Abstract
Mannheimia haemolytica infection of the lower respiratory tract of cattle results in a bronchofibrinous pneumonia characterized by massive cellular influx and lung tissue remodeling and scarring. Since altered levels of gelatinases and their inhibitors have been detected in a variety of inflammatory conditions and are associated with tissue remodeling, we examined the presence of gelatinases in lesional and nonlesional lung tissue obtained from calves experimentally infected with M. haemolytica. Lesional tissue had elevated levels of progelatinase A and B and active gelatinase A and B when compared with nonlesional tissue obtained from the same lung lobe. In vitro, M. haemolytica products stimulated production of gelatinase B, but not its activation, by bovine monocytes. Alveolar macrophages showed constitutive production of gelatinase B but no change in response to M. haemolytica products. Bovine neutrophils exposed to M. haemolytica products also released gelatinase B, and there was a significant increase in the activated form of this enzyme. These effects were virtually identical when recombinant O-sialoglycoprotease was used to stimulate these cells. M. haemolytica products also enhanced the expression by bovine monocytes and alveolar macrophages of the tissue inhibitor of metalloproteinase 1. Our results provide evidence that matrix metalloproteinases are activated in lung lesions from cattle with shipping fever and that M. haemolytica virulence products induce production, release, and especially activation of gelatinase B by bovine inflammatory cells in vitro.
Bronchofibrinous pneumonia (shipping fever) is a respiratory disease in cattle that is characterized by cellular influx and interalveolar and interlobular fibrosis in the lungs of affected animals (3, 7, 18, 20, 64, 65). Lung lesions consistently show areas of coagulation necrosis, extensive fibrin deposits, and intense cellular infiltration into the alveoli (5, 63, 65). Economic losses to the beef industry due to shipping fever are estimated at over one billion dollars per year in North America alone (64). Causes of this pneumonic condition are multifactorial, including a combination of stress or viral infection, with a final acute illness due most often to the bacteria Mannheimia (Pasteurella) haemolytica serotype A1 (16, 39, 64). M. haemolytica serotype A1 is a normal resident of the upper respiratory flora of most cattle, but when aspirated into the lower respiratory tract of immunocompromised animals, it is able to colonize the lungs and induce an inflammatory reaction (65).
M. haemolytica was originally classified in the genus Pasteurella (50), but more recent genetic characterization has resulted in reclassification of 11 serotypes of biotype A in the new genus Mannheimia (4, 9). M. haemolytica is a trehalose-negative coccobacillus in which heat-stable lipopolysaccharide (LPS) constitutes 12 to 25% of the dried cell wall (66). During logarithmic growth, the bacteria produce and release a heat-labile exotoxin, termed leukotoxin (Lkt), and a heat-labile enzyme, termed O-sialoglycoprotease (Gcp) (1, 31). Both LPS and Lkt have been implicated in initiating an inflammatory response in cattle in vivo, likely through their activities on leukocytes (20, 57, 59, 65).
LPS activates bovine alveolar macrophage expression and production of the proinflammatory cytokines tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-8 (25, 37, 67, 68), all of which are elevated in the lung tissue and bronchoalveolar lavage fluid of cattle affected with shipping fever (8, 33, 46, 68). Moreover, LPS complexes with and seems to act synergistically with Lkt (13, 28). M. haemolytica Lkt is a member of the RTX (repeat in toxin) family of toxins, which also includes cytolysins produced by Escherichia coli and Actinobacillus species (13, 60). The RTX toxins are characterized by the presence of glycine-rich repeats at the C termini, comparable mechanisms of secretion, genetic homology, and similar bioactivities (28, 62). The 105-kDa Lkt protein is produced as an inactive precursor that is activated by posttranslational acylation (28, 31, 62).
Lkt binds target cells via the β2 integrin lymphocyte function-associated antigen 1 (21). High doses of Lkt cause the lysis of ruminant leukocytes by membrane pore formation when paralleled by a rise in intracellular calcium levels (11, 12, 45, 52). Lower doses of Lkt are chemotactic and stimulate active degranulation of bovine neutrophils in vitro (32, 36). Mutation experiments showed that if Lkt is not produced by M. haemolytica or if an inactive Lkt is produced, cattle infected with the bacteria have reduced lung pathology, indicating the importance of Lkt in this disease pathogenesis (20, 59). Unlike the information available about LPS and Lkt, there has been little indication to date of the role of Gcp in bronchofibrinous pneumonia, although a recently published study showed that vaccination with a recombinant Gcp fusion protein induced some protection against experimental challenge with M. haemolytica (54). Gcp is a 35-kDa protein that selectively cleaves O-glycosylated glycoproteins from cell surfaces (2, 58). There is evidence that bovine platelets have altered adhesive properties (40) and human platelets have altered aggregation and degranulation responses (23) when incubated in the presence of Gcp. Further work is required to investigate the potential role of Gcp as an M. haemolytica virulence factor.
An inflammatory response is initiated to combat the activities of virulence factors during M. haemolytica infection in the lung. The inflammatory process generally begins when resident macrophages initiate a cascade of events that recruit leukocytes, including neutrophils and monocytes, from the circulation through the endothelium and basement membrane and into infected tissue, an event requiring matrix proteolysis (24, 42). Once in the tissue, leukocytes interact with bacteria and virulence factors and inflammatory mediators, become activated, and produce or release additional enzymes. These are able to, but may do more than, destroy invading pathogens. Damaged areas of tissue are replaced by abnormal scar tissue that in the case of animals affected with M. haemolytica leads to chronic health problems. Despite an understanding of the general processes of inflammatory cell recruitment and activation, there has been little work examining how M. haemolytica virulence factors are involved in stimulating host cell damage and remodeling tissue within the lungs of affected cattle.
Recent studies in other species have implicated matrix metalloproteinases (MMPs), in inflammatory disease-associated lung tissue damage. The family of MMPs comprises a group of zinc-binding proteases, released in a zymogen form, that are able to degrade at least one component of the extracellular matrix or basement membrane (39). Two MMPs of particular importance to lung tissue damage are gelatinase A (MMP-2; 72-kDa type IV collagenase) and gelatinase B (MMP-9; 92-kDa type IV collagenase), collectively termed the gelatinases. Gelatinase A is constitutively produced (at low levels) by macrophages, lymphocytes, dendritic cells, and fibroblasts, whereas gelatinase B is inducible in these cell types (44). Conversely, neutrophils do not produce gelatinase A but rather store gelatinase B in their granules; in bovine neutrophils gelatinase B is stored in tertiary granules (44, 29). As with other MMPs, the gelatinases are released as progelatinases that are activated after the removal of an 80-amino-acid residue propeptide by proteases such as plasmin or other MMPs (38, 43). Active gelatinases target type IV collagen and destroy basement membrane tissue and are also able to degrade other matrix components (43).
