Abstract
Several neuropsychiatric disorders are associated with cognitive and social dysfunction. Post-mortem studies of patients with schizophrenia have revealed specific changes in area CA2, a long over-looked region of the hippocampus recently found to be critical for social memory formation. To examine how area CA2 is altered in psychiatric illness, we used the Df(16)A+/− mouse model of the 22q11.2 microdeletion, a genetic risk factor for developing several neuropsychiatric disorders, including schizophrenia. We report several age-dependent CA2 alterations: a decrease in the density of parvalbumin-stained interneurons, a reduction in the amount of feed-forward inhibition and a change in CA2 pyramidal neuron intrinsic properties. Furthermore, we found that area CA2 is less plastic in Df(16)A+/− mice, making it nearly impossible to evoke action potential firing in CA2 pyramidal neurons. Finally, we show that Df(16)A+/− mice display impaired social cognition, providing a potential mechanism and a neural substrate for this impairment in psychiatric disorders.
INTRODUCTION
While much recent progress has been made in understanding the genetic causes of psychiatric illnesses, there remain many unresolved questions pertaining to the neural substrates at the cellular and circuitry level underlying specific symptoms and cognitive deficits. One area in particular that merits further study is the long overlooked area CA2 of the hippocampus. It was shown recently that area CA2 is critical for social memory formation (Hitti and Siegelbaum, 2014; Stevenson and Caldwell, 2014), likely plays little role in spatial coding (Lee et al., 2015; Lu et al., 2015; Mankin et al., 2015) and may serve to detect conflicts between memory-driven and sensory information converging on the hippocampus (Wintzer et al., 2014). Before the contribution of area CA2 to hippocampal function was appreciated, numerous post-mortem studies of schizophrenic and psychotic patients had revealed that this relatively small hippocampal region undergoes disease-related changes in size and composition (Benes et al., 1998; Narr et al., 2004). A meta-analysis study reported that a decrease in parvalbumin-expressing (PV+) interneuron (IN) density in area CA2 was one of the few measures, of more than 200, to be significantly altered in schizophrenia and bipolar disorder in the hippocampus (Knable et al., 2004). A decrease in PV+ INs in CA2 has also been reported in Alzheimer’s disease (Brady and Mufson, 1997). Therefore, understanding cellular alterations that occur in area CA2 in psychiatric disorders is likely to provide invaluable information about the pathogenesis of these diseases. To this end, we analyzed CA2 in a mouse model of the 22q11.2 deletion syndrome (22q11.2DS), as this allows a more reliable and comprehensive examination of the consequences of the disease on cellular function and circuitry dynamics.
Individuals with the 22q11.2 deletion are sometimes given other diagnoses early in life, including attention-deficit hyperactivity disorder, generalized anxiety disorder, obsessive-compulsive disorder and autism spectrum disorders (ASD) (Karayiorgou et al., 2010). In late adolescence and early adulthood, up to one-third of all individuals carrying the 22q11.2 deletion develop schizophrenia or schizoaffective disorder, an approximately 30-fold increase in disease risk. Moreover, de novo 22q11.2 deletions account for up to 1–2% of sporadic schizophrenia cases (International Schizophrenia Consortium, 2008; Karayiorgou et al., 1995; Xu et al., 2008). Most affected individuals carry a 3-Mb hemizygous deletion, whereas 7% have a nested 1.5-Mb deletion spanning 27 known genes (Karayiorgou et al., 2010). A mouse model, Df(16)A+/− mice (Stark et al., 2008), carrying an engineered orthologous deletion on mouse chromosome 16 encompassing all but one of the genes encoded in the 22q11.2 critical region, is a particularly powerful tool for deciphering how this genetic lesion increases risk for neuropsychiatric disorders. Df(16)A+/− mice have deficits in sensorimotor gating, emotional learning (Stark et al., 2008) and altered performance and long-range synchrony between the hippocampus and prefrontal cortex during a spatial working memory task (Sigurdsson et al., 2010; Stark et al., 2008). However, besides the impairment in long-range connectivity between brain structures, the local cellular changes at the level of the hippocampal microcircuit are subtle in area CA1 (Drew et al., 2011; Earls et al., 2010) and completely unknown in areas CA2 and CA3. Given the reported alterations in area CA2 in patients with schizophrenia and other neuropsychiatric disorders, we decided to examine area CA2 of Df(16)A+/− mice, and in particular, the inhibitory transmission and activity-dependent plasticity mediated by PV+ INs.
We found that the density of PV+ INs in the hippocampus of Df(16)A+/− mice is specifically reduced in area CA2. Accompanying this reduction is an impairment of feed-forward inhibition onto CA2 PNs and a larger excitatory drive from CA3 inputs. These effects are only observed after maturity to adulthood, paralleling the disease onset in humans. The intrinsic properties of CA2 PNs are also affected, resulting in a decreased action potential firing in response to proximal and distal excitatory inputs stimulation. The Df(16)A+/− mice display social memory impairment similar to that observed following specific silencing of CA2 PNs. These results show that the specific alterations reported in hippocampal CA2 in humans with schizophrenia are also present in Df(16)A+/− mice and may underlie impaired social cognition in this disorder.
RESULTS
The density of PV+ INs in the hippocampus is decreased specifically in area CA2 of adult Df(16)A+/− mice
In the hippocampus, both individual and meta-analysis of postmortem studies of individuals with schizophrenia have reported a significant decrease in PV+ IN density specifically in area CA2 (Benes et al., 1998; Berretta et al., 2009; Knable et al., 2004; Zhang and Reynolds, 2002). Therefore, we first asked whether a similar change in PV+ IN density occurred in area CA2 of Df(16)A+/− mice. To this end, we performed immunostaining against PV and quantified the density of PV+ cells in the different subdivisions of the hippocampus in adult mice (8-week old). Consistent with previous findings (Botcher et al., 2014; Piskorowski and Chevaleyre, 2013), the density of PV+ INs in wild-type littermate control mice (WT) was higher in area CA2 stratum oriens (SO) and stratum pyramidale (SP) than in the other hippocampal areas (Figure 1A,B). In Df(16)A+/− mice, the density of PV+ INs in area CA2 was significantly lower than in WT mice (in SO: 6650 ± 525 for WT vs. 4691 ± 239 for Df(16)A+/− mice, p = 0.0068; in SP: 24664 ± 1307 for WT vs. 16110 ± 2071 for Df(16)A+/− mice, p = 0.0058, n = 6 mice for both WT and Df(16)A+/− mice). Strikingly, this decrease in PV+ cell density was specific to area CA2 as no changes were observed in areas CA1 and CA3 (Figure 1B). To ensure that the quantification of PV+ density in CA2 was not biased by a change in the size of area CA2 in Df(16)A+/− mice, we also performed co-staining with the CA2-specific marker regulator of G-protein signaling 14 (RGS14) (Lee et al., 2010). We found no difference between WT and Df(16)A+/− mice in the area of the hippocampus stained by the RGS14 antibody, indicating that the size of area CA2 is unchanged. Moreover, with the boundaries between the CA areas defined by RGS14 staining alone, we confirmed the significant decrease in the density of PV+ cells in CA2 area of Df(16)A+/− mice (Supl Figure 1, in SP: p = 0.008; n = 3 and 4 mice for WT and Df(16)A+/− mice respectively).
Figure 1. The density of parvalbumin-expressing INs in the hippocampus is decreased in area CA2 of adult Df(16)A+/− mice.
(A) Immunohistochemical staining for parvalbumin in the different hippocampal areas of the hippocampus from a 8-week old wild-type and Df(16)A+/− mouse. The different hippocampal layers are indicated (SO: stratum oriens, SP: stratum pyramidale, SR: stratum radiatum, SL: stratum lucidum). (B) Quantification of the density of parvalbumin positive soma (PV+) in the different strata of areas CA1, CA2 and CA3 of 8-week old WT and Df(16)A+/− mice (n = 6 mice for both genotypes). (C) Immunohistochemical staining for parvalbumin in the different hippocampal areas of the hippocampus from a 4-week old wild-type and Df(16)A+/− mouse. (D) Quantification of the density of PV+ soma in the different strata of CA1, CA2 and CA3 areas in 4-week old WT (n = 6) and Df(16)A+/− mice (n = 6). Error bars show SEM. See also figure S1.
