechanism of SpeB-induced apoptosis, particularly that occurring in vivo, is not fully understood. SpeB is produced extracellularly and functions as an exotoxin, and similarly, proMMPs are released extracellularly by various host cells during infections, as discussed below. Therefore, extracellular events caused by SpeB, such as MMP activation, rather than intracellular signaling, are considered to be critical for SpeB-induced apoptosis occurring during GAS infection. In our present study, we clearly showed that SpeB activated human proMMP-9 and -2, with subsequent release of TNF-α and sFasL, and that the levels of these molecules were increased in vivo during severe GAS infection in mice. TNF-α and sFasL are known to cause apoptosis and an enhanced inflammatory response (18, 39). We therefore speculate that enhanced extracellular release of TNF-α and sFasL stimulated by SpeB may contribute to the pathogenesis of GAS infections via induction of apoptosis.
sFasL has been detected in several diseases, including cancer, pulmonary fibrosis, and acute respiratory distress syndrome (31), and the Fas/FasL system was suggested to play an important role in acute lung injury and fibrosis (28). Although other work indicated potent cytotoxic activity of sFasL (31, 48, 50), Josephs et al. reported that apoptotic liver injury in mice after LPS and d-galactosamine treatment was due primarily to TNF-α release, whereas increased sFasL made a minor contribution to mortality and liver injury (20). Thus, the functions of sFasL may differ in various diseases, and the pathological consequence of sFasL induction during infections may be determined by a complex interaction between host and pathogen. Further investigations should explore the precise role of sFasL in the pathogenesis of severe invasive GAS infections, such as TSLS.
MMPs constitute a family of zinc-dependent enzymes that are involved in a variety of physiological and pathological processes and diseases, such as arteriosclerosis, arthritis, cancer, inflammation, and infections. Activation of proMMPs can be achieved either by limited proteolysis of the zymogen or by effects of chemicals, such as active oxygen species and reactive nitrogen metabolites, including nitrogen dioxide and peroxynitrite (44). Because many proMMPs are secreted as inactive precursors (proMMPs) from connective tissue cells and inflammatory cells, there should be enough chance for proMMPs to be activated by SpeB that is also produced extracellularly from GAS. MMPs thus activated in the extracellular milieu may cause subsequent sFasL and TNF-α release. MMP-2 and MMP-9 have been demonstrated to be associated with acute lung injury (6). proMMP-2 is synthesized by fibroblasts, endothelial cells, and alveolar epithelial cells; proMMP-9 is produced by inflammatory cells, such as polymorphonuclear neutrophils, monocytes, macrophages, and lymphocytes. Epithelial cells and inflammatory cells, such as neutrophils and macrophages, are the major sources for MMP production in the septic foci. Also, resident and monocyte-derived activated macrophages are known to contribute to the pathology of bacterial infection by releasing MMPs and proinflammatory cytokines, such as TNF-α. In fact, an appreciable level of macrophage infiltration was observed with the mouse lungs of our severe GAS infection. It is now well accepted that TNF-α can stimulate the expression of proMMP-2 and proMMP-9 in these cells and that, conversely, MMPs can proteolytically process TNF-α in cell membranes and enhance the extracellular release of TNF-α. In addition, it was previously reported that proteinases from pathogenic bacteria, particularly enzymes belonging to the thermolysin family, effectively activated various proMMPs by means of limited proteolysis (45). It is therefore logical to expect that the proMMP-activating potential of bacterial proteinases may be a trigger for the extracellular release of TNF-α from host cells during bacterial infections. A similar mechanism involving MMP-7 is said to occur during proteolytic processing of sFasL (47), although the exact nature of this processing is unidentified. Our present work is the first demonstration of the potential role of MMPs activated by a bacterial proteinase, SpeB, in apoptotic tissue injury mediated by the proapoptotic molecules TNF-α and sFasL. This MMP-dependent pathogenesis may contribute in a critical manner to tissue damage and the dysfunction of host defenses during infections.
Cell death is believed to occur through both necrotic and apoptotic mechanisms. Necrotic cell death is considered to be an accidental type of death, caused by global cell and tissue injury. Apoptotic cell death is specifically induced, typically as a preprogrammed cellular event in individual cells. In this context, SpeB-induced MMP activation may cause nonspecific global cell death rather than specifically induced cell death via total tissue disintegration caused by ECM degradation. Certain reports have shown, however, that both apoptotic and necrotic pathways could be activated through a mechanism mediated by the Fas and TNF death receptors (30, 53). It is therefore conceivable that extensive necrotic and apoptotic tissue degradation observed in TSLS patients may result not only from ECM destruction, which eliminates structural and functional intercellular integrity, but also from specific processes and extracellular release of proapoptotic molecules, such as sFasL and TNF-α, via limited proteolysis caused by MMPs and bacterial proteinases.
