Abstract
Background
Non-typeable Haemophilus influenzae (NTHi) is a ubiquitous bacterial pathogen which accounts for a majority of human upper respiratory tract infections. Laboratory lysate preparations from this bacterium are commonly utilized to investigate the promulgation of inflammatory responses in respiratory and middle ear epithelium both in vivo and in vitro. We undertook an unbiased proteomics based analysis of NTHi lysate preps to: a) identify abundant bacterial proteins present in these lysates that could play a role in NTHi biological effects and b) determine the protein content variability in different lysate prep batches from the same NTHI strain.
Study Design
Proteomic analysis of laboratory NTHi lysate preparations from clinical strain 12.
Methods
NTHi lysates were denatured, gel-fractionated, digested by trypsin and the generated peptides were identified using a liquid chromatography tandem mass spectrometry (LC-MS/MS). Western blot analyses for the important proinflammatory enhancer, outer membrane protein 6 (OMP6), was performed to validate the MS findings. Luciferase assays for NF-kB activation were used to measure the pro-inflammatory biologic effects from each NTHi lysate preparation.
Results
The MS identified 793 unique NTHi proteins. Most common and abundant proteins found have been described to either contribute to biofilm formation, elude the innate immune system, or activate epithelial pro-inflammatory pathways such as Toll Like Receptor 2 (TLR-2) signaling and NF-kB transcription factor. Strong positive signal for OMP6 was found in each of the NTHi lysate preparations. Significant NF-kB promoter response activation as expected with NTHi stimulation over control was also noted for each NTHi lysate preparation.
Conclusions
Proteomics was a successful technique to broadly define the protein content of NTHi lysates. This is the first report of the proteome of NTHi lysates widely used in laboratories to study the biological effect of NTHi. Despite the variability of the protein composition from different preps, all the batches of NTHi lysates induced similar NFκB activation.
LEVEL OF EVIDENCE
NA
Keywords: NTHi lysates, proteomics, OMP6, NF-kappaB
Introduction
Non-typeable Haemophilus influenzae (NTHi) is a gram negative bacterium lacking capsular polysaccharides. A ubiquitous human respiratory pathogen, it has become the most common infectious pathogen in upper airway diseases such as acute otitis media (AOM) 1,2 and acute sinusitis. Although less systemically virulent and invasive than encapsulated H. influenzae strains, NTHi strains contribute to a majority of respiratory tract infections3 primarily due to its ability to adhere to respiratory mucosa4, it’s high pediatric nasopharynx carrier rates5 and through its ability to form biofilms over respiratory mucosal surfaces6. NTHi clinical strain 12 in is one of the most studied strains, particularly in terms of its ability to adhere to respiratory surfaces4, and is a common pathogen in acute otitis media 7. Importantly, nearly complete genomic data is now available for this strain of NTHi8,9.
Bacterial lysis due to innate immunity defense molecules such as lactoferrin or defensins, due to the bactericidal action of antibiotics, or due to autolysis, can result in the release of a plethora of proteins and bacterial products which have been shown to further potentiate a proinflammatory response including an activation of MAP kinase signaling 10 and NF-kappaB11,12 in epithelial cells. Laboratory preparations of NTHi bacterial lysates, most often from clinical strain 12, are commonly used to investigate the substantiation of inflammatory responses in respiratory and middle ear epithelium by us and other groups11,13–15, yet the actual protein content of lysates from this clinical strain has not been profiled. A comprehensive analysis of the lysates would help understand variation in protein presence from lysate to lysate that could account for subsequent experimental effect variablity.
In order to better understand the global composition of NTHi lysates from clinical strain 12 we undertook an unbiased proteomics based analysis of 3 separate lysate preps from this bacterial strain. Our goal was to potentially elucidate and identify novel bacterial mediators of inflammatory regulation along with defining a list of abundant bacterial proteins in this clinical strain.
Materials and Methods
Preparation of NTHi lysates
NTHi clinical strain 12 was generously provided by Dr. Xin-Xing Gu (NIDCD, Bethesda, MD). Bacteria were grown on chocolate agar at 37°C in 5% CO2 overnight and inoculated in brain heart infusion (BHI) broth supplemented with 10 mg of nicotinamide adenine dinucleotide per ml. After overnight incubation, bacteria were subcultured into 500 ml of fresh brain heart infusion (BHI) and upon reaching log phase growth NTHi were washed and suspended in phosphate-buffered saline (PBS) followed by sonication for lysis.
