Abstract
Background
Health-related quality of life (HRQoL) is affected by numerous variables including depression and anxiety. However, these associations have not yet been described among patients with systemic lupus erythematosus (SLE) and the impact of SLE-related clinical variables remains unknown.
Objectives
In this cross-sectional study, the prevalence of depression and anxiety among Iranian patients with SLE living in Kermanshah province, Iran, has been estimated and the determinants of HRQoL in comparison with healthy subjects have been identified.
Patients and Methods
Sampling was performed based on recruitment of subjects according to inclusion and exclusion criteria. Systemic lupus erythematosus-related variables including cutaneous manifestations, pericarditis, arthritis, history of seizure and psychosis were recorded. Blood samples were taken to measure antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), Anti-Smith (Anti-SM), anticardiolipin antibody. Matched healthy subjects in demographic characteristics were selected from general population of Kermanshah province, Iran. Depression and anxiety and HRQoL were assessed using the Beck depression inventory-II, Beck anxiety inventory and short-form 36 health survey, respectively.
Results
A total of 310 individuals (160 patients with SLE and 150 healthy subjects) participated in this study. The prevalence of depression and anxiety was about 20% among people with SLE, which was noticeably high but not significantly different from healthy individuals. More severe depression was associated with lower scores in domains of physical functioning (PF), role limitation due to physical problems (RP) and subsequently physical component summary (PCS) in the SLE group (P < 0.0001 for all). A higher anxiety level was negatively correlated with PF, RP, social functioning (SF), general health (GH) and PCS in the SLE group (P = 0.01, < 0.0001, 0.004, 0.02 and 0.005, respectively). Scores of PF and PCS were significantly lower among patients with SLE compared to the control group (P = 0.001 for both). Malar rash, photosensitivity, discoid rash, pleuritis, pericarditis, history of seizure and positive Anti-SM Ab were associated with poorer SF (P = 0.003, 0.003, 0.018, 0.001, < 0.0001, 0.021 and 0.002, respectively).
Conclusions
The results of this study show that patients with SLE have poorer HRQoL in physical components whereas the mental component of QoL is relatively similar to healthy individuals. Depression and anxiety were not related to clinical manifestations of SLE. However, the SF domain of HRQoL was the most susceptible component of QoL, which was affected by SLE clinical variables. The high estimated prevalence of depression and anxiety among patients with SLE requires attention.
Keywords: Depression, Anxiety, Quality of Life, Systemic Lupus Erythematosus
1. Background
Multiple organs can be affected by systemic lupus erythematous (SLE). Psychiatric symptoms are also commonly observed among affected individuals (1, 2) which leads to impairment of health-related quality of life (HRQoL) (3). Many investigations have tried to determine the impact of disease status and psychiatric symptoms on HRQoL among patients with SLE (4, 5). Moreover, the close correlation between depression and SLE has been demonstrated (6-8). Depression can also be the initial symptom presented in patients with SLE (9). Due to multifactorial construct of depression and HRQoL, the mutual relationship between psychiatric symptoms and quality of life (QoL) in people with SLE can be influenced by many variables. The role of disease status as a predictor of QoL and its impact on the association between depression, anxiety and HRQoL still needs to be clarified. Previous studies have tried to identify the predictors of depression and anxiety in SLE (10). High prevalence of depression and anxiety among Iranian patients with SLE has been reported (11); however, the impact of depression, anxiety and disease-related variables on HRQoL has not yet been described in Iranian population.
Although depression and anxiety are treatable complications accompanied with SLE, inadequate treatment of psychiatric symptoms has been shown (12). Neglected depression and anxiety not only can lead to decreased QoL (13, 14), but also severe comorbidities including increased incidence of cardiovascular diseases (15) and suicidal ideation (16) may occur. Therefore, identifications of determinants of depression and anxiety and their impact on QoL have noticeable clinical importance. Depression and anxiety are also closely related with each other since they are both associated with negative affectivity (17). Furthermore, the significant correlations between depression, anxiety and QoL have been demonstrated in various populations of patients (18-20). In the present study, we tried to determine the prevalence of depression and anxiety among Iranian patients with SLE living in Kermanshah province and their association with QoL has been discussed. Moreover, the impact of disease-related variables on severity of depression and anxiety has been assessed. Whether depression, anxiety and disease-related variables can be determinants of QoL among affected individuals have also been investigated.
2. Objectives
This is the first study which determines the impact of mutual correlations between depression, anxiety and disease-related variables on QoL among Iranian population with SLE in Kermanshah province, Iran. Identifications of predictors of QoL can help to determine those patients susceptible to severe depression and poor QoL. Thus; patients at risk of mood deterioration can be classified according to defined priorities and subsequently, preventive and therapeutic strategies can be implemented for those patients tended to poor HRQoL and severe psychiatric symptoms.
3. Patients and Methods
3.1. Study Design and Participants
In this observational cross-sectional study, two groups of patients with SLE and healthy controls were defined to assess and compare the prevalence of depression and QoL status. Patients with confirmed diagnosis of SLE referred to the rheumatology clinic, which is a governmental referral center affiliated to Kermanshah University of Medical Sciences, were invited to participate in this investigation. The diagnosis of SLE was based on criteria described by American College of Rheumatology and was assessed by a rheumatologist. Healthy controls were selected from general population of Kermanshah province. An informed consent was obtained from each individual before enrollment. The protocol of the study was approved by ethics committee of Kermanshah University of Medical Sciences. Data were collected between November 2012 and April 2015. Patients were selected according to the following inclusion criteria: confirmed SLE diagnosis, age > 18, consciousness and stable mental status for proper measurement of subjective depression and self-reported QoL. Exclusion criteria were: other coincidental chronic deteriorative diseases such as cancer, AIDS, endocrine disorders, renal failure, liver dysfunction and mental disorders. Those patients who were experiencing acute psychotic episodes at the time of interview were also excluded due to unreliable responses for assessment of subjective depression and QoL. Since assessment of depression, anxiety and QoL is subjective and dependent to participants’ responses, mental status of patients should not be under influence of illegal drugs and alcohol. Therefore, those subjects with history of addiction and alcoholism were excluded as well. Cochran’s sample size formula was used to estimate sample size. According to Davatchi et al. (21) the prevalence of SLE is approximately 40 in 100,000 persons. By considering the total population of Kermanshah province which is about 2,000,000 people, it can be estimated that approximately 800 people are affected with SLE in Kermanshah. Since our center was a referral center, we could cover a large number of this population. Using Cochran’s sample size formula with error level of 5% and confidence interval of 5, a sample of 260 patients were required. We recruited 60 more patients in case of drop outs and exclusions (total of 320 patients). Only ten patients were excluded according to the exclusion criteria and the remained 310 patients voluntarily entered the investigation. The used formula was as follows:
| (1) |
3.2. Beck Depression Inventory-II
Beck depression inventory II (BDI-II) which contains 21 items was used to assess depressive mood. This instrument renders scores with range of 0 to 63 and higher scores are indicative of more severe depression (22). According to the scoring of this measurement tool, patients are classified as follows: normal (score: 1 - 10), mild mood disturbance (score: 11 - 16), borderline clinical depression (score: 21 - 30), moderate depressive mood (score: 31 - 40) and severe depression (score > 40) (22). This questionnaire has also been validated in Farsi language (23) and has acceptable internal consistency (Cronbach's alpha = 0.87) and test-retest reliability (r = 0.74).