The typical onset of behavioral symptoms of schizophrenia occurs during early adulthood. We wondered whether the change we observed in PV+ INs density might also be age-dependent. Therefore, we quantified the density of PV+ INs in 4-week-old mice. The density of PV+ cells in 4-week-old WT mice was similar to that observed in older mice. Notably however, the density of PV+ cells in area CA2 was identical between WT and Df(16)A+/− mice (Figure 1C,D; in SO: 6662 ± 839 for WT vs. 6703 ± 1203 for Df(16)A+/− mice, p = 0.97; in SP: 22430 ± 1465 for WT vs. 22728 ± 1496 for Df(16)A+/− mice, p = 0.89, n = 6 for WT and 6 for Df(16)A+/− mice). Overall, our results show that the Df(16)A+/− mice recapitulate one of the most consistent cellular phenotypes observed in the hippocampus of patients with schizophrenia, i.e., an age-dependent decrease in PV+ cell number in area CA2.
The inhibitory control of excitatory drive from CA3 is reduced in adult Df(16)A+/− mice
Inhibitory transmission in area CA2 powerfully controls the strength of excitatory transmission from the Schaeffer collateral (SC) inputs, and completely prevents CA3 neurons to evoke action potential firing in CA2 pyramidal neurons (PNs). To address whether the decrease in PV+ neurons observed in Df(16)A+/− mice could affect the control of the excitatory drive from CA3 PNs, we monitored the post-synaptic potentials (PSPs) in CA2 PNs in response to stimulation of SC inputs before and after blockade of GABA receptors. The depolarizing phase of the PSP in control conditions was very small in WT mice and was truncated by a large hyperpolarizing component. The addition of GABA blockers markedly increased the amplitude of the excitatory PSP (EPSP; Figure 2A,B). In the absence of GABA blockers, the depolarizing component of the PSP was significantly larger in Df(16)A+/− mice than in WT mice (Figure 2B, n = 8, 3 mice for WT, n = 17, 6 mice for Df(16)A+/− mice; ANOVA 2 way RM: genotype: F(1,7) = 6.89, p = 0.03; stimulation: F(2.06,14.4) = 50.3, p = 0.00000027; genotype x stimulation: F(2.6,18.4) = 2.03, p = 0.14). However, in the presence of GABA blockers, the EPSPs in WT and Df(16)A+/− mice were not different (Figure 2A,B, n = 8, 3 mice for WT, n = 17, 6 mice for Df(16)A+/− mice; ANOVA 2 way RM: genotype: F(1,6) = 0.00032, p = 0.98; stimulation: F(1.7,10.24) = 185.9, p = 1.37×10−8; genotype x stimulation: F(1.42,8.56) = 0.23, p = 0.72). When we examined the change in PSP amplitude before and after blocking inhibition, the 4–5 fold increase observed in WT mice was significantly reduced to 2–3 fold in Df(16)A+/− mice (Figure 2C, n = 8, 3 mice for WT, n = 17, 6 mice for Df(16)A+/− mice; ANOVA 2 way RM: genotype: F(1,7) = 6.89, p = 0.03; stimulation: F(2.1,14.4) = 50.3, p = 2.7×10−7; genotype x stimulation: F(2.6,18.4) = 2.03, p = 0.14). Finally, to isolate the inhibitory component evoked during the stimulation, we subtracted the responses in the presence of GABA blockers from the responses with intact inhibition. Infering the inhibitory PSP (IPSP) size from a compound EPSP-IPSP has previously been validated in other studies (Basu et al., 2013; Pouille and Scanziani, 2001). We found that the deduced IPSP (IPSP) was significantly smaller in Df(16)A+/− mice (Figure 2D, n = 8, 3 mice for WT, n = 17, 6 mice for Df(16)A+/− mice; ANOVA 2 way RM: genotype: F(1,7) = 7.19, p = 0.03; stimulation: F(2.6,18.2) = 169.4, p = 3.7×10−13; genotype x stimulation: F(1.99,13.97) = 7.0, p = 0.0078). These results show that the depolarizing component of the compound EPSP-IPSP is larger in Df(16)A+/− mice, and that this increased PSP in the absence of GABA receptor blockers results from a decrease in the level of inhibitory transmission.
Figure 2. Inhibitory transmission onto CA2 PNs is decreased in adult Df(16)A+/− mice.
(A) Top; Schematic representation of the experimental conditions in which a compound EPSP/IPSP sequence was recorded in CA2 pyramidal neurons (PNs) following stimulation of Schaffer collaterals (SC). Bottom; Sample traces of the compound EPSP/IPSP (Control, black traces) and the EPSP obtained after blocking inhibition (SR/CGP, grey traces) in CA2 PNs in response to stimulation of SC inputs in WT and Df(16)A+/− mice. Below: the inhibitory component obtained after subtracting the control traces from the traces with GABA receptor blockers is shown in grey. (B) Summary graph of the input-output curves of the PSP in control conditions and after blocking inhibition (SR/CGP) in response to SC stimulation in adult WT (n = 9) and Df(16)A+/− mice (n = 19). (C) Summary graph of the fold-increase in PSP amplitude after blocking inhibition in WT and Df(16)A+/− mice.
(D) Summary graph of the input-output curves of the IPSP amplitude obtained by subtraction of control traces from the traces with GABA receptors blockers in WT and in Df(16)A+/− mice. (E) Summary graph of the input-output curves of the PSPs in response to SC stimulation in control conditions and following blockade of inhibitory transmission in young (4–5-week old) WT (n = 6) and Df(16)A+/− mice (n = 5). (F) Summary graph of the input-output curves of the IPSP in response to CA3 input stimulation obtained by subtracting control traces from traces with GABA receptor blockers in 4–5-week old WT and Df(16)A+/− mice. Error bars show SEM. See also figure S2.
Because the change in PV+ IN density is age-dependent, we measured the amplitude of the PSPs with and without inhibition and quantified the amplitude of deduced IPSPs for 4–5 week-old mice. We observed no difference in the amplitude of the PSP between WT and Df(16)A+/− mice (Figure 2E; n = 6, 4 mice for WT and n = 5, 5 mice for Df(16)A+/− mice, ANOVA 2 way RM: genotype: F(1,4) = 1.38×10−7, p = 0.99; stimulation: F(3.0,12.0) = 57.3, p = 2.14×10−7; genotype x stimulation: F(1.3,5.21) = 0.39, p = 0.61 for the PSP before blockers and ANOVA 2 way RM: genotype: F(1,3) = 0.12, p = 0.74; stimulation: F(1.9,5.8) = 148.2, p = 1.08×10−5; genotype x stimulation: F(1.3,3.9) = 0.25, p = 0.70 after GABA blockers). We also observed no difference in the amplitude of the deduced IPSP (Figure 2F; n = 6, 4 mice for WT and n = 5, 5 mice for Df(16)A+/− mice; ANOVA 2 way RM: genotype: F(1,3) = 0.036, p = 0.86; stimulation: F(1.7,5.17) = 66.5, p = 2.3×10−4; genotype x stimulation: F(1.6,4.9) = 0.22, p = 0.76). Therefore, these results indicate that the decrease in PV+ IN number and in recruited inhibition results from of a change during peri-adolescent development.
The inhibition onto the pyramidal cell comes from the recruitment of feed-forward inhibition engaged by the SC, but also potentially from a direct activation of inhibitory cells and axons by the stimulating electrode. To address whether the feed-forward inhibition is impaired in Df(16)A+/− mice, we directly monitored inhibitory post-synaptic currents (IPSCs) at a holding potential of 0 mV (the reversal potential for excitatory currents) before and after blocking excitatory transmission. In control conditions, both the directly-activated inhibition and the feed-forward inhibition will be present, while only directly activated inhibition will be monitored after blocking excitation. We quantified the amount of feed-forward inhibition by subtracting IPSCs in AMPA/NMDA blockers from the IPSCs in control conditions. We found that the level of feed-forward inhibition was ~30% larger in WT mice compared to Df(16)A+/− mice (Figure S2A,B; for instance at 20V stimulation: WT: 1764.8 ± 262.1 pA, n = 17, 7 mice; Df(16)A+/− mice: 1274.1 ± 211.9 pA, n = 15, 7 mice, ANOVA 2 way RM: genotype: F(1,13) = 5.59, p = 0.034; stimulation: F(1.6,21.0) = 51.47, p = 2.79×10−8; genotype x stimulation: F(1.96,25.5) = 0.95, p = 0.39).