The proteolytic actions of MMPs are inhibited by noncovalent interaction with tissue inhibitors of metalloproteinases (TIMPs) (17). The regulation of gelatinase activity is complex, but generally it has been recognized that an upset in the balance of MMPs with their inhibitors is involved in the pathogenesis of inflammatory, pulmonary, and neoplastic diseases. Therefore, this study was designed to investigate the presence of gelatinases and their inhibitors in the lung tissue of animals infected with M. haemolytica and to determine if gelatinase or inhibitor levels are altered by M. haemolytica virulence factors in an in vitro model of bovine inflammation.
MATERIALS AND METHODS
M. haemolytica products.
M. haemolytica culture supernatant (CS) was prepared as previously described (52, 53). The 50% effective dose of the CS was 3 mg/ml; this amount of CS resulted in lysis of 50% of cells from the bovine lymphocyte line BL3 as determined by neutral red viability (52, 53). Lyophilized CS was resuspended to a concentration of 20 mg of CS per ml of sterile water. Recombinant Gcp (Cederlane Laboratories, Hornby, Ontario, Canada) was resuspended in sterile water to a concentration of 2.4 mg/ml. Heat-labile components of the preparations were inactivated by incubating aliquots of the CS and of Gcp at 55°C for 45 min to result in heat-treated (HT) culture supernatant (HTCS) and HTGcp, respectively. Aliquots of all preparations were stored at −20°C.
Calf challenge.
The calf challenge protocol was approved by the University of Guelph Local Animal Care Committee, as per guidelines of the Canadian Council on Animal Care. Six male Holstein calves (aged 3 to 4 months) were inoculated by intrabronchial infusion with 4.4 × 1011 CFU of M. haemolytica as previously described (54) (although high, this dose is necessary to reliably induce disease in healthy, unstressed animals). Six days postinoculation, animals were euthanized with barbiturate overdoses, lungs were scored for lesions according to established criteria (53, 54), and samples were taken from both lesion areas (presence of lesions and necrosis upon gross examination) and nonlesion areas (healthy tissue, no lesions visible upon gross examination) of lungs from each calf. Tissue samples were immediately frozen in liquid nitrogen and stored at −80°C. Frozen lung samples were homogenized in lysis buffer (48 mM Tris-HCl [pH 7.5], 144 mM NaCl2, 0.48 mM MgCl2, 0.19 mM EGTA, 1% Triton X-100, 0.96 mM phenylmethylsulfonyl fluoride, 0.019 mg of aprotinin per ml, 0.29 mg of dithiothreitol per ml). Samples were incubated on ice for 30 min and then centrifuged to remove cellular debris. The protein-containing supernatant was aliquoted and stored at −80°C.
Monocyte assays.
Whole blood from healthy adult Holstein cows was centrifuged, and the buffy coat layer was isolated. Red cells in the buffy coat were lysed with 9 volumes of 4 M ammonium chloride for 2 min. The suspension was centrifuged and the white cell pellet was isolated. After two washes with Ca2+- and Mg2+-free Hanks’ balanced salt solution (HBSS), 108 white cells were plated in 100-mm culture dishes in RPMI medium plus 2% fetal bovine serum and cultured for 1 h at 37°C and 5% CO2. Dishes were washed twice to remove nonadherent cells, leaving adherent monocytes. Examination of cytospins from plated material and from culture washes confirmed that greater than 90% of the cells adherent to the plates were monocytes. Monocytes were incubated for 0, 1, 2, 4, and 8 h in fresh medium alone, in medium with CS or HTCS at a final concentration of 0.1 mg/ml, or in medium with 10 μg of Gcp or HTGcp per ml. Following incubation, cell-free monocyte releasate was collected, concentrated five times by lyophilization, resuspended in sterile water, and stored at −80°C.
Alveolar macrophage assays.
Lungs were obtained from healthy adult cattle and lavaged by using phosphate-buffered saline. The lavage fluid was collected and filtered through a 22-μm-pore-size nylon membrane and centrifuged, and the cell pellet was isolated and resuspended in a smaller volume of Ca2+- and Mg2+-free HBSS. The cells were separated by using a continuous gradient (5 to 30%) of Optiprep (MJS Biolynx Inc., Brockville, Ontario, Canada). The resuspended cells were applied to the gradient and centrifuged at 800 × g for 20 min, and the cell layer containing alveolar macrophages was isolated. After two washes with Ca2+- and Mg2+-free HBSS, 107 cells were plated in 100-mm culture dishes in phenol red-free RPMI medium plus 2% fetal bovine serum and cultured for 1 h at 37°C and 5% CO2. Dishes were washed twice to remove nonadherent cells. An examination of cytospins from plated material and from culture washes confirmed that the majority of cells adherent to the plates were alveolar monocytes. Macrophages were incubated for a further 4 h in fresh medium alone or in medium with CS or HTCS at a final concentration of 0.1 mg/ml. Following incubation, cell-free alveolar macrophage releasate was collected, concentrated five times by lyophilization, resuspended in sterile water, and stored at −80°C.
Neutrophil assays.
Whole blood from healthy adult Holstein cows was centrifuged, and the buffy coat and two-thirds of the red cell layer were removed. Red cells in the remaining layer were lysed with 9 volumes of 4 M ammonium chloride for 2 min. The suspension was centrifuged, and the granulocyte pellet was isolated. After two washes with Ca2+- and Mg2+-free HBSS, neutrophils were resuspended in HBSS containing 0.1% bovine serum albumin. Cytospins confirmed that isolates contained greater than 95% neutrophils. A total of 2.5 × 106 cells were added to each reaction tube and incubated in 0.1 mg of CS or HTCS per ml or 10 μg of Gcp or HTGcp per ml for 30 min at 37°C. Tubes were then centrifuged to pellet cells, and cell-free neutrophil releasate was removed, aliquoted, and stored at −80°C.
Gelatin zymography.
Protein concentrations of inflammatory cell releasates were determined by Bradford assay and confirmed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) prior to zymography. Zymography for progelatinase A and B and active gelatinase A and B was performed as described previously (55). Briefly, 15 μg of total lung protein or 45 μg of conditioned-media protein samples from monocytes, alveolar macrophages, or neutrophils was separated by electrophoresis by nonreducing SDS-10% PAGE containing 0.5% gelatin. After electrophoresis, gels were washed twice for 30 min with 2.5% Triton X-100 and then incubated for 18 h at 37°C in buffer (pH 8.0) containing 50 mM Trizma base, 5 mM CaCl2, and 1 μM ZnCl2. Alternatively, the buffer also contained 10 mM EDTA (which chelates zinc ions) to confirm MMP activity (49). After incubation, gels were stained with 0.1% Coomassie blue G-250 and destained in a solution of 5% methanol and 7% acetic acid. The presence of gelatinase was indicated by clear lysis bands on a darkly stained gel. The molecular weights of gelatinolytic bands were estimated by using prestained molecular weight markers (Kaleidoscope; Bio-Rad).
Reverse zymography.