3.3. Beck Anxiety Inventory
The Beck anxiety inventory (BAI) is a reliable instrument to measure anxiety, which focuses on somatic symptoms of anxiety (24). This measure has admissible psychometric properties in rheumatologic diseases (25). It has also been demonstrated that this questionnaire can be used to measure the severity of anxiety (26). The Persian version of BAI has proved to have good reliability (r = 0.72, P < 0.001), validity (r = 0.83, P < 0.001), and an excellent internal consistency (Alpha = 0.92) (27). This questionnaire contains 21 items and higher scores indicate more severe anxiety. The interpretations of scores are as follow: 0 - 7: minimal level of anxiety, 8 - 15: mild anxiety, 16 - 25: moderate anxiety, and 26 - 63: severe anxiety (24).
3.4. Assessment of Health-Related Quality of Life
Health-related quality of life (HRQoL) was assessed with Short-Form 36 (SF-36) health survey. This questionnaire is a standard measurement tool for assessment of QoL. The acceptable psychometric properties of the Iranian version of SF-36 questionnaire are well-documented (28). This questionnaire contains 36 items which assesses QoL in the following eight domains: Physical Functioning (PF), Role limitation due to physical problems (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitation due to emotional problems (RE) and mental health (MH). Two major component summaries are obtained by this measurement tool (physical component summary (PCS) and mental component summary (MCS)). The scores in each domain range between 0 to 100 and higher scores are representative of better QoL in that domain (29). The average scores of PF, RP, BP and GH contribute to the construct of the PCS. Mental component summary includes the average of the following domains: VT, SF, RE and MH.
3.5. Demographic and Systemic Lupus Erythematous-Related Variables
Patients’ age, gender, marital status, educational level and occupation were recorded in designed forms. Systemic lupus erythematous-related clinical symptoms including malar rash, discoid rash, photosensitivity, mouth ulcer, pericarditis, arthritis and psychosis were examined and indexed by an expert rheumatologist. Urine analysis was performed to assess proteinuria and existence of abnormal casts. Venous blood samples were taken to measure hemoglobin (Hb), white blood cell (WBC) counts, antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), Anti-Smith (Anti-SM), anticardiolipin antibody and platelet count. The enzyme-linked immunosorbent assay kits were used to measure ANA, anti-dsDNA, Anti-SM and anticardiolipin antibody.
3.6. Statistical Analysis
All the statistical analyses were performed using IBM SPSS software (IBM Corp, Armonk, New York). The chi-square test (Fisher’s exact test) was used to compare categorical variables in univariate analysis. The comparison of SF-36 scores between groups was performed using one-way Analysis of Variance (ANOVA). Categorical data are reported using percentages and continuous quantitative values are reported by Mean ± Standard Deviation (SD). Correlation analysis was used to evaluate the relationship between continuous variables. The Kolmogorov-Smirnov test was used to evaluate normal distribution of continuous variables (age and scores of SF-36 domains). P Value < 0.05 was considered as statistically significant.
4. Results
A total of 310 individuals (160 patients with SLE and 150 healthy subjects) participated in this investigation. Most of the participants were female in both groups (n: 148 (92.5%) and n: 133 (88.7%) in the SLE and control groups, respectively). Individuals in both groups had relatively similar age (P = 0.86) and there was no difference in demographic characteristics including marital status, employment, educational level and income between the two groups (P = 0.36, 0.19, 0.08 and 0.35, respectively). Table 1 shows that the SLE and control groups were matched in demographic characteristics. Healthy individuals had significantly better QoL in domain of PF (P = 0.001) and subsequently PCS (P = 0.001). Thirty three patients in the SLE group (20.6%) and 21 individuals in the control group (14.0%) had severe depression according to BDI-II classification; however, the difference was not statistically significant (P = 0.6). The prevalence of severe depression was estimated to be about 20%. The prevalence of severe anxiety was also similar between the two groups (29 (18.1%) and 24 (16.0%) subjects in the SLE and control groups, respectively; P = 0.88).
Table 1. Baseline Characteristics, Depression, Anxiety and Quality of Life Among Patients With Systemic Lupus Erythematosus Compared to Healthy Subjectsa,b.
