Abstract
Dendritic cells (DCs) are critical for initiating a pathogen-specific T-cell response. During chronic infections the pool of tissue DCs must be renewed by recruitment of both circulating DC progenitors and in loco differentiating monocytes. However, the interaction of monocytes with pathogens could affect their differentiation. Mycobacterium tuberculosis has been shown to variably interfere with the generation and function of antigen-presenting cells (APCs). In this study we found that when alpha interferon (IFN-α) is used as an inductor of monocyte differentiation, M. tuberculosis inhibits the generation of DCs, forcing the generation of immunoprivileged macrophage-like cells instead. Cells derived from M. tuberculosis-infected monocyte-derived macrophages (M. tuberculosis-infected MoMφ) retained CD14 without acquiring CD1 molecules and partially expressed B7.2 but did not up-regulate B7.1 and major histocompatibility complex (MHC) class I and II molecules. They synthesized tumor necrosis factor alpha and interleukin-10 (IL-10) but not IL-12. They also showed a reduced ability to induce proliferation and functional polarization of allogeneic T lymphocytes. Thus, in the presence of IFN-α, M. tuberculosis may hamper the renewal of potent APCs, such as DCs, generating a safe habitat for intracellular growth. M. tuberculosis-infected MoMφ, in fact, showed reduced expression of both signal 1 (CD1, MHC classes I and II) and signal 2 (B7.1 and B7.2), which are essential for mycobacterium-specific T-lymphocyte priming and/or activation. These data further suggest that M. tuberculosis has the ability to specifically interfere with monocyte differentiation. This ability may represent an effective M. tuberculosis strategy for eluding immune surveillance and persisting in the host.
Mycobacterium tuberculosis is the etiological agent of tuberculosis, a disease that affects nearly one-third of the world's population and causes the death of almost 3 million people per year (11, 18). M. tuberculosis infection is acquired essentially through inhalation of infectious bacilli, which are internalized by alveolar macrophages, where they survive and replicate. At the infection site, recruitment of monocytes and lymphocytes leads to the formation of granulomas, which seem to prevent dissemination of the infection (25). By mechanisms that are still not completely understood, M. tuberculosis may persist by suppressing the microbicidal activities of host macrophages and by ultimately subverting cell-mediated immune responses that eradicate the infection (34, 42).
The T-cell response to pathogens requires that antigens must be presented by professional antigen-presenting cells (APCs), such as dendritic cells (DCs), in order for antigenic peptides to be recognized on major histocompatibility complex (MHC) or CD1 molecules and to activate naïve T cells. A large body of evidence has demonstrated that DCs display multiple and contrasting properties according to the maturation stage or the nature of their precursors (5, 16, 21, 44). Upon exposure to inflammatory mediators and/or pathogens, the efficient antigen-capturing immature DCs (imDCs) are transformed into strongly stimulatory mature DCs (mDCs), which migrate with high efficiency into draining lymph nodes (6, 20, 29). In these compartments, DCs can prime T lymphocytes, which ultimately leads to both memory T-cell expansion and effector T-cell differentiation, which in turn confer immediate protection against pathogens in peripheral tissues (22-24). In lifelong chronic infections, however, the relative paucity of tissue DCs implies that they are renewed in order to sustain T-lymphocyte activation in the lymph nodes. Monocyte-derived DCs are the best candidates for this renewal, after the recruitment of monocytes to the inflammatory milieu induced by pathogens and their differentiation into DCs. Indeed, alveolar macrophages infected by M. tuberculosis secrete several cytokines, as well as chemokines, that rapidly recruit monocytes (39, 42).
A variety of mechanisms have been proposed to explain the survival of M. tuberculosis within the macrophage, including inhibition of phagosome-lysosome fusion, inhibition of the acidification of phagosomes, and resistance to killing by oxygenated metabolites. The containment of viable M. tuberculosis within specialized phagosomes may also be considered an escape mechanism; by interfering with intracellular degradation, M. tuberculosis can substantially block the processing of antigens, the loading of immunodominant peptides onto MHC class II molecules, and/or the transport of MHC-peptide complexes to the cell surface (11). Because CD4+ T lymphocytes are critically involved in host defense against M. tuberculosis, limitation of class II-dependent antigen presentation may be a significant mechanism by which M. tuberculosis evades immune surveillance. Of particular relevance, at high infection ratios M. tuberculosis has been shown to down-modulate CD1 molecules on APCs, thus also limiting CD1-restricted T-cell recognition (45). Recent studies have further underlined the ability of M. tuberculosis to interfere with APC functions through the signaling generated by engagement of the DC-SIGN receptor on the DC membrane (14, 17, 46). In line with these findings, it has recently been shown that M. tuberculosis can interfere not only with DC function but also with generation of DCs. By taking advantage of the commonly used method to generate DCs from human monocytes (i.e., culture with granulocyte-macrophage colony-stimulating factor [GM-CSF] and interleukin-4 [IL-4]), it was demonstrated that monocytes infected with live M. tuberculosis can differentiate into mDCs, but these cells displayed a unique phenotype and a defective APC function (28). However, granulomas from different patients, as well as single granulomas from the same patient, appear to be microenvironments with distinctive patterns of cytokine production (8, 9). Thus, analysis of the strategies used by M. tuberculosis to elude the immune response should take into account the notion that in the local inflammatory environment there may be significant variations in the cellular and cytokine contents during the different stages of a lifelong chronic disease such as tuberculosis. In this light, it is interesting that following infection with M. tuberculosis, both monocytes and DCs produce alpha interferon (IFN-α) (38, 50) and that among the wide spectrum of biological activities, IFN-α has also been described as a potent stimulus, in association with GM-CSF, for the differentiation of monocytes into DCs (41).
