Abstract
Ami is an autolytic amidase from Listeria monocytogenes that is targeted to the bacterial surface via its C-terminal cell wall anchoring (CWA) domain. We recently showed that the CWA domain from Ami of L. monocytogenes EGD (serovar 1/2a) (Ami 1/2a) mediated bacterial binding to mammalian cells. Here we studied the sequence and binding properties of Ami from CHUT 82337 (serovar 4b) (Ami 4b). The Ami 4b polypeptide is predicted to be 770 amino acids long (compared with the 917 amino acids of Ami 1/2a from EGD). Ami 1/2a and Ami 4b are almost identical in the N-terminal enzymatic domain (∼98% amino acid identity), but the sequence is poorly conserved in the C-terminal CWA domain, with only ∼54% amino acid identity and eight GW modules in Ami 1/2a compared with six GW modules in Ami 4b. The purified Ami 4b CWA domain efficiently bound serovar 4b bacterial cells and only poorly bound serovar 1/2a bacterial cells. The Ami 4b CWA domain was also significantly less able to bind Hep-G2 human hepatocytic cells than the Ami 1/2a CWA domain. We sequenced the ami regions encoding CWA domains of reference strains belonging to the 12 L. monocytogenes serovars. The phylogenic tree constructed from the sequences yielded a binary division into group I (serovars 1/2a, 1/2b, 1/2c, 3a, 3b, 3c, and 7) and group II (serovars 4a, 4b, 4c, 4d, and 4e). This is the first direct evidence of divergence between serovars 1/2a and 4b in a gene involved in the adhesion of L. monocytogenes to mammalian cells, as well as the first demonstration of allelic polymorphism correlated with the somatic antigen in this species.
Listeria monocytogenes is a gram-positive bacillus that is widespread in the environment and causes life-threatening infections in humans and animals, including meningoencephalitis, bacteremia, and perinatal infections (7, 15). In humans, it is transmitted by contaminated food and may be responsible for large outbreaks in industrialized countries (7). L. monocytogenes is a facultative intracellular pathogen that is able to infect both professional (25) and nonprofessional phagocytes, such as epithelial cells (10, 11) and hepatocytes (5, 13, 43). The molecular basis of its intracellular life cycle has largely been elucidated (40). Early in the cycle, L. monocytogenes lyses the phagosomal vacuole and is released into the cytoplasm. It then divides and spreads into adjacent cells by mediating actin assembly.
Listeria strains can be classified by their antigenic properties according to the serological scheme introduced by Paterson (31) and modified by Seeliger and Höhne (36). This scheme distinguishes various serovars on the basis of their somatic (O) and flagellar (H) antigens. It is based on hemagglutination tests with different sera designated A to E for flagellar antigens and I to XV for somatic antigens. At least 13 serovars are recognized within L. monocytogenes, and they are designated serovars 1/2a, 1/2b, 1/2c, 3a, 3b, 3c, 4a, 4ab, 4b, 4c, 4d, 4e, and 7 (38). While several serovars can be recovered from the environment and from foods, serovars 1/2a, 1/2b, and 4b account for most human infections (7, 26, 34). Serovar 4b strains are especially overrepresented among clinical isolates, accounting for 40 to 60% of sporadic cases of human listeriosis. Serovar 4b is also the serovar that is most frequently involved in major outbreaks (7).
Ami is an autolytic amidase that was originally identified in L. monocytogenes serovar 1/2a (2, 27). Ami 1/2a, the predicted Ami of strain EGD (serovar 1/2a), the strain whose genome has been sequenced (14), is a 917-amino-acid protein with three characteristic domains: (i) a 30-amino-acid putative signal sequence; (ii) a 179-amino-acid N-terminal domain similar to the alanine amidase domain of the Atl autolysin of Staphylococcus aureus; and (iii) a C-terminal cell wall anchoring (CWA) domain (amino acids 262 to 917) containing four repeats, each composed of two approximately 80-amino-acid modules called GW modules because of the presence of the dipeptide GW (2, 4). Ami 1/2a is exposed at the Listeria surface (2). Like other GW autolysins, the molecule is likely to be secreted and targeted to the bacterial surface via its CWA domain (4).
Ami 1/2a is believed to contribute to the attachment of L. monocytogenes to eukaryotic cells. We recently showed that ami null mutants constructed with serovar 1/2a strains lacking InlA or InlB or both are five to 10 times less adherent than the parental strains in various cell types (28, 29). We also showed that the adhesive properties of Ami 1/2a are carried by the C-terminal CWA domain of the molecule (29). Expression of this domain by complementation fully restores the adhesion capacity of ami null mutants in inlA and/or inlB backgrounds. Moreover, the purified CWA domain of Ami 1/2a binds eukaryotic cells in a cell adhesion assay.
Paradoxically, to date there is no information about the properties of Ami 4b, the Ami from L. monocytogenes serovar 4b, the most prevalent serovar in human listeriosis. Here we studied the sequence and binding activity of Ami 4b. We found that the CWA domains of Ami 4b and Ami 1/2a are not similar and have distinct patterns of binding to eukaryotic cells. We also sequenced the Ami CWA domains of strains belonging to each L. monocytogenes serovar. We showed that the Ami CWA sequences can be divided into two groups correlated with the somatic antigen and the structure of teichoic acids (TA).