The decrease in the level of feed forward inhibition could result from a decrease in the number of recruited INs or from a decrease in GABA release probability. We performed a paired stimulation to quantify the paired-pulse ratio (PPR) of two consecutive IPSCs, a parameter inversely proportional to release probability. When IPSCs were isolated in presence of AMPA/NMDA blockers, we found that the PPR (with a stimulation interval of 100 ms) was identical for WT and Df(16)A+/− mice over the entire stimulation intensity range (Figure S2C, n = 15, 6 mice for WT and n = 15, 6 mice for Df(16)A+/− mice, ANOVA 2 way RM: genotype: F(1,6) = 2.25, p = 0.18; stimulation: F(3.6,21.9) = 1.34, p = 0.28; genotype x stimulation: F(2.3,14.2) = 0.70, p = 0.53). We also measured the PPR at different stimulation intervals (between 20 and 800 ms) and again found no difference between WT and Df(16)A+/− mice (Figure S2D, n = 10, 5 mice for WT and 11, 5 mice for Df(16)A+/− mice, ANOVA 2 way RM: genotype: F(1,8) = 0.27, p = 0.61; stimulation: F(2.2,17.9) = 4.4, p = 0.02; genotype x stimulation: F(2.3,18.7) = 1.7, p = 0.20). Finally, because an increase in summation of synaptic responses during a train at CA3-CA1 excitatory synapses had previously been reported in Df(16)A+/− mice (Earls et al., 2010), we looked at the EPSP summation during a train of 5 pulses at the CA3-CA2 synapse. We found no difference between WT and Df(16)A+/− mice (Supl. Figure S2E; n = 6, 3 mice for WT and 7, 4 mice for Df(16)A+/− mice, ANOVA 2 way RM: genotype: F(1,3) = 0.10 p = 0.77; stimulation: F(1.8,5.6) = 102.6, p = 3.6×10−5; genotype x stimulation: F(1.2,3.6) = 0.15, p = 0.76). These results suggest that release probability at inhibitory synapses is not significantly altered in Df(16)A+/− mice and that the decrease in feed-forward inhibition is likely a consequence of the decrease in PV+ IN function.
Distal excitatory transmission is not affected in Df(16)A+/− mice
CA2 PNs receive excitatory input from CA3 SCs on proximal apical dendrites, and excitatory input from the entorhinal cortex on distal apical dendrites. To address whether the change we observed in Df(16)A+/− mice on the inhibitory control of excitatory transmission is input specific or a general feature in these mice, we stimulated in stratum lacunosum moleculare (SLM), and recorded PSPs before and after blocking inhibitory transmission. As reported previously, EPSPs from distal inputs were much less affected by blockade of inhibition than EPSPs from proximal inputs (Chevaleyre and Siegelbaum, 2010). We observed no difference in the PSP amplitude between adult WT and Df(16)A+/− mice, both in control conditions and following inhibition block (Figure 3A,B; n = 7, 3 mice for WT and 17, 6 mice for Df(16)A+/− mice, ANOVA 2 way RM: genotype: F(1,5) = 0.07, p = 0.79; stimulation: F(1.7,8.5) = 51.5, p = 2.25×10−5; genotype x stimulation: F(1.7,8.8) = 0.15, p = 0.83 before GABA blockers and ANOVA 2 way RM: genotype: F(1,5) = 0.0018, p = 0.96; stimulation: F(1.6,8.0) = 101.05, p = 3.04×10−6; genotype x stimulation: F(1.7,8.7) = 0.52, p = 0.58 after GABA blockers). Similarly, the increase in PSP amplitude after blocking inhibition and the deducted IPSP responses with and without GABA blockers were also unaffected in Df(16)A+/− mice (Figure 3C,D; n = 7, 3 mice for WT and 17, 6 mice for Df(16)A+/− mice; ANOVA 2 way RM: genotype: F(1,2) = 0.019, p = 0.90; stimulation: F(1.2,2.4) = 4.07, p = 0.16; genotype x stimulation: F(1.7,3.4) = 0.039, p = 0.94 for the fold increase in PSP and ANOVA 2 way RM: genotype: F(1,5) = 0.31, p = 0.59; stimulation: F(1.9,9.9) = 22.3, p = 226×10−4; genotype x stimulation: F(2.9,14.5) = 0.37, p = 0.76 for the IPSP).
Figure 3. Synaptic transmission of distal inputs onto CA2 PNs is not altered in adult Df(16)A+/− mice.
(A) Top; Schematic representation of the experimental conditions in which a compound EPSP/IPSP sequence was recorded in CA2 PNs following stimulation of distal inputs. Bottom; sample traces of the compound EPSP/IPSP (Control, black trace) and the EPSP obtained after blocking inhibition (SR/CGP, grey trace) in CA2 PNs in response to stimulation of distal inputs in WT and Df(16)A+/− mice. The inhibitory component obtained after subtracting the control traces from the traces with GABA receptor blockers is shown in grey.
(B) Summary graph of the input-output curves of the PSP amplitude in response to distal input stimulation in control conditions and after blocking inhibition in adult WT (n = 8) and Df(16)A+/− mice (n = 18). (C) Summary graph of the fold-increase in PSP amplitude after blocking inhibition in WT and Df(16)A+/− mice. (D) Summary graph of the input-output curves of the IPSP obtained after subtraction of the control traces from traces with GABA receptor blockers in WT and in Df(16)A+/− mice. (E) Summary graph of the input-output curves of PSPs in response to distal input stimulation in control conditions and after blocking inhibitory transmission in 4–5-week old WT (n = 6) and Df(16)A+/− mice (n = 6). (F) Summary graph of the input-output curves of the IPSP in response to distal input stimulation obtained by subtracting control traces from traces with GABA receptor blockers in 4–5-week old WT and Df(16)A+/− mice. Error bars show SEM.
We quantified the amplitude of the PSPs with and without inhibition and the amplitude of the deduced IPSPs in 4–5-week-old mice. We observed no difference in the amplitude of the PSP between WT and Df(16)A+/− mice (Figure 3E; n = 6, 3 mice for WT and n = 6, 4 mice for Df(16)A+/− mice, ANOVA 2 way RM: genotype: F(1,4) = 0.001, p = 0.97; stimulation: F(2.1,8.5) = 40.9, p = 3.85×10−5; genotype x stimulation: F(1.2,4.6) = 0.062, p = 0.84 for the PSP before GABA blockers and ANOVA 2 way RM: genotype: F(1,4) = 0.002, p = 0.97; stimulation: F(2.0,8.2) = 77.22, p = 4.5×10−6; genotype x stimulation: F(1.2,4.9) = 0.053, p = 0.87 after GABA blockers), and no difference in the amplitude of the deduced IPSP (Figure 3F; ANOVA 2 way RM: genotype: F(1,3) = 0.42, p = 0.56; stimulation: F(2.2,6.7) = 26.3, p = 5.8×10−4; genotype x stimulation: F(1.9,5.9) = 0.32, p = 0.73). These results indicate that the change in inhibitory transmission in Df(16)A+/− mice is input specific, with a decrease in feed-forward inhibition recruited by SC proximal inputs, but no change in feed-forward inhibition recruited by distal inputs.
Adult Df(16)A+/− mice CA2 pyramidal neurons have a more hyperpolarized resting membrane potential
A large feed-forward inhibition prevents CA3 SC inputs from evoking action potential firing in CA2 PNs. However, when inhibition is pharmacologically blocked, stimulation of SC inputs can easily evoke action potential firing in CA2 PNs. Because feed-forward inhibition is reduced in Df(16)A+/− mice, SC stimulation should more easily evoke action potential firing in these mice. However, this only holds true if other parameters such as the intrinsic properties of CA2 PNs are identical in Df(16)A+/− mice. Therefore, we measured intrinsic properties of pyramidal neurons in CA2, as well as the depolarizing sag observed during a hyperpolarizing pulse and the action potential threshold. We found that mature (≥ 6 week old), but not young Df(16)A+/− mice had a more hyperpolarized resting potential (Fig. 4A; at postnatal week 5: −76.6 ± 1.22 mV for WT, n = 12; −77.4 ± 0.9 mV for Df(16)A+/− mice, n = 9, T test: p = 0.63; at postnatal week ≥ 6–7: −76.4 ± 0.5 mV for WT, n =39; −79.8 ± 0.5 mV for Df(16)A+/− mice, n = 51, ANOVA 2 way: genotype: F(1,81) = 20.45, p = 2.06×10−5; age: F(3,81) = 0.12, p = 0.94; genotype x age: F(3,81) = 0.33, p = 0.79). A lower membrane resistance in Df(16)A+/− mice was also observed at W9–10 (Figure 4B), but this effect was transient and not observed in older animals. The other parameters measured, including membrane capacitance, depolarizing sag and action potential threshold were not different between WT and Df(16)A+/− mice (Figure 4C–E). Paralleling the change in synaptic transmission occurring in mature Df(16)A+/− mice, the membrane potential of CA2 pyramidal neurons becomes more hyperpolarized in Df(16)A+/− mice over the 6th to 7th week of life.
Figure 4. Adult CA2 PNs in Df(16)A+/− mice have a more hyperpolarized resting potential.