| Variable | SLE Group (n = 160) | Control Group (n = 150) | P Value |
|---|---|---|---|
| Age, y | 30.09 ± 6.19 | 30.20 ± 5.57 | 0.86 |
| Gender | 0.16 | ||
| Male | 12 (7.5) | 17 (11.3) | |
| Female | 148 (92.5) | 133 (88.7) | |
| Marital status | 0.36 | ||
| Single | 52 (32.5) | 45 (30.0) | |
| Married | 108 (67.5) | 105 (70.0) | |
| Educational level | 0.08 | ||
| Illiterate | 5 (3.1) | 1 (0.7) | |
| Primary school | 69 (43.1) | 60 (40.0) | |
| Middle school | 68 (42.5) | 65 (43.3) | |
| Diploma | 18 (11.3) | 19 (12.7) | |
| Academic educations | 0 (0) | 5 (3.3) | |
| Occupation | 0.19 | ||
| Employed | 45 (28.1) | 50 (33.3) | |
| Unemployed | 115 (71.9) | 100 (66.7) | |
| Income | 0.35 | ||
| High | 30 (18.8) | 34 (22.7) | |
| Medium | 69 (43.1) | 70 (46.7) | |
| Low | 61 (38.1) | 46 (30.7) | |
| Quality of life (as assessed by SF-36 questionnaire) | |||
| PF | 50.46 ± 15.61 | 56.46 ± 14.68 | 0.001 c |
| RP | 44.53 ± 28.13 | 48.33 ± 24.69 | 0.20 |
| RE | 52.35 ± 33.26 | 51.40 ± 33.92 | 0.80 |
| VT | 52.40 ± 29.33 | 55.80 ± 27.76 | 0.29 |
| SF | 62.43 ± 26.15 | 64.80 ± 24.65 | 0.41 |
| BP | 55.26 ± 32.17 | 61.96 ± 31.44 | 0.06 |
| GH | 34.65 ± 22.82 | 37.23 ± 22.54 | 0.31 |
| MH | 66.43 ± 29.77 | 65.03 ± 28.06 | 0.67 |
| PCS | 46.23 ± 12.50 | 51.0 ± 11.63 | 0.001 c |
| MCS | 58.40 ± 17.53 | 59.25 ± 16.46 | 0.66 |
| Depression (as assessed by BDI-II) | 0.60 | ||
| Normal | 11 (6.9) | 9 (6.0) | |
| Mild mood disturbance | 38 (23.8) | 37 (24.7) | |
| Borderline clinical depression | 49 (30.6) | 51 (34.0) | |
| moderate depressive mood | 29 (18.1) | 32 (21.3) | |
| Severe depression | 33 (20.6) | 21 (14.0) | |
| Anxiety (as assessed by BAI) | 0.88 | ||
| Minimal | 30 (18.8) | 28 (18.7) | |
| Mild | 47 (29.4) | 50 (33.3) | |
| Moderate | 54 (33.8) | 48 (32.0) | |
| Severe | 29 (18.1) | 24 (16.0) |
aAbbreviations: BAI: Beck anxiety inventory; BDI: Beck depression inventory; BP: bodily pain; GH: general health; MCS: mental component summary; MH: mental health; PCS: physical component summary; PF: physical functioning; RE: role limitation due to emotional problems; RP: role limitation due to physical problems; SD: standard deviation; SF: social functioning; SLE: systemic lupus erythematosus; VT: vitality.
bValues are presented as frequency (%) or mean ± SD.
cSignificance at the level of P < 0.01.
Photosensitivity and mouth ulcer were found in 113 (70.6%) and 61 (38.1%) patients. While malar rash was detected in most of the patients with SLE (n: 100, 62.5%), discoid rash was found only in 46 (28.8%) subjects. Table 2 illustrates the prevalence of SLE symptoms and signs detected in the participants of this investigation. History of psychosis and seizure was detected in 67 (41.9%) and 50 (31.3%) patients, respectively. Complaints from weakness and arthritis were observed in most of the patients (69.4% and 75.0%, respectively). Urine analysis was positive for protein and abnormal casts in 69 (43.1%) and 84 (52.5%) subjects, respectively. Positive status of ANA, anti-ds DNA antibody, anti-SM antibody and anti-cardiolipin antibody were detected in 104 (65.0%), 105 (65.6%), 56 (35.0%) and 59 (36.9%) patients, respectively (Table 2).
Table 2. Systemic Lupus Erythematosus-Related Variables of the Patients Participated in the Studya,b.
| Symptom/sign | Results |
|---|---|
| Malar rash | |
| Yes | 100 (62.5) |
| No | 60 (37.5) |
| Discoid rash | |
| Yes | 46 (28.8) |
| No | 114 (71.3) |
| Photosensitivity | |
| Yes | 113 (70.6) |
| No | 47 (29.4%) |
| Mouth ulcer | |
| Yes | 61 (38.1) |
| No | 99 (61.9) |
| Arthritis | |
| Yes | 120 (75.0) |
| No | 40 (25.0) |
| Pericarditis | |
| Yes | 68 (42.5) |
| No | 92 (57.5) |
| Psychosis | |
| Yes | 67 (41.9%) |
| No | 93 (58.1%) |
| Seizure | |
| Yes | 50 (31.3) |
| No | 110 (68.8) |
| Fever | |
| Yes | 116 (72.5) |
| No | 44 (27.5) |
| Weakness | |
| Yes | 111 (69.4) |
| No | 49 (30.6) |
| Proteinuria | |
| Yes | 69 (43.1) |
| No | 91 (56.9) |
| Anti-double stranded DNA | |
| Positive | 105 (65.6) |
| Negative | 55 (34.4) |
| Antinuclear antibodies | |
| Positive | 104 (65.0) |
| Negative | 56 (35.0) |
| Anti-Smith antibody | |
| Positive | 56 (35.0) |
| Negative | 104 (65.0) |
| Anticardiolipin antibody | |
| Positive | 59 (36.9) |
| Negative | 101 (63.1) |
| Pleuritis | |
| Yes | 64 (40.0) |
| No | 96 (60.0) |
| Urine abnormal cast | |
| Yes | 84 (52.5) |
| No | 76 (47.5) |
aValues are presented as frequency (%).
bN = 160.
The Kolmogorov-Smirnov test showed that age and obtained scores of SF-36 domains were normally distributed.
The effect of demographic characteristics including gender, marital status, employment, and the income level on QoL has been evaluated. Being single was associated with better scores of GH in both SLE and control groups. Among healthy subjects, male individuals had better scores in domain of SF whereas gender difference in patients with SLE had completely another pattern. Women had better MH and MCS in the SLE group (P = 0.002 and 0.012, respectively). However, since the number of male individuals was noticeably low, the results on gender differences should be interpreted cautiously. Employment was a major determinant of BP domain among patients with SLE (P = 0.009) whereas no significant effect of employment on QoL could be detected among healthy controls. Furthermore, higher income was related to better GH in both groups (P = 0.007 and 0.024 in the SLE and control groups, respectively). Although better SF was observed among patients with SLE with higher incomes (P = 0.036), no such correlation was detected in the control group (Table 3). Age was not related to any domains of HRQoL in both groups.
Table 3. The Effect of Demographic Characteristics on Quality of Life Assessed With the Short-Form 36 Health Survey in Patients With Systemic Lupus Erythematosus Compared to Healthy Subjectsa.