In conclusion, our present work revealed a unique pathogenic function of SpeB related to its proMMP-activating and proapoptotic potential, which may support and accelerate invasion by bacteria and intensify cell and tissue injury in the host. Thus, MMPs, as well as SpeB, may become targets for therapeutic agents, particularly in combination with conventional antimicrobial agents. Further investigation is warranted to develop new tactics for treatment not only of severe invasive GAS infections, such as TSLS and necrotizing fasciitis, but also of other microbial diseases whose pathogeneses involve endogenous and microbial proteinases.
Acknowledgments
We thank Judith B. Gandy for excellent editorial work on the manuscript.
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and from the Ministry of Health, Labor and Welfare of Japan.
Editor: J. N. Weiser
REFERENCES
- 1.Akaike, T., A. Molla, M. Ando, S. Araki, and H. Maeda. 1989. Molecular mechanism of complex infection by bacteria and virus analyzed by a model using serratial protease and influenza virus in mice. J. Virol. 63:2252-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belani, K., M. Schlievert, E. L. Kaplan, and P. Ferrieri. 1991. Association of exotoxin-producing group A streptococci and severe disease in children. Pediatr. Infect. Dis. J. 10:351-354. [DOI] [PubMed] [Google Scholar]
- 3.Black, R. A., C. T. Rauch, C. J. Kozlosky, J. J. Peschon, J. L. Slack, M. F. Wolfson, B. J. Castner, K. L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K. A. Schooley, M. Gerhart, R. Davis, J. N. Fitner, R. S. Johnson, R. J. Paxton, C. J. March, and D. P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385:729-733. [DOI] [PubMed] [Google Scholar]
- 4.Burns, E. H., Jr., A. M. Marciel, and J. M. Musser. 1996. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect. Immun. 64:4744-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cone, L. A., D. R. Woodard, P. M. Schlievert, and G. S. Tomory. 1987. Clinical and bacteriologic observations of a toxic-shock like syndrome due to Streptococcus pyogenes. N. Engl. J. Med. 317:146-149. [DOI] [PubMed] [Google Scholar]
- 6.Corbel, M., S. Caulet-Maugendre, N. Germain, S. Molet, V. Lagente, and E. Boichot. 2001. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J. Pathol. 193:538-545. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yesinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of Bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 9.Gearing, A. J. H., P. Christodoulou, M. Churchill, J. Clements, A. H. Davidson, A. H. Drummond, W. A. Galloway, R. Gilbert, J. L. Gordon, T. M. Leber, M. Mangan, K. Miller, P. Nayee, K. Owen, S. Patel, W. Thomas, G. Wells, L. M. Wood, and K. Woolley. 1994. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 370:555-557. [DOI] [PubMed] [Google Scholar]
- 10.George, H. J., P. Marchand, K. Murphy, B. H. Wiswall, R. Dowling, J. Giannaras, G. F. Hollis, J. M. Trzaskos, and R. A. Copeland. 1997. Recombinant human 92-kDa type IV collagenase/gelatinase from baculovirus-infected insect cell: expression, purification, and characterization. Protein Expr. Purif. 10:154-161. [DOI] [PubMed] [Google Scholar]
- 11.Grassmé, H., S. Kirschnek, J. Riethmueller, A. Riehle, G. von Kürthy, F. Lang, M. Weller, and E. Gulbins. 2000. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290:527-530. [DOI] [PubMed] [Google Scholar]
- 12.Gubba, S., D. E. Low, and J. M. Musser. 1998. Expression and characterization of group A Streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human invasive disease episodes. Infect. Immun. 66:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauser, A. R., and P. M. Schlievert. 1990. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J. Bacteriol. 172:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hersh, D., D. M. Monach, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herward, H., M. Collin, W. Müller-Esterl, and L. Björck. 1996. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J. Exp. Med. 184:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 17.Holm, S. E., A. Norrby, M. Bergholm, and M. Norgren. 1992. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988-1989. J. Infect. Dis. 166:31-37. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, H., H. B. Shu, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 19.Huhtala, P., L. T. Chow, and K. Tryggvason. 1990. Structure of the human type IV collagenase gene. J. Biol. Chem. 265:11077-11082. [PubMed] [Google Scholar]