Sodium Dodecyl Sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and peptide preparation for mass spectrometry (MS) analysis
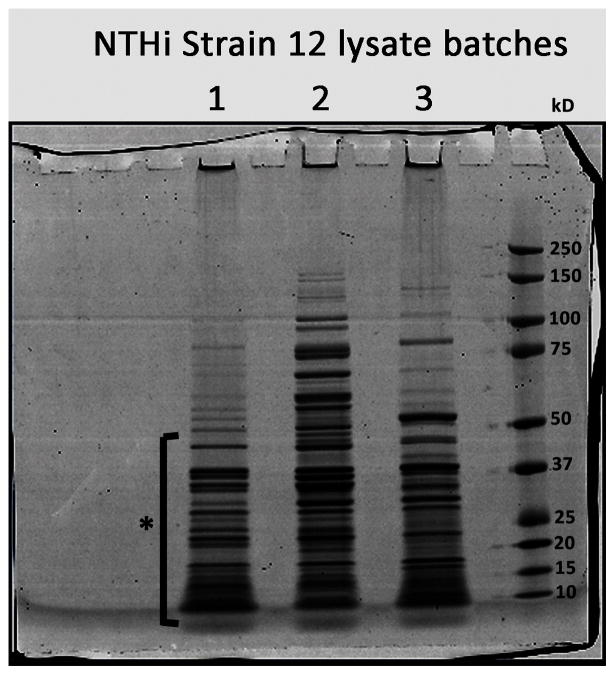
NTHi lysates (50 μg) were dissolved in Laemili buffer containing 0.1 mM DTT and were run in a one-dimensional SDS gel electrophoresis gel at 200 V for 50 min. The gel was fixed with methanol and stained with Coomassie for protein visualization (Figure 1). Each gel lane was sliced into 30 segments, and each slice was digested with trypsin as follows. Briefly, the gel cuts were placed in 100 μL of water and then subjected to two washes with a 1:1 by volume solution of water and acetonitrile. The gel pieces were then dehydrated with acetonitrile and rehydrated using 100 mM ammonium bicarbonate, followed by a 1:1 by volume wash of 100 mM ammonium bicarbonate and acetonitrile. The gels were then dehydrated with acetonitrile, resuspended in digestion buffer containing 12.5 ng/μL of MS grade Trypsin Gold (Promega Corp., Madison, WI), and incubated overnight at 37°C. Extraction of peptides from the gel was then conducted via two washes with 25 mM of ammonium bicarbonate, followed by two washes with a 1:1 by volume solution of 5% formic acid and acetonitrile. The extracted peptides were then completely dried in a SpeedVac (ThermoScientific, Waltham, MA).
Figure 1. SDS-PAGE of NTHi lysate batches stained by Coomassie blue.
SDS-PAGE was performed with three lysate preparations, the gel was fixed with a mixture of acetone and methanel and then stained with Coomassie to visualize the proteins. Each individual sample gel lane was cut into 30 gel segment bins where protein bands were noted with the staining. The cuts were performed for each lysate sample prior to in gel digestion and LC-MS/MS analysis for bacterial peptide identification as described in the Methods section. Notably, a majority of the common protein bands were identifed for proteins 50kD and under in size (as denoted by the asterisk).
Mass spectometry (MS) and protein identification
Dried peptides were resuspended in 10μL of 0.1% trifluoroacetic acid (TFA). Each sample (6 μL) was injected via an autosampler and loaded onto a C18 trap column (5 μm, 300μm i.d. X 5 mm, LC Packings) for 10 min at a flow rate of 10 L/min, 100% A. The sample was subsequently separated by a C18 reverse-phase column (3.5 μm, 75 μm X 15 cm, LC Packings) at a flow rate of 250 nL/min using an Eksigent Nano-HPLC System (Dublin, CA). The mobile phases consisted of water with 0.1% formic acid (A) and 90% acetonitrile with 0.1% formic acid (B). A 65-min linear gradient from 5 to 60% B was used. Eluted peptides were introduced into the mass spectrometer via a 10-μm silica tip (New Objective Inc., Ringoes, NJ) adapted to a nano-electrospray source (Thermo Fisher Scientific). The spray voltage was set at 1.2 kV and the heated capillary at 200°C. The linear trap quadrupole (LTQ) mass spectrometer (ThermoFisher Scientific) was operated in data-dependent mode with dynamic exclusion in which one cycle of experiments consisted of a full-MS (300–2000 m/z) survey scan and five subsequent MS/MS scans of the most intense peaks. Proteins with more than 2 peptide hits in at least one of the batches were considered unique and positively identified. The reasoning for this cut-off is that 2 peptides per protein is the standard minimum number of peptide ‘hits’ needed to confidently state the protein has been identified by MS, and is the cutoff previously used in protemics reports from our group16–18.