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. All bacteria were grown at 37°C. Listeria and Escherichia coli strains were grown in brain heart infusion (Difco Laboratories, Detroit, Mich.) and Luria-Bertani (Difco Laboratories) broth and agar, respectively. For E. coli strains harboring pET28a+ derivatives kanamycin was added to the medium at a final concentration of 30 mg/liter.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Serovar | Genotype or description | Reference or source |
|---|---|---|---|
| L. monocytogenes strains | |||
| EGD (BUG600) | 1/2a | Virulent, clinical isolate | 25 |
| LO28 | 1/2c | Virulent, clinical isolate | 41 |
| ATCC 19111 | 1/2a | Virulent, clinical isolate | 39 |
| CNL 880203 | 1/2b | Virulent, clinical isolate | 39 |
| CHUT 861141 | 1/2c | Virulent, food environment isolate | 39 |
| CHUT 82337 | 4b | Virulent, clinical isolate | 39 |
| CHUT 850212 | 4b | Virulent, clinical isolate | 39 |
| INRA 76 | 4b | Virulent, clinical isolate | 39 |
| EGD ami | 1/2a | ami null mutant | 29 |
| CLIP 74902 | 1/2a | Reference strain | 36 |
| CLIP 74903 | 1/2b | Reference strain | 36 |
| CLIP 74904 | 1/2c | Reference strain | 36 |
| CLIP 74905 | 3a | Reference strain | 36 |
| CLIP 74906 | 3b | Reference strain | 36 |
| CLIP 74907 | 3c | Reference strain | 36 |
| CLIP 74908 | 4a | Reference strain | 36 |
| CLIP 74910 | 4b | Reference strain | 36 |
| CLIP 74911 | 4c | Reference strain | 36 |
| CLIP 74912 | 4d | Reference strain | 36 |
| CLIP 74913 | 4e | Reference strain | 36 |
| CLIP 74917 | 7 | Reference strain | 36 |
| E. coli strains | |||
| BUG1756 | E. coli BL21(DE3)/pET28.a-5 | 29 | |
| BUG1963 | E. coli BL21(DE3)/pET28.a-6 | This study | |
| Plasmids | |||
| pUC18 | 44 | ||
| pET28a+ | Expression vector | Novagen | |
| pET28.a-5 | pET28 Ωamicwa 1/2a | 29 | |
| pET28.a-6 | pET28 Ωamicwa 4b | This study |
General genetic manipulations, nucleotide sequencing, and sequence analyses.
Total DNA was isolated from Listeria cells as previously described (32). Plasmid DNA was prepared from E. coli by the rapid alkaline lysis method (1). Standard techniques were used for DNA fragment isolation, DNA cloning, and restriction analysis (35). Restriction enzymes and ligase were purchased from New England Biolabs Inc. (Beverly, Mass.) and were used as recommended by the manufacturer. DNA was amplified with DyNazyme EXT DNA polymerase (Finnzymes) or Vent DNA polymerase (New England Biolabs). The PCR conditions were as follows: 35 cycles of 30 s at 95°C, 45 s at 55°C, and 90 s at 72°C in a PTC-200 thermal cycler (MJ Research). The ami 4b gene was amplified by using primers AMI-F1 (5′-CAAGTGCTGCTTCCATTG-3′) and AMI-R1 (5′-CTGATTGATCACCCTTGG-3′). The CWA domain portion of the ami gene was amplified by using primers CWAsv1-F (5′-GATGGCAAAGGAACAGTC-3′) and CWAsv1-R (5′-CTGATTGATCACCCTTGG-3′) for serovars 1/2a, 1/2b, 1/2c, 3a, 3b, 3c, and 7 and primers CWAsv4-F (5′-GTCTGGTCCCACGATGC-3′) and CWAsv4-R (5′-CTCGTCCAAATCGTCCGC-3′) for serovars 4a, 4b, 4c, 4d, and 4e. Nucleotide sequencing was carried out with a Big Dye terminator sequencing kit (Applied Biosystems, Perkin-Elmer) with fluorescently labeled dideoxynucleotides and primers. Labeled extension products were analyzed with a 3700 DNA sequencer (Perkin-Elmer). Multiple alignments were constructed with the Clustal W program. Phylogenetic trees were constructed by using the NJplot program.
Preparation of bacterial extracts, SDS-PAGE, and Western blot analysis.
Total bacterial extracts were prepared as follows. Listeria was grown in brain heart infusion broth to the exponential phase (A600, 0.8), and then 1 ml was centrifuged. The resulting pellet was washed twice in cold water and sonicated three times for 5 min. The lysate was collected by centrifugation and suspended in 1× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer (130 mM Tris-HCl [pH 6.8], 1% SDS, 7% 2-β-mercaptoethanol, 7% sucrose, 0.01% bromophenol blue). To prepare a 1% SDS extract, 10 ml of a culture was centrifuged, and the bacterial pellet was washed twice in phosphate-buffered saline (PBS) and suspended in 0.2 ml of PBS containing 1% SDS. The bacterial cells were incubated for 15 min at 37°C. The supernatant collected after centrifugation was filtered and solubilized in 1× SDS-PAGE sample buffer. SDS-PAGE was carried out as previously described (21) in 10% polyacrylamide minigels (Mini Protean II; Bio-Rad, Ivry sur Seine, France). Proteins were stained with Coomassie brilliant blue. Western blotting was carried out as previously described (29). Western blots were probed with rabbit affinity-purified anti-Ami antibodies or rabbit anti-InlB antibodies (2) diluted 1/1,000 and anti-rabbit horseradish peroxidase-conjugated secondary antibodies. Antibody binding was revealed by adding 0.05% diaminobenzidine tetrahydrochloride (Sigma) and 0.03% hydrogen peroxide.
Expression and purification of recombinant Amicwa-His6 polypeptides.
The CWA domain of Ami (Amicwa) of CHUT 82337 was produced as a recombinant His-tagged polypeptide as previously described (29). A 1,582-bp PCR fragment was produced by using genomic DNA of CHUT 82337 as the template and primers 5′-GGAATTCCATATGTTGATTAATGAAAAGTACAAAGCG-3′ and 5′-CGCGGATCCATAATTGGCTGGGAG-3′, which introduced NdeI and BamHI sites (underlined), respectively. The resulting fragment was digested with NdeI and BamHI and inserted in frame upstream from the His tag sequence in the expression vector pET28a+ (Novagen). The resulting plasmid, pET28.a-6, was verified by sequencing the insert from both junctions. It was used to transform E. coli BL21(DE3) (Novagen), giving rise to BUG 1963. Recombinant Amicwa-His6 polypeptides originating from either EGD (29) or CHUT 82337 (this study) were purified by using a two-step chromatographic procedure. The first step, involving metal affinity chromatography (Novagen), was carried out as previously described (3). The fractions containing Amicwa-His6 were pooled and subjected to cation-exchange chromatography with a POROS HS column (Pharmacia). The loading buffer contained 50 mM HEPES (pH 7.6) and 200 mM NaCl. Elution was performed with a 0.2 to 0.4 M NaCl gradient. The Amicwa-His6 polypeptide that eluted in the presence of 280 mM NaCl was dialyzed for 18 h against loading buffer and concentrated by using Centriprep 50 devices (Amicon). The His tag was removed by thrombin digestion. The purified Amicwa polypeptide was stored in 10% glycerol at −80°C. Protein concentrations were determined with the bicinchoninic acid system (Pierce).