Here we show that in the presence of GM-CSF and IFN-α, M. tuberculosis inhibits the generation of DCs from monocytes, forcing the differentiation of cells displaying features of macrophages. The data presented here further expand our understanding of M. tuberculosis's interference with APC generation in infected organs.
MATERIALS AND METHODS
Growth of mycobacteria.
M. tuberculosis H37Rv (ATCC 27294) and Mycobacterium avium strain 485 were grown in Middlebrook 7H10 agar (Difco Laboratories, Detroit, Mich.) at 37°C under a humidified 5% CO2 atmosphere for 2 weeks and 1 week, respectively. Bacterial suspensions were prepared by dispersing colonies with glass beads in RPMI 1640. The tubes were vortexed and left to stand for 30 min to allow larger particles to settle. The upper supernatant was stored at −80°C until it was used. CFU were counted by the standard viable count technique in Middlebrook 7H10 agar plates (28).
Monocyte isolation, infection, and generation of DCs from infected monocytes.
Peripheral blood mononuclear cells were isolated with a Ficoll density gradient (1, 32). Monocytes were then positively sorted by using anti-CD14-labeled magnetic beads (MACS; Miltenyi Biotech, Bergesh Gladbach, Germany) and resuspended in RPMI 1640-based complete medium (31). Monocytes were infected with single-cell suspensions of M. tuberculosis at multiplicities of infection (MOIs) ranging from 0.5:1 to 10:1; an MOI of 3:1 was used unless otherwise indicated (15). In control experiments, M. tuberculosis was heat killed at 80°C for 30 min before incubation with monocytes. In some experiments, opsonization of mycobacteria was performed by 30 min of incubation at 37°C with normal human serum (7). Latex particles (Sigma, St. Louis, Mo.) were used at particle/monocyte ratios ranging from 1:1 to 5:1. The efficiency of infection or phagocytosis was quantitated by counting intracellular mycobacteria or particles in cells stained by the Kinyoun method (15). DCs were generated by culturing infected and noninfected monocytes for 6 days in complete medium containing GM-CSF (200 U/ml; Sandoz, Basel, Switzerland) and IFN-α (1,000 U/ml; Roche, Nutley, N.J.) plus, in some experiments, neutralizing anti-IL-10 antibodies or an appropriate isotype control (BD Pharmingen) at a concentration of 10 μg/ml. Lipopolysaccharide (LPS) (from Escherichia coli; Sigma) at a concentration of 0.1 μg/ml was added on the fifth day of culture to induce DC maturation. Cultures were grown both in the presence and in the absence of kanamycin (100 U/ml). Adherent cells were harvested following the gentle use of a cell scraper (Costar). The viability of infected cells was determined by trypan blue exclusion. Virtually all living cells (>95%) infected at an MOI of 3:1 contained >10 mycobacteria/cell 6 days after infection irrespective of the use of kanamycin.
Fluorescence-activated cell sorting analysis.
We used anti-HLA class I, anti-HLA class II, anti-CD1a, anti-CD1b, anti-CD3, anti-CD11c, anti-CD14, anti-CD25, anti-CD40, anti-CD80, anti-CD83, anti-CD86, anti-CD123, and anti-CCR7 antibodies and appropriate isotype controls, all purchased from BD Pharmingen (San Diego, Calif.). Biotin-conjugated anti-CD1c antibodies were obtained from Cymbus Biotechnology Ltd. (Ghandlers Ford, Hampshire, Great Britain). Human-adsorbed, fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G and goat anti-mouse immunoglobulin M (Southern Biotechnology Associates, Inc., Birmingham, Ala.) were used as the secondary antibodies. FITC-conjugated streptavidin (Sigma) was used in association with anti-CD1c. Cells were harvested and washed in phosphate-buffered saline containing 1% fetal calf serum and 0.1%NaN3 (staining buffer) and were stained by using the above-mentioned antibodies or appropriate isotype controls for background determination. After incubation and washing in staining buffer, cells were resuspended in medium suitable for cytometric analysis (FACSFlow; Becton Dickinson, Mountain View, Calif.) supplemented with 5 mg of propidium iodide per ml to exclude dead cells. Staining of intracellular cytokines in T cells was performed by using phycoerythrin-conjugated rat anti-human IL-4 or IL-10 and FITC-conjugated mouse anti-human IFN-γ in PerCp-conjugated CD3+ cells (BD Pharmingen) after fixation and permeabilization with Cytofix/Cytoperm (BD Pharmingen) used according to the manufacturer's instructions.
Stained cells were analyzed by flow cytometry by using a FACScan cytometer (Becton Dickinson) equipped with Cellquest software (Becton Dickinson). The fluorescence intensity was evaluated by computerized analysis of dot plots or histograms generated with 5,000 viable cells
Measurement of cytokine secretion.
IL-10, IL-12, and tumor necrosis factor alpha concentrations were determined by using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Abingdon, Oxon, United Kingdom) and were expressed in picograms per milliliter.