Summary graphs and diagrams illustrating how the measurements were made of the resting membrane potential (A), the membrane resistance (Rm) (B), the membrane capacitance (C), the depolarizing sag during a hyperpolarizing current step (D), and the action potential threshold (E) measured in CA2 PNs in WT and in Df(16)A+/− mice at different postnatal weeks. The number of cells is shown for each data point. (F) The fluoxetine-sensitive current evoked by a voltage ramp for WT (n = 13) and Df(16)A+/− mice (n = 13). Grey shading indicates the SEM. Inset, the estimated conductance of this current, likely due to TREK channels. (G) A plot of the TREK-1 conductance versus Resting membrane potential for all of the recorded cells, showing the correlation between the TREK conductance and resting membrane potential (Pearson’s R = −0.53). Error bars show SEM. See also figure S3.
We next explored the molecular mechanism underlying the change in membrane potential of CA2 PNs in Df(16)A+/− mice. Among the channels known to control resting membrane potential, the two-pore-domain potassium channel TREK is a strong candidate because it is highly expressed in area CA2 (Talley et al., 2001) and can be modulated by numerous second messengers (Honoré, 2007). To isolate TREK current, we blocked voltage-activated Ca2+, K+, Na+ and HCN channels (with Cd2+, Ni2+, TEA, 4AP, TTX and Cs+) and applied a ramp protocol while recording in whole-cell voltage clamp mode before and after application of 100 μM fluoxetine, a potent blocker of TREK channels (Kennard et al., 2005). We restricted the analysis of the ramp between −130 and −50 mV because a large inward current was evoked at more depolarized potentials (Supl Figure S3). We found that the fluoxetine-sensitive current was much larger in Df(16)A+/− mice as compared to WT control mice (Fig. 4F). We calculated the conductance of the fluoxetine-sensitive current by fitting the slope of the I/V curves and found that it was over three times larger in Df(16)A+/− mice (Fig. 4F inset; 0.76 pS, n = 13, 4 mice for WT and 2.77 pS, n = 13, 5 mice for Df(16)A+/− mice, T test: p = 0.018). We also observed that a significant correlation between the resting membrane potential of the cells and the amount of fluoxetine-sensitive current when the values from WT and Df(16)A+/− mice were plotted together (Fig. 4G; Pearson’s R = −0.53, ANOVA F(1,23) = 9.03, p = 0.006). Together, these data show that the current mediated by TREK channels is up-regulated in Df(16)A+/− mice and potentially contributes to the more hyperpolarized PN resting potential.
Decreased action potential firing of pyramidal neurons in Df(16)A+/− mice in response to proximal and distal inputs stimulation
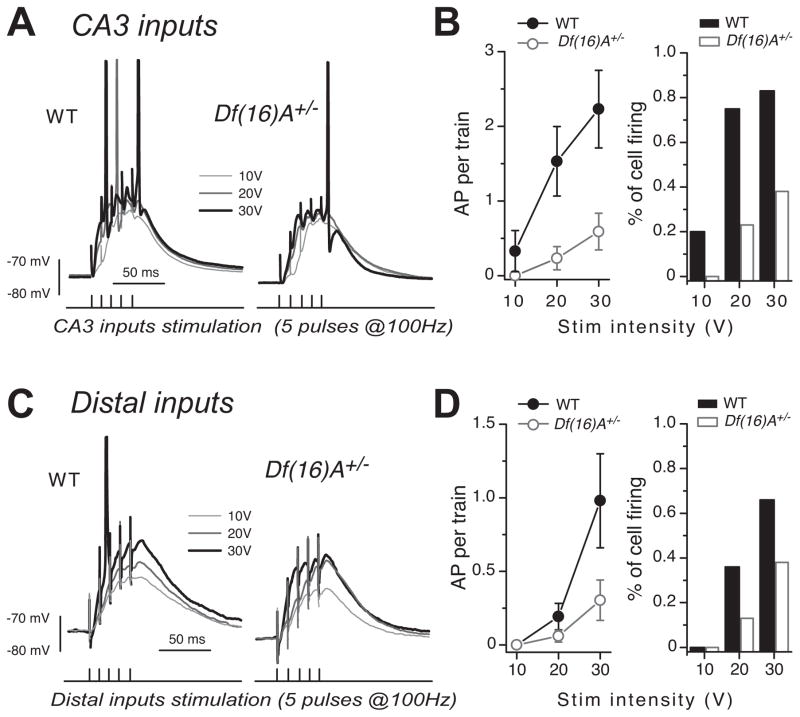
Because of alterations in intrinsic properties, it is difficult to predict which of the changes observed Df(16)A+/− mice, i.e., the decrease in inhibition or the lower resting membrane potential, will have a stronger impact on the ability of CA2 PNs to fire action potentials in response to stimulation of proximal and distal inputs. Therefore, we directly addressed this question by applying a train of 5 pulses at 100 Hz to proximal and distal inputs, and quantified the number of action potentials evoked in CA2 PNs in WT and Df(16)A+/− mice. With inhibition intact, no action potentials were observed when stimulating proximal inputs, both in WT and Df(16)A+/− mice. Only a few cells (2/11) in WT fired action potentials in response to distal input stimulation, indicating that despite the decrease in inhibition, excitatory input does not drive action potential firing more readily in CA2 PNs of Df(16)A+/− mice. We then performed the same experiment after blocking inhibition to increase the percentage of cells firing an action potential. In response to SC stimulation, both the number of action potentials during the train and the percentage of cells firing at least one action potential were significantly smaller in Df(16)A+/− mice (Figure 5A,B. At 30V stimulation: WT mice: 2.23 ± 0.52 AP per train, 83.3% of cell firing, n = 12, 9 mice; Df(16)A+/− mice: 0.56 ± 0.38 AP per train, 38.8% of cell firing, n = 18, 11 mice, at 20 and 30V: ANOVA 2 way RM: genotype: F(1,11) = 6.01, p = 0.03; stimulation: F(1.11) = 7.21, p = 0.02; genotype x stimulation: F(1,11) = 0.32, p = 0.58). Because the threshold for action potential firing was not different between WT and Df(16)A+/− mice, this result suggests that the decreased ability to fire action potential results from the more hyperpolarized membrane potential of PNs in Df(16)A+/− mice. Indeed, the resting potential of PNs in Df(16)A+/− mice was more hyperpolarized even after blocking inhibitory transmission (Figure S4A. WT: −73.3 ± 0.5 mV, n = 13, 8 mice; Df(16)A+/− mice: −75.4 ± 0.7 mV, n = 15, 9 mice, T test p = 0.04), and injection of a small depolarizing current in PNs of Df(16)A+/− mice to compensate for the more hyperpolarized resting potential was sufficient to restore the number of action potential to a level similar to WT mice (Supl. Figure S4B. At 30V stimulation: 0.66 ± 0.55 AP per train at resting membrane potential, 2.21 ± 0.73 AP per train after depolarization, n = 9, 7 mice ANOVA 2 way RM: depolarization: F(1,8) = 11.1, p = 0.01; stimulation: F(1.2,10.2) = 12.1, p = 0.004; depolarization x stimulation: F(1.1,9.2) = 12.4, p = 0.005 and ANOVA 2 way RM: genotype: F(1,7) = 5.9×10−4, p = 0.0.98; stimulation: F(1.4,10.0) = 21.4, p = 4.6×10−4; genotype x stimulation: F(1.2,8.3) = 0.17, p = 0.73 between WT and Df(16)A+/− mice after depolarization). We then looked at action potential firing in response to stimulation of distal inputs. Again, both the number of action potentials per train and the percentage of cells firing at least one action potential were reduced in Df(16)A+/− mice (Figure 5C,D. At 30V stimulation: WT mice: 0.98 ± 0.32 AP per train, 66.6% of cell firing, n = 12, 9 mice; Df(16)A+/− mice: 0.30 ± 0.13 AP per train, 38.8% of cell firing, n = 17, 9 mice, at 30V: ANOVA 1 way, F(1,27) = 4.6, p = 0.04). These results show that the decrease in feed-forward inhibition did not facilitate action potential firing in response to proximal and distal inputs stimulation. Instead, the more hyperpolarized resting potential of CA2 PNs resulted in a non-specific decrease in action potential firing, affecting the excitatory drive of both proximal and distal inputs.
Figure 5. Action potential firing of CA2 PNs is decreased in adult Df(16)A+/− mice.
(A) Sample traces of the depolarization of CA2 PNs in response to stimulation of SC inputs (5 pulses at 100Hz) in WT and Df(16)A+/− mice. (B) Summary graph of the number of action potentials (left) and of the percentage of cells firing at least one action potential (right) during the train of stimulation of CA3 inputs at different intensities (10, 20 and 30V) in WT (n = 12) and Df(16)A+/− mice (n = 18). (C) Sample traces of the depolarization of CA2 PNs in response to a stimulation of distal inputs (5 pulses at 100Hz) in WT and in Df(16)A+/− mice. (D) Summary graph of the number of action potentials (left) and of the percentage of cells firing at least one action potential (right) during the train of stimulation of distal inputs at different intensities (10, 20 and 30V) in WT (n = 12) and in Df(16)A+/− mice (n = 17). Error bars show SEM. See also figure S4.