Binding of Amicwa to bacterial cells.
Binding assays were performed as previously described (2), with minor modifications. One milliliter of an exponential-phase culture (A600, 0.8) of L. monocytogenes was washed twice in PBS, pelleted, and resuspended in 200 μl of 220 mM NaCl-PBS. Various concentrations of the purified Amicwa-His6 polypeptide were added, and the mixtures were incubated for 45 min at room temperature with gentle agitation. Bacterial cells were then washed twice in 220 mM NaCl-PBS to remove unbound material and resuspended in 1× SDS-PAGE sample buffer. Bound proteins were visualized by Coomassie staining after SDS-PAGE.
Culture of cell lines.
The human colon carcinoma cell line Caco-2 (ATCC HTB 37), used between passages 25 and 35, was propagated as described previously (12). The human hepatocellular carcinoma cell line Hep-G2 (ATCC HB 8065) was propagated as described by Dramsi et al. (5). All incubations were carried out in a 10% CO2 atmosphere at 37°C.
Cell binding assay with Amicwa-coated surface.
A cell binding assay was performed as described previously (29). Maxisorp microtiter plates (Nunc) were coated for 18 h at 4°C with 50 μl of purified Amicwa 1/2a or Ami 4b, with various concentrations of bovine serum albumin (BSA) (0.07, 0.14, 0.35, and 0.7 μM), or with 10 μg of poly-l-lysine in 50 mM carbonate buffer (pH 9.6). Wells were treated for 2 h at 37°C with 0.5% BSA in PBS for blocking and washed three times with PBS. The adhesion assay was performed as follows. Wells were filled with 50 μl of a cell suspension (approximately 106 cells per ml) in Dulbecco modified Eagle medium containing 0.4% BSA and incubated for 1 h at 37°C in a 10% CO2 atmosphere. After washing, bound cells were quantified by the hexosaminidase assay (22). The results are expressed below relative to the cell binding obtained with poly-l-lysine-coated wells, which was arbitrarily defined as 100.
Statistical analysis.
The Student t test was used to compare values, and P values of <0.05 were considered to be statistically significant.
RESULTS
CWA domains of Ami 4b and Ami 1/2a are not similar.
We sequenced the ami gene from L. monocytogenes CHUT 82337 (serovar 4b) and compared it with that of L. monocytogenes EGD (serovar 1/2a). The two ami gene sequences were similar in the region encoding the enzymatic domain (97.2% nucleotide identity) but not in the region encoding the CWA domain (43.2% nucleotide identity). Moreover, the gene encoding the CWA domain of CHUT 82337 contained only three DNA repeats, compared to the four DNA repeats in the EGD gene. We verified that this difference was not due to a cloning artifact. The ami region encoding the CWA domain of CHUT 82337 was amplified with the external primers AMIsv4-F and PYRGsv4-R. The resulting PCR product was about 0.5 kb smaller than the EGD PCR product. To ensure that the results obtained with CHUT 82337 were not strain specific, the ami regions encoding the CWA domains of three other serovar 4b strains (ATCC 19115, CHUT 850212, and INRA 76) were amplified by using the same primers. Each PCR product was the same size as the CHUT 82337 product (data not shown). The DNA repeat nearest the C-terminal extremity (588 bp) of each of the three strains was sequenced on both strands. The sequences were 99.9 and 43.2% identical to those of CHUT 82337 and EGD, respectively (data not shown), thus confirming the previous results obtained with CHUT 82337.
The deduced Ami polypeptide of L. monocytogenes CHUT 82337 is 770 amino acids long, whereas that of EGD is 917 amino acids long (Fig. 1). CHUT 82337 Ami is organized like Ami of EGD. It contains an N-terminal domain with putative alanine amidase activity and a C-terminal CWA domain composed of repeats, each made up of two GW modules (the N-terminal and C-terminal GW modules of each repeat are conventionally referred to as a and b, respectively; the a and b modules of repeat 1 are designated R1a and R1b, respectively [4]). However, although the two proteins have almost identical enzymatic domains (98.1% amino acid identity), their CWA domains are different (54.2% amino acid identity). Moreover, the CWA domain of CHUT 82337 contains only three repeats (six GW modules), compared to four repeats (eight GW modules) in the EGD CWA domain. In addition, the b modules of CHUT 82337 are each four or five amino acids longer than the b modules of EGD. Another striking difference is the presence of the short sequence RTSXTFI upstream from the GW dipeptide of the b modules in CHUT 82337. In EGD, this motif is found at a similar location in InlB (6, 11) but not in Ami (Fig. 1).
FIG. 1.