T-lymphocyte proliferation and functional polarization.
Decreasing numbers of APCs were added to 5 × 104 allogeneic cord blood T lymphocytes (CB-T lymphocytes)/well. The proliferative response was measured after 6 days of growth by using a 16-h pulse of [3H]thymidine (1 μCi/well; Amersham, Little Chalfont, Great Britain) (31). The intracellular accumulation of cytokines in allogeneic CB-T lymphocytes was analyzed at the end of a 6-day coculture with cells derived from M. tuberculosis-infected or noninfected monocytes treated or not treated with LPS. T cells were then further stimulated with 10−7 M phorbol myristate acetate and 0.5 of μg of ionomycin (Sigma) per ml for 5 h in the presence of brefeldin (Golgi plug; BD Pharmingen) at a concentration of 2 μg/ml for the last 2 h. Cells were then washed and treated as described above for fluorescence-activated cell sorting analysis.
Statistical analysis.
Data were analyzed by using the SPSS program (SPSS Inc., Chicago, Ill.). The statistical significance of the difference between groups of data with a normal distribution was determined by the analysis of variance test with Bonferroni-Dunn posttests.
RESULTS
The phenotype of cells derived from M. tuberculosis-infected monocytes differs from that of uninfected controls.
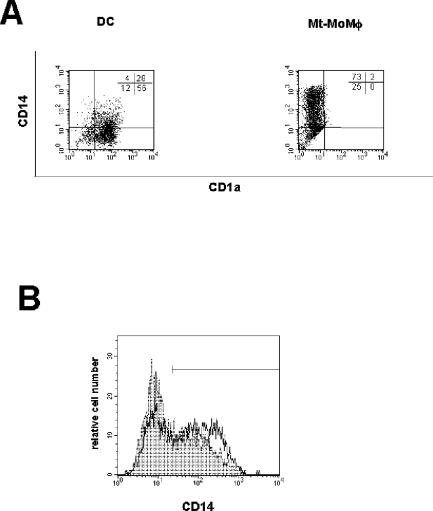
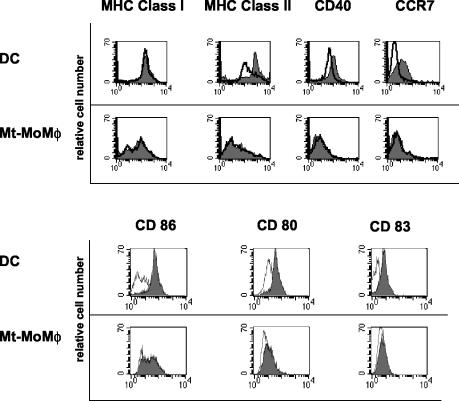
Monocytes infected with M. tuberculosis and cultured for 6 days with GM-CSF and IFN-α displayed a phenotype completely different from that of uninfected control DCs (Fig. 1A and 2). They expressed a macrophage-like phenotype since they were CD14+ CD123− (data not shown) and did not express molecules of the CD1 family (CD1a, CD1b, and CD1c). In addition, both B7.1 (CD80) and MHC classes I and II were not up-regulated, while B7.2 (CD86) was expressed in the majority of the cells. These cells were CD83 and CCR7 negative even after LPS stimulation, suggesting that upon infection with M. tuberculosis they were unable to differentiate into mDCs and thus to acquire migratory capacities. These data, together with the cellular morphology and the cytometric side and forward scatter parameters, suggest that these cells were bona fide macrophages derived from M. tuberculosis-infected monocytes (M. tuberculosis-infected MoMφ). Anti-IL-10 neutralizing antibodies induced a reduction in CD14 expression (Fig. 1B) not associated with acquisition of molecules of the CD1 family, suggesting that secretion of IL-10 may be involved in promoting differentiation of M. tuberculosis-infected monocytes cultured with IFN-α into macrophage-like cells. Notably, inhibition of DC generation by M. tuberculosis was observed at an MOI of 3:1, while infection at MOIs of ≤1:1 and ≥5:1 resulted in cells phenotypically indistinguishable from uninfected controls and in progressively greater cell mortality, respectively, demonstrating that a critical M. tuberculosis burden is required for inhibition of DC generation. Importantly, monocytes that had phagocytosed heat-killed M. tuberculosis and then were cultured with the same cytokine cocktail developed into macrophages (heat-killed M. tuberculosis-containing MoMφ) that displayed a phenotype identical to that of M. tuberculosis-infected MoMφ. These results suggest that structural mycobacterial molecules, rather than the metabolic products from living bacteria, are probably responsible for the observed effects on monocyte differentiation in the presence of IFN-α.
FIG. 1.
M. tuberculosis-infected monocytes cultured with GM-CSF and IFN-α fail to develop into DCs. M. tuberculosis-infected (MOI, 3:1) and noninfected monocytes from the same donor were cultured for 6 days with GM-CSF and IFN-α. One-half of the cultures were treated with LPS in the last 18 h. Cells were then stained with the monoclonal antibodies indicated and analyzed by flow cytometry. (A) Dot plots generated by analyzing events from 5,000 living cells. DC, DCs derived from uninfected monocytes; Mt-MoMφ, macrophage-like cells derived from M. tuberculosis-infected monocytes. Markers were set to exclude >95% of the cells stained with the appropriate isotype control antibody. (B) M. tuberculosis-infected monocytes were cultured for 6 days with GM-CSF and IFN-α in the presence of neutralizing anti-IL-10 antibodies or an appropriate isotype control antibody. Expression of CD14 on M. tuberculosis-infected monocyte-derived cells cultured with the isotype control antibody (open histogram; percentage of positive cells, 67%) or neutralizing anti-IL-10 monoclonal antibody (filled histogram; percentage of positive cells, 35%) was determined. Representative results of one of three independent experiments are shown.