Plasticity at inhibitory synapses and the resulting dis-inhibitory increase in SC-CA2 synaptic drive are impaired in Df(16)A+/− mice
While it is well-established that CA2 PNs cannot undergo a post-synaptic LTP (Zhao et al., 2007), the inhibitory transmission from PV+ INs in this region express a long-term depression (iLTD) dependent on Delta-opioid receptor activation (Piskorowski and Chevaleyre, 2013). Because the density of PV+ INs and inhibitory transmission are reduced in Df(16)A+/− mice, we first asked whether the magnitude of iLTD might also be reduced in these mice. For this, we directly monitored inhibitory transmission in CA2 PNs in the presence of AMPA/NMDA blockers (NBQX, D-APV). After collecting a stable baseline, we provided a high frequency stimulation (HFS; two trains of 100 pulses at 100 Hz). As shown in Figure 6A, this protocol led to a robust and lasting depression of IPSCs in WT mice (63.4 ± 3.0% of baseline, n = 9, 5 mice). Consistent with a presynaptic locus of expression, iLTD resulted in a significant increase in the PPR (Figure 6B, 116.6 ± 5.6%, from 0.47 ± 0.02 to 0.55 ± 0.03, paired T test: p = 0.001). Although the same stimulation also led to a lasting depression in Df(16)A+/− mice, the magnitude of the iLTD (Fig. 6A; 83.3 ± 3.1% of baseline, n = 9, 5 mice, p = 0.0003 compared to WT) and the change in PPR (109.7 ± 3.8%, from 0.47 ± 0.03 to 0.51 ± 0.03, paired T test: p = 0.03) were strongly reduced in these mice. These results show that plasticity at inhibitory synapses in area CA2 is impaired in Df(16)A+/− mice, likely because the sub-population of inhibitory neurons that express iLTD, i.e. PV+ INs, is also reduced in Df(16)A+/− mice.
Figure 6. Long-term depression at inhibitory transmission onto CA2 PNs is decreased in adult Df(16)A+/− mice.
(A) Summary graph of normalized IPSCs recorded before and after delivery of tetanic stimulation (100 pulses at 100Hz twice) in WT (n = 9) and Df(16)A+/− mice (n = 9). Sample traces corresponding to the time points before (a) and after (b) tetanus are shown on top. (B) The paired-pulse ratio for individual experiments before (a) and after (b) tetanic stimulation for WT and Df(16)A+/− mice. Error bars show SEM.
The iLTD in area CA2 allows for an increase in the net excitatory drive of SC inputs onto CA2 PNs (Nasrallah et al., 2015), resulting in an increased PSP amplitude following iLTD induction. Because iLTD is reduced in Df(16)A+/− mice, we wondered whether the dis-inhibitory-mediated increase in PSP might also be impaired. In order to test this, we performed whole cell current-clamp recordings, and injected current as necessary to maintain the same resting membrane potential for WT and Df(16)A+/− mice. Under these conditions, we found a much smaller increase in the PSP amplitude following HFS in Df(16)A+/− mice (Figure 7A, WT: 236.1 ± 10.1%, n = 6, 4 mice; Df(16)A+/− mice: 137.6 ± 4.5%, n = 8, 6 mice, p < 0.00001 with WT). As previously reported, we verified that this increase in PSP was mediated by a dis-inhibition as it was abolished in presence of GABA receptor blockers, both in WT and Df(16)A+/− mice (Figure 7A, WT: 105.9 ± 7.9%, n = 4, 4 mice; Df(16)A+/− mice: 111.0 ± 2.4%, n = 5, 5 mice, p = 0.52 with WT).
Figure 7. The dis-inhibitory increase in PSP amplitude and in action potential firing is impaired in adult Df(16)A+/− mice.
(A) Summary graph of the change in the amplitude of the PSP recorded in whole-cell current clamp configuration in response to SC input stimulation following a high frequency stimulation (100 pulses at 100Hz repeated twice). The lasting increase in the depolarizing component of the PSP in WT mice (black filled circles, n = 6) is significantly smaller in Df(16)A+/− mice (red filled circles n = 8). This increase is dependent on inhibitory transmission and is blocked by GABA receptor blockers (SR/CGP) both in WT (black open circles, n = 4) and in Df(16)A+/− mice (red open circles, n = 5). Cells were held at −70 mV.
(B) Summary graph of the change in the amplitude of the field PSP recorded extracellularly in response to CA3 input stimulation following a high frequency stimulation (HFS: 100 pulses at 100Hz repeated twice) in WT (black, n = 6) and in Df(16)A+/− mice (red, n = 5). (C) Summary graphs of the amplitude of the population spike monitored extracellularly in the somatic layer of area CA2 in response to SC input stimulation before (a) and after (b) HFS in WT (left, n = 14) and in Df(16)A+/− mice (right, n = 14). Error bars show SEM.
Because CA2 PNs are more hyperpolarized in Df(16)A+/− than WT mice, the impact of inhibition on the EPSP might also be reduced due to a smaller driving force for Cl−. Therefore, in order to test the effect of HFS without affecting the resting membrane potential and Cl− concentration in the PNs, we performed extracellular recordings to monitor the effect of induction of iLTD by HFS on SC-CA2 field PSP (fPSP) amplitude. We found that the lasting increase in fPSP amplitude was completely abolished in Df(16)A+/− mice (Figure 7B, WT: 165.7 ± 20.8 %, n = 6, 5 mice; Df(16)A+/− mice 110.2 ± 6.9 %, n = 5, 4 mice, p = 0.04 between WT and Df(16)A+/− mice). Therefore, this data suggests that the more profound impairment in the dis-inhibitory-mediated plasticity observed using extracellular recordings results from both the reduced plasticity of inhibitory transmission and the more hyperpolarized resting potential of pyramidal cells in Df(16)A+/− mice.
Under basal conditions, area CA2 PNs are primarily inhibited by SC input stimulation. However, following the induction of iLTD in area CA2, stimulation of SC inputs are able to drive action potential firing in CA2 PNs (Nasrallah et al., 2015). To address whether action potential firing is impaired after induction of plasticity in Df(16)A+/− mice, we monitored action potential firing extracellularly in the somatic layer of area CA2 before and after HFS. In the majority of experiments in both WT and Df(16)A+/− mice (20/28 slices), there was no detection of action potential firing before HFS, which would be observed in the extracellular recording configuration as a population spike (PS) (Fig. 8C). This confirms that most CA2 PNs are not firing action potentials in response to SC stimulation in basal conditions. Following a HFS, a large PS was observed in WT mice in response to SC stimulation (Figure 7C left; for instance from 92.8 ± 19.9 to 200.6 ± 64.8 μV at 30V stimulation, ANOVA 2 way RM: HFS: F(1,13) = 5.8, p = 0.03; stimulation: F(1.0,13.7) = 4.65, p = 0.04; HFS x stimulation: F(1.0,13.7) = 2.7, p = 0.11, n = 14, 4 mice). In Df(16)A+/− mice, HFS did not reveal a PS even at the highest stimulation setting (Figure 7C right; for instance from 103.7 ± 24.8 to 136.9 ± 34.2 μV at 30V stimulation, ANOVA 2 way RM: HFS: F(1,13) = 2.9, p = 0.11; stimulation: F(1.1,14.6) = 9.7, p = 0.005; HFS x stimulation: F(1.1,14.3) = 0.65, p = 0.44, n = 14, 4 mice). These results show that the activity-dependent ability of CA3 PNs to engage CA2 PNs is strongly impaired in Df(16)A+/− mice.
Figure 8. Social memory is impaired in adult Df(16)A+/− mice.
(A) Top: Experimental setup for the direct interaction test in which a different stimulus animal was presented in trials 1 and 2. Bottom left: the two groups (Df(16)A+/−, n = 8; WT, n = 8) engaged in social interaction with the two stimulus animals for a similar amount of time. Bottom right: Df(16)A+/− mice and their WT littermates have similar difference scores when interacting with two novel juvenile mice (One-way ANOVA F(1,15) = 0.469, P = 0.504). (B) Top: experimental setup for the direct interaction test in which the same stimulus animal was presented for both trials 1 and 2. Bottom left: Unlike WT, Df(16)A+/− mice fail to show significant recognition of the familiar animal (two-way RM ANOVA: Genotype x Trial F(1,14) = 13.503, P = 0.0025). Social memory in the WT mice is evidenced by a decrement in social investigation on trial 2, which is not the case for the Df(16)A+/− mice. Bottom right: the difference score of the Df(16)A+/− group was not only less than that of the WT group, but even had a negative value, indicating that the Df(16)A+/− show no social memory when tested with this paradigm. Error bars show SEM.