Ami 4b molecule. (A) Amino acid sequence of the ami gene product from CHUT 82337. The signal peptide is underlined, as is the short RTSXTFI sequence present in InlB (see panel C). The amidase domain (positions 1 to 261) and the CWA domain (positions 262 to 770) are indicated. The a and b modules of the three GW repeats (positions 277 to 770), designated R1a to R3a and R1b to R3b, are aligned. The repeat consensus sequence (con) shows the amino acids that are identical in at least two of three repeats. (B) Amino acid sequence of the CWA domain of Ami 1/2a (strain EGD). The a and b modules of the four GW repeats (positions 274 to 917), designated R1a to R4a and R1b to R4b, are aligned. Dashes represent gaps introduced to maximize matching (see panel C). The repeat consensus sequence (con) shows the amino acids that are identical in at least three of four repeats. Asterisks indicate identity with the repeat consensus sequence of Ami 4b. (C) Amino acid sequence of the CWA domain of InlB 1/2a (strain EGD). The a an b modules of the two GW repeats (positions 399 to 630) are designated R′1a, R′1b, R′2a, and R′2b. R′1a is missing, and R′1b is incomplete (68 amino acids). Asterisks indicate amino acids that are identical in R′2 and in the repeat consensus sequence of Ami 4b. The short RTSXTFI sequence, common to InlB and Ami 4b and absent from Ami 1/2a (see panel B), is underlined.
L. monocytogenes serovar 4b produces an 85-kDa Ami polypeptide found in total cell extracts and SDS extracts.
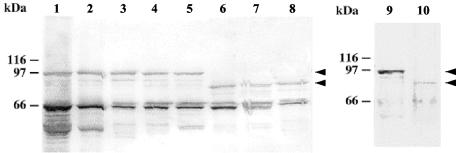
We used Western blotting to analyze the Ami proteins from serovar 1/2a and 4b strains. Western blots were probed with rabbit affinity-purified anti-Ami antibodies or anti-InlB antibodies, which recognize Ami and give lower background values (2). In accordance with previous observations made with Ami 1/2a from EGD (2, 29), Ami 4b was detected in total cell extracts and SDS extracts but not in culture supernatants. However, as expected from sequence data, the Ami molecules were clearly shorter in strains of serovar 4b than in strains of serovar 1/2a (∼85 kDa versus ∼100 kDa) (Fig. 2). Similar data were reported in a recent study (18).
FIG. 2.
Immunoblot analysis of Ami molecules produced by serovar 1/2 and 4b strains. Blots prepared from SDS bacterial extracts were probed with anti-InlB (lanes 1 to 8) or anti-Ami (lanes 9 and 10) polyclonal antibodies. Lane 1, EGD as a control serovar 1/2a strain; lane 2, LO28 (serovar 1/2c); lane 3, ATCC 19111 (serovar 1/2a); lane 4, CNL 880203 (serovar 1/2b); lane 5, CHUT 861141 (serovar 1/2c); lane 6, CHUT 82337 (serovar 4b); lane 7, CHUT 850212 (serovar 4b); lane 8, INRA 76 (serovar 4b); lane 9, EGD (serovar 1/2a); lane 10, CHUT 82337 (serovar 4b). Note that the main band, corresponding to the complete form of Ami (arrowheads), is at ∼85 kDa in serovar 4b strains and at ∼100 kDa in serovar 1/2 strains. The ∼65-kDa band present in all extracts probed with anti-InlB antibodies is InlB.
CWA domain of Ami 4b binds to the surface of serovar 4b cells more efficiently than to the surface of serovar 1/2a cells.
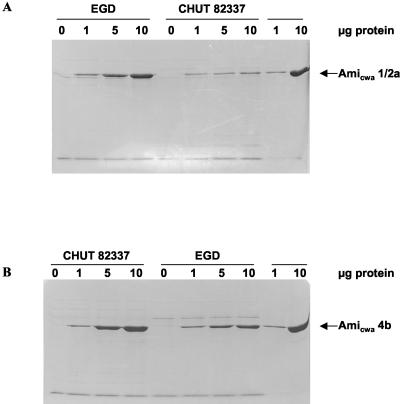
We compared the abilities of the CWA domains of Ami 1/2a and Ami 4b to bind to the surfaces of serovar 1/2a and 4b bacterial cells using purified polypeptides. Binding experiments were performed by incubating EGD and CHUT 82337 bacterial cells (serovar 1/2a and 4b cells) with the purified Ami CWA domain originating from either strain. After the preparations were washed to remove nonspecifically bound material, bacterial protein extracts were separated by SDS-PAGE and stained with Coomassie blue (Fig. 3).
FIG. 3.
Binding efficiencies of purified Amicwa 1/2a and Amicwa 4b added exogenously to L. monocytogenes bacterial cells. Equivalent numbers of either EGD (serovar 1/2a) or CHUT 82337 (serovar 4b) cells were incubated with 0, 1, 5, or 10 μg of purified Ami CWA domains. After washing, bound protein was detected by Coomassie blue staining. (A) Binding of Amicwa 1/2a. (B) Binding of Amicwa 4b.
The purified Amicwa 1/2a bound efficiently to the serovar 1/2a bacterial cells in a dose-dependent manner. Much less Amicwa 1/2a bound to the serovar 4b cells, and binding was not dose dependent. This experiment was repeated with purified Amicwa 4b. Amicwa 4b bound to the serovar 4b cells very efficiently and in a dose-dependent manner. Less Amicwa 4b bound to the serovar 1/2a cells, but the binding remained clearly dose dependent; overall, Amicwa 4b bound to the serovar 1/2a cells slightly better than Amicwa 1/2a bound to the serovar 4b cells. Thus, the CWA domains of Ami 1/2a and Ami 4b appear to bind autologous bacterial surfaces most efficiently. However, the CWA domain of Ami 4b may have less selective binding capacity.
CWA domains of Ami 4b and Ami 1/2a show different patterns of binding to eukaryotic cells.