FIG. 2.
M. tuberculosis-infected monocytes cultured with GM-CSF and IFN-α display features of activated macrophages with low expression of MHC molecules. M. tuberculosis-infected (MOI, 3:1) and noninfected monocytes from the same donor were cultured for 6 days with GM-CSF and IFN-α. One-half of the cultures were treated with LPS in the last 18 h. Histograms for single stained cells that were stimulated (filled histograms) or not stimulated (open histograms) with LPS are shown. DC, DCs derived from uninfected monocytes; Mt-MoMφ, macrophage-like cells derived from M. tuberculosis-infected monocytes. Representative results of one of three independent experiments are shown.
No differences in the phenotypes of cells derived from M. tuberculosis-infected monocytes were observed when the cells were cultured in the absence and in the presence of kanamycin, an aminoglycoside antibiotic with reduced efficacy against intracellular mycobacteria (47, 48). In the latter case, however, the level of recovery of living cells was more than 50%. The increase in cell viability was probably caused by the kanamycin-dependent killing of extracellular mycobacteria spreading from infected cells, which died during the culture period. Released mycobacteria would, in fact, increase randomly the MOI of single monocytes and, consequently, cell death. However, the viability of monocytes cultured in the presence of GM-CSF and IFN-α was similar to the viability of monocytes cultured in the presence of GM-CSF and IL-4 (28) after infection with M. tuberculosis at MOIs greater than 3:1, suggesting that the differentiation methods used did not produce distinct effects on cell death due to M. tuberculosis infection.
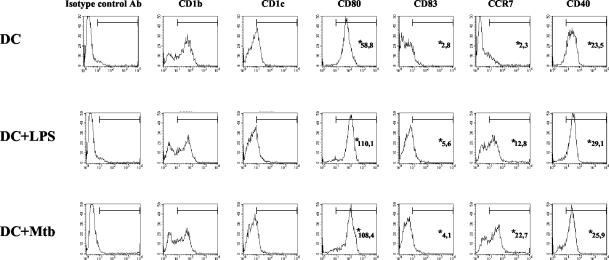
Interference with differentiation was observed in monocytes infected with live M. tuberculosis or after phagocytosis of heat-killed M. tuberculosis but not in monocytes infected with opsonized or nonopsonized M. avium used at MOIs of 3:1 and 33:1 (data not shown). We confirmed that monocytes allowed to phagocytose latex particles differentiated into DCs with a phenotype and function identical to those of DCs derived from untreated monocytes, indicating that phagocytosis per se does not interfere with monocyte differentiation (28). We also investigated whether infection with mycobacteria of differentiated imDCs induced the same phenotypic modifications as infection before differentiation. imDCs derived from uninfected monocytes cultured with GM-CSF and IFN-α for 5 days were subsequently infected at an MOI of 3:1 and cultured for an additional 48 h. These cells became strongly positive for CD40, CD80, and CD86, as well as MHC class I and II molecules, and expressed CD83 and CCR7 like LPS-stimulated mDCs (Fig. 3). This result emphasizes the unique phenotype of M. tuberculosis-infected MoMφ and indicates that M. tuberculosis interferes at early stages of DC differentiation from infected monocytes.
FIG. 3.
M. tuberculosis induces maturation of DCs cultured with GM-CSF and IFN-α. Monocytes were cultured for 5 days with GM-CSF and IFN-α to obtain imDCs. Some of the DCs were infected with M. tuberculosis (MOI, 3:1) and cultured for an additional 48 h. LPS was added to a portion of the noninfected DCs in the last 18 h of culture. Histograms were generated by analyzing events from 5,000 living cells. DC, DCs derived from uninfected monocytes; DC+LPS, DCs treated with 0.1 μg of LPS per ml; DC+Mtb, M. tuberculosis-infected DCs. Representative results of one of two independent experiments are shown, *, mean intensity of fluorescence.
Differences in cytokine secretion by DCs and M. tuberculosis-infected MoMφ.
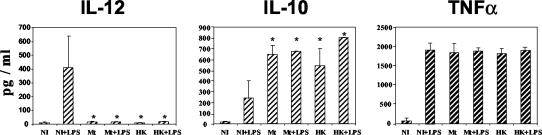
The secretion of cytokines by M. tuberculosis-infected MoMφ differed markedly from that of control LPS-stimulated DCs. M. tuberculosis-infected MoMφ did not produce IL-12 but secreted three- to fourfold-larger amounts of IL-10 than control DCs and equal amounts of tumor necrosis factor alpha (Fig. 4). This pattern of cytokine secretion differed from that of imDCs, obtained by culturing monocytes with GM-CSF and IFN-α and then infected with M. tuberculosis for 48 h. In this case, in fact, IL-12 production was not inhibited by M. tuberculosis infection (mean IL-12 concentration, 250 pg/ml [data not shown]).
FIG. 4.