| Variable | PF | RP | RE | VT | SF | BP | GH | MH | PCS | MCS |
|---|---|---|---|---|---|---|---|---|---|---|
| SLE group | ||||||||||
| Gender | 0.49 | 0.48 | 0.08 | 0.24 | 0.81 | 0.57 | 0.56 | 0.002b | 0.41 | 0.012b |
| Age, y | 0.46 | 0.12 | 0.22 | 0.50 | 0.30 | 0.52 | 0.10 | 0.51 | 0.60 | 0.71 |
| Marital status | 0.54 | 0.041 c | 0.55 | 0.58 | 0.37 | 0.012 c | 0.04 c | 0.97 | 0.10 | 0.77 |
| Employment | 0.60 | 0.73 | 0.53 | 0.89 | 0.16 | 0.009 d | 0.42 | 0.09 | 0.34 | 0.38 |
| Income | 0.08 | 0.07 | 0.89 | 0.18 | 0.036 e | 0.41 | 0.007 e | 0.52 | 0.96 | 0.15 |
| Education | 0.36 | 0.30 | 0.55 | 0.22 | 0.14 | 0.24 | 0.63 | 0.76 | 0.34 | 0.98 |
| Control group | ||||||||||
| Gender | 0.60 | 0.18 | 0.32 | 0.12 | 0.022 f | 0.57 | 0.84 | 0.14 | 0.79 | 0.16 |
| Age, y | 0.68 | 0.35 | 0.93 | 0.78 | 0.83 | 0.88 | 0.61 | 0.64 | 0.98 | 0.78 |
| Marital status | 0.63 | 0.28 | 0.15 | 0.81 | 0.92 | 0.22 | 0.019 g | 0.73 | 0.90 | 0.51 |
| Employment | 0.58 | 0.32 | 0.22 | 0.73 | 0.83 | 0.07 | 0.91 | 0.88 | 0.36 | 0.45 |
| Income | 0.06 | 0.60 | 0.80 | 0.40 | 0.05 | 0.55 | 0.024 h | 0.46 | 0.69 | 0.29 |
| Education | 0.94 | 0.73 | 0.21 | 0.30 | 0.36 | 0.08 | 0.10 | 0.96 | 0.10 | 0.32 |
aAbbreviations: BP: bodily pain; GH: general health; MCS: mental component summary; MH: mental health; PCS: physical component summary; PF: physical functioning; RE: role limitation due to emotional problems; RP: role limitation due to physical problems; SF: social functioning; VT: vitality.
bSignificant higher MH and MCS were observed in females in the SLE group.
cHigher RP and BP rates were detected among married patients whereas GH was higher in single individuals in the SLE group.
dBP was significantly higher among employed patients compared to unemployed individuals in SLE group.
eSF and GH were significantly better among patients with high income in SLE group.
fSF scores were significantly higher in men in control group.
gGH scores were significantly higher in single individuals compared to married people in control group.
hPeople with high income had better scores in domain of GH in control group.
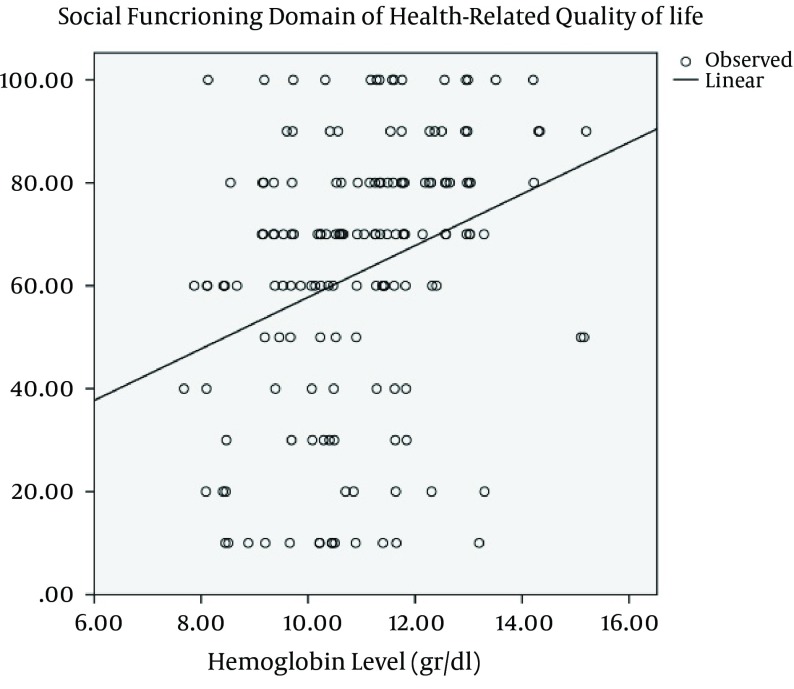
We also evaluated that in what extent QoL is influenced by SLE clinical features. Our results showed that the domain of SF is commonly affected by clinical signs and symptoms of SLE. Malar rash, photosensitivity, discoid rash, pleuritis, pericarditis, history of seizure and positive Anti-SM Ab were associated with poorer SF (P = 0.003, 0.003, 0.018, 0.001, < 0.0001, 0.021 and 0.002, respectively). Physical functioning was only influenced by discoid rash (P = 0.001). Role limitation due to physical problems, BP and subsequently PCS were higher among patients with proteinuria (P = 0.01, 0.03 and 0.003, respectively). Positive status of Anti-ds DNA was a determinant of poorer MH and RP (P = 0.01 for both). Positive ANA status was significantly correlated with lower scores in a domain of RE (P < 0.0001) and subsequently MCS (P = 0.002) (Table 4). None of the SLE-related variables were associated with depression and anxiety (Table 5). The level of Hb had a significant positive correlation only with the domain of SF among patients with SLE (P < 0.0001, r = 0.30) (Figure 1).
Table 4. The Impact of Systemic Lupus Erythematosus-Related Variables on Quality of Life (P Values Stand for Comparison of Means With One-Way Analysis of Variance) a.