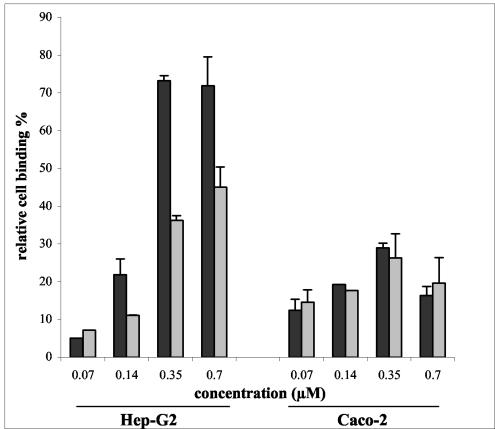
It was recently shown that the efficiencies with which purified Amicwa 1/2a binds to eukaryotic cells are different for different cell lines (29). For example, in identical experimental conditions, Amicwa 1/2a binds to Hep-G2 hepatocytic cells with significantly greater efficiency than it binds to Caco-2 enterocytic cells. Thus, we studied whether the CWA domains of Ami 1/2a and Ami 4b display distinct patterns of binding to eukaryotic cells. Microtiter plates were coated with various amounts of purified Amicwa 1/2a or Amicwa 4b and incubated with either Hep-G2 or Caco-2 cells. After washing, cell binding was evaluated by a hexosaminidase colorimetric assay.
As previously reported (29), Amicwa 1/2a bound to Hep-G2 cells much more efficiently than it bound to Caco-2 cells (2.5- to 5.0-fold-greater efficiencies at concentrations of 0.35 and 0.70 mM, respectively; P < 0.001) (Fig. 4). This may have been due to the fact that Hep-G2 cells express particularly high levels of surface glycosaminoglycans (9, 30), which are the cell receptors of InlB and possibly of Ami molecules (20). However, interestingly, Amicwa 4b bound to Hep-G2 cells 1.5 to 2 times less efficiently than Amicwa 1/2a bound to these cells (P < 0.001), whereas the two polypeptides bound to Caco-2 cells similarly. Thus, Amicwa 4b and Amicwa 1/2a differ in the ability to bind to Hep-G2 cells but not in the ability to bind to Caco-2 cells.
FIG. 4.
Binding of Amicwa 1/2a and Amicwa 4b to eukaryotic cells. Wells coated with various concentrations of purified Amicwa 1/2a or Amicwa 4b were incubated with Caco-2 or Hep-G2 cells for 2 h at 37°C. After washing, bound cells were quantified by a colorimetric hexosaminidase assay. Values are expressed relative to the binding value obtained with poly-l-lysine-coated wells, defined arbitrarily as 100. The values are means and standard errors for two independent experiments.
Sequences of the Ami CWA domain in various serovars of L. monocytogenes correlate with the somatic antigen.
The dissimilarity of the Ami 1/2a and Ami 4b CWA domains suggested that the CWA domains of the Ami molecules may vary among the serovars of L. monocytogenes. To approach this question, we amplified and sequenced the part of the ami gene encoding the CWA domain (amicwa) in strains belonging to the 12 L. monocytogenes serovars. To prevent errors in serovar assignment, we sequenced the panel of strains that are used by the National Reference Center for Listeria (Institut Pasteur, Paris, France) for the preparation of specific antisera.
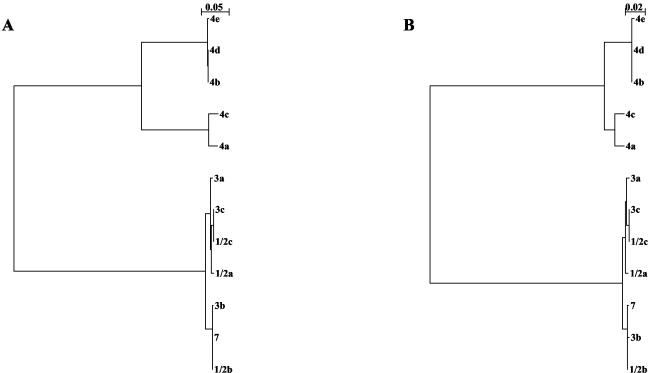
The sizes of PCR products allowed us to distinguish two groups, one that yielded products about 2.2 kb long (serovars 1/2a, 1/2b, 1/2c, 3a, 3b, 3c, 4a, 4c, and 7) and one that yielded products about 1.7 kb long (serovars 4b, 4d, and 4e). DNA sequence analysis showed that the difference in size between the two groups was mainly due to the presence of four DNA repeats in the first group and only three DNA repeats in the second group. A phylogenetic analysis based on the nucleotide sequence also allowed us to distinguish two groups; group I consisted of serovars 1/2a, 1/2b, 1/2c, 3a, 3b, 3c, and 7 (serogroups 1/2 and 3 and serovar 7), and group II consisted of serovars 4a, 4b, 4c, 4d, and 4e (serogroup 4) (Fig. 5A). All members of group I had an amicwa region consisting of about 2.2 kb containing four DNA repeats. Group II could be further divided into two subgroups; members of subgroup IIa (serovars 4a and 4c) had an amicwa region consisting of about 2.2 kb containing four DNA repeats, whereas members of subgroup IIb (serovars 4b, 4d, and 4e) had an amicwa region consisting of about 1.7 kb containing only three repeats. The sequence similarity was very high within each group (97.1 to 100% nucleotide identity in group I and 89.3 to 100% nucleotide identity in group II) but very low between the two groups (37.3 to 46.2% nucleotide identity) (Table 2).
FIG. 5.
Phylogenetic trees based on the nucleotide (A) and amino acid (B) sequences of the Ami CWA domains from the 12 L. monocytogenes serovars. The trees were constructed with the NJplot program. The bar above each tree indicates genetic distance.
TABLE 2.