M. tuberculosis causes inhibition of IL-12 and an increase in IL-10 secretion in cells derived from infected monocytes. Cytokine secretion was determined in culture supernatants of cells derived from noninfected (NI), M. tuberculosis-infected (Mt), or heat-killed M. tuberculosis-treated (HK) monocytes after 6 days of culture in the presence of GM-CSF and IFN-α. Some of the cultures were stimulated with LPS (+LPS) for the last 18 h. An asterisk indicates that the P value is <0.0167 for a comparison with the cells that were derived from noninfected monocytes that were stimulated with LPS, as determined by the analysis of variance test with Bonferroni-Dunn posttests. The results are the means and standard deviations for three independent experiments.
Antigen presentation functions of M. tuberculosis-infected MoMφ.
DCs can be distinguished from monocyte-macrophages not only by the pattern of their membrane markers but also by their superior capacity to induce T-cell priming and proliferation. First, the APC properties of M. tuberculosis-infected MoMφ and heat-killed M. tuberculosis-containing MoMφ were studied by using a mixed leukocyte reaction as an assay in which the responder cells were naïve allogeneic CB-T lymphocytes. Uninfected DCs, with and without LPS treatment, and imDCs infected for 48 h with M. tuberculosis were also included in the test. Both M. tuberculosis-infected MoMφ and heat-killed M. tuberculosis-containing MoMφ generated from the same donor induced reduced proliferation of CB-T lymphocytes compared to uninfected mDCs (Fig. 5A) or DCs infected after differentiation from normal monocytes (Fig. 5B).
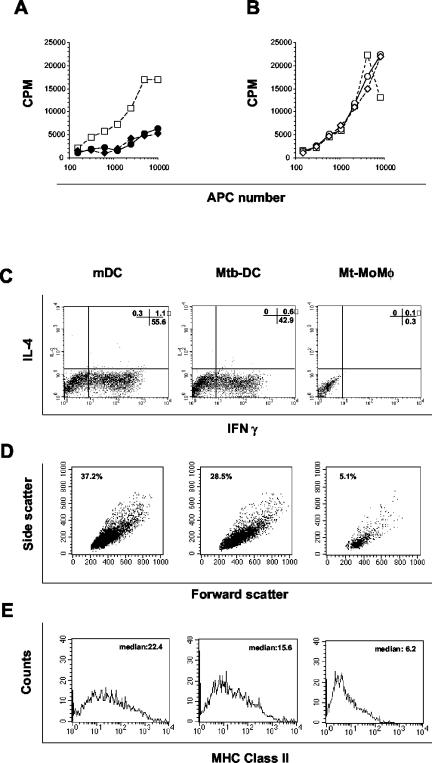
FIG. 5.
M. tuberculosis causes differentiation of monocytes into cells with hampered antigen presentation functions. (A) M. tuberculosis-infected and noninfected monocytes from the same donor were cultured for 6 days with GM-CSF and IFN-α. One-half of the cultures were treated with LPS in the last 18 h. Cells were then washed and used in decreasing numbers as APCs for allogeneic CB-T lymphocytes. Proliferation of CB-T lymphocytes stimulated with uninfected mDCs (□), M. tuberculosis-infected MoMφ (•), or heat-killed M. tuberculosis-containing MoMφ (⧫) was measured by determining [3H]thymidine incorporation after 6 days of coculture of APCs and CB-T lymphocytes. (B) imDCs were infected for 48 h with M. tuberculosis or treated with LPS and then used in decreasing numbers as APCs for CB-T lymphocytes. Proliferation of CB-T lymphocytes stimulated with uninfected mDCs (□), M. tuberculosis-infected DCs (○), or heat-killed M. tuberculosis-treated DCs (◊) was measured by determining [3H]thymidine incorporation after 6 days of coculture of APCs and CB-T lymphocytes. The results are expressed in mean counts per minute for triplicate cultures. The standard deviations were not greater than 10% of the means. (C) CB-T lymphocytes were cocultured with allogeneic mDCs generated in the presence of GM-CSF and IFN-α (mDC), imDCs infected for 48 with M. tuberculosis (Mtb-DC), or M. tuberculosis-infected MoMφ (Mt-MoMφ) for 6 days. Cells were then stimulated with phorbol myristate acetate and ionomycin for 5 h in the presence of brefeldin in the last 2 h. The intracellular cytokine accumulation was measured by flow cytometry in membrane CD3+ cells. (D) CB-T lymphocytes were cocultured with allogeneic mDCs, M.tuberculosis-infected DCs, or M. tuberculosis-infected MoMφ for 6 days and then analyzed by flow cytometry. The dot plots represent cells in the dimensional gate of lymphocytes after exclusion of propidium iodide-positive dead cells. The values indicate the percentages of gated (living) lymphocytes. (E) MHC class II expression in T lymphocytes primed by mDCs, M. tuberculosis-infected DCs, or M. tuberculosis-infected MoMφ after 6 days of coculture. The APCs used were generated by culturing monocytes from the same healthy donor. Representative results of one of three independent experiments are shown.