| Variable | PF | RP | RE | VT | SF | BP | GH | MH | PCS | MCS |
|---|---|---|---|---|---|---|---|---|---|---|
| Malar rash | 0.97 | 0.78 | 0.31 | 0.82 | 0.003 | 0.44 | 0.06 | 0.58 | 0.60 | 0.76 |
| Discoid rash | 0.001 | 0.54 | 0.05 | 0.83 | 0.018 | 0.79 | 0.09 | 0.77 | 0.72 | 0.74 |
| Photosensitivity | 0.68 | 0.30 | 0.46 | 0.12 | 0.003 | 0.64 | 0.76 | 0.45 | 0.58 | 0.83 |
| Mouth ulcer | 0.80 | 0.16 | 0.21 | 0.38 | 0.95 | 0.46 | 0.33 | 0.18 | 0.95 | 0.41 |
| Arthritis | 0.96 | 0.31 | 0.83 | 0.87 | 0.28 | 0.08 | < 0.0001 | 0.91 | 0.94 | 0.70 |
| Pericarditis | 0.05 | 0.15 | 0.55 | 0.24 | < 0.0001 | 0.42 | 0.85 | 0.07 | 0.33 | 0.16 |
| Psychosis | 0.48 | 0.60 | 0.16 | 0.78 | 0.05 | 0.65 | 0.28 | 0.40 | 0.75 | 0.79 |
| Proteinuria | 0.93 | 0.015 | 0.77 | 0.66 | 0.86 | 0.031 | 0.70 | 0.81 | 0.003 | 0.87 |
| Anti-ds DNA | 0.67 | 0.011 | 0.66 | 0.94 | 0.82 | 0.13 | 0.26 | 0.010 | 0.27 | 0.32 |
| ANA | 0.25 | 0.17 | < 0.0001 | 0.44 | 0.33 | 0.25 | 0.68 | 0.07 | 0.09 | 0.002 |
| Pleuritis | 0.27 | 0.66 | 0.29 | 0.37 | 0.001 | 0.13 | 0.37 | 0.35 | 0.14 | 0.47 |
| Seizure | 0.29 | 0.37 | 0.93 | 0.66 | 0.021 | 0.52 | 0.86 | 0.18 | 0.61 | 0.23 |
| Cast | 0.47 | 0.63 | 0.08 | 0.52 | 0.38 | 0.28 | 0.17 | 0.52 | 0.68 | 0.62 |
| Anti-SM Ab | 0.43 | 0.07 | 0.52 | 0.20 | 0.002 | 0.50 | 0.19 | 0.025 | 0.07 | 0.06 |
| Anti-cardiolipin Ab | 0.06 | 0.05 | 0.37 | 0.12 | 0.22 | 0.95 | 0.23 | 0.41 | 0.98 | 0.90 |
aAbbreviations: ANA: antinuclear antibody; BP: bodily pain; GH: general health; MCS: mental component summary; MH: mental health; PCS: physical component summary; PF: physical functioning; RE: role limitation due to emotional problems; RP: role limitation due to physical problems; SF: social functioning; VT: vitality.
Table 5. The Effect of Systemic Lupus Erythematosus-Related Variables on the Extent of Depression and Anxiety Assessed by Beck Depression Inventory-II and Beck Anxiety Inventory, Respectively. (P Values Stand for Fischer Exact’s Test (the Chi-Square Test) to Evaluate the Relationship Between Categorical Variables)a.
| Variable | Depression (BDI-II) | Anxiety (BAI) |
|---|---|---|
| Malar rash | 0.69 | 0.53 |
| Discoid rash | 0.94 | 0.51 |
| Photosensitivity | 0.93 | 0.87 |
| Mouth ulcer | 0.13 | 0.73 |
| Arthritis | 0.72 | 0.26 |
| Pericarditis | 0.94 | 0.77 |
| Psychosis | 0.77 | 0.41 |
| Proteinuria | 0.42 | 0.09 |
| Anti-double strand DNA antibody | 0.40 | 0.39 |
| ANA a | 0.80 | 0.92 |
| Pleuritis | 0.16 | 0.88 |
| Seizure | 0.30 | 0.08 |
| Abnormal cast | 0.72 | 0.52 |
| Anti-Smith antibody | 0.30 | 0.36 |
| Anti cardiolipin antibody | 0.83 | 0.18 |
aAbbreviation: ANA: antinuclear antibody.
Figure 1. The Positive Correlation Between the Hemoglobin Level and Social Functioning Domain of Health-Related Quality of Life Among Patients With Systemic Lupus Erythematosus.
As shown in Table 1, SF was significantly lower among patients with malar rash (57.70 ± 26.0 vs. 70.33 ± 24.63). Moreover, SF was significant lower among patients with discoid rash (54.78 ± 26.5 vs. 65.52 ± 25.45). PF was significantly lower among patients with discoid rash (44.0 ± 12.67 vs. 53.07 ± 15.98). SF was significantly lower among patients with photosensitivity (52.97 ± 26.7 vs. 66.37 ± 25.0). GH was significantly lower among patients with arthritis (30.95 ± 21.63 vs. 45.75 ± 22.94). SF was significantly lower among patients with pericarditis (53.67 ± 27.03 vs. 68.91 ± 23.60). RP, BP and subsequently PCS were higher among patients with proteinuria (PCS: 49.57 ± 12.27 vs. 43.7 ± 12.13). RP was significantly higher among patients with negative Anti-ds DNA (52.27 ± 30.53 vs. 40.47 ± 26.03). MH was significantly higher among patients with negative Anti-ds DNA (70.76 ± 26.73 vs. 58.18 ± 33.57). RE was significantly higher among patients with negative ANA status (65.0 ± 29.84 vs. 45.54 ± 33.14). MCS was significantly higher among patients with negative ANA status (64.30 ± 17.09 vs. 55.23 ± 17.01). SF was significantly lower among patients with pleuritic (54.37 ± 25.06 vs. 67.81 ± 25.59). SF was significantly lower among patients with history of seizure (55.40 ± 30.91 vs. 65.63 ± 23.12). SF was significantly higher among patients with negative Anti-SM status (67.01 ± 22.93 vs. 53.92 ± 29.64). MH was significantly higher among patients with negative Anti-SM status (70.28 ± 28.49 vs. 59.28 ± 31.0).
More severe depression was associated with lower scores in domains of PF, RP and PCS in the SLE group (P < 0.0001 for all). Similarly, depression was related to poorer outcomes in PF domain and PCS in the control group (P = 0.01 and 0.03, respectively). However, depression was a determinant of poor scores in RP among patient with SLE whereas such association could not be detected among heathy individuals. Furthermore, depression was significantly associated with BP in healthy people (P = 0.04) whereas the discriminating value of depression in determining poorer scores of BP was insignificant among patients with SLE (Table 6). A higher anxiety level was a determinant of poorer physical functioning and PCS in both groups (P = 0.018 and 0.002 for PF in the SLE and control groups, respectively). However, anxiety was indicative of poorer RP, SF and GH in patients with SLE whereas the effect of anxiety on RP, SF and GH domain among healthy subjects was insignificant (Table 6). These results showed that the impact of anxiety and depression on QoL among patients with SLE has a different pattern compared to healthy subjects.
Table 6. The Discriminating Values of Depression and Anxiety as Determinants of Quality of Life Among Patients With Systemic Lupus Erythematosus Compared to Healthy Subjectsa.
| Quality of Life | SLE Group | Control Group | ||
|---|---|---|---|---|
| Depression | Anxiety | Depression | Anxiety | |
| Physical functioning | < 0.0001 | 0.018 | 0.015 | 0.002 |
| Role limitation due to physical problems | < 0.0001 | < 0.0001 | 0.17 | 0.06 |
| Role limitation due to emotional problems | 0.33 | 0.22 | 0.14 | 0.26 |
| Vitality | 0.61 | 0.29 | 0.67 | 0.014 |
| Social functioning | 0.09 | 0.004 | 0.54 | 0.82 |
| Bodily pain | 0.08 | 0.06 | 0.04 | 0.06 |
| General health | 0.24 | 0.020 | 0.14 | 0.33 |
| Mental health | 0.32 | 0.05 | 0.82 | 0.36 |
| Physical component summary | < 0.0001 | 0.005 | 0.03 | 0.04 |
| Mental component summary | 0.11 | 0.61 | 0.59 | 0.05 |
aNumbers in the table express P Values obtained by comparison of means between groups with one-way ANOVA.