Cell lines
The mouse middle ear epithelial cell line mMEEC was graciously provided by Dr. Jizhen Lin (University of Minnesota, Minneapolis, MN). These cells are immortalized by a temperature sensitive simian virus 40 (SV40), allowing for a proliferative phenotype at 33 C and for differentiation at 37 C 19. mMEEC were maintained and passaged in full growth media (FGM) as previously described 20. Prior to experimentation, cells were transferred to a 37°C, 5% CO2 humidified atmosphere to inactivate the SV-40 virus.
Transient Transfection and Luciferase Assays
The pIgκBLuc reporter construct containing three immunoglobulin G-κ chain NF-κB binding sites upstream of the luciferase gene has been previously described 21 and was generously provided by Frank Ondrey MD, PhD, University of Minnesota, Minneapolis, Minnesota. This NF-κB reporter plasmid was transiently transfected into mMEEC cells for luciferase assays as follows. Cell line cultures at 50 to 60% confluence were co-transfected with the luciferase plasmids (2 μg/ml) and a pCMV-βGal reporter construct (0.05 μg/ml) (Clontech, Mountainview, CA) in Opti-MEM medium containing 3 μg/ml of Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 16 h, the medium was removed, and the cells were placed in FGM. The next day the cells were treated with NTHi lysates at 150 and 300 μg/ml in RPMI medium, or RPMI alone for 16 hours. After stimulation, the relative luciferase activity was determined with the Dual Light reporter gene assay (Tropix, Medford MA) and a Mythras plate luminometer (Berthold, Oak Ridge, TN) according to the manufacturers’ instructions. Results for relative luciferase units (RLU) were determined as a ratio of the luciferase constructs over the pCMV-βGal reporter to normalize for harvesting efficiency.
Western Blotting
Western blot analysis was performed using established protocols in our laboratory16,22. For blotting, 40 μg total protein were separated by electrophoresis in NuPAGE Novex 4-12% Bis-Tris gels (Life technologies, Carlsbad, CA). The proteins were then transferred to a nitrocellulose membrane (Invitrogen). The OMP6 antibody used was purchased from DSHB (Developmental Studies Hybridoma Bank, Iowa City, IA). This monoclonal antibody IgG2a, kappa light chain recognizes outer membrane protein P6 from NTHi and was created with Haemophilus influenzae strain 2019. It was used at 1:20 dilution of the provided antibody supernatant solution.
Statistical Analysis
The statistical difference between experimental and control groups for all experiments was determined by two-tailed Student T-tests, ANOVA test followed by Dunnet test or Wilcoxon tests. Significance level was set at p<0.05.
Results
Proteomic characterization of NTHi lysates
In order to interrogate potential mediators of pro-inflammatory responses, the global protein composition of three separate lysate batches was analyzed by LTQ-MS/MS proteomic techniques.