Reverse zymography for TIMPs was performed as described previously (55), utilizing crude gelatinase A and purified mouse TIMPs (Dylan Edwards, University of East Anglia, Norwich, United Kingdom). Samples of 45 μg of total protein in nonreducing buffer were separated by SDS-12.5% PAGE containing 0.2% gelatin and 6.5% crude gelatinase A. Gels were washed twice in 2.5% Triton X for 30 min following electrophoresis and then incubated for 18 h at 37°C in a buffer of 50 mM Tris and 5 mM CaCl2. After incubation gels were stained and destained as for zymography. The activity of TIMPs was identified as dark bands on a light background and corresponded in size to purified mouse TIMPs. Additionally, the molecular weights of the bands were confirmed by using prestained molecular weight markers (Kaleidoscope; Bio-Rad).
Image processing and densitometric analysis.
Images of zymography and reverse zymography gels were digitized by scanning. Bands corresponding to gelatinases A and B from gelatin zymography and to TIMPs from reverse zymography were analyzed by using Molecular Analyst software (Bio-Rad Laboratories). Background measurements were subtracted from the integration area measured for each band. Thus, results were standardized for each gel and expressed in dimensionless units. Lung tissue protein results were obtained from six separate animals; inflammatory cell results were obtained from three to six separate experiments for each condition and cell type.
Statistical analysis.
Densitometry results were compared from replicated experiments for inflammatory cell releasates and from replicate animals for lung protein, and means were calculated for each experimental system. All data are presented as the mean ± standard error of the means (SEM). The significance of the differences among the means was determined by one-way analysis of variance, followed by Tukey's multiple comparisons method, and differences were considered significant at a P value of <0.05.
RESULTS
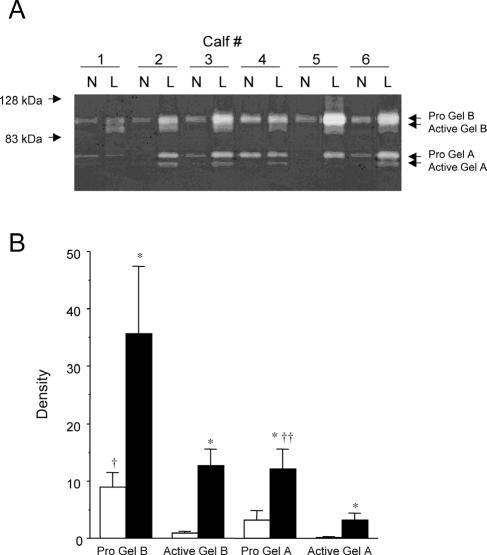
Levels of progelatinase A and B and active gelatinase A and B were found to be increased in affected areas of lung tissue. All animals challenged with M. haemolytica displayed lesions and inflammation that were visible upon gross examination at necropsy. Histological specimens collected from lesion areas indicated extensive mononuclear infiltrate, with regions of necrosis, collagen accumulation, and fibrin deposition, while specimens from nonlesion areas of the same lung did not display evidence of inflammation (results not shown). As detected by zymography (Fig. 1A), bands corresponding to approximately 92, 82, 72, and 68 kDa were evident in samples of lesional and nonlesional lung tissue. These bands were not evident in zymography gels incubated in the presence of EDTA (results not shown), indicating that the gelatin lysis was due to the activities of progelatinase A and B and of active gelatinase A and B (49). The mean levels of progelatinase A and B and active gelatinase A and B in lesional tissue were significantly higher than in nonlesional tissue (P < 0.05) (Fig. 1B).
FIG. 1.
(A) Zymography showing gelatinase activity in tissue lysates from nonlesional (N) and lesional (L) lung tissue obtained from calves 6 days after challenge with M. haemolytica. Lysis bands representing both progelatinase A and B and activated gelatinase A (MMP-2) and B (MMP-9) are more prominent in samples from lesion areas of lung. (B) Densitometric analysis of zymography from lesion and nonlesion areas of lungs from six animals. Open bar, mean density (± SEM) of gelatinases in nonlesional tissue; filled bar, mean density (± SEM) of gelatinases in lesional tissue; *, significantly higher levels compared to nonlesional tissue for each enzyme (P < 0.05); †, significantly higher levels of progelatinase B than active gelatinase B in nonlesional tissue (P < 0.05); ††, significantly higher levels of progelatinase A than active gelatinase A in lesional tissue (P < 0.05). Gel, gelatinase; Pro Gel, progelatinase.
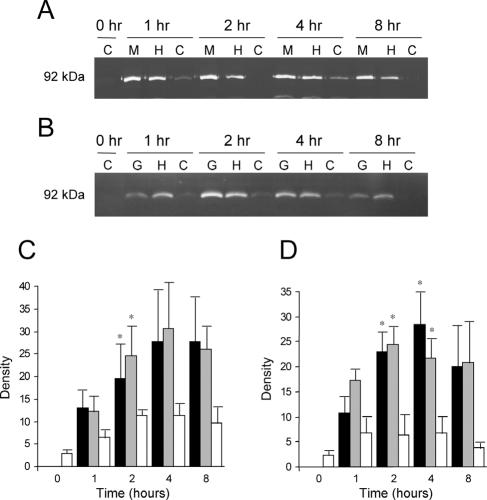
We used gelatin zymography to determine the potential of recruited monocytes to contribute to MMP levels and activation in affected areas of lung and found that the predominant gelatinolytic bands were consistently found only at 92 kDa in samples from monocytes stimulated with CS or HTCS (Fig. 2A) or with Gcp or HTGcp (Fig. 2B). Occasional faint bands were also visible at approximately 72 kDa, but this was not reproducible and did not correspond to cell treatments. Releasates from monocytes stimulated with either CS or with HTCS had higher levels of the 92-kDa gelatinolytic protein than did controls at each time point tested (Fig. 2C), and both levels were significantly higher than those of controls at the 2-h time point. Similarly, releasates from monocytes stimulated with Gcp or HTGcp had significantly higher 92-kDa gelatinolytic protein levels than controls at 2 and 4 h (Fig. 2D).
FIG. 2.
(A) Representative zymography of gelatinase B activity in monocytes stimulated with M. haemolytica products. M, M. haemolytica supernatant; H, HTCS; C, control (vehicle only) conditions. (B) Representative zymography of gelatinase B activity in monocytes stimulated with the following: G, M. haemolytica recombinant Gcp; H, HTGcp; C, control conditions. (C) Densitometric analysis of zymography gels from four separate experiments, showing the mean density (± SEM) of gelatinase B from monocytes stimulated with CS (black), HTCS (gray), or control (vehicle only) conditions (white). (D) Densitometric analysis of zymography gels from four separate experiments, showing the mean density (± SEM) of gelatinase B from monocytes stimulated with recombinant Gcp (black), HTGcp (gray), or under control conditions (white). *, significant difference from control at the same time point (P < 0.05).