Social memory is impaired in Df(16)A+/− mice
Social dysfunction is a hallmark of several psychiatric diseases. Interestingly, it was recently shown that targeted genetic silencing of CA2 PNs results in a strong deficit in social memory formation (Hitti and Siegelbaum, 2014). Because our results show that AP firing in CA2 PNs is strongly reduced in Df(16)A+/− mice, both under basal conditions and following activity-dependent plasticity at inhibitory synapses, we wondered if this reduced level of CA2 PN activity would have a similar effect on social learning as the complete silencing of CA2 PNs.
In order to test this hypothesis, we use the direct interaction test. For this test, a subject mouse is first exposed to an unfamiliar mouse during trial 1. During trial 2, the subject mouse is either exposed to a second novel mouse (Figure 8A) or re-exposed to the same mouse encountered in trial 1 (Figure 8B). We found that exploration time for trial 1 and 2, when the subject mouse encountered two different novel mice, was similar in WT and Df(16)A+/− mice (two-way RMs ANOVA: Genotype x Trial F(1,14) = 0.602, P = 0.451; Genotype F(1,14) = 0.510, P = 0.487; Trial F(1,14) = 1.806, P = 0.200). As a result, the difference score (difference in time that the subject mouse spends exploring the other mouse between trial 1 and 2) was also similar between WT and Df(16)A+/− mice (Figure 8A; −7.0 ± 6.4, n = 8 for WT and −2.6 ± 1.4, n = 8 for Df(16)A+/− mice, one-way ANOVA F(1,15) = 0.469, p = 0.5). This result suggests that sociability is not impaired in Df(16)A+/− mice.
We then tested whether social memory was affected in Df(16)A+/− mice. As previously reported, when the subject mouse was re-exposed to the same mouse encountered in trial 1, the interaction time for trial 2 was strongly decreased in WT mice (Figure 8B; from 44.5s to 25.3s, p<0.01, Bonferroni post-test, n = 8), leading to a difference score of 19.1 ± 5.5. However, social memory was strongly impaired in Df(16)A+/− mice. Indeed, unlike WT mice, Df(16)A+/− mice did not spend less time exploring a previously encountered mouse (from 47.0s to 58.3s, p > 0.05, Bonferroni post-test, n = 8) and failed to show significant recognition of the familiar mouse (two-way RM ANOVA: Genotype x Trial F(1,14) = 13.503, P = 0.0025, WT: p<0.01; Df(16)A+/−; p > 0.05 Bonferroni post-tests; Trial F(1,14) = 1.998, p = 0.79; Genotype F(1,14) = 1.236, p = 0.285). The difference score was not only lower than that of WT mice (−11.3 ± 4.9, one way ANOVA p = 0.002), but also had a negative value, indicating no social learning of the Df(16)A+/− mice with this test.
These results indicate that social learning, but not sociability, is impaired in Df(16)A+/− mice, hence mimicking the phenotype observed in mice with complete CA2 PN silencing. Thus, our data provide a potential cellular mechanism for the impairment in social memory observed in patients with schizophrenia.
DISCUSSION
In this study, we reveal cellular alterations in the 22q11.2 mouse model of schizophrenia that occur uniquely in area CA2 of the hippocampus, and highlight social memory impairment in these mice, a behavior critically dependent on area CA2. In detail, we have shown that the density of PV+ INs is reduced in area CA2 of Df(16)A+/− mice, and the level of feed-forward inhibitory transmission onto CA2 PNs is also reduced. In addition, similar to the disease onset in early adulthood in humans, these differences were not present in 4-week old mice. We also found age-dependent changes in the intrinsic properties of CA2 PNs in Df(16)A+/− mice, causing the cells to be more hyperpolarized. As a consequence, CA2 PNs in Df(16)A+/− mice displayed fewer action potentials in response to both proximal and distal excitatory input stimulation. Furthermore, the unique plasticity of inhibitory synapses in area CA2 that typically undergoes an activity-dependent iLTD is reduced in Df(16)A+/− mice, disrupting the dis-inhibitory mechanism that allows CA3 to drive action potential firing in CA2 PNs. Thus, information transfer and activity-dependent modulation of the excitatory drive between CA3 and CA2 is strongly impaired in Df(16)A+/− mice. Finally, Df(16)A+/− mice display a deficit in social memory, a phenotype similar to the one observed after complete silencing of CA2 PNs (Hitti and Siegelbaum, 2014).
Loss of PV+ INs: significance, consequences and potential causes
Consistent with our findings, a decrease in PV+ IN density uniquely in area CA2 has been observed both in human post-mortem studies and pharmacological animal models (Benes et al., 1998; Berretta et al., 2009; Knable et al., 2004; Zhang and Reynolds, 2002), recapitulating one of the most consistent changes observed in the hippocampus during schizophrenia. A decrease in PV+ IN number in area CA2 has also been reported in bipolar disorder (Benes et al., 1998) and in Alzheimer’s disease affected brains (Brady and Mufson, 1997).
The overall importance of PV+ INs is quite clear: global impairment of PV+ IN function has been shown to disrupt hippocampal network synchrony and was accompanied by profound changes in working and spatial memory (Fuchs et al., 2007; Korotkova et al., 2010). Furthermore, it has recently been shown that hippocampal PV+ basket cells play a pivotal role in regulating memory formation in an experience-dependent manner (Donato et al., 2013). However, previous investigations of hippocampal properties and plasticity of Df(16)A+/− mice have revealed only fairly modest changes in inhibitory transmission and plasticity at the SC-CA1 synapse (Drew et al., 2011; Earls et al., 2010). Furthermore, an examination of the theta oscillation and hippocampal synchrony of the Df(16)A+/− mice found no difference with control animals (Sigurdsson et al., 2010).
The major aim of this study was to test the hypothesis that the PV+ INs in area CA2 in Df(16)A+/− mice are reduced and to examine the resulting consequences in the local network. It has recently been shown that the density of PV+ INs in mice is much higher in area CA2 compared to CA1 and CA3 (Botcher et al., 2014; Piskorowski and Chevaleyre, 2013), and the age-dependent reduction of PV+ staining uniquely in area CA2 of the hippocampus that we observe suggests that inhibitory transmission from PV+ cells in this region may be playing a peculiar function. These INs undergo a unique long-lasting delta opioid-mediated plasticity (Piskorowski and Chevaleyre, 2013) that allows the otherwise non-plastic CA2 PNs (Zhao et al., 2007) to be incorporated into the hippocampal tri-synaptic circuit (Nasrallah et al., 2015).
The most predictable consequence of the reduction in CA2 PV+ staining is the reduction in feed-forward inhibition between CA3 and CA2 PNs. It is difficult to establish a quantitative link between the decrease in PV+ IN density, the decrease in inhibitory transmission and the decrease in plasticity, due to uncertainty in the subclass of PV+ cells that are affected and in the effect that PV+ cell reduction has in iLTD and controlling the PSP amplitude. Nevertheless, the ~35% decrease in PV+ INs can account for the ~30% decease in inhibitory transmission, and the resulting increase in EPSP amplitude (by ~ 60%) can account for the decrease in dis-inhibitory LTP (due to an occlusion effect). Furthermore, we have shown that PV+ INs undergo iLTD (Piskorowski and Chevaleyre, 2013) and the dis-inhibitory increase in PSP is entirely mediated through a decrease in inhibition resulting from iLTD (Nasrallah et al., 2015). Therefore, although it is difficult to make a quantitative link, we believe that the change in PV+ cell density, the change in IPSP and EPSP amplitude, and the change in plasticity are causally linked. A potential additional outcome of the loss of inhibition is the parallel age-dependent change in intrinsic properties of CA2 PNs. Although an exhaustive study would be required to carefully examine the precise time course of the change in inhibitory transmission, we postulate that the more hyperpolarized membrane potential of CA2 PNs is a compensatory effect of the decrease in inhibition. Indeed, a persistent decrease in inhibitory transmission in CA2 might have a damaging effect on the homeostasis of the hippocampus, as several studies have reported a decrease or loss of inhibition in CA2 during epilepsy (Andrioli et al., 2007; Cohen-Gadol et al., 2004; Olney et al., 1983) and it has been shown that epileptic bursts in human hippocampus are generated in area CA2 (Wittner et al., 2009).
Interestingly, similar to findings that have revealed disinhibition dysfunction in the neocortex (O’Donnell, 2011), our results indicate that periadolescent changes in hippocampal dis-inhibitory networks are also disrupted. The cause of the loss of PV staining and feed-forward inhibition after 4 weeks is an intriguing and pertinent question given the parallel nature of disease onset in humans.