- 20.Josephs, M. D., F. R. Bahjat, K. Fukuzawa, R. Ksontini, C. C. Solorzano, C. K. Edwards III, C. L. Tannahhill, S. L. D. MacKay, E. M. Copeland III, and L. L. Moldawer. 2000. Lipopolysaccharide and d-galactosamine-induced hepatic injury is mediated by TNF-α and not by Fas ligand. Am. J. Physiol. 278:R1196-R1201. [DOI] [PubMed] [Google Scholar]
- 21.Kapur, V., M. W. Majesky, L.-L. Li, R. A. Black, and J. M. Musser. 1993. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 90:7676-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapur, V., S. Topouzis, M. W. Majesky, L.-L. Li, M. R. Hamrick, R. J. Hamill, J. M. Patti, and J. M. Musser. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 15:327-346. [DOI] [PubMed] [Google Scholar]
- 23.Kayagaki, N., A. Kawasaki, T. Ebata, H. Ohmoto, S. Ikeda, S. Inoue, K. Yoshino, K. Okumura, and H. Yagita. 1995. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. 182:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo, C.-F., J.-J. Wu, K.-Y. Lin, P.-J. Tsai, S.-C. Lee, Y.-T. Jin, H.-Y. Lei, and Y.-S. Lin. 1998. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect. Immun. 66:3931-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo, C. F., J. J. Wu, P. J. Tsai, F. J. Kao, H. Y. Lei, M. T. Lin, and Y. S. Lin. 1999. Streptococcal pyrogenic exotoxin B induces apoptosis and reduces phagocytic activity in U937 cells. Infect. Immun. 67:126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukomski, S., E. H. Burns, Jr., P. R. Wyde, A. Podbielski, J. Rurangirwa, D. K. Moore-Poveda, and J. M. Musser. 1998. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect. Immun. 66:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukomski, S., C. A. Montgomery, J. Rurangirwa, R. S. Geske, J. P. Barrish, G. J. Adams, and J. M. Musser. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, T. R., M. Nakamura, and G. Matute-Bello. 2003. The role of apoptosis in acute lung injury. Crit. Care Med. 31:S184-S188. [DOI] [PubMed] [Google Scholar]
- 29.Masure, S., G. Nys, P. Fiten, J. Van Damme, and G. Opdenakker. 1993. Mouse gelatinase B. cDNA cloning, regulation of expression and glycosylation in WEHI-3 macrophages and gene organisation. Eur. J. Biochem. 218:129-141. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura, H., Y. Shimizu, Y. Ohsawa, A. Kuwahara, Y. Uchiyama, and S. Nagata. 2000. Necrotic death pathway in Fas receptor signaling. J. Cell Biol. 151:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matute-Bello, G., W. C. Liles, K. P. Steinberg, P. A. Kiener, S. Mongovin, E. Y. Chi, M. Jonas, and T. R. Martin. 1999. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J. Immunol. 163:2217-2225. [PubMed] [Google Scholar]
- 32.McDonnell, S., V. Chaudhry, J. Mansilla-Soto, Z. S. Zeng, W. P. Shu, and J. G. Guillem. 1999. Metastatic and non-metastatic colorectal cancer (CRC) cells induce host metalloproteinase production in vivo. Clin. Exp. Metastasis 17:341-349. [DOI] [PubMed] [Google Scholar]
- 33.McGeehan, G. M., J. D. Becherer, R. C. Bast, Jr., C. M. Boyer, B. Champion, K. M. Connolly, J. G. Conway, P. Furdon, S. Karp, S. Kidao, A. B. McElroy, J. Nichols, K. M. Pryzwansky, F. Schoenen, L. Sekut, A. Truesdale, M. Verghese, J. Warner, and J. P. Ways. 1994. Regulation of tumour necrosis factor-α processing by a metalloproteinase inhibitor. Nature 370:558-561. [DOI] [PubMed] [Google Scholar]
- 34.Mitsiades, N., V. Poulaki, V. Kotoula, A. Leone, and M. Tsokos. 1998. Fas ligand is present in tumors of the Ewing's sarcoma family and is cleaved into a soluble form by a metalloproteinase. Am. J. Pathol. 153:1947-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyajima, S., T. Akaike, K. Matsumoto, T. Okamoto, J. Yoshitake, K. Hayashida, A. Negi, and H. Maeda. 2001. Matrix metalloproteinases induction by pseudomonal virulence factors and inflammatory cytokines in vitro. Microb. Pathog. 31:271-281. [DOI] [PubMed] [Google Scholar]