Sequence similarity for group I and group II Ami CWA domains
| Serovar | % Identitya
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I
|
Group II
|
|||||||||||
| Serovar 1/2a | Serovar 1/2b | Serovar 1/2c | Serovar 3a | Serovar 3b | Serovar 3c | Serovar 7 | Serovar 4a | Serovar 4b | Serovar 4c | Serovar 4d | Serovar 4e | |
| 1/2a | 98.9 | 99.4 | 99.4 | 98.8 | 99.4 | 98.8 | 54.0 | 54.4 | 43.9 | 54.4 | 54.4 | |
| 1/2b | 97.2 | 98.6 | 98.9 | 99.8 | 98.6 | 99.8 | 43.9 | 54.4 | 43.9 | 54.4 | 54.4 | |
| 1/2c | 99.2 | 97.2 | 99.4 | 98.5 | 100 | 98.5 | 54.1 | 54.4 | 43.9 | 54.4 | 54.4 | |
| 3a | 99.0 | 97.3 | 99.0 | 98.8 | 99.4 | 98.8 | 43.9 | 54.0 | 43.75 | 54.0 | 54.0 | |
| 3b | 97.1 | 99.8 | 97.1 | 97.3 | 98.5 | 99.7 | 43.75 | 54.2 | 43.75 | 54.2 | 54.2 | |
| 3c | 99.2 | 97.2 | 100 | 99 | 97.1 | 98.5 | 54.1 | 54.4 | 43.9 | 54.4 | 54.4 | |
| 7 | 97.1 | 99.9 | 97.2 | 97.3 | 99.8 | 97.2 | 54.0 | 54.4 | 43.9 | 54.4 | 54.4 | |
| 4a | 46.1 | 38.0 | 46.2 | 46.2 | 38.0 | 46.2 | 38.0 | 95.1 | 98.1 | 95.1 | 94.9 | |
| 4b | 43.2 | 43.1 | 43.0 | 46.1 | 43.1 | 43.0 | 43.1 | 90.4 | 94.1 | 100 | 99.8 | |
| 4c | 37.3 | 37.5 | 37.3 | 37.4 | 37.6 | 37.3 | 37.6 | 96.8 | 89.6 | 94.1 | 93.9 | |
| 4d | 43.2 | 43.1 | 43.0 | 43.1 | 43.1 | 43.0 | 43.1 | 90.4 | 100 | 89.5 | 99.8 | |
| 4e | 43.3 | 43.1 | 43.0 | 43.3 | 43.1 | 43.0 | 43.2 | 90.1 | 99.5 | 89.3 | 99.5 | |
The levels of amino acid and nucleotide identity are shown on the upper right and the lower left, respectively.
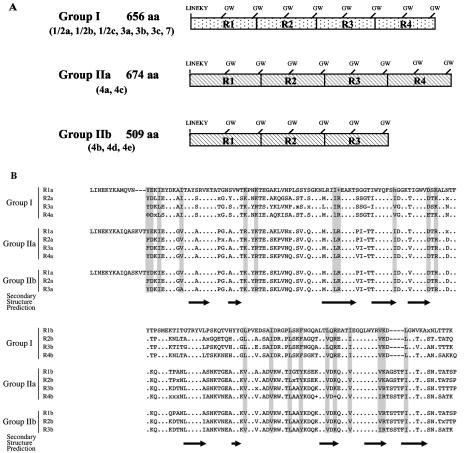
Analysis of deduced amino acid sequences yielded the same division into group I (serogroups 1/2 and 3 and serovar 7) and group II (serogroup 4) and the same subdivision of group II into subgroup IIa (serovars 4a and 4c) and subgroup IIb (serovars 4b, 4d, and 4e) (Fig. 5B). The levels of amino acid identity ranged from 98.5 to 100% within group I and from 93.9 to 100% within group II but did not exceed 54.4% when comparisons were made between the two groups (Table 2). Figure 6A shows the structural organization of the CWA domains within each group. The Ami CWA domain is 656 amino acids long and contains four 160-amino-acid repeats (eight GW modules) in group I, is 674 amino acids long and contains four 165-amino-acid repeats (eight GW modules) in subgroup IIa, and is 509 amino acids long and contains three 165-amino-acid repeats (six GW modules) in subgroup IIb. These data need to be confirmed with a larger number of strains. However, they are fully consistent with a recent study showing that Ami has an apparent molecular mass of ∼80 kDa in serovar 4b and ∼98 kDa in serovars 1/2a, 1/2b, 1/2c, 3c, and 7 (18). Sequence alignments revealed that the distribution of charged and hydrophobic amino acid residues is remarkably conserved (Fig. 6B). The analysis also revealed a short sequence of variable length located just upstream of the GW dipeptide of b modules, which presumably corresponds to a loop. Finally, the comparison of secondary structures predicted from consensus sequences suggested that Ami proteins produced by the various serovars have a common structure (Fig. 6B).
FIG. 6.
Ami CWA domains from the 12 serovars of L. monocytogenes. (A) General organization of the Ami CWA domains in the various serovars. (B) Sequence alignments. We defined a consensus sequence (threshold, 96%) from the repeat region of each group. Dots indicate residues that are completely conserved in all repeats; positions are shaded if the chemical nature of the residues was conserved in all the sequences within the repeats, as follows: h, aliphatic residues (ILV); φ, aromatic residues (YFWH) (of the hydrophobic residues); +, basic residues (KHR); and -, acidic residues (ED) (of the polar residues). Secondary structure predictions are shown below the sequences; β-strands are represented by arrows.
DISCUSSION
A number of previous studies have attempted to identify a correlation between virulence gene allelic diversity and serovars. However, analyses of polymorphism of hly (also called hlyA or lisA previously), mpl, plcA, inlA, and inlB have failed to find factors involved in L. monocytogenes pathogenicity that are serovar dependent (6, 33, 41, 42). We provide here the first example of allelic polymorphism correlated with the somatic antigen in a gene involved in the pathogenicity of L. monocytogenes. Our results indicate that O antigens I, II, IV, XII, and XIII occur exclusively in group I of Ami sequences (serogroups 1/2 and 3 and serovar 7) and O antigens V, VI, VII, VIII, IX, and X occur exclusively in group II (serogroup 4), while H antigens A, B, C, and D occur in both group I and group II. This clearly shows that the sequence of the CWA domain of Ami molecules correlates with the somatic antigen and not with the flagellar antigen.