Area CA2 and neuropsychiatric disorders
Our analysis of a mouse model of the 22q11.2DS, revealed that CA2 PNs are essentially rendered silent due to change in their inherent properties, and the loss of inhibitory transmission prevents activity-dependent plasticity from increasing excitatory drive onto these cells. This finding potentially holds great significance when one considers the output of area CA2. It has been shown recently that CA2 PNs project to several extra-hippocampal structures such as the medial entorhinal cortex (Rowland et al., 2013), the medial and lateral septum, the diagonal band of broca, and the hypothalamic supramammillary nucleus (Cui et al., 2013). Thus, if one also considers the diverse and numerous inputs of area CA2, which include but are not limited to cortical, hypothalamic and intra-hippocampal origins (Chevaleyre and Siegelbaum, 2010; Cui et al., 2013; Hitti and Siegelbaum, 2014; Kohara et al., 2014), CA2 is poised to be a hub connecting the hippocampus with multiple brain regions. Previously, it has been reported that the Df(16)A+/− mice have reduced performance during a working memory task and reduced synchrony between the hippocampus and prefrontal cortex (Sigurdsson et al., 2010; Stark et al., 2008) in the absence of any changes in oscillations in the neocortex or hippocampus. While there are multiple ways in which long-range connections can be disrupted, we speculate that the significant changes we see in CA2 PN output may potentially play a role in altering the long-range communication between the hippocampus and numerous other brain regions in the Df(16)A+/− mice.
The interesting role of area CA2 in social learning and hippocampal function has only recently emerged. Recent studies have shown that CA2 is essential for social memory (Hitti and Siegelbaum, 2014; Stevenson and Caldwell, 2014). In addition, vasopressin 1b receptor, which is selectively expressed in CA2 PNs, has been shown to be a key player in modulating social memory and aggression in rodents (Stevenson and Caldwell, 2012; Young et al., 2006). In fact, rescue of vasopressin 1b receptor expression specifically in area of CA2 in the hippocampus restored socially motivated attack responses in vasopressin 1b receptor knockout mice (Pagani et al., 2014). Thus, it seems that mice with compromised CA2 function are unable to appropriately assess social situations.
Is there a broader role for area CA2? Recent reports investigating place cell dynamics indicate this region likely does not encode spatial information but rather displays marked instability over time in the same environment (Lee et al., 2015; Lu et al., 2015; Mankin et al., 2015). A study using immediate early gene expression revealed that area CA2 is more sensitive than area CA1 and CA3 to changes in context, and may be set to detect conflicts between memory and experience (Wintzer et al., 2014). Remarkably, area CA2 is altered in a number of psychiatric disorders including schizophrenia and bipolar disorder as well as in neurodegenerative diseases (Jones and McHugh, 2011). CA2 is connected to subcortical structures including amygdala, raphe nucleus and hypothalamic nuclei, and projects to higher cortical structures. Bridging primitive and higher-level structures, the integrity of area CA2 might be necessary to finely tune the interplay between primitive drives (i.e. hypothalamic signals) and higher-level cognition. Thus, we speculate that a compromised area CA2 will result in cognitive dysfunction. Indeed, the level of dementia during Parkinson’s disease is associated with the extent of alpha synuclein and of amyloid Beta peptide in area CA2 (Kalaitzakis et al., 2009), and the degree of cognitive impairment is correlated with the density of Lewy neurites in area CA2 (Churchyard and Lees, 1997).
Disruption in social cognition is a core symptom of schizophrenia, autism spectrum disorder and neurodegenerative diseases. In schizophrenia it is among the earlier onset features and is highly correlated with poor functional outcome (Brüne, 2005; Penn et al., 2008). The reciprocal relation of social cognition to both positive (paranoia and delusions) and negative symptoms (social withdrawal and reduced motivation) (Foussias et al., 2014), place it central in current translational strategies (Millan and Bales, 2013). Associations of social cognition impairments with executive function and negative symptoms are particularly evident in the 22q11.2DS (Campbell et al., 2015).
Obviously, rodents do not display all features of human social cognition and comparable information on cross-species circuit recruitment in social interactions is scarce. What dimensions of altered social cognition are measurable in experimental animal models remains unknown, but there is a need for identification of common neural substrates engaged in animals and humans to facilitate adoption of comparable procedures and common readouts in drug evaluation. In that respect, our results, taken together with previous post-mortem studies in patients, suggest that altered circuitry functionality within the CA2 hippocampal area, and its possible interactions with other relevant brain areas, such as the amygdala from which it receives abundant projections (Pikkarainen et al., 1999), might underlie parts of the social cognition deficits seen in some psychiatric and neurodevelopmental disorders. This region of the hippocampus is consistently overlooked or merged with other CA areas in human imaging studies (Small et al., 2011). Clearly, our results provide strong evidence that this region of the brain merits further study both in animal models of psychiatric diseases, but also in humans. Furthermore, given the unusual property of neurons in area CA2 to be modulated by numerous neuropeptides (Pagani et al., 2014; Piskorowski and Chevaleyre, 2013; Simons et al., 2012), our results suggest that this pharmacologically unexplored region may be a fruitful therapeutic target for psychiatric diseases.
METHODS
Slice preparation
400 μM transverse hippocampal slices were prepared from 6- to 17-week-old C57BL6 or Df(16)A+/− male mice. Animals were euthanized in accordance with institutional regulations under anesthesia with isofluorane. Hippocampi were removed and placed upright into an agar mold and cut with a vibratome in ice-cold extracellular slicing solution (for solution compositions see Detailed Experimental Procedures in Supplemental Information.) The slices were then transferred to 30°C artificial cerebral spinal fluid, ACSF, for 30 min and kept at room temperature for at least 1.5 hr before recording. All experiments were performed at 33°C.
Electrophysiological recordings and analysis
Field recordings of PSPs were performed in current clamp mode with a recording patch pipette (3–5 MΩ) containing 1M of NaCl and positioned in the middle of stratum radiatum of CA2. Whole-cell recordings were obtained from CA2 PNs in current clamp mode held at −73 mV with a patch pipette (3–5 MΩ) containing a KMethylSulfate-based solution. Inhibitory currents were recorded with pipette solution containing CesiumMethylSulfate. The liquid junction potential was ~2 mV and membrane potentials were corrected for this junction potential. Series resistance (typically 12–18 MΩ) was monitored throughout each experiment and cells with more than 15% change were excluded from analysis. The K+ current mediated by TREK channels was recorded with a modified ACSF containing reduced sodium and blockers for voltage-gated Ca2+, K+, Na+ and cationic channels. Cell capacity current and access resistance was compensated with the amplifier circuitry, and series resistance compensation was set at 80–95% and frequently checked during the experiment. We performed a ramp from −120 to +20 mV (liquid junction potential was measured and corrected post-hoc) before and after application of fluoxetine to block TREK channels (Figure S3). The subtracted current is shown in figure 4F the I/V curves and the conductance was estimated between −130 and −90mV.
We identified the CA2 PNs by somatic location and size. The cell type was confirmed by several electrophysiological properties (input resistance, membrane capacitance, resting membrane potential, sag amplitude, action potential amplitude and duration) as previously described (Chevaleyre and Siegelbaum, 2010).
Synaptic potentials were evoked by mono-polar stimulation with a patch pipette filled with ACSF and positioned in the middle of CA1 SR. The amplitudes of the PSPs or PSCs were normalized to the baseline amplitude. A HFS (100 pulses at 100Hz repeated twice) was applied following stable baseline. The magnitude of plasticity was estimated by comparing averaged responses at 30–40 min for whole cell and at 50–60 min for extracellular recordings after the induction protocol with baseline-averaged responses from 0 to10 min before the induction protocol. We used pClamp10 and Axograph X software for data acquisition and Origin Pro for data analysis. Statistical comparisons were performed using Student’s t-test or two-way ANOVA with repeated measure (RM) and we used a Greenhouse-Geiser for correction of degrees of freedom when sphericity was not assumed. Results are reported as mean ± SEM.
Immunohistochemistry
For histology experiments, 4 or 8-week old male mice were transcardially perfused, the brains dissected and post-fixed and 30 μm floating coronal sections were prepared. 8 serial sections were selected spanning bregma −1.8 to −2.1. A rabbit anti-parvalbumin antibody (Swant) was used at a dilution of 1:2000, the mouse monoclonal anti-RGS14 antibody (Neuromab) was used at dilution of 1:300. Images were collected with a Zeiss 710 laser-scanning confocal microscope. Z-series images consisting of two channels were collected every 5 μm over a total distance of 35 μm per slice. RGS14 staining was used to define areas CA2.
All image analysis was performed with ImageJ. All experimenters were blind to the genotype of the animals for all recordings, imaging and analysis (including quantification of PV+ IN density).