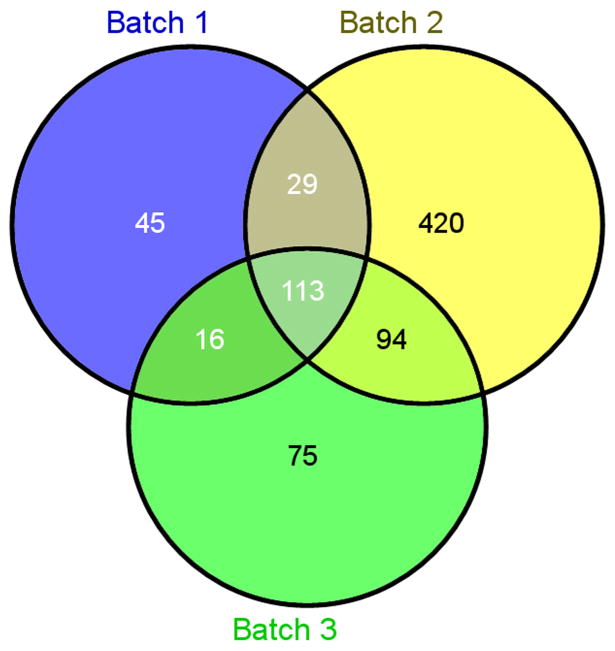
The MS identified 793 unique NTHi proteins with a cutoff of 3 peptides minimum per protein (Sup. Data Table 1). Of these, 113 were present in all 3 NTHi lysate preparations (Figure 2), signifying a fair amount of identifiable protein variability per each lysate batch preparation. Importantly, of the commonly identified proteins (present in all preparations) a functional Uniprot analysis of the list revealed 12 to be classified as purely outer membrane/cell surface associated, 7 as metabolic, 6 as a biosynthesis mediator, 3 protease/peptidases, 8 chaperone/DNA binding, 18 as transporters or metal binding, 6 reductases, 3 hydrolases, and 11 ribosomal. The remaining identified proteins were uncharacterized (Table 1). The putative role of each of the mediators is described in Table 1 as well. Most of these have been described to either contribute to biofilm formation, elude the innate immune system, or activate epithelial pro-inflammatory pathways such as TLR-2 signaling and NF-kB. Table 2 lists the overall number of proteins identified in each batch by functional category as defined by Uniprot. Notably, although the Venn Diagram shows significant variation in the protein lists, the overall pattern of functional distribution was very similar among the batches. The most common functional category of proteins in each of the batches were comprised by catalytic enzymes. The second and third most common functional categories in each of the batches were membrane binding proteins and transporter proteins respectively.
Figure 2. Venn diagram representing unique proteins identified in each lysate batch preparation.
The Venn diagram was generated by the website.
Table 1. List of proteins common to all of the NTHI lysate preparations.
Table lists the Uniprot assigned functional category of each protein, along with the putative role for each.
| accession | description | category | putative role |
|---|---|---|---|
| E3GV36 | Adhesin HMW2A | cell surface membrane | absence of HMW1 and/or 2 is associated with decreased adherence among NTHi clinical isolates |
| Q48031 | Adhesin | cell surface membrane | HMW1 and HMW2 adhesins are major virulence factors in NTHi" |
| E3GSE4 | Galactoside ABC transporter, periplasmic binding protein | Transporter | Transmembrane protein; utilizes energy of ATP binding and hydrolysis to carry out translocation of substrates across membranes and non-transport-related processes such as translation of RNA and DNA repair |
| A4NKQ0 | Chaperone protein DnaK | Chaperone/DNA binding | stress response; heat shock protein (hsp 70) --> TLR-2 & TLR-4 --> inflammation |
| E3GVD7 | Outer membrane protein P5 | cell surface membrane | OMP P5 --> CECAM --> increased expression of CD 105 |
| E3GUI5 | Phosphate-binding protein PstS | Transporter | Part of the ABC transporter complex PstSACB involved in phosphate import |
| E3GVW8 | Immunoglobulin A1 protease | Peptidase | cleaves human IgA1 and interacts with other host components |
| E3GTM2 | 23-cyclic-nucleotide 2-phosphodiesterase | hydrolase | belongs to family of hydrolases, specifically those acting on phosphoric diester bonds; purine metabolism and pyrimidine metabolism |
| E7A808 | Iron-utilization periplasmic protein hFbpA | Transporter | transporter activity |
| A5UHW1 | Phosphoenolpyruvate carboxykinase [ATP] | Metabolic | Involved in gluconeogenesis; Catalyzes the conversion of oxaloacetate (OAA) to phosphoenolpyruvate (PEP) through direct phosphoryl transfer between the nucleoside triphosphate and OAA |
| Q9S6A6 | Outer membrane protein 26 (Fragment) | cell surface membrane | skp family; role is speculative |
| E3GRV4 | Outer membrane protein P1 | cell surface membrane | |
| A4NGN0 | 60 kDa chaperonin | Chaperone/DNA binding | Prevents misfolding and promotes the refolding and proper assembly of unfolded polypeptides generated under stress conditions |
| O68197 | HtrA (Fragment) | Peptidase | serine-type endopeptidase activity |