More severe depression was associated with lower scores in domains of PF, RP and PCS in the SLE group. Higher levels of anxiety were associated with lower scores in domains of PF, RP, SF, GH and PCS in the SLE group. More severe depression was related to lower scores in domains of PF, BP and PCS in the control group. Higher levels of anxiety were related to lower scores in domains of PF, VT and PCS in the control group.
5. Discussion
According to this study, the prevalence of depression and anxiety is about 20% among people with SLE in Kermanshah province, Iran. Although this prevalence rate is noticeable, it was not significantly different from general population of Kermanshah. Previously, Shen et al. (4) showed that Chinese patients with SLE are more likely to suffer from depression and anxiety than healthy people. Our study demonstrates the noticeable but similar prevalence rate of depression and anxiety among Iranian patients with SLE in Kermanshah compared to healthy individuals. Similar to our study, Shen et al. (4) reported that PF and RP are major contributors to anxiety in patients with SLE. Furthermore, our study indicated the significant contributory role of PF to the construct of depression among patients with SLE which is in line with Shen et al.’s findings. However, the correlation between SF, RE and VT, and depression which was reported by Shen et al. (4) was insignificant in our investigation.
There are several studies that have shown poorer QoL and overall wellbeing among patients with SLE compared to healthy individuals(30-32). Our study showed that only physical component (PF and PCS) are affected by SLE. In fact, Iranian patients with SLE in Kermanshah have poorer HRQoL in physical components whereas the mental component of QoL is relatively spared. This finding indicates that patients with SLE have the potential for proper mental adaptability to cope with their mental preoccupations.
Previously, Sutcliffe et al. (33) reported that the socioeconomic status is a determinant of disease outcome among patients with SLE. The results of the present study show that higher income which is representative of better socioeconomic status is significantly associated with better SF and GH components of QoL. Previously, it has been shown that health status of patients with SLE is improved by increasing satisfaction with health care (34). Furthermore, Da Costa et al. demonstrated that social support is a more important variable than clinical status in determining patients’ satisfaction with medical care (35). Therefore, it seems that better QoL among patients with higher income can be due to better social support and higher satisfaction with health care.
The incidence of SLE is six times higher among women compared to male individuals (36). Our study showed that healthy men had better QoL status in domain of SF in comparison with healthy women. However, women with SLE had better MH than male patients. The findings of this study show the higher coping capabilities to maintain mental stability and MH among women with SLE. To understand the reasons behind this difference, the role of disease clinical characteristics in determining QoL should be considered. The existence of different clinical manifestations of SLE between men and women has been previously shown (37). Men are known to be more likely to develop discoid rash and type IV lupus nephritis whereas arthritis and leukopenia occur more frequently among women (37). These differences may contribute to the diverse patterns of HRQoL among men and women with SLE.
A higher educational level has been shown to be associated with better QoL among people with SLE (38) which contradicts with our results. Here, no significant relationship was found between education and HRQoL in both groups (Iranian healthy individuals and patients with SLE). It seems that the contributory role of educational level in determining QoL can be affected by other factors including community characteristics. In developing countries, such as Iran, even highly educated individuals may face difficulties to have proper job with adequate income. Therefore, the effect of education on QoL is relatively attenuated. The results of the present study do not confirm the effect of the educational level on QoL in Iranian population in Kermanshah province.
Previously, Adam et al. (39) found a relationship between psychological factors (stress, depression, and anxiety) and SLE symptoms. Further investigation by Ward et al. (40) showed that scores of depression and anxiety are related to patients’ assessment of their SLE activity. Our study showed no relationship between SLE clinical manifestations and severity of depression and anxiety. In fact, it seems that while patients’ assessment of SLE activity can be influenced by depression and anxiety (41), the actual clinical findings are not related to psychological factors.
Since QoL assessed by SF-36 questionnaire is a subjective measurement, it is closely related to patients’ perception of their QoL. It has been shown that SF is severely affected by SLE compared to general population (34) and the extent of impairment was even higher when compared to patients with other common chronic diseases (42). Social functioning is defined by normative behaviors in social situations. Our study showed that SF is the most vulnerable domain of QoL which is affected by SLE clinical status. Malar rash, discoid rash, photosensitivity, pericarditis, pleuritic, history of seizure and positive status of Anti-SM Ab were all associated with lower scores in domain of SF. Our results are in line with Alarcon et al. (43) findings in which higher disease activity was a major determinant of poorer SF.
A linear association between the Hb level and QoL has been shown among patients with chronic obstructive pulmonary disease (44). Reduced Hb levels also have been shown to be related to poorer QoL among populations susceptible to anemia such as women with heavy menstrual bleeding (45), hemodialysis patients (46) and individuals with heart failure (47). Our study demonstrated a significant positive correlation between the Hb level and social function domain of HRQoL which approves the effect of a higher Hb level in maintaining better QoL. However, Coyne et al. showed that therapeutic interventions to increase Hb do not improve QoL (48). The influence of increasing Hb by proper treatment strategies on QoL among patients with SLE needs to be investigated in future clinical trials.
The strong points of this study are the large sample size and assignment of a control group which increases the reliability of analysis and accuracy of detected findings. The weak point of this investigation is that no follow-up was designed through time and the changes of SLE-related variables through time and their impact on depression and anxiety during remissions and exacerbation of disease is unclear.
In this study the mutual impact of depression, anxiety and HRQoL among patients with SLE has been compared to general population in Kermanshah Province, Iran. Furthermore, the effect of SLE-related clinical variables on depression, anxiety and HRQoL has been discussed. The prevalence of anxiety and depression is estimated to be 20% among Iranian patients with SLE. More severe anxiety and depression were associated with lower physical component summary of QoL. Patients with SLE had poorer HRQoL in physical components whereas the mental component of QoL was relatively similar to healthy individuals. Depression and anxiety were not related to clinical manifestations of SLE. On the other hand, the SF domain was the most susceptible component of QoL which was affected by SLE clinical variables. Higher income was a predictor of better SF among patients with SLE.