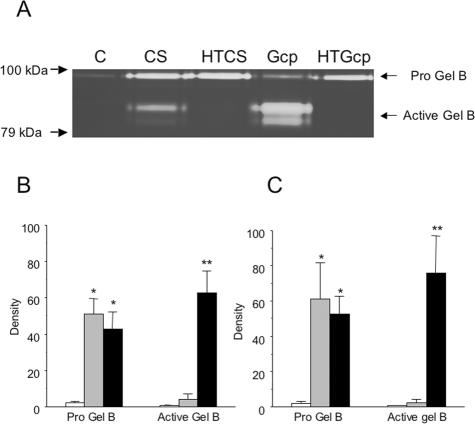
We also analyzed samples from alveolar macrophages stimulated with CS or HTCS and found detectable levels of progelatinase B in all samples, with no evidence that M. haemolytica products influenced production of this enzyme (data not shown). However, bovine neutrophils exposed to M. haemolytica products in vitro released extensive amounts of gelatinase B (Fig. 3). Cells released equal amounts of progelatinase B when exposed to the control or heat-treated products (or to E. coli LPS; data not shown) for 30 min. Over the same time course we saw statistically significant (P < 0.05) conversion of progelatinase B to its cleaved, active form but only in samples treated with native (versus heat-treated) forms of CS or recombinant Gcp (Fig. 3B and C).
FIG. 3.
(A) Representative zymography of gelatinase B activity in bovine neutrophil releasates from cells stimulated with M. haemolytica CS, HTCS, Gcp, HTGcp, or control conditions (vehicle only) for 30 min in vitro. Bands of approximately 82 kDa, indicative of activated gelatinase B, were prominent in releasates from cells stimulated with active but not heat-treated M. hemolytic products. (B) Densitometric analysis of zymography gels from six separate experiments, showing the mean density (± SEM) of gelatinase B from cells stimulated under control conditions (white bars) or with HTCS (gray bars) or CS (black bars). Stimulation with M. haemolytica products induced extensive release of gelatinase B in relation to the level released under control conditions. (C) Densitometric analysis of zymography gels from four separate experiments, showing the mean density (± SEM) of gelatinase B from cells stimulated with control (white bars), HTGcp (gray bars), or Gcp (black bars). *, significantly higher levels of progelatinase B than under control conditions (P < 0.05); **, significantly higher levels of active gelatinase B than for control or under heat-treated conditions (P < 0.05). Gel, gelatinase; Pro Gel, progelatinase.
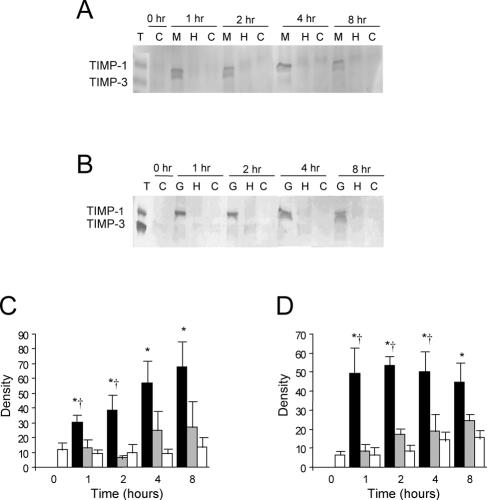
Reverse zymography was employed to investigate the presence of TIMPs in lung tissue isolated from M. haemolytica-challenged calves and the production of TIMPs by stimulated monocytes and alveolar macrophages. Only control mouse TIMPs were evident in reverse zymography gels with samples from nonlesional and lesional lung tissue (results not shown). Figure 4A and Fig. 5A are representative gels of CS-stimulated monocytes and macrophages, respectively. While purified mouse TIMP-1 and TIMP-3 standards were detectable by reverse zymography, only a band corresponding to TIMP-1 was evident in monocyte- and macrophage-conditioned media. The band occasionally appeared as a doublet; no detectable levels of TIMP-2 or TIMP-3 were found (Fig. 4A and B). Densitometry revealed that the levels of TIMP-1 from monocytes stimulated with CS were significantly higher at all time points tested when compared with control cells and higher at 1 and 2 h when compared with HTCS-stimulated cells (P < 0.05) (Fig. 4C). There was no significant difference in TIMP-1 observed between HTCS-stimulated and control monocyte-cultured media. Similarly, the levels of TIMP-1 produced by monocytes stimulated with Gcp were significantly higher (P < 0.05) at all times tested compared to levels in control cells and higher at 1, 2, and 4 h than levels in cells stimulated with HTGcp (Fig. 4D). Significantly increased TIMP-1 levels were also seen in alveolar macrophages stimulated with CS versus HTCS for 4 h (P < 0.05) (Fig. 5B).
FIG. 4.
(A) Composite reverse zymography of TIMP activity in monocytes stimulated with M. haemolytica products. M, M. haemolytica supernatant; H, HTCS; C, control (vehicle) conditions. Purified mouse TIMPs (T) were run as standards. (B) Composite reverse zymography of TIMP activity in monocytes stimulated with recombinant M. haemolytica Gcp (G) or HTGcp (H) or under control (vehicle) conditions (C). Purified mouse TIMPs (T) were run as standards. (C) Densitometric analysis of reverse zymography gels from four separate experiments. Columns represent the mean density (± SEM) of TIMP-1 from monocytes stimulated with CS (black), HTCS (gray), or under control conditions (white). (D) Densitometric analysis of reverse zymography gels from four separate experiments. Columns represent the mean density (± SEM) of TIMP-1 from monocytes stimulated with Gcp (black), HTGcp (gray), or under control conditions (white). *, significant difference from control at the same time point; †, significant difference from heat-treated counterpart at the same time point (P < 0.05).
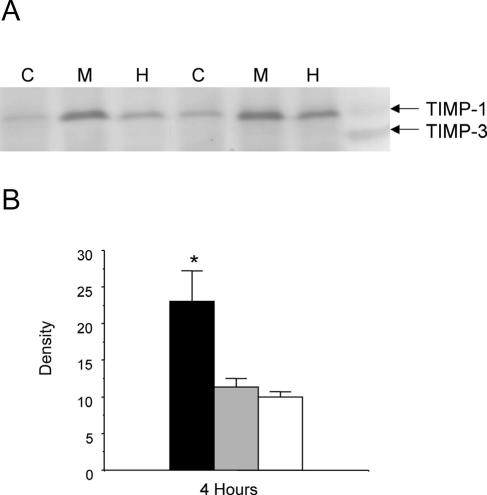
FIG. 5.
(A) Representative reverse zymography of TIMP activity in alveolar macrophages stimulated with M. haemolytica products for 4 h. M, M. haemolytica supernatant; H, HTCS; C, control conditions. Purified mouse TIMPs (T) were run on the gel as standards. (B) Densitometric analysis of reverse zymography gels from four separate experiments. Columns represent the mean density (± SEM) of TIMP-1 from alveolar macrophages stimulated with CS (black), HTCS (gray), or under control conditions (white). *, significant difference from control and heat-treated sample at the same time point (P < 0.05).