| E3GRS7 | DNA-binding protein HU-alpha | Chaperone/DNA binding | Belongs to the bacterial histone-like protein family; DNA condensation |
| Q4QMS6 | 50S ribosomal protein L7/L12 | ribosomal | Forms part of ribosomal stalk which helps ribosome interact with GTP-bound translation factors; essential for accurate translation |
| P45173 | Uncharacterized protein HI_1349 | uncharacterized | |
| A4NMK0 | Putative uncharacterized protein | uncharacterized | |
| A4MYE9 | D-ribose transporter subunit | Transporter | |
| E3GSX2 | Trigger factor | Chaperone/DNA binding | Involved in protein export. acts as chaperone by maintaining the newly synthesized protein in an open conformation |
| E3GSP4 | Heme-hemopexin utilization protein A | Transporter | utilization of heme-hemopexin as a source of heme |
| A4NL98 | Enolase | Metabolic | Catalyzes reversible conversion of 2-phosphoglycerate into phosphoenolpyruvate; essential for the degradation of carbohydrates via glycolysis |
| A4NB90 | Putative uncharacterized protein | uncharacterized | |
| E3GV87 | Heme-utilization protein Hup | Transporter | NTHI vesicles contain DNA, adhesin P5, IgA endopeptidase, serine protease, and heme utilization protein, suggesting a multifaceted role in virulence; receptor & transporter activity |
| Q48032 | Putative accessory processing protein | uncharacterized | |
| A5UFJ0 | Autonomous glycyl radical cofactor | Metabolic | Acts as a radical domain for damaged PFL (enzyme that helps regulate anaerobic glucose metabolism) & possibly other radical proteins, cytoplasmic protein |
| H1LNX1 | 6,7-dimethyl-8-ribityllumazine synthase | uncharacterized | |
| A5UDR6 | Ribosome-recycling factor | ribosomal | Release of ribosomes from mRNA at termination of protein biosynthesis; increase efficiency of translation by recycling ribosomes from one round of translation to another |
| A4NC32 | Glycerophosphoryl diester phosphodiesterase | Metabolic | catalyzes the chemical reaction, glycerophosphodiester + H2O = alcohol + sn-glycerol 3- phosphate; glycerophospholipid metabolism |
| E3GSX3 | Hemoglobin and hemoglobin-haptoglobin binding protein C | Transporter | Receptor for hemoglobin or hemoglobin/haptoglobin complex of human host; required for heme uptake; located in cell outer membrane; Belongs to the TonB-dependent receptor family |
| E7A6V0 | 30S ribosomal protein S7 | ribosomal | One of the primary rRNA binding proteins; binds directly to 16S rRNA & nucleates assembly of head domain of 30S subunit; located at subunit interface close to decoding center, probably blocks exit of E-site tRNA |
| A4N5F1 | Ferritin | Transporter | iron storage & transport |
| E3GSP5 | Heme-hemopexin utilization protein B | Transporter | binds heme-hemopexin complexes |
| C4F222 | Lipoprotein | cell surface membrane | belongs to the nlpa family (probable D-methoione binding protein) |
| M4PH69 | Outer membrane protein P6 (Fragment) | cell surface membrane | Potent & selective inducer of human macrophage proinflammatory cytokines |
| E3GT93 | Hemoglobin and hemoglobin-haptoglobin binding protein B | Transporter | Receptor for hemoglobin or hemoglobin/haptoglobin complex of human host and is required for heme uptake |
| P43707 | Probable ferritin-1 | iron storage & transport | |
| E3GVC3 | Arginine ABC transporter, periplasmic-binding protein ArtI | Transporter | outer membrane bounded periplasmic space, up takes L-arginine from extracellular space |
| E3GSU1 | L-asparaginase II | hydrolase | catalyzes hydrolysis of asparagine to aspartic acid |
| E3GSW6 | Putative uncharacterized protein yjgF | uncharacterized | |
| A4NPQ5 | Heme-binding lipoprotein | Transporter | Heme binding & transport |
| A4MVC3 | Malate dehydrogenase | Metabolic | Catalyzes reversible oxidation of malate to oxaloacetate; cellular carbohydrate metabolism |
| A4NBL5 | Putative sialic acid transporter, TRAP-type C4-dicarboxylate | Transporter | transporter activity |