Acknowledgments
We would like to thank all the staff of Farabi Hospital in Kermanshah province, Iran, that cooperated with us in the process of data collection.
Footnotes
Authors’ Contribution:Jalal Shakeri contributed to study design, interpretation of data, and editing of the manuscript. Hania Shakeri contributed to data collection and writing the manuscript. Farid Arman contributed to data collection, statistical analysis and editing the final manuscript. Monir Omrani contributed to data collection. Hamid Reza Omrani contributed to interpretation of data and editing of the manuscript. Ali Vahdani contributed to data collection and obtained the ethical approval.
Funding/Support:The study was financially supported by Kermanshah University of Medical Sciences.
References
- 1.Covey TJ, Shucard JL, Shucard DW, Stegen S, Benedict RH. Comparison of neuropsychological impairment and vocational outcomes in systemic lupus erythematosus and multiple sclerosis patients. J Int Neuropsychol Soc. 2012;18(3):530–40. doi: 10.1017/S1355617712000057. [DOI] [PubMed] [Google Scholar]
- 2.Asano NM, Coriolano M, Asano BJ, Lins OG. Psychiatric comorbidities in patients with systemic lupus erythematosus: a systematic review of the last 10 years. Rev Bras Reumatol. 2013;53(5):431–7. [PubMed] [Google Scholar]
- 3.Choi ST, Kang JI, Park IH, Lee YW, Song JS, Park YB, et al. Subscale analysis of quality of life in patients with systemic lupus erythematosus: association with depression, fatigue, disease activity and damage. Clin Exp Rheumatol. 2012;30(5):665–72. [PubMed] [Google Scholar]
- 4.Shen B, Tan W, Feng G, He Y, Liu J, Chen W, et al. The correlations of disease activity, socioeconomic status, quality of life, and depression/anxiety in Chinese patients with systemic lupus erythematosus. Clin Dev Immunol. 2013;2013:270878. doi: 10.1155/2013/270878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yilmaz-Oner S, Oner C, Dogukan FM, Moses TF, Demir K, Tekayev N, et al. Anxiety and depression predict quality of life in Turkish patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2015;33(3):360–5. [PubMed] [Google Scholar]
- 6.Huang X, Magder LS, Petri M. Predictors of incident depression in systemic lupus erythematosus. J Rheumatol. 2014;41(9):1823–33. doi: 10.3899/jrheum.140111. [DOI] [PubMed] [Google Scholar]
- 7.van Exel E, Jacobs J, Korswagen LA, Voskuyl AE, Stek M, Dekker J, et al. Depression in systemic lupus erythematosus, dependent on or independent of severity of disease. Lupus. 2013;22(14):1462–9. doi: 10.1177/0961203313508443. [DOI] [PubMed] [Google Scholar]
- 8.Lemaire B, Geron D, Malaise O, Krzesinski JM, Ansseau M, Scantamburlo G. [Depression as a common complication of systemic lupus erythematosus]. Rev Med Liege. 2015;70(4):215–8. [PubMed] [Google Scholar]
- 9.Marian G, Nica EA, Ionescu BE, Carlogea DG. Depression as an initial feature of systemic lupus erythematosus? A case report. J Med Life. 2010;3(2):183–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Maneeton B, Maneeton N, Louthrenoo W. Prevalence and predictors of depression in patients with systemic lupus erythematosus: a cross-sectional study. Neuropsychiatr Dis Treat. 2013;9:799–804. doi: 10.2147/NDT.S44248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheirandish M, Faezi ST, Paragomi P, Akhlaghi M, Gharibdoost F, Shahali A, et al. Prevalence and severity of depression and anxiety in patients with systemic lupus erythematosus: An epidemiologic study in Iranian patients. Mod Rheumatol. 2015;25(3):405–9. doi: 10.3109/14397595.2014.962241. [DOI] [PubMed] [Google Scholar]
- 12.Meszaros ZS, Perl A, Faraone SV. Psychiatric symptoms in systemic lupus erythematosus: a systematic review. J Clin Psychiatry. 2012;73(7):993–1001. doi: 10.4088/JCP.11m07043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak A, Tang CS, Ho RC. Serum tumour necrosis factor-alpha is associated with poor health-related quality of life and depressive symptoms in patients with systemic lupus erythematosus. Lupus. 2013;22(3):254–61. doi: 10.1177/0961203312471872. [DOI] [PubMed] [Google Scholar]
- 14.Pettersson S, Lovgren M, Eriksson LE, Moberg C, Svenungsson E, Gunnarsson I, et al. An exploration of patient-reported symptoms in systemic lupus erythematosus and the relationship to health-related quality of life. Scand J Rheumatol. 2012;41(5):383–90. doi: 10.3109/03009742.2012.677857. [DOI] [PubMed] [Google Scholar]
- 15.Greco CM, Li T, Sattar A, Kao AH, Danchenko N, Edmundowicz D, et al. Association between depression and vascular disease in systemic lupus erythematosus. J Rheumatol. 2012;39(2):262–8. doi: 10.3899/jrheum.110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie LF, Chen PL, Pan HF, Tao JH, Li XP, Zhang YJ, et al. Prevalence and correlates of suicidal ideation in SLE inpatients: Chinese experience. Rheumatol Int. 2012;32(9):2707–14. doi: 10.1007/s00296-011-2043-3. [DOI] [PubMed] [Google Scholar]
- 17.Gulley LD, Hankin BL, Young JF. Risk for Depression and Anxiety in Youth: The Interaction between Negative Affectivity, Effortful Control, and Stressors. J Abnorm Child Psychol. 2015. doi: 10.1007/s10802-015-9997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grech LB, Kiropoulos LA, Kirby KM, Butler E, Paine M, Hester R. The effect of executive function on stress, depression, anxiety, and quality of life in multiple sclerosis. J Clin Exp Neuropsychol. 2015;37(5):549–62. doi: 10.1080/13803395.2015.1037723. [DOI] [PubMed] [Google Scholar]
- 19.Kouris A, Armyra K, Christodoulou C, Katoulis A, Potouridou I, Tsatovidou R, et al. Quality of life, anxiety, depression and obsessive-compulsive tendencies in patients with chronic hand eczema. Contact Dermatitis. 2015;72(6):367–70. doi: 10.1111/cod.12366. [DOI] [PubMed] [Google Scholar]
- 20.Olagunju AT, Campbell EA, Adeyemi JD. Interplay of anxiety and depression with quality of life in endstage renal disease. Psychosomatics. 2015;56(1):67–77. doi: 10.1016/j.psym.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Davatchi F, Jamshidi AR, Banihashemi AT, Gholami J, Forouzanfar MH, Akhlaghi M, et al. WHO-ILAR COPCORD Study (Stage 1, Urban Study) in Iran. J Rheumatol. 2008;35(7):1384. [PubMed] [Google Scholar]