DISCUSSION
Here we demonstrate that the MMPs gelatinase A and B are detectable as both progelatinases and active forms in bovine lung tissue lysates and that activities of these gelatinases are increased in lung lesions produced by M. haemolytica challenge. Gelatinase B was produced by bovine inflammatory cells exposed to M. haemolytica products in vitro, and active gelatinase B was generated by stimulated neutrophils. The expression and production of a gelatinase moderator, TIMP-1, was also altered in bovine monocytes and alveolar macrophages cultured with M. haemolytica products.
Several studies have associated MMPs with lung damage observed in other pulmonary infectious and inflammatory conditions (10, 15, 22, 26, 27, 48). The ratio of active gelatinase A to progelatinase A is significantly higher in lung tissue homogenate from human patients suffering from bronchiolitis obliterans/organizing pneumonia or from idiopathic pulmonary fibrosis when compared with control lungs (14). Similarly, increased levels of gelatinase A and B were observed in bronchoalveolar lavage fluid obtained from human patients with adult respiratory distress syndrome (60). In a gene knockout study, immune complex-induced lung injury resulted in less severe damage to lungs of gelatinase B-deficient mice than in the control counterparts (61).
In our study, zinc-dependent gelatinolytic activity in lung tissue was observed at approximately 92, 82, 72, and 68 kDa, which suggests that these bands correspond to progelatinase B, active gelatinase B, progelatinase A, and active gelatinase A, respectively (29, 55). Gelatinase A is produced by alveolar epithelial cells (6, 14), as well as lung vascular endothelial cells (14). Levels of both gelatinase A and B were higher in lesional tissue from our study compared to levels in nonlesional tissue. Moreover, the amount of active gelatinase B was also significantly higher than that of progelatinase B in lesional tissue, suggesting that enzyme activation, as well as enhanced synthesis, is occurring in lesion areas. Gelatinase B activation follows cleavage of the propeptide by the enzymatic activities of other MMPs, such as gelatinase A and stromelysin 1 (MMP-3), and also by hypochlorous acid, a product of the respiratory burst of activated neutrophils (43). Gelatinase B, in addition to degrading components of the basement membrane, is also able to activate the cytokines IL-1 and IL-8, thereby enhancing the inflammatory response (44).
Our lung tissue homogenate contains both resident and recruited cells such as alveolar cells, macrophages, endothelial cells, monocytes, neutrophils, and lymphocytes. Studies in other species have implicated the alveolar macrophage production of gelatinases in pulmonary disease pathogenesis. Human alveolar macrophages isolated from patients suffering from pulmonary sarcoidosis or pneumonia produce gelatinase A at levels exceeding that from healthy counterparts (22). Similarly, gelatinase B production is elevated in human alveolar macrophages obtained from patients with idiopathic pulmonary fibrosis (28). Gibbs and coworkers (15) investigated the potential of rat lung fibroblasts, endothelial cells, type II epithelial cells, neutrophils, and alveolar macrophages as sources of MMPs identified in bronchoalveolar lavage fluid from injured rat lungs. Only alveolar macrophages had a profile of activity similar to that seen in the lavage fluid (15). Thus, in our study, bovine alveolar macrophages and blood monocytes seemed a likely source of the gelatinases observed in the lung tissue, and so we applied an in vitro model system to investigate this possibility.
Cultured media from monocytes stimulated with CS, HTCS, Gcp, or HTGcp demonstrated zinc-dependent gelatinolytic activity at approximately 92 kDa, indicating that the enzyme present in the cultured media was progelatinase B (29). In addition, these findings indicate that it is a heat-stable product of M. haemolytica CS and of the Gcp preparation that stimulates progelatinase B release by bovine monocytes. Through preincubation with polymyxin B agarose beads, we estimate that LPS composes approximately 75% of the heat-stable portion of our CS preparation. Thus, the heat-stable component of the CS responsible for stimulating progelatinase B production is likely M. haemolytica LPS. In the case of monocytes stimulated with recombinant Gcp or HTGcp, the heat-stable factor is likely E. coli LPS. This finding is consistent with previous studies that found that bacterial LPS stimulates gelatinase B production by macrophages and monocytes (44).
We found no detectable progelatinase A or active gelatinase A produced by the monocytes and macrophages stimulated with M. haemolytica factors within the 8-h time period of our study. Additionally, the lack of active gelatinase B in the monocyte- and macrophage-conditioned media indicates that, while stimulation of these cells with M. haemolytica products induces progelatinase B release, enzymes capable of cleaving the propeptide to produce active gelatinase B are not functional or not present in our in vitro model.
Li and coworkers showed that gelatinase B is released (but not activated) by bovine neutrophils in a degranulation response to phorbol myristate acetate (29), indicating that neutrophils may also be a source of the gelatinase B observed in our lung tissue. Of the cells we examined, only neutrophils seemed capable of activating this MMP upon exposure to M. haemolytica products. Moreover, unlike the stimulation of progelatinase B production, a heat-labile component of the CS and of the recombinant Gcp appears to participate in neutrophil-associated activation of gelatinase B. Exactly what is involved in activation of gelatinase B once the progelatinase form is secreted is an area of active research. There is evidence in other systems that tissue-associated proteases (19) and matrix adhesion (30) are both required for the activation of gelatinase B. It is also possible that protein complexes (composed of progelatinase B, membrane type MMPs, and TIMPs) associated with the surfaces of cells are responsible for activation of this protease (41, 56).
Elevated levels of gelatinases and other MMPs are frequently associated with the concomitant production of their inhibitors (43); both pro-MMPs and active MMPs are inhibited by TIMPs in a 1:1 ratio (41) TIMP-1 is constitutively produced by macrophages, though production can also be induced (44). In our study, conditioned media from monocytes and alveolar macrophages stimulated with CS or with Gcp contained significantly more TIMP-1 activity than their counterparts exposed to HTCS or HTGcp or than cells incubated under control conditions, an expected result given the increased gelatinase production in stimulated cells.
Surprisingly, despite maximal loading of protein, TIMP activity was not detected by reverse zymography in any of the lung tissue samples. Elevated TIMP-1 levels have been associated with numerous pulmonary diseases in other species, including adult respiratory distress syndrome (60), cryptogenic organizing pneumonia (10), idiopathic pulmonary fibrosis (34, 35, 51), and experimentally induced lung diseases (47, 48). It is therefore difficult to ascertain why there was no evidence of TIMP-1 in lesional or nonlesional lung tissue from calves challenged with M. haemolytica. We are unaware of any studies that have investigated TIMP levels in the bovine lung; further work must be completed to determine whether the results obtained herein are specific to M. haemolytica infection or are a feature of the bovine lung itself.