| A4NKN2 | Thioredoxin | oxidoreductase | Facilitates reduction of other proteins by cysteine thiol-disulfide exchange; participates in various redox reactions through reversible oxidation of its active center dithiol to a disulfide; catalyzes dithiol-disulfide exchange reactions |
| A4NUY8 | Peptidyl-prolyl cis-trans isomerase | oxidoreductase | interconverts cis and trans isomers of peptide bonds with the amino acid proline; protein folding |
| D5MUB8 | Uncharacterized protein | uncharacterized | |
| E3GVV1 | Thiamin ABC transporter, periplasmic-binding protein | Transporter | involved in the specific translocation of thiamine and its phosphoesters across the inner membrane |
| A4N217 | CTP synthetase | Biosynthesis | enzyme involved in pyrimidine biosynthesis that interconverts UTP and CTP; catalyzes last committed step in pyrimidine nucleotide biosynthesis |
| Q4QMP8 | Cell division protein ZapB | Biosynthesis | Non-essential, abundant cell division factor required for proper Z-ring formation; recruited early to divisome by direct interaction with FtsZ, stimulating Z-ring assembly & promoting cell division earlier in cell cycle; recruitment to Z-ring requires functional FtsA or ZipA; belongs to ZapB family |
| E1X880 | DNA binding protein, nucleoid-associated | Chaperone/DNA binding | histone-like protein family |
| A4N9R0 | Spermidine/putrescine ABC transporter periplasmic- binding protein | Transporter | Required for activity of bacterial periplasmic transport system of putrescine |
| C4F629 | Uncharacterized protein | uncharacterized | |
| E3GSP6 | Heme-hemopexin utilization protein C | Transporter | binds heme-hemopexin complexes |
| E3GUL8 | Putative uncharacterized protein | uncharacterized | |
| C4F2R8 | GTP cyclohydrolase 1 | hydrolase | hydrolysis of GTP to form 7,8-dihydroneopterin triphosphate |
| E3GSY5 | Peptidyl-prolyl cis-trans isomerase | Metabolic | interconverts cis and trans isomers of peptide bonds with the amino acid proline |
| A4N906 | Protein hfq | Chaperone/DNA binding | RNA chaperone; binds small regulatory RNA (sRNAs) and mRNAs to facilitate mRNA translational regulation in response to envelope stress, environmental stress & changes in metabolite concentrations |
| A5UAA5 | Protein TolB | Transporter | Involved in the TonB-independent uptake of proteins; protein import |
| C9MF43 | GrxA family Glutaredoxin | oxidoreductase | protein disulfide oxidoreductase |
| C4F6I3 | RNA polymerase-binding transcription factor DksA | ribosomal | Transcription factor; acts by binding directly to RNA polymerase (RNAP); required for negative regulation of rRNA expression & positive regulation of several amino acid biosynthesis promoters; required for regulation of fis expression |
| A4NWM8 | 3-hydroxyacyl-[acyl-carrier-protein] dehydratase FabZ | Metabolic | Involved in unsaturated fatty acids biosynthesis. Catalyzes the dehydration of short chain beta-hydroxyacyl-ACPs and long chain saturated and unsaturated beta-hydroxyacyl-ACPs. |
| A5UBD9 | DNA-directed RNA polymerase subunit omega | Biosynthesis | Promotes RNA polymerase assembly. Latches the N- and C-terminal regions of the beta' subunit thereby facilitating its interaction with the beta and alpha subunits. |
| E3GU64 | Putative uncharacterized protein yraP | uncharacterized | Uncharacterized protein |
| P43734 | 10 kDa chaperonin | Chaperone/DNA binding | Binds to Cpn60 in the presence of Mg-ATP and suppresses the ATPase activity of the latter. |
| C4F588 | Uncharacterized protein | uncharacterized | Uncharacterized protein |
| A4N582 | Outer membrane protein assembly factor BamD | cell surface membrane | Part of the outer membrane protein assembly complex, which is involved in assembly and insertion of beta-barrel proteins into the outer membrane. |
| E3GTX7 | Formate dehydrogenase-N, major subunit | oxidoreductase | Formate dehydrogenase-N, major subunit fdnG; catalyzes the conversion of formate to CO2, and donates the electrons to a second electron carrier |
| C4EXL4 | Uncharacterized protein | uncharacterized | Uncharacterized protein |
| A5UC74 | Putative uncharacterized protein | uncharacterized | Uncharacterized protein |
