Summary
Using both direct neural recordings and electrical microstimulation, Joshi et al. (2016) show that locus coeruleus (LC) activity closely matches moment-to-moment changes in pupil size. But what causes these two measures to be related is not straightforward.
Based on the observation that changes in pupil size coincide with various arousal states, philosophers, poets, and artists have long posited the pupil as a window into the mind. Scientists’ interest in pupil size has been no less passionate. In recent years, researchers in cognitive neuroscience and psychology have increasingly turned to measuring pupil size to obtain a read out of physiological arousal during cognitive (Eldar et al., 2013) and emotional challenge (Bradley et al., 2008). This measure of autonomic arousal has proven popular in part because pupil size changes rapidly (~250 ms) in response to external stimuli or internal mentation, and the relative ease at which it can be measured using modern eye trackers. Which brain areas influence pupil size and how activity in each area is related to instantaneous fluctuations in pupil size is not well understood.
In recent years the idea that pupil size is directly related to moment-to-moment fluctuations in the activity of noradrenergic neurons in the locus coeruleus (LC) has gained significant attention (Aston-Jones and Cohen, 2005; Nassar et al., 2012). This notion is enormously appealing, as it would allow a simple physiological measure to be related to the activity of a specific brain area and neuromodulator. The allure of this idea has, however, been slightly tempered by the lack of any mechanistic and causal studies to prove the relationship. Until recently, the most direct evidence relating LC activity and pupil size was from an intriguing observation that the activity of an LC neuron closely aligned with simultaneously recorded changes in pupil size (Aston-Jones and Cohen, 2005). What has been needed is an in depth study to directly assess the link between LC activity and pupil size.
To begin filling this void, a study by Joshi and colleagues set out to determine the relationship between spontaneous and event driven changes in pupil size and LC activity in rhesus macaques (Joshi et al., 2015). The authors recorded activity in LC as well as a number of other parts of the brain that have recently been implicated in controlling pupil size, the inferior colliculus (IC), superior colliculus (SC), anterior cingulate cortex (ACC), and posterior cingulate cortex (CGp) in macaque monkeys (Ebitz and Platt, 2015; Varazzani et al., 2015; Wang et al., 2012). By recording across this set of areas they could assess not only whether LC activity is related to pupil size, but also how it compares to these other areas.
First, Joshi and colleagues assessed how pupil size is related to spontaneous neural activity. To avoid any confounds from either the cognitive demands of a task or visual stimuli that might cause changes in pupil size, they trained five monkeys to fix their gaze on a red spot on a computer monitor while they recorded ongoing neural activity and pupil size. In these situations the size of the pupil slightly oscillates. Because neural activity also shows subtle moment-to-moment fluctuations this allowed the authors to see if these two time-varying signals oscillated in step. During passive fixation they found that fluctuations in the firing rate of many of the neurons that they recorded from correlated with the oscillations in pupil size. Interestingly, these correlations occurred on two different timescales. When spiking activity was aggregated over seconds (1–5 s) on a trial-by-trial basis, changes in pupil size were positively correlated only with the spiking activity of individual cells recorded in LC and IC.
A very different pattern emerged when the relationship between pupil size and neuronal activity from all five recorded brain regions were analyzed on a finer timescale. When changes in pupil size were aligned to individual spikes there was clear evidence of an association between activity in LC, IC and SC, and changes in pupil size—dilation followed by constriction. Spike-triggered pupil responses for the two cortical sites, ACC and CGp, were more variable. Most strikingly, when neuronal activity was temporally aligned to instances of pupil dilation and these were compared to instances of pupil constriction, Joshi et al. were able to demonstrate that spiking activity was greater prior to dilation than constriction (Figure 1). This occurred in all five brain regions, but was particularly evident in LC, IC and SC. Similarly, changes in the gamma band of the local field potential (LFP), likely reflecting local processing in an area, preceded instances of dilation versus constriction at relatively short time scales (<0.5 s) in LC, IC, and SC. Intriguingly, changes in gamma band LFP power, preceding pupil dilation versus constriction, occurred much earlier in ACC and CGp (> 1 s), potentially indicating that these cortical areas function upstream from brainstem sites.
Figure 1.
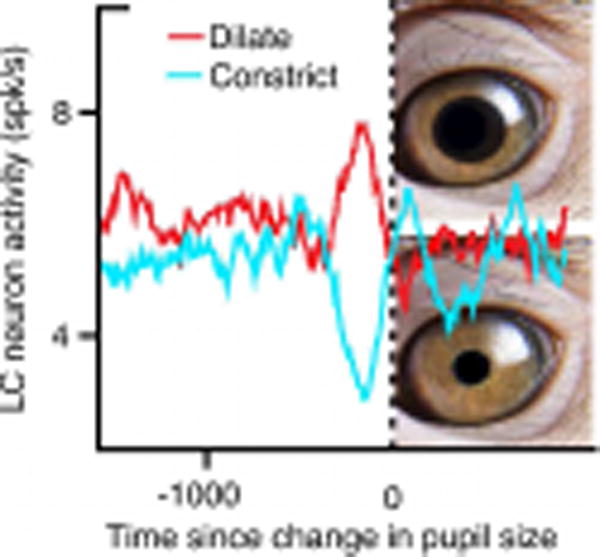
Higher Spike Rates in LC Precede Pupil Dilation than Constriction
These findings are noteworthy, as they show that independent of the timescale on which LC activity is measured, it is the region most strongly linked to pupil size fluctuations and does so in the absence of any task or visual stimuli. This last point is especially important as many of the previous studies implicating LC in pupil size did so within a task, which could have affected the results. It also supports the view that pupil size can be used to index changes in LC activity regardless of external stimulation.