- 22.Siegert RJ, Tennant A, Turner-Stokes L. Rasch analysis of the Beck Depression Inventory-II in a neurological rehabilitation sample. Disabil Rehabil. 2010;32(1):8–17. doi: 10.3109/09638280902971398. [DOI] [PubMed] [Google Scholar]
- 23.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory--Second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21(4):185–92. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 25.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467–72. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muntingh AD, van der Feltz-Cornelis CM, van Marwijk HW, Spinhoven P, Penninx BW, van Balkom AJ. Is the Beck Anxiety Inventory a good tool to assess the severity of anxiety? A primary care study in the Netherlands Study of Depression and Anxiety (NESDA). BMC Fam Pract. 2011;12:66. doi: 10.1186/1471-2296-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaviani H, Mousavi AS. Psychometric properties of the Persian version of Beck Anxiety Inventory (BAI). Tehran Univ Med J. 2008;66(2):136–40. [Google Scholar]
- 28.Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B. The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Qual Life Res. 2005;14(3):875–82. doi: 10.1007/s11136-004-1014-5. [DOI] [PubMed] [Google Scholar]
- 29.Ware JEKM, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston: The Health Institute; 1994. [Google Scholar]
- 30.Kiani AN, Strand V, Fang H, Jaranilla J, Petri M. Predictors of self-reported health-related quality of life in systemic lupus erythematosus. Rheumatology (Oxford). 2013;52(9):1651–7. doi: 10.1093/rheumatology/ket171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorsey RR, Andresen EM, Moore TL. Health-related quality of life and support group attendance for patients with systemic lupus erythematosus. J Clin Rheumatol. 2004;10(1):6–9. doi: 10.1097/01.rhu.0000111311.38407.15. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi S, Doria A, Salaffi F, Ermani M, Iaccarino L, Ghirardello A, et al. Health-related quality of life in Italian patients with systemic lupus erythematosus. I. Relationship between physical and mental dimension and impact of age. Rheumatology (Oxford). 2004;43(12):1574–9. doi: 10.1093/rheumatology/keh397. [DOI] [PubMed] [Google Scholar]
- 33.Sutcliffe N, Clarke AE, Gordon C, Farewell V, Isenberg DA. The association of socio-economic status, race, psychosocial factors and outcome in patients with systemic lupus erythematosus. Rheumatology (Oxford). 1999;38(11):1130–7. doi: 10.1093/rheumatology/38.11.1130. [DOI] [PubMed] [Google Scholar]
- 34.Sutcliffe N, Clarke AE, Levinton C, Frost C, Gordon C, Isenberg DA. Associates of health status in patients with systemic lupus erythematosus. J Rheumatol. 1999;26(11):2352–6. [PubMed] [Google Scholar]
- 35.Da Costa D, Clarke AE, Dobkin PL, Senecal JL, Fortin PR, Danoff DS, et al. The relationship between health status, social support and satisfaction with medical care among patients with systemic lupus erythematosus. Int J Qual Health Care. 1999;11(3):201–7. doi: 10.1093/intqhc/11.3.201. [DOI] [PubMed] [Google Scholar]
- 36.RG L. Systemic Lupus Erythematosus. New York: Academic Press; 2004. [Google Scholar]
- 37.Faezi ST, Hosseini Almodarresi M, Akbarian M, Gharibdoost F, Akhlaghi M, Jamshidi A, et al. Clinical and immunological pattern of systemic lupus erythematosus in men in a cohort of 2355 patients. Int J Rheum Dis. 2014;17(4):394–9. doi: 10.1111/1756-185X.12268. [DOI] [PubMed] [Google Scholar]
- 38.dos Reis MG, da Costa IP. Health-related quality of life in patients with systemic lupus erythematosus in Midwest Brazil. Rev Bras Reumatol. 2010;50(4):408–22. [PubMed] [Google Scholar]
- 39.Adams Jr SG, Dammers PM, Saia TL, Brantley PJ, Gaydos GR. Stress, depression, and anxiety predict average symptom severity and daily symptom fluctuation in systemic lupus erythematosus. J Behav Med. 1994;17(5):459–77. doi: 10.1007/BF01857920. [DOI] [PubMed] [Google Scholar]
- 40.Ward MM, Marx AS, Barry NN. Psychological distress and changes in the activity of systemic lupus erythematosus. Rheumatology (Oxford). 2002;41(2):184–8. doi: 10.1093/rheumatology/41.2.184. [DOI] [PubMed] [Google Scholar]
- 41.Yen JC, Abrahamowicz M, Dobkin PL, Clarke AE, Battista RN, Fortin PR. Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30(9):1967–76. [PubMed] [Google Scholar]
- 42.Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol. 2005;32(9):1706–8. [PubMed] [Google Scholar]
- 43.Alarcon GS, McGwin G, Uribe A, Friedman AW, Roseman JM, Fessler BJ, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum. 2004;51(3):465–74. doi: 10.1002/art.20409. [DOI] [PubMed] [Google Scholar]
- 44.Ferrari M, Manea L, Anton K, Bruzzone P, Meneghello M, Zamboni F, et al. Anemia and hemoglobin serum levels are associated with exercise capacity and quality of life in chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:58. doi: 10.1186/s12890-015-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Souza SS, Camargos AF, Ferreira MC, de Assis Nunes Pereira F, de Rezende CP, Araujo CA, et al. Hemoglobin levels predict quality of life in women with heavy menstrual bleeding. Arch Gynecol Obstet. 2010;281(5):895–900. doi: 10.1007/s00404-009-1207-9. [DOI] [PubMed] [Google Scholar]
- 46.Hirakata H, Tsubakihara Y, Gejyo F, Nishi S, Iino Y, Watanabe Y, et al. Maintaining high hemoglobin levels improved the left ventricular mass index and quality of life scores in pre-dialysis Japanese chronic kidney disease patients. Clin Exp Nephrol. 2010;14(1):28–35. doi: 10.1007/s10157-009-0212-4. [DOI] [PubMed] [Google Scholar]
- 47.Adams KF, Pina IL, Ghali JK, Wagoner LE, Dunlap SH, Schwartz TA, et al. Prospective evaluation of the association between hemoglobin concentration and quality of life in patients with heart failure. Am Heart J. 2009;158(6):965–71. doi: 10.1016/j.ahj.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Coyne DW. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney Int. 2012;82(2):235–41. doi: 10.1038/ki.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]