| A4NIV9 | Putative uncharacterized protein | uncharacterized | Putative uncharacterized protein |
| A5UE96 | 50S ribosomal protein L10 | ribosomal | Forms part of the ribosomal stalk, playing a central role in the interaction of the ribosome with GTP-bound translation factors. |
| C4F0T1 | Keto-hydroxyglutarate-aldolase/keto-deoxy- phosphogluconate aldolase | Metabolic | predicted protein, could be implicated in biofilm formation. |
| A4MW79 | 50S ribosomal protein L33 | ribosomal | ribosmal protein |
| A4NZK2 | 15 kDa peptidoglycan-associated lipoprotein | cell surface membrane | |
| C4F270 | Conserved ABC-type transport system protein | Transporter | Transmembrane protein; utilizes energy of ATP binding and hydrolysis to carry out translocation of substrates across membranes and non-transport-related processes such as translation of RNA and DNA repair [wiki] |
| A4NM76 | Flavodoxin | oxidoreductase | Low-potential electron donor to a number of redox enzymes. |
| A5UG40 | Arsenate reductase | oxidoreductase | |
| A4NA91 | Arginine transporter permease subunit ArtM | Transporter | arginine transporter |
| A4N731 | Peptide deformylase | ribosomal | Removes the formyl group from the N-terminal Met of newly synthesized proteins. Requires at least a dipeptide for an efficient rate of reaction. N-terminal L-methionine is a prerequisite for activity but the enzyme has broad specificity at other positions. |
| A4NAZ0 | Protein ProQ homolog | ribosomal | proQ homologue, RNA chaperone that controls ProP levels |
| E3GTH5 | Lipoprotein, putative | uncharacterized | uncharacterized lipoprotein |
| A4MXW3 | Nitrate reductase | oxidoreductase | Catalytic subunit of the nitrate reductase (NAP). Only expressed at high levels during aerobic growth. NapAB complex receives electrons from the membrane-anchored tetraheme protein NapC. Essential function for nitrate assimilation and may have a role in anaerobic metabolism. |
| A4NVH8 | Conserved hypothetical lipoprotein | uncharacterized | Conserved hypothetical lipoprotein |
| C4F4Z5 | 6-carboxy-5,6,7,8-tetrahydropterin synthase | Biosynthesis | involved in purine metabolism, 7-cyano-7-deazaguanine biosynthesis (homologous to E. coli) |
| E3GV91 | Protein disulfide isomerase II | ribosomal | Protein disulfide isomerase II; Catalysis of the rearrangement of both intrachain and interchain disulfide bonds in proteins. |
| A4NIV2 | Putative uncharacterized protein | uncharacterized | Putative uncharacterized protein |
| E3GSS6 | Adhesion and penetration protein | cell surface membrane | Catalysis of the hydrolysis of internal, alpha-peptide bonds in a polypeptide chain by a catalytic mechanism that involves a catalytic triad consisting of a serine nucleophile that is activated by a proton relay involving an acidic residue (e.g. aspartate or glutamate) and a basic residue (usually histidine). [Elastase activity] |
| E7A856 | Single-stranded DNA-binding protein | Biosynthesis | Single-stranded DNA-binding protein:binds to single-stranded regions of DNA to prevent premature annealing, to protect the single-stranded DNA from being digested by nucleases, and to remove secondary structure from the DNA to allow other enzymes to function effectively upon it. |
| A4N8Y4 | Membrane spanning protein in TolA-TolQ-TolR complex | cell surface membrane | transmembrane protein, interacts between inner and outer membrane proteins? (E. coli) |
| E3GTA4 | Phosphopantetheine adenylyltransferase | Metabolic | Reversibly transfers an adenylyl group from ATP to 4'-phosphopantetheine, yielding dephospho-CoA (dPCoA) and pyrophosphate. |
| A4N344 | 50S ribosomal protein L17 | ribosomal | ribonucleuoprotein complex, structural constituent of ribosome, translation |
| E3GUC5 | Putative NAD(P)H-flavin oxidoreductase | oxidoreductase | Catalysis of an oxidation-reduction (redox) reaction, a reversible chemical reaction in which the oxidation state of an atom or atoms within a molecule is altered. One substrate acts as a hydrogen or electron donor and becomes oxidized, while the other acts as hydrogen or electron acceptor and becomes reduced. |