- 36.Mohler, K., P. R. Sleath, J. N. Fitzner, D. P. Cerretti, M. Alderson, S. S. Kerwar, D. S. Torrance, C. Otten-Evans, T. Greenstreet, K. Weerawarna, S. R. Knonheim, M. Petersen, M. Gerhart, C. J. Kozlosky, C. J. March, and R. A. Black. 1994. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 370:218-220. [DOI] [PubMed] [Google Scholar]
- 37.Moss, M. L., S.-L. Jin, M. E. Milla, W. Burkhart, D. M. Bickett, H. L. Carter, W. J. Chen, W. C. Clay, J. R. Didsbury, D. Hassler, C. R. Hoffman, T. A. Kost, M. H. Lambert, M. A. Leesnitzer, P. McCauley, G. McGeehan, J. Mitchell, M. Moyer, G. Pahel, W. Rocque, L. K. Overton, F. Schoenen, T. Seaton, J.-L. Su, J. Warner, D. Willard, and J. D. Becherer. 1997. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 385:733-736. [DOI] [PubMed] [Google Scholar]
- 38.Musser, J. M., K. Stockbauer, V. Kapur, and G. W. Rudgers. 1996. Substitution of cysteine 192 in a highly conserved Streptococcus pyogenes extracellular cysteine protease (interleukin 1β convertase) alters proteolytic activity and ablates zymogen processing. Infect. Immun. 64:1913-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 40.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 41.Negoescu, A., P. Lorimier, F. Labat-Moleur, C. Drouet, C. Robert, C. Guillermet, C. Brambilla, and E. Brambilla. 1996. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J. Histochem. Cytochem. 44:959-968. [DOI] [PubMed] [Google Scholar]
- 42.Norrby-Teglund, A., S. Chatellier, D. E. Low, A. McGeer, K. Green, and M. Kotb. 2000. Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur. J. Immunol. 30:3247-3255. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto, S., S. Kawabata, I. Nakagawa, Y. Okuno, T. Goto, K. Sano, and S. Hamada. 2003. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J. Virol. 77:4104-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto, T., T. Akaike, T. Nagano, S. Miyajima, M. Suga, M. Ando, K. Ichimori, and H. Maeda. 1997. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch. Biochem. Biophys. 342:261-274. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto, T., T. Akaike, M. Suga, S. Tanase, H. Horie, S. Miyajima, M. Ando, Y. Ichinose, and H. Maeda. 1997. Activation of human matrix metalloproteinases by various bacterial proteinases. J. Biol. Chem. 272:6059-6066. [DOI] [PubMed] [Google Scholar]
- 46.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 47.Powell, W. C., B. Fingleton, C. L. Wilson, M. Boothby, and M. Matrisian. 1999. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 9:1441-1447. [DOI] [PubMed] [Google Scholar]
- 48.Serrao, K. L., J. D. Fortenberry, M. L. Owens, F. L. Harris, and L. A. Brown. 2001. Neutrophils induce apoptosis of lung epithelial cells via release of soluble Fas ligand. Am. J. Physiol. 280:L298-L305. [DOI] [PubMed] [Google Scholar]
- 49.Shiraki, K., N. Tsuji, T. Shioda, K. Isselbacher, and H. Takahashi. 1997. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc. Natl. Acad. Sci. USA 94:6420-6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka, M., T. Suda, T. Takahashi, and S. Nagata. 1995. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 14:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal integration shuttle vectors for gene cloning and inactivation. Gene 120:105-110. [DOI] [PubMed] [Google Scholar]
- 52.Tsai, P. J., Y. S. Lin, C. F. Kuo, H. Y. Lei, and J. J. Wu. 1999. Group A streptococcus induces apoptosis in human epithelial cells. Infect. Immun. 67:4334-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vercammen, D., R. Beyaet, G. Denecker, V. Goossens, G. Van Loo, W. Declercq, J. Grooten, W. Fiers, and P. Vandenabeele. 1998. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 55.Wolf, B. B., C. A. Gibson, V. Kapur, I. M. Hussaini, J. M. Musser, and S. L. Gonias. 1994. Proteolytically active streptococcal pyrogenic exotoxin B cleaves monocytic cell urokinase receptor and releases an active fragment of the receptor from the cell surface. J. Biol. Chem. 269:30682-30687. [PubMed] [Google Scholar]
- 56.Wu, J., T. Akaike, K. Hayashida, T. Okamoto, A. Okumaya, and H. Maeda. 2001. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn. J. Cancer Res. 92:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zychlinsky, A., and P. Sansonetti. 1997. Apoptosis in bacterial pathogenesis. J. Clin. Investig. 100:493-495. [DOI] [PMC free article] [PubMed] [Google Scholar]