The low proliferative response observed in the [3H]thymidine incorporation assay was paralleled by the observation that an extremely low number of living CB-T lymphocytes were detectable after 6 days of coculture with M. tuberculosis-infected MoMφ. In addition, the low number of CB-T lymphocytes expanded by allogeneic M. tuberculosis-infected MoMφ were unable to synthesize IFN-γ, IL-4 (Fig. 5C), or IL-10 (data not shown), irrespective of LPS treatment of APCs. By contrast, control LPS-treated (uninfected) DCs and imDCs infected with M. tuberculosis after differentiation with GM-CSF and IFN-α induced significant CB-T lymphocyte secretion of IFN-γ but not secretion of IL-4 (Fig. 5C) and IL-10 (data not shown). These data suggest that mDCs and M. tuberculosis-infected DCs have the capacity to promote a typical Th1-like cell response. CB-T lymphocytes stimulated with mDCs had the morphology of blasts, as determined by microscopy and flow cytometry scatter parameters (Fig. 5D), and were CD25 positive (data not shown) and MHC class II positive (Fig. 5E). On the other hand, lymphocytes stimulated with M. tuberculosis-infected MoMφ had the morphology of resting cells with low side and forward scatter in flow cytometry and were CD25 and MHC class II negative. Altogether, these data indicate that infection of monocytes with M. tuberculosis leads to the development of cells with a markedly altered capacity for naïve T-cell priming and a substantial inability to promote the expansion of effector cells. Thus, these data further suggest that cells derived from infected monocytes are not DCs but are macrophage-like cells.
DISCUSSION
To date, it has not been clearly established whether the pool of tissue DCs is renewed through the recruitment into normal and inflamed tissues of DC progenitors or monocytes that subsequently differentiate into DCs. However, previous studies have clearly demonstrated that phagocytosing monocytes may develop into DCs and migrate to lymph nodes. It has been suggested that the phagocytosis and presentation of particulate (as opposite to soluble) antigens, such as bacteria, may represent a major function of DCs derived from monocytes (36, 37). In this light, we were interested in establishing whether the function of DCs derived from monocytes after the phagocytosis of M. tuberculosis should be considered protective or altered and potentially detrimental for the host. Along these lines, it has recently been demonstrated that M. tuberculosis-infected monocytes cultured in the presence of GM-CSF and IL-4 differentiate into mDCs, which are, however, unable to expand effector T lymphocytes (28). This method for DC generation in vitro does not necessarily reflect the actual conditions needed for monocyte differentiation in vivo. Nonetheless, it represents a widely used in vitro model that is now available, and moreover, the cytokines used may in principle be produced in the inflammatory milieu induced by M. tuberculosis (4, 10, 43). Recently, a new in vitro method for DC differentiation from human peripheral blood monocytes that uses IFN-α in association with GM-CSF has been described (41). IFN-α is readily released as a danger signal in several infections caused by viruses or bacteria, including M. tuberculosis (19, 30). It has been reported that following infection with M. tuberculosis, both monocytes and DCs produce IFN-α (38, 50). Thus, the inflammatory milieu induced by M. tuberculosis might be enriched with IFN-α that, in turn, could favor the differentiation of recruited monocytes into DCs (40). The autocrine secretion of IFN-α has also been suggested to be a modulator of monocyte differentiation in a model tissue setting in which monocytes were cocultured with endothelium grown on a type I collagen matrix (35). In this regard, in vivo experiments clearly indicated that M. tuberculosis strains with the ability to induce IFN-α displayed increased virulence and a capacity to induce disease (26, 27). The production of IFN-α was associated with the failure of induction of the IL-12-mediated protective Th1 response. These results were paralleled by the marked reduction in the survival of M. tuberculosis-infected mice intranasally inoculated with IFN-α/β but not IFN-γ. However, possible cellular mechanisms responsible for the IFN-α-associated virulence remain undefined. Thus, we asked whether M. tuberculosis interferes with the generation of DCs from infected monocytes in an IFN-α-containing culture medium that could reflect an in vivo microenvironment modulated during the chronic infection by virulent M. tuberculosis (10, 19, 30).
We demonstrated that infected monocytes were inhibited in their differentiation into DCs. In fact, M. tuberculosis skewed monocyte differentiation into a subset with macrophage-like characteristics (M. tuberculosis-infected MoMφ). M. tuberculosis-infected MoMφ retained CD14 and remained CD83 and CCR7 negative, even after LPS treatment, but, unlike monocytes, the majority of cells had up-regulated B7.2. In agreement with their macrophage-like phenotype, M. tuberculosis-infected MoMφ showed a severely reduced capacity to induce proliferation of allogeneic CB-T lymphocytes compared to mDCs. There was no expansion of alloreactive T cells, and the few living lymphocytes that it was possible to analyze were not polarized.
We noted that M. tuberculosis, while suppressing IL-12 production, strongly amplified IL-10 secretion of IFN-α-cultured monocytes, suggesting that this effect may have a major role in inhibiting the differentiation of monocytes into DCs and favoring the generation of macrophages instead (2, 3). This possible autocrine effect is also supported by previous results obtained with IL-10 added to cultures of M. tuberculosis-infected DCs (13). Moreover, in the presence of anti-IL-10 antibodies, we observed reduced macrophage differentiation, as measured by CD14 expression. This result is consistent with the possible role of IL-10 in the inhibition of DC differentiation.