| A5UA40 | DNA polymerase III subunit delta' | Biosynthesis | Catalysis of the reaction: deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1); the synthesis of DNA from deoxyribonucleotide triphosphates in the presence of a DNA template and a 3'hydroxyl group. |
| A5UIA7 | 30S ribosomal protein S20 | ribosomal | RNA binding, structural constitiuent of ribosome, translation |
| E3GVV2 | Putative DNA uptake protein ComE1 | Biosynthesis | DNA uptake: homolog of E. coli genes that confer a competitive advantage during long-term stationary-phase incubation to utilize extracellular DNA as source of carbon and energy |
| E3GUY1 | Protease IV | Peptidase | integral to cellular inner membrane, signal specific peptidase, might be implicated in pili adhesion |
| A4N7P6 | Outer membrane protein assembly factor BamE | cell surface membrane | Part of the outer membrane protein assembly complex, which is involved in assembly and insertion of beta-barrel proteins into the outer membrane |
| A4NVQ4 | Hydroxyethylthiazole kinase | Biosynthesis | Catalyzes the phosphorylation of the hydroxyl group of 4-methyl-5-beta- hydroxyethylthiazole (THZ), with cofactor of magnesium. Part of thiamine synthesis pathway |
| P45148 | Carbonic anhydrase 2 | Biosynthesis | lyase with metal binding activity. Converts CO2 to bicarbonate for carbon utilization |
| A4NM15 | 3-dehydroquinate dehydratase | Biosynthesis | Catalyzes a trans-dehydration via an enolate intermediate, biosynthesis of a secondary metabolite. (shikimate pathway) |
| D1NHL7 | Translation initiation inhibitor | ribosomal | |
| A5UGZ3 | ATP synthase subunit b | Metabolic | F1F0 ATP synthase produces ATP from ADP in the presence of a proton or sodium gradient. F-type ATPases consist of two structural domains, F1 containing the extramembraneous catalytic core and F0 containing the membrane proton channel, linked together by a central stalk and a peripheral stalk. During catalysis, ATP synthesis in the catalytic domain of F1 is coupled via a rotary mechanism of the central stalk subunits to proton translocation. |
| C4F189 | Molybdate-binding periplasmic protein | cell surface membrane | outer membrane/periplasmic protein |
| A4NQJ5 | Virulence-associated protein D | cell surface membrane | CRISPR associated protein Cas2: (clustered regularly interspaced short palindromic repeats) associated proteins, conferring resistance to infection by certain bacteriophages, can show similarity to helicases and repair proteins. |
| A4N5Z4 | Lipoprotein | cell surface membrane | lipoprotein (similar) |
| A4N654 | N-acetylmannosamine kinase | Biosynthesis | amino acid sugar metabolism, Catalyzes the phosphorylation of N-acetylmannosamine (ManNAc) to ManNAc-6-P |
| A5UE07 | Heat shock protein HtpX | cell surface membrane | outer membrane protein, heat shock protein |
| E3GSD8 | Putative uncharacterized protein yciI | uncharacterized | |
| H1LQB2 | 50S ribosomal protein L23 | ribosomal | One of the early assembly proteins it binds 23S rRNA. One of the proteins that surrounds the polypeptide exit tunnel on the outside of the ribosome. Forms the main docking site for trigger factor binding to the ribosome. Conserved across Haemophilus spp. |
| C9MD86 | Translation initiation factor Sui1 | ribosomal | |
| A4NJ59 | Putative uncharacterized protein | uncharacterized | iron-sulfer cluster assembly: the incorporation of iron and exogenous sulfur into a metallo-sulfur cluster. |
| C4F2L6 | Conserved predicted lipoprotein | cell surface membrane | Together with LptD, is involved in the assembly of lipopolysaccharide (LPS) at the surface of the outer membrane. Required for the proper assembly of LptD. Binds LPS and may serve as the LPS recognition site at the outer membrane. Conserved across Haemophilus spp. |
| D1NCA9 | Putative uncharacterized protein | uncharacterized |
Table 2. Molecular function of each of the