To our knowledge, this study is the first to investigate gelatinases and TIMPs in the context of bovine bronchofibrinous pneumonia. We have identified increased levels of gelatinase A and B in lesional lung tissue from calves after M. haemolytica challenge and were able to replicate enhanced gelatinase B production in an in vitro model. During inflammation, MMPs may act directly to damage alveolar epithelial and vascular endothelial cells, and MMP destruction of the basement membrane enables enhanced inflammatory cell recruitment (15). Enzymes, free radicals, and other products released by these recruited cells subsequently damage host tissue. There is significant cellular influx into the lungs of cattle suffering from shipping fever (18, 64-66). Bovine neutrophils degranulate and generate free radicals in response to M. haemolytica factors in vitro (32), and our experiments provide evidence that heat-stable M. haemolytica products stimulate gelatinase production and release by bovine monocytes, macrophages, and neutrophils and that heat-labile factors are involved in neutrophil-associated gelatinase activation. Therefore, all three cell types investigated likely contribute to the tissue damage seen in shipping fever. Moreover, our experiments indicate that M. haemolytica heat-labile products alter TIMP-1 production in vitro. The lack of TIMP-1 in bovine lung tissue suggests a possible inability of the M. haemolytica-challenged bovine lung to compensate for increased MMP production. An imbalance between MMPs and their inhibitors is involved in the progression of other inflammatory pulmonary diseases (35), consistent with the possibility that gelatinases and their inhibitors are also involved in shipping fever pathogenesis.
Acknowledgments
This work was supported in part by grants from Ontario Cattlemen's Association; the Natural Sciences and Engineering Research Council of Canada; the Ontario Ministry of Food and Rural Affairs; and the Food, Science and Biotechnology Centre, University of Guelph.
We thank the staff at Ponsonby Research Station, University of Guelph, and members of the Shewen laboratory for animal care. We also thank Michelle Ross, Amy Cook, and Lukasz Rzepiel for their technical assistance.
Editor: J. N. Weiser
REFERENCES
- 1.Abdullah, K. M., R. Y. Lo, and A. Mellors. 1991. Cloning, nucleotide sequence, and expression of the Pasteurella haemolytica A1 glycoprotease gene. J. Bacteriol. 173:5597-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdullah, K. M., E. A. Udoh, P. E. Shewen, and A. Mellors. 1992. A neutral glycoprotease of Pasteurella haemolytica A1 specifically cleaves O-sialoglycoproteins. Infect. Immun. 60:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann, M. R., and K. A. Brogden. 2000. Response of the ruminant respiratory tract to Mannheimia (Pasteurella) haemolytica. Microbes Infect. 2:1079-1088. [DOI] [PubMed] [Google Scholar]
- 4.Angen, O., M. Quirie, W. Donachie, and M. Bisgaard. 1999. Investigations on the species specificity of Mannheimia (Pasteurella) haemolytica serotyping. Vet. Microbiol. 65:283-290. [DOI] [PubMed] [Google Scholar]
- 5.Bryson, D. G. 1995. Calf pneumonia. Vet. Clin. N. Am. Food Anim. Pract. 1:237-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley, S., B. Driscoll, W. Shi, K. Anderson, and D. Warburton. 2001. Migration and gelatinases in cultured fetal, adult, and hyperoxic alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L427-L434. [DOI] [PubMed] [Google Scholar]
- 7.Cardella, M. A., M. A. Adveinto, and R. M. Nervig. 1987. Vaccination studies against experimental bovine Pasteurella haemolytica. Can. J. Vet. Res. 51:204-211. [PMC free article] [PubMed] [Google Scholar]
- 8.Caswell, J. L., D. M. Middleton, S. D. Sorden, and J. R. Gordon. 1998. Expression of the neutrophil chemoattractant interleukin-8 in the lesions of bovine pneumonic pasteurellosis. Vet. Pathol. 35:124-131. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y. F., H. W. Renshaw, and R. Young. 1987. Pneumonic pasteurellosis: examination of typable and untypable Pasteurella haemolytica strains for leukotoxin production, plasmid content, and antimicrobial susceptibility. Am. J. Vet. Res. 48:378-384. [PubMed] [Google Scholar]
- 10.Choi, K. H., H. B. Lee, M. Y. Jeong, Y. K. Rhee, M. J. Chung, Y. G. Kwak, and Y. C. Lee. 2002. The role of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in cryptogenic organizing pneumonia. Chest 121:1478-1485. [DOI] [PubMed] [Google Scholar]
- 11.Clinkenbeard, K. D., D. A. Mosier, A. L. Timko, and A. W. Confer. 1989. Effects of Pasteurella haemolytica leukotoxin on cultured bovine lymphoma cells. Am. J. Vet. Res. 50:271-275. [PubMed] [Google Scholar]
- 12.Cudd, L., C. Clarke, K. Clinkenbeard, M. Shelton, P. Clinkenbeard, and G. Murphy. 1999. Role of intracellular calcium in Pasteurella haemolytica leukotoxin-induced bovine neutrophil leukotriene B4 production and plasma membrane damage. FEMS Microbiol. Lett. 172:123-129. [DOI] [PubMed] [Google Scholar]
- 13.Czuprynski, C. J., and R. A. Welch. 1995. Biological effects of RTX toxins: the possible role of lipopolysaccharide. Trends Microbiol. 3:480-483. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda, Y., M. Ishizaki, S. Kudoh, M. Kitaichi, and N. Yamanaka. 1998. Localization of matrix metalloproteinases-1, -2 and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab. Investig. 78:687-698. [PubMed] [Google Scholar]
- 15.Gibbs, D. F., T. P. Shanley, R. L. Warner, H. S. Murphy, J. Varani, and K. J. Johnson. 1999. Role of matrix metalloproteinases in models of macrophage-dependent acute lung injury. Am. J. Respir. Cell Mol. Biol. 20:1145-1154. [DOI] [PubMed] [Google Scholar]
- 16.Gilmour, N. J. L. 1993. Pasteurellosis: the disease, p. 79-82. In B. E. Patten, T. L. Spenser, R. B. Johnson, D. Hoffmann, and L. Lehane (ed.), Pasteurellosis in production animals. Australian Centre for International Agricultural Research, Canberra, Australia.