Social memory-Direct interaction with juveniles
All mice were housed two to five in each cage and given ad libitum access to food and water. They were kept on a 12-h (6:00 to 18:00) light–dark cycle with the room temperature regulated between 21–23°C. Behavioral tests were performed during the light cycle in a testing room adjunct to the mouse housing room, which minimizes any transportation stress. Immediately prior to the experimental sessions, 10–12-week-old Df16A+/− and WT littermates were transferred to the testing room and placed into individual cages, identical to the ones used for housing, where they were allowed to habituate to the new environment for 15 min. Male juvenile mice (C57BL/6J, 4–5-week-old) were also placed in the testing room in their home cages and allowed to habituate a similar amount of time. Testing began when a novel juvenile mouse was introduced to a cage with one of the adult experimental mice. Activity was monitored for 5 min (trial 1) and scored online by a trained observer blind to the genotype of the test mice for social behavior (anogenital and nose-to-nose sniffing, close following and allogrooming) initiated by the experimental subject, as described by (Hitti and Siegelbaum, 2014). After an inter-trial interval of 1h, the experimental mice were introduced with either the previously encountered mouse or a novel mouse again for 5 min (Trial 2). The time spent in social interaction during trial 1 was subtracted from the social interaction time during trial 2 to obtain the difference score. Statistical significance was assessed by one-way ANOVA, or two-way RM ANOVA where appropriate.
Supplementary Material
Acknowledgments
We would like to thank Yan Sun and Rachel Waldman for taking care of the mouse colony at Columbia University. This work was supported by the CNRS ATIP-Avenir (VC), Agence Nationale de la Recherche ANR-12-BSV4-0021-01 (VC), ANR-13-JSV4-0002-01 (RAP), the Ville de Paris (RAP) and NIH R01MH097879 (J.A.G).
Footnotes
Author contributions
Conceptualization: RAP, VC, SAS and JAG; Investigation: RAP, KN, VC performed all electrophysiology with SIH contributing to TREK recordings, RAP and JM performed immunohistology, RAP and VC completed imaging and quantification, AD completed behavioral experiments; Formal Analysis, VC and AD; Visualization, RAP and VC; Writing – Original Draft, RAP and VC; Writing – Review and Editing, RAP. VC, AD and JAG; Funding Acquisition, RAP, VC, SAS and JAG; Resources, RAP, VC, SAS and JAG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrioli A, Alonso-Nanclares L, Arellano J, DeFelipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Berretta S, Gisabella B, Benes FM. A rodent model of schizophrenia derived from postmortem studies. Behav Brain Res. 2009;204:363–368. doi: 10.1016/j.bbr.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Botcher NA, Falck JE, Thomson AM, Mercer A. Distribution of interneurons in the CA2 region of the rat hippocampus. Front Neuroanat. 2014;8:104. doi: 10.3389/fnana.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady DR, Mufson EJ. Parvalbumin-immunoreactive neurons in the hippocampal formation of Alzheimer’s diseased brain. Neuroscience. 1997;80:1113–1125. doi: 10.1016/s0306-4522(97)00068-7. [DOI] [PubMed] [Google Scholar]
- Brüne M. Emotion recognition, “theory of mind,” and social behavior in schizophrenia. Psychiatry Res. 2005;133:135–147. doi: 10.1016/j.psychres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Campbell LE, McCabe KL, Melville JL, Strutt PA, Schall U. Social cognition dysfunction in adolescents with 22q11.2 deletion syndrome (velo-cardio-facial syndrome): relationship with executive functioning and social competence/functioning. J Intellect Disabil Res. 2015;59:845–859. doi: 10.1111/jir.12183. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Siegelbaum SA. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron. 2010;66:560–572. doi: 10.1016/j.neuron.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchyard A, Lees AJ. The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson’s disease. Neurology. 1997;49:1570–1576. doi: 10.1212/wnl.49.6.1570. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Pan JW, Kim JH, Spencer DD, Hetherington HH. Mesial temporal lobe epilepsy: a proton magnetic resonance spectroscopy study and a histopathological analysis. J Neurosurg. 2004;101:613–620. doi: 10.3171/jns.2004.101.4.0613. [DOI] [PubMed] [Google Scholar]
- Cui Z, Gerfen CR, Young WS. Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J Comp Neurol. 2013;521:1844–1866. doi: 10.1002/cne.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Stark KL, Fénelon K, Karayiorgou M, Macdermott AB, Gogos JA. Evidence for altered hippocampal function in a mouse model of the human 22q11.2 microdeletion. Mol Cell Neurosci. 2011;47:293–305. doi: 10.1016/j.mcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls LR, Bayazitov IT, Fricke RG, Berry RB, Illingworth E, Mittleman G, Zakharenko SS. Dysregulation of presynaptic calcium and synaptic plasticity in a mouse model of 22q11 deletion syndrome. Journal of Neuroscience. 2010;30:15843–15855. doi: 10.1523/JNEUROSCI.1425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussias G, Agid O, Fervaha G, Remington G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur Neuropsychopharmacol. 2014;24:693–709. doi: 10.1016/j.euroneuro.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FEN, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JNP, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, McHugh TJ. Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci. 2011;34:526–535. doi: 10.1016/j.tins.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RKB, Gentleman SM. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:196–204. doi: 10.1016/j.parkreldis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF Stanley Neuropathology Consortium. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609-20-544. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nature Neuroscience. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Lee H, Wang C, Deshmukh SS, Knierim JJ. Neural Population Evidence of Functional Heterogeneity along the CA3 Transverse Axis: Pattern Completion versus Pattern Separation. Neuron. 2015;87:1093–1105. doi: 10.1016/j.neuron.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Simons SB, Heldt SA, Zhao M, Schroeder JP, Vellano CP, Cowan DP, Ramineni S, Yates CK, Feng Y, et al. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci U S A. 2010;107:16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Igarashi KM, Witter MP, Moser EI, Moser MB. Topography of Place Maps along the CA3-to-CA2 Axis of the Hippocampus. Neuron. 2015;87:1078–1092. doi: 10.1016/j.neuron.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S, Leutgeb JK. Hippocampal CA2 Activity Patterns Change over Time to a Larger Extent than between Spatial Contexts. Neuron. 2015;85:190–201. doi: 10.1016/j.neuron.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neuroscience and Biobehavioral Reviews. 2013;37:2166–2180. doi: 10.1016/j.neubiorev.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Nasrallah K, Piskorowski RA, Chevaleyre V. Inhibitory Plasticity Permits the Recruitment of CA2 Pyramidal Neurons by CA3. eNeuro. 2015;2:1–12. doi: 10.1523/ENEURO.0049-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, deGubareff T, Sloviter RS. “Epileptic” brain damage in rats induced by sustained electrical stimulation of the perforant path. II. Ultrastructural analysis of acute hippocampal pathology. Brain Res Bull. 1983;10:699–712. doi: 10.1016/0361-9230(83)90038-2. [DOI] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Avram SKW, Caruana DA, Dudek SM, Young WS. Role of the vasopressin 1b receptor in rodent aggressivebehavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry. 2014:1–17. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Piskorowski RA, Chevaleyre V. Delta-Opioid Receptors Mediate Unique Plasticity onto Parvalbumin-Expressing Interneurons in Area CA2 of the Hippocampus. Journal of Neuroscience. 2013;33:14567–14578. doi: 10.1523/JNEUROSCI.0649-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Rowland DC, Weible AP, Wickersham IR, Wu H, Mayford M, Witter MP, Kentros CG. Transgenically Targeted Rabies Virus Demonstrates a Major Monosynaptic Projection from Hippocampal Area CA2 to Medial Entorhinal Layer II Neurons. Journal of Neuroscience. 2013;33:14889–14898. doi: 10.1523/JNEUROSCI.1046-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SB, Caruana DA, Zhao M, Dudek SM. Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nature Neuroscience. 2012;15:23–25. doi: 10.1038/nn.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav. 2012;61:277–282. doi: 10.1016/j.yhbeh.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014 doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. Journal of Neuroscience. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintzer ME, Boehringer R, Polygalov D, McHugh TJ. The Hippocampal CA2 Ensemble Is Sensitive to Contextual Change. Journal of Neuroscience. 2014;34:3056–3066. doi: 10.1523/JNEUROSCI.2563-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittner L, Huberfeld G, Clémenceau S, Eross L, Dezamis E, Entz L, Ulbert I, Baulac M, Freund TF, Maglóczky Z, et al. The epileptic human hippocampal cornu ammonis 2 region generates spontaneous interictal-like activity in vitro. Brain. 2009;132:3032–3046. doi: 10.1093/brain/awp238. [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- Zhao M, Choi YS, Obrietan K, Dudek SM. Synaptic plasticity (and the lack thereof) in hippocampal CA2 neurons. J Neurosci. 2007;27:12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.