Up to this point, the experiments in this study only show that fluctuations in pupil size correlate with spontaneous activity in LC, but what would happen when external events drive changes in pupil size? Previous work has shown that spiking activity in LC, but not substantia nigra, is positively correlated with changes in pupil size during decision-making tasks (Varazzani et al., 2015). Building on these findings, Joshi and colleagues demonstrate a similar specificity of LC neuron activity in responding to external events that cause changes in arousal. While monkeys passively fixated, a startling tone was randomly delivered. By using auditory tones, researchers eliminated any influence of the light reflex on pupil size. While the unexpected tones elicited transient neural responses in the LC, IC and ACC, only LC activity correlated with pupil size on a trial-by-trial basis. Responses in SC and PCC were not clearly locked to the startling stimuli. This nicely complements evidence that correlations between LC activity and pupil size are not necessarily task related (Varazzani et al., 2015).
While the correlation between activity in LC and pupil size is convincing, this doesn’t show that LC activity is causally related to pupil size. To address this question the Joshi and colleagues electrically stimulated the LC, IC, and SC while monkeys passively fixated a red spot. While stimulation of each brain region evoked transient increases in pupil size, the effects were most consistent in the LC, with maximal pupil change occurring 250–700 ms following the onset of stimulation. More variable effects were found when stimulating IC or SC. Viewed alongside evidence that fluctuations in pupil size are correlated with LC activity and changes in LC spiking activity precede changes in pupil size, these stimulation results would appear to offer some of the most convincing evidence to date that pupil size and LC activity are directly related.
There is, however, a catch. At present there are no known direct connections between the LC and brainstem nuclei regulating pupil size in macaques. This means that either: 1) an as yet unidentified projection from LC to brain stem nuclei exists, 2) projections from the LC to autonomic control centers in the hypothalamus drive changes in pupil size (Smith et al., 2006), or 3) the effects of stimulation are due to antidromic activation of upstream areas that do control pupil size. This final possibility suggests that both LC and the nuclei controlling pupil size share a common input. Under such a mechanism, stimulation of the LC could cause back propagation of action potentials to the hypothesized common input area, driving changes in pupil size. One likely source is the nucleus paragigantocellularis of the ventral medulla. This nucleus receives widespread cortical and subcortical inputs, projects to both the Edinger-Westphal nucleus, which controls pupil constriction, and LC (Breen et al., 1983) and functionally interacts with LC (Ennis and Aston-Jones, 1988). Uncertainty around the precise mechanisms through which pupil size and LC activity are related highlights the need for further investigation into this circuitry. This question would seem to be ripe for interrogation using modern pathway specific techniques, particularly since optogenetic modulation of LC neurons can be used to regulate cortical and behavioral arousal (Carter et al., 2010).
If the LC does not directly control pupil size, how might it interact with the network of areas that do control arousal and ultimately pupil size? One potential mechanism is through interaction with the ACC, especially when arousal needs to be modulated during ongoing behavior. The LC and ACC are bi-directionally connected (Arnsten and Goldman-Rakic, 1984), and the ACC is part of a network involved in controlling autonomic arousal (An et al., 1998). Indeed lesions of subcallosal ACC, the part of the cortex high levels of noradrenergic transporter (Smith et al., 2006), disrupt the normal patterns of sustained pupil dilation in anticipation of rewards (Rudebeck et al., 2014). One exciting possibility is that coordinated activity between LC and ACC is vital for synchronizing and sustaining arousal states across the brain and periphery during behavior.
The idea that pupil size provides a moment-to-moment index of LC neural activity has received considerable attention. Here Joshi et al provide some compelling evidence that LC activity and pupil size are indeed closely correlated, but the mechanisms are far from clear. Hopefully their findings will arouse others to take a closer look at this issue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual review of neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen LA, Burde RM, Loewy AD. Brainstem connections to the Edinger-Westphal nucleus of the cat: a retrograde tracer study. Brain Res. 1983;261:303–306. doi: 10.1016/0006-8993(83)90633-9. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Platt ML. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 2015;85:628–640. doi: 10.1016/j.neuron.2014.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat Neurosci. 2013;16:1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci. 1988;8:3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SYL, Kalwani R, Gold JI. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron. 2015 doi: 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar MR, Rumsey KM, Wilson RC, Parikh K, Heasly B, Gold JI. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat Neurosci. 2012;15:1040–1046. doi: 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SE, Murray EA. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc Natl Acad Sci U S A. 2014;111:5391–5396. doi: 10.1073/pnas.1317695111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138:703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Varazzani C, San-Galli A, Gilardeau S, Bouret S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J Neurosci. 2015;35:7866–7877. doi: 10.1523/JNEUROSCI.0454-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CA, Boehnke SE, White BJ, Munoz DP. Microstimulation of the monkey superior colliculus induces pupil dilation without evoking saccades. J Neurosci. 2012;32:3629–3636. doi: 10.1523/JNEUROSCI.5512-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