Finally, the recent observation that M. tuberculosis inhibits selectively the activation of STAT-1 by IFN-α, but not by IFN-γ, in infected monocytes (33) suggests a possible mechanism of M. tuberculosis interference with monocyte differentiation into DCs that deserves further investigation. Irrespective of the mechanism, if the M. tuberculosis-mediated shift of monocyte differentiation observed in the presence of IFN-α operates in vivo, the net result would be, on the one hand, a reduced availability of professional APCs qualified to reach secondary lymphoid organs and capable of activating specific T lymphocytes and, on the other hand, the generation of immunoprivileged macrophage-like cells which have reduced expression of both T-lymphocyte stimulatory and costimulatory molecules. Macrophages are critical cells in the pathogenesis of tuberculosis, representing the host cell type for M. tuberculosis. To safely persist within macrophages, however, M. tuberculosis must prevent the conversion of macrophages into effector cells capable of killing intracellular bacteria (12, 49). In this regard, the ability of M. tuberculosis to induce the differentiation of macrophage-like cells lacking both signal 1 and signal 2 required for T-cell activation could be considered a powerful mechanism that M. tuberculosis uses to circumvent immune recognition and persist as an intracellular pathogen.
The composition of the cellular infiltrate within tubercular lesions varies with the evolution of the disease. In addition, in different patients, as well as in different granulomas within the same patient, the immunophenotype of infiltrating cells and, as a consequence, the local cytokine production vary markedly. Thus, every granuloma appears to be a microenvironment with its own pattern of cytokine production (8, 9). The present data, together with the data described previously (28), suggest that M. tuberculosis has the ability to interfere with monocyte differentiation irrespective of the composition of the cytokines in the local microenvironment. Thus, we propose that the interference with monocyte differentiation represents a major strategy of M. tuberculosis for eluding immune surveillance and/or for creating an ideal habitat in which to persist and eventually cause disease.
Acknowledgments
We thank Vincenzo Barnaba, Filippo Belardelli, and Cristiana Chelucci for criticism and discussions. We also thank Antonio Cassone and Gennaro De Libero for their helpful comments and suggestions during the project and for their critical reading of the manuscript.
This work was supported by Istituto Superiore di Sanità grants 50B/C and 502.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Accapezzato, D., R. Nisini, M. Paroli, G. Bruno, F. Bonino, M. Houghton, and V. Barnaba. 1998. Generation of an MHC class II-restricted T cell epitope by extracellular processing of hepatitis delta antigen. J. Immunol. 160:5262-5266. [PubMed] [Google Scholar]
- 2.Allavena, P., L. Piemonti, D. Longoni, S. Bernasconi, A. Stoppacciaro, L. Ruco, and A. Mantovani. 1998. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 28:359-369. [DOI] [PubMed] [Google Scholar]
- 3.Aman, M. J., T. Tretter, I. Eisenbeis, G. Bug, T. Decker, W. E. Aulitzky, H. Tilg, C. Huber, and C. Peschel. 1996. Interferon-alpha stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood 87:4731-4736. [PubMed] [Google Scholar]
- 4.Baliko, Z., L. Szereday, and J. Szekeres-Bartho. 1998. Th2 biased immune response in cases with active Mycobacterium tuberculosis infection and tuberculin anergy. FEMS Immunol. Med. Microbiol. 22:199-204. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 6.Cella, M., F. Sallusto, and A. Lanzavecchia. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10-16. [DOI] [PubMed] [Google Scholar]
- 7.Fattorossi, A., R. Nisini, J. G. Pizzolo, and R. D'Amelio. 1989. New, simple flow cytometry technique to discriminate between internalized and membrane-bound particles in phagocytosis. Cytometry 10:320-325. [DOI] [PubMed] [Google Scholar]
- 8.Fenhalls, G., G. R. Squires, L. Stevens-Muller, J. Bezuidenhout, G. Amphlett, K. Duncan, and P. T. Lukey. 2002. Associations between Toll-like receptors and IL-4 in the lungs of patients with tuberculosis. Am. J. Respir. Cell Mol. Biol. 29:28-38. [DOI] [PubMed] [Google Scholar]
- 9.Fenhalls, G., L. Stevens, J. Bezuidenhout, G. E. Amphlett, K. Duncan, P. Bardin, and P. T. Lukey. 2002. Distribution of IFN-gamma, IL-4 and TNF-alpha protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology 105:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenhalls, G., A. Wong, J. Bezuidenhout, P. van Helden, P. Bardin, and P. T. Lukey. 2000. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect. Immun. 68:2827-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 12.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortsch, D., M. Rollinghoff, and S. Stenger. 2000. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J. Immunol. 165:978-987. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 16.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J. V. Ravetch, R. M. Steinman, and M. C. Nussenzweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann, S. H., and U. E. Schaible. 2003. A dangerous liaison between two major killers: Mycobacterium tuberculosis and HIV target dendritic cells through DC-SIGN. J. Exp. Med. 197:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann, S. H. E. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 19.Lande, R., E. Giacomini, T. Grassi, M. E. Remoli, E. Iona, M. Miettinen, I. Julkunen, and E. M. Coccia. 2003. IFN-alphabeta released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J. Immunol. 170:1174-1182. [DOI] [PubMed] [Google Scholar]