- 17.Gomez, D. E., D. F. Alonso, G. Yoshiji, and U. P. Thorgirsson. 1997. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur. J. Cell Biol. 74:111-122. [PubMed] [Google Scholar]
- 18.Griffen, D. 1993. Feedlot disease. Vet. Clin. N. Am. Food Anim. Pract. 14:199-231. [DOI] [PubMed] [Google Scholar]
- 19.Han, Y. P., Y. D. Nien, and W. L. Garner. 2002. Tumor necrosis factor-alpha-induced proteolytic activation of pro-matrix metalloproteinase-9 by human skin is controlled by down-regulating tissue inhibitor of metalloproteinase-1 and mediated by tissue-associated chymotrypsin-like proteinase. J. Biol. Chem. 277:27319-27327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Highlander, S. K., N. D. Fedorova, D. M. Dusek, R. Panciera, L. E. Alvarez, and C. Rinehart. 2000. Inactivation of Pasteurella (Mannheimia) haemolytica leukotoxin causes partial attenuation of virulence in a calf challenge model. Infect. Immun. 68:3916-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeyaseelan, S., S. L. Hsuan, M. S. Kannan, B. Walcheck, J. F. Wang, M. E. Kehrli, E. T. Lally, G. C. Sieck, and S. K. Maheswaran. 2000. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect. Immun. 68:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John, M., U. Oltmanns, I. Fletze, and C. Witt. 2002. Increased production of matrix metalloproteinase-2 in alveolar macrophages and regulation by interleukin-10 in patients with acute pulmonary sarcoidosis. Exp. Lung Res. 25:55-68. [DOI] [PubMed] [Google Scholar]
- 23.Kinlough-Rathbone, R. L., D. W. Perry, M. L. Rand, and M. A. Packham. 2000. Responses to aggregating agents after cleavage of GPIb of human platelets by the O-sialoglycoprotein endoprotease from Pasteurella haemolytica. Thromb. Res. 99:165-172. [DOI] [PubMed] [Google Scholar]
- 24.Kvietys, P. R., and M. Sandig. 2001. Neutrophil diapedesis: paracellular or transcellular? News Phys. Sci. 16:15-19. [DOI] [PubMed] [Google Scholar]
- 25.Lafleur, R. L., M. S. Abrahamsen, and S. K. Maheswaran. 1998. The biphasic mRNA expression pattern of bovine interleukin-8 in Pasteurella haemolytica lipopolysaccharide-stimulated alveolar macrophages is primarily due to tumor necrosis factor alpha. Infect. Immun. 66:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, Y. C., H. B. Lee, Y. K. Rhee, and C. H. Song. 2001. The involvement of matrix metalloproteinase-9 in airway inflammation of patients with acute asthma. Clin. Exp. Allergy 31:1623-1630. [DOI] [PubMed] [Google Scholar]
- 27.Lemjabbar, H., P. Gosset, E. Lechapt-Zalcman, M. L. Franco-Montoya, B. Wallaert, A. Harf, and C. Lafuma. 1999. Overexpression of alveolar macrophage gelatinase B (MMP-9) in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 20:903-913. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., and K. D. Clinkenbeard. 1999. Lipopolysaccharide complexes with Pasteurella haemolytica leukotoxin. Infect. Immun. 67:2920-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, X., X. Zhao, and S. Ma. 1999. Secretion of 92 kDa gelatinase (MMP-9) by bovine neutrophils. Vet. Immunol. Immunopathol. 67:247-258. [DOI] [PubMed] [Google Scholar]
- 30.Lindsey, M., K. Wedin, M. D. Brown, C. Keller, A. J. Evans, J. Smolen, A. R. Burns, R. D. Rossen, L. Michael, and M. Entman. 2001. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation 103:2181-2187. [DOI] [PubMed] [Google Scholar]
- 31.Lo, R. Y., C. A. Strathdee, and P. E. Shewen. 1987. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica leukotoxin. Infect. Immun. 55:1987-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maheswaran, S. K., D. J. Weiss, M. S. Kannan, E. L. Townsend, K. R. Reddy, L. O. Whiteley, and S. Srikumaran. 1992. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet. Immunol. Immunopathol. 33:51-68. [DOI] [PubMed] [Google Scholar]
- 33.Malazdrewich, S. K., T. R. Ames, M. S. Abrahamsen, and S. K. Maheswaran. 2001. Pulmonary expression of tumor necrosis factor alpha, interleukin-1 beta, and interleukin-8 in the acute phase of bovine pneumonic pasteurellosis. Vet. Pathol. 38:297-310. [DOI] [PubMed] [Google Scholar]
- 34.Mautino, G., C. Henriquet, D. Jaffuel, J. Bousquet, and F. Capony. 1999. Tissue inhibitor of metalloproteinase-1 levels in bronchoalveolar lavage fluid from asthmatic subjects. Am. J. Respir. Crit. Care Med. 160:324-330. [DOI] [PubMed] [Google Scholar]
- 35.Mautino, G., C. Henriquet, C. Gougat, A. Le Cam, J. M. Dayer, J. Bousquet, and F. Capony. 1999. Increased expression of tissue inhibitor of metalloproteinase-1 and loss of correlation with matrix metalloproteinase-9 by macrophages in asthma. Lab. Investig. 79:39-47. [PubMed] [Google Scholar]
- 36.Mdurvwa, E. G., and C. J. Brunner. 1994. Bovine neutrophil activation by culture fluid from Pasteurella haemolytica serotypes A1 and A11. Vet. Microbiol. 41:311-319. [DOI] [PubMed] [Google Scholar]
- 37.Morsey, M. A., A. G. Van Kessel, Y. Mori, Y. Popowych, D. Godson, M. Campos, and L. A. Babiuk. 1999. Cytokine profiles following interaction between bovine alveolar macrophages and Pasteurella haemolytica. Microb. Pathog. 26:325-331. [DOI] [PubMed] [Google Scholar]
- 38.Nagase, H., and J. F. Woessner. 1999. Matrix metalloproteinases. J. Biol. Chem. 274:21491-21494. [DOI] [PubMed] [Google Scholar]
- 39.Narita, M., K. Kimura, N. Tanimura, S. Arai, T. Tsuboi, and K. Katsuda. 2000. Immunohistochemical characterization of calf pneumonia produced by the combined endobronchial administration of bovine herpesvirus 1 and Pasteurella haemolytica. J. Comp. Pathol. 123:126-134. [DOI] [PubMed] [Google Scholar]
- 40.Nyarko, K. A., B. L. Coomber, A. Mellors, and P. A. Gentry. 1998. Bovine platelet adhesion is enhanced by leukotoxin and sialoglycoprotease isolated from Pasteurella haemolytica A1 cultures. Vet. Microbiol. 61:81-91. [DOI] [PubMed] [Google Scholar]
- 41.Ogata, Y., Y. Itoh, and H. Nagase. 1995. Steps involved in activation of the pro-matrix metalloproteinase 9 (progelatinase B)-tissue inhibitor of metalloproteinases-1 complex by 4-aminophenylmercuric acetate and proteinases. J. Biol. Chem. 270:18506-18511. [DOI] [PubMed] [Google Scholar]
- 42.Opdenakker, G., W. E. Fibbe, and J. Van Damme. 1998. The molecular basis of leukocytosis. Immunol. Today 19:182-189. [DOI] [PubMed] [Google Scholar]