These data suggest that the anchoring of Ami involves a bacterial factor that varies with the somatic antigen. This factor might be the cell wall TA as these molecules are major components of the somatic antigens (8). TA consist of polyribitolphosphate chains that may be decorated with various sugar or N-acetylamino sugar residues. In serogroups 1/2 and 3 and serovar 7, the polyribitolphosphate chains are replaced by rhamnose and/or N-acetylglucosamine at C-2 and/or C-4 of ribitolphosphate. In serogroup 4 (and serogroups 5 and 6 in the genus Listeria), N-acetylglucosamine is integrated into the polymer chains and attached to the C-2 or C-4 hydroxyl group of ribitolphosphate; replacement by glucosyl or galactosyl residues may occur at C-3 and/or C-6 of N-acetylglucosamine. Given the differences between the Ami CWA domains in groups I and II and the strong similarities of these domains within each group, it is more likely that the structure of the polyribitolphosphate backbone plays an important role in the anchoring of Ami rather than TA-associated decorating sugars. Similar observations have been made with L. monocytogenes phage endolysins Ply118 and Ply500 (23), which bind to the surfaces of serogroup 1/2 and 3 and serovar 7 Listeria cells and to serogroup 4 and 6 and serovar 5cells, respectively, via the C-terminal binding domain (24).
The supposed role of bacterial factors correlated with the somatic antigen for the targeting of Ami molecules may explain why, when added exogenously to bacterial cells, the CWA domains of Ami 4b and Ami 1/2a bind more efficiently to bacterial cells belonging to the same serovar. This is also consistent with recent experiments comparing expression of the CWA domains of Ami 1/2a and Ami 4b in Listeria innocua serovar 6a, which displays TA similar to serogroup 4 TA; in this bacterial host, the Ami 4b CWA domain is expressed much better than the Ami 1/2a CWA domain at the bacterial surface (Milohanic, unpublished data). These data are reminiscent of a previous study in which the workers compared production of an InlB-Ami hybrid comprising the first 398 amino acids of InlB and the eight GW modules of Ami 1/2a in L. monocytogenes EGD (serovar 1/2a) and L. innocua BUG 499 (serovar 6a) (3, 19). This study showed that the hybrid protein was entirely surface associated when it was produced in L. monocytogenes and was mostly secreted into the supernatant in L. innocua. Thus, the Ami CWA domain clearly needs molecules that are linked to or part of the autologous somatic antigen to efficiently target Ami to the bacterial surface.
It was recently shown that Ami contributes to the adhesion of L. monocytogenes serovar 1/2a to mammalian cells via its CWA domain (29). We now present evidence that the Ami molecules produced by serovar 1/2a and 4b strains display distinct adhesion patterns in two cell models widely used to evaluate the interaction of Listeria with human cells, the hepatocytic Hep-G2 model (5) and the enterocytic Caco-2 model (10, 11); while the two CWA domains bind to Caco-2 human enterocytic cells similarly, the Ami 1/2a CWA domains bind to Hep-G2 human hepatocytic cells about two times more efficiently. The molecular basis of the different adhesion patterns of the CWA domains of Ami 1/2a and Ami 4b remains to be elucidated. It is possible that Ami 4b binds to cells less efficiently because it contains fewer GW modules. Adhesion activity is strong with the unprocessed form of the staphylococcal autolysin-adhesin AtlE from Staphylococcus epidermidis, which has six GW modules, and is weaker with the amidase- and glucosaminidase-processing products, which only have four and two GW modules, respectively (16). Similar observations have been made with the autolysin-adhesin Aas from Staphylococcus saprophyticus (17).
Whether the differences in the adhesion patterns of Ami 1/2a and Ami 4b have a significant impact in terms of pathogenicity remains an open question. Ami is not per se a major actor in the interaction between Listeria and host cells. The loss of adhesion resulting from inactivation of ami is significant only in inlA and/or inlB mutants, probably because InlA and InlB largely overcome the defect in the Ami cell adhesion function (28, 29). However, we believe that Ami contributes to the fine-tuning of the molecular events that occur in the Listeria-cell interaction process. It was shown previously that overexpression of Ami CWA severely inhibited the entry of L. monocytogenes into Hep-G2 cells but not the entry into Caco-2 cells (29). This surprising result suggests that if the association between bacteria and the cell surface is too strong, as is the case with Hep-G2 cells, the entry process may be hindered. Alternatively, since the CWA domains of Ami 1/2a and InlB both have a strong affinity for glycosaminoglycans, Ami 1/2a may hinder the binding of InlB to its glycosylated receptor, the Met receptor tyrosine kinase (20, 37). Ami 1/2a may thus interfere with the entry process mediated by InlB, which is more important for the invasion of Hep-G2 cells than for the invasion of Caco-2 cells (3). Whatever the underlying mechanism involved, the overall weaker adhesion of Ami 4b may be an advantage for the invasion of certain host cells. This may result in a somewhat different behavior of L. monocytogenes serovar 4b in the living host. To approach this question, it is necessary to evaluate the pathogenicity of ami null mutants constructed in serovar 1/2a and 4b backgrounds.
Acknowledgments
We thank H. Bierne for kindly providing pET28.a-5, P. Velge for the gift of strains, and C. Buch and I. Sénégas for critical reading of the manuscript.
This work was supported by the Institut Pasteur, the Paris V University, the Ministère de l'Education Nationale, de la Recherche et de la Technologie, the EU (grant BMH4CT96 0659/RA03813), the Fondation pour la Recherche Médicale, GlaxoWellcome, and SmithKline Beecham. P.C. is an international investigator of the Howard Hughes Medical Institute.