- 20.Lanzavecchia, A. 1999. Dendritic cell maturation and generation of immune responses. Haematologica 84(Suppl. EHA 4):23-25. [PubMed]
- 21.Lanzavecchia, A. 1998. From antigen presentation to T-cell activation. Res. Immunol. 149:626. [DOI] [PubMed] [Google Scholar]
- 22.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 290:92-97. [DOI] [PubMed] [Google Scholar]
- 23.Lanzavecchia, A., and F. Sallusto. 2001. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr. Opin. Immunol. 13:291-298. [DOI] [PubMed] [Google Scholar]
- 24.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263-266. [DOI] [PubMed] [Google Scholar]
- 25.Lauzardo, M., and D. Ashkin. 2000. Phthisiology at the dawn of the new century. Chest 117:1455-1473. [DOI] [PubMed] [Google Scholar]
- 26.Manca, C., L. Tsenova, C. E. Barry III, A. Bergtold, S. Freeman, P. A. Haslett, J. M. Musser, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 162:6740-6746. [PubMed] [Google Scholar]
- 27.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc. Natl. Acad. Sci. USA 98:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariotti, S., R. Teloni, E. Iona, L. Fattorini, F. Giannoni, G. Romagnoli, G. Orefici, and R. Nisini. 2002. Mycobacterium tuberculosis subverts the differentiation of human monocyte into dendritic cell. Eur. J. Immunol. 32:3050-3058. [DOI] [PubMed] [Google Scholar]
- 29.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 30.Muller, H., and S. Kruger. 1994. Immunohistochemical analysis of cell composition and in situ cytokine expression in HIV- and non-HIV-associated tuberculous lymphadenitis. Immunobiology 191:354-368. [DOI] [PubMed] [Google Scholar]
- 31.Nisini, R., A. Fattorossi, C. Ferlini, and R. D'Amelio. 1996. One cause for the apparent inability of human T cell clones to function as professional superantigen-presenting cells is autoactivation. Eur. J. Immunol 26:797-803. [DOI] [PubMed] [Google Scholar]
- 32.Nisini, R., M. Paroli, D. Accapezzato, F. Bonino, F. Rosina, T. Santantonio, F. Sallusto, A. Amoroso, M. Houghton, and V. Barnaba. 1997. Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. J. Virol. 71:2241-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhakar, S., Y. Qiao, Y. Hoshino, M. Weiden, A. Canova, E. Giacomini, E. Coccia, and R. Pine. 2003. Inhibition of response to alpha interferon by Mycobacterium tuberculosis. Infect. Immun. 71:2487-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-875. [DOI] [PubMed] [Google Scholar]
- 35.Qu, C., T. M. Moran, and G. J. Randolph. 2003. Autocrine type I IFN and contact with endothelium promote the presentation of influenza A virus by monocyte-derived APC. J. Immunol. 170:1010-1018. [DOI] [PubMed] [Google Scholar]
- 36.Randolph, G. J., S. Beaulieu, S. Lebecque, R. M. Steinman, and W. A. Muller. 1998. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282:480-483. [DOI] [PubMed] [Google Scholar]
- 37.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 38.Remoli, M. E., E. Giacomini, G. Lutfalla, E. Dondi, G. Orefici, A. Battistini, G. Uze, S. Pellegrini, and E. M. Coccia. 2002. Selective expression of type I IFN genes in human dendritic cells infected with Mycobacterium tuberculosis. J. Immunol. 169:366-374. [DOI] [PubMed] [Google Scholar]
- 39.Sadek, M. I., E. Sada, Z. Toossi, S. K. Schwander, and E. A. Rich. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513-521. [DOI] [PubMed] [Google Scholar]
- 40.Santini, S. M., T. Di Pucchio, C. Lapenta, S. Parlato, M. Logozzi, and F. Belardelli. 2003. A new type I IFN-mediated pathway for the rapid differentiation of monocytes into highly active dendritic cells. Stem Cells 21:357-362. [DOI] [PubMed] [Google Scholar]
- 41.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schluger, N. W., and W. N. Rom. 1998. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157:679-691. [DOI] [PubMed] [Google Scholar]
- 43.Seah, G. T., G. M. Scott, and G. A. Rook. 2000. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J. Infect. Dis. 181:385-389. [DOI] [PubMed] [Google Scholar]
- 44.Steinman, R. M., S. Turley, I. Mellman, and K. Inaba. 2000. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenger, S., K. R. Niazi, and R. L. Modlin. 1998. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J. Immunol. 161:3582-3588. [PubMed] [Google Scholar]
- 46.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Broek, P. J. 1989. Antimicrobial drugs, microorganisms, and phagocytes. Rev. Infect. Dis. 11:213-245. [DOI] [PubMed] [Google Scholar]
- 48.Van der Auwera, P. 1990. Interactions between antibiotics and phagocytosis in bacterial killing. Scand. J. Infect. Dis. Suppl. 74:42-48. [PubMed] [Google Scholar]
- 49.Vanham, G., Z. Toossi, C. S. Hirsch, R. S. Wallis, S. K. Schwander, E. A. Rich, and J. J. Ellner. 1997. Examining a paradox in the pathogenesis of human pulmonary tuberculosis: immune activation and suppression/anergy. Tuber. Lung Dis. 78:145-158. [DOI] [PubMed] [Google Scholar]
- 50.Weiden, M., N. Tanaka, Y. Qiao, B. Y. Zhao, Y. Honda, K. Nakata, A. Canova, D. E. Levy, W. N. Rom, and R. Pine. 2000. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J. Immunol. 165:2028-2039. [DOI] [PubMed] [Google Scholar]