- 43.Opdenakker, G., P. E. Van den Steen, and J. Van Damme. 2001. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 22:571-579. [DOI] [PubMed] [Google Scholar]
- 44.Opdenakker, G., P. E. Van den Steen, B. Dubois, I. Nelissen, E. Van Coillie, S. Masure, P. Proost, and J. Van Damme. 2001. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 69:851-859. [PubMed] [Google Scholar]
- 45.Ortiz-Carranza, O., and C. J. Czuprynski. 1992. Activation of bovine neutrophils by Pasteurella haemolytica leukotoxin is calcium dependent. J. Leukoc. Biol. 52:558-564. [DOI] [PubMed] [Google Scholar]
- 46.Pace, L. W., J. M. Kreeger, K. L. Bailey, S. E. Turnquist, and W. H. Fales. 1993. Serum levels of tumor necrosis factor-alpha in calves experimentally infected with Pasteurella haemolytica A1. Vet. Immunol. Immunopathol. 35:353-364. [DOI] [PubMed] [Google Scholar]
- 47.Pardo, A., R. Barrios, V. Maldonado, J. Meléndez, J. Pérez, V. Ruiz, L. Segura-Valdez, J. Sznajder, and M. Selman. 1998. Gelatinases A and B are up-regulated in rat lungs by subacute hyperoxia. Am. J. Pathol. 153:833-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez-Ramos, J., L. Segura-Valdez, B. Vanda, M. Selman, and A. Pardo. 1999. Matrix metalloproteinases 2, 9, and 13, and tissue inhibitors of metalloproteinases 1 and 2 in experimental lung silicosis. Am. J. Respir. Crit. Care Med. 160:1274-1282. [DOI] [PubMed] [Google Scholar]
- 49.Rao, V. H., J. A. Bridge, J. R. Neff, G. B. Schaefer, B. A. Buehler, J. K. Vishwanatha, R. E. Pollock, G. L. Nicolson, M. Yamamoto, and Z. L. Gokaslam. 1995. Expression of 72 kDa and 92 kDa type IV collagenases from human giant-cell tumor of bone. Clin. Exp. Metastasis 13:420-426. [DOI] [PubMed] [Google Scholar]
- 50.Rimler, R. B. 1993. Pasteurella: laboratory techniques for typing and diagnosis of infection, p. 199-202. In B. E. Patten, T. L. Spenser, R. B. Johnson, D. Hoffmann, and L. Lehane (ed.), Pasteurellosis in production animals. Australian Centre for International Agricultural Research, Canberra, Australia.
- 51.Selman, M., V. Ruiz, S. Cabrera, L. Segura, R. Ramírez, R. Barrios, and A. Pardo. 2000. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L562-L574. [DOI] [PubMed] [Google Scholar]
- 52.Shewen, P. E., and B. N. Wilkie. 1982. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect. Immun. 35:91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shewen, P. E., and B. N. Wilkie. 1987. Vaccination of calves with leukotoxic culture supernatant from Pasteurella haemolytica. Can. J. Vet. Res. 52:30-36. [PMC free article] [PubMed] [Google Scholar]
- 54.Shewen, P. E., C. W. Lee, A. Perets, D. C. Hodgins, K. Baldwin, and R. Y. C. Lo. 2003. Efficacy of recombinant sialogylcoprotease in protection of cattle against pneumonic challenge with Mannheimia (Pasteurella) haemolytica A1. Vaccine 21:1901-1906. [DOI] [PubMed] [Google Scholar]
- 55.Song, L., D. G. Porter, and B. L. Coomber. 1999. Production of gelatinases and tissue inhibitors of matrix metalloproteinases by equine ovarian stromal cells in vitro. Biol. Reprod. 60:1-7. [DOI] [PubMed] [Google Scholar]
- 56.Sternlicht, M. D., and Z. Werb. 2001. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17:463-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun, Y., K. D. Clinkenbeard, L. A. Cudd, C. R. Clarke, and P. A. Clinkenbeard. 1999. Correlation of Pasteurella haemolytica leukotoxin binding with susceptibility to intoxication of lymphoid cells from various species. Infect. Immun. 67:6264-6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutherland, D. R., K. M. Abdullah, P. Cyopick, and A. Mellors. 1992. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. J. Immunol. 148:1458-1464. [PubMed] [Google Scholar]
- 59.Tatum, F. M., R. E. Briggs, S. S. Sreevatsan, E. S. Zehr, H. S. Ling, L. O. Whiteley, T. R. Ames, and S. K. Maheswaran. 1998. Construction of an isogenic leukotoxin deletion mutant of Pasteurella haemolytica serotype 1: characterization and virulence. Microb. Pathog. 24:37-46. [DOI] [PubMed] [Google Scholar]
- 60.Torii, K., K. I. Iida, Y. Miyazaki, S. Saga, Y. Kondoh, H. Taniguchi, F. Taki, K. Takagi, M. Matsuyama, and R. Suzuki. 1997. Higher concentration of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 155:43-46. [DOI] [PubMed] [Google Scholar]
- 61.Warner, R. L., L. Beltran, E. M. Younkin, C. S. Lewis, S. J. Weiss, J. Varani, and K. J. Johnson. 2001. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am. J. Respir. Cell Mol. Biol. 24:537-544. [DOI] [PubMed] [Google Scholar]
- 62.Welch, R. A. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85-111. [DOI] [PubMed] [Google Scholar]
- 63.Whiteley, L. O., S. K. Maheswaran, D. J. Weiss, and T. R. Ames. 1991. Morphological and morphometrical analysis of the acute response of the bovine alveolar wall to Pasteurella haemolytica A1-derived endotoxin and leucotoxin. J. Comp. Pathol. 104:23-32. [DOI] [PubMed] [Google Scholar]
- 64.Whiteley, L. O., S. K. Maheswaran, D. J. Weiss, T. R. Ames, and M. S. Kannan. 1992. Pasteurella haemolytica A1 and bovine respiratory disease: pathogenesis. J. Vet. Intern. Med. 6:11-22. [DOI] [PubMed] [Google Scholar]
- 65.Wikse, S. E. 1985. Feedlot cattle pneumonias. Vet. Clin. N. Am. Food Anim. Pract. 1:289-309. [DOI] [PubMed] [Google Scholar]
- 66.Woolcock, J. B. 1993. The biology of Pasteurella multocida and Pasteurella haemolytica, p. 25-34. In B. E. Patten, T. L. Spenser, R. B. Johnson, D. Hoffmann, and L. Lehane (ed.), Pasteurellosis in production animals. Australian Centre for International Agricultural Research, Canberra, Australia.
- 67.Yoo, H. S., S. K. Maheswaran, G. Lin, E. L. Townsend, and T. R. Ames. 1995. Induction of inflammatory cytokines in bovine alveolar macrophages following stimulation with Pasteurella haemolytica lipopolysaccharide. Infect. Immun. 63:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo, H. S., S. K. Maheswaran, S. Srinand, T. R. Ames, and M. Suresh. 1995. Increased tumor necrosis factor-alpha and interleukin-1 beta expression in the lungs of calves with experimental pneumonic pasteurellosis. Vet. Immunol. Immunopathol. 49:15-28. [DOI] [PubMed] [Google Scholar]