Editor: V. J. DiRita
REFERENCES
- 1.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285-294. [DOI] [PubMed] [Google Scholar]
- 3.Braun, L., H. Ohayon, and P. Cossart. 1998. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077-1087. [DOI] [PubMed] [Google Scholar]
- 4.Cabanes, D., P. Dehoux, O. Dussurget, L. Frangeul, and P. Cossart. 2002. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 10:238-245. [DOI] [PubMed] [Google Scholar]
- 5.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of Listeria monocytogenes into hepatocytes requires expression of InIB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 6.Ericsson, H., H. Unnerstad, J. G. Mattsson, M. L. Danielsson-Tham, and W. Tham. 2000. Molecular grouping of Listeria monocytogenes based on the sequence of the inIB gene. J. Med. Microbiol. 49:73-80. [DOI] [PubMed] [Google Scholar]
- 7.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiedler, F. 1988. Biochemistry of the cell surface of Listeria strains: a locating general view. Infection 16(Suppl. 2):S92-S97. [DOI] [PubMed] [Google Scholar]
- 9.Frevert, U., P. Sinnis, C. Cerami, W. Shreffler, B. Takacs, and V. Nussenzweig. 1993. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J. Exp. Med. 177:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard, J. L., B. B. and Finlay. 1996. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect. Immun. 64:1299-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard, J. L., F. Jaubert, and P. Berche. 1996. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J. Exp. Med. 183:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaert, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. Mata Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, T., Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 15.Gray, M. L., and A. H. Killinger. 1966. Listeria monocytogenes and listeric infections. Bacteriol. Rev. 30:309-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilmann, C., M. Hussain, G. Peters, and F. Gotz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 17.Hell, W., H. G. Meyer, and S. G. Gatermann. 1998. Cloning of aas, a gene encoding a Staphylococcus saprophyticus surface protein with adhesive and autolytic properties. Mol. Microbiol. 29:871-881. [DOI] [PubMed] [Google Scholar]
- 18.Jacquet, C., E. Gouin, D. Jeannel, P. Cossart, and J. Rocourt. 2002. Expression of ActA, Ami, InlB, and listeriolysin O in Listeria monocytogenes of human and food origin. Appl. Environ. Microbiol. 68:616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonquieres, R., H. Bierne, F. Fiedler, P. Gounon, and P. Cossart. 1999. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of gram-positive bacteria. Mol. Microbiol. 34:902-914. [DOI] [PubMed] [Google Scholar]
- 20.Jonquieres, R., J. Pizarro-Cerda, and P. Cossart. 2001. Synergy between the N- and C-terminal domains of InlB for efficient invasion of non-phagocytic cells by Listeria monocytogenes. Mol. Microbiol. 42:955-965. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Landegren, U. 1984. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J. Immunol. Methods 67:379-388. [DOI] [PubMed] [Google Scholar]
- 23.Loessner, M. J., G. Wendlinger, and S. Scherer. 1995. Heterogeneous endolysins in Listeria monocytogenes bacteriophages: a new class of enzymes and evidence for conserved holin genes within the siphoviral lysis cassettes. Mol. Microbiol. 16:1231-1241. [DOI] [PubMed] [Google Scholar]
- 24.Loessner, M. J., K. Kramer, F. Ebel, and S. Scherer. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44:335-349. [DOI] [PubMed] [Google Scholar]
- 25.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 26.McLauchlin, J. 1990. Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis. Eur. J. Clin. Microbiol. Infect. Dis. 9:210-213. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlan, A. M., and S. J. Foster. 1998. Molecular characterization of an autolytic amidase of Listeria monocytogenes EGD. Microbiology 144:1359-1367. [DOI] [PubMed] [Google Scholar]
- 28.Milohanic, E., B. Pron, P. Berche, and J. L. Gaillard. 2000. Identification of new loci involved in adhesion of Listeria monocytogenes to eukaryotic cells. European Listeria Genome Consortium. Microbiology 146:731-739. [DOI] [PubMed] [Google Scholar]
- 29.Milohanic, E., R. Jonquieres, P. Cossart, P. Berche, and J. L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 30.Pancake, S. J., G. D. Holt, S. Mellouk, and S. L. Hoffman. 1992. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 117:1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson, J. 1940. The antigenic structure of organisms of the genus Listeria. J. Pathol. Bacteriol. 51:427-436. [Google Scholar]
- 32.Poyart-Salmeron, C., P. Trieu-Cuot, C. Carlier, A. MacGowan, J. McLauchlin, and P. Courvalin. 1992. Genetic basis of tetracycline resistance in clinical isolates of Listeria monocytogenes. Antimicrob. Agents Chemother. 36:463-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen, O. F., T. Beck, J. E. Olsen, L. Dons, L. and Rossen. 1991. Listeria monocytogenes isolates can be classified into two major types according to the sequence of the listeriolysin gene. Infect. Immun. 59:3945-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocourt, J., and H. P. Seeliger. 1985. Distribution of species of the genus Listeria. Zentralbl. Bakteriol. Mikrobiol. Hyg. Ser. A 259:317-330. [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Seeliger, H., K. and Höhne. 1979. Serotyping of Listeria monocytogenes and related species. Methods Microbiol. 13:33-48.
- 37.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501-510. [DOI] [PubMed] [Google Scholar]
- 38.Slutsker, L., and A. Schuchat. 1999. Subtyping Listeria monocytogenes, p. 75-95. In E. Ryser and E. Marth (ed.), Listeria, listeriosis and food listeriosis. Marcel Dekker, Inc., New York, N.Y.
- 39.Van Langendonck, N., E. Bottreau, S. Bailly, M. Tabouret, J. Marly, P. Pardon, and P. Velge. 1998. Tissue culture assays using Caco-2 cell line differentiate virulent from non-virulent Listeria monocytogenes strains. J. Appl. Microbiol. 85:337-346. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vicente, M. F., F. Baquero, and J. C. Pérez-Diaz. 1985. Cloning and expression of the Listeria monocytogenes haemolysin in Escherichia coli. FEMS Microbiol. Lett. 30:77-79. [Google Scholar]
- 42.Vines, A., and B. Swaminathan. 1998. Identification and characterization of nucleotide sequence differences in three virulence-associated genes of Listeria monocytogenes strains representing clinically important serotypes. Curr. Microbiol. 36:309-318. [DOI] [PubMed] [Google Scholar]
- 43.Wood, S., N. Maroushek, and C. J. Czuprynski. 1993. Multiplication of Listeria monocytogenes in a murine hepatocyte cell line. Infect. Immun. 61:3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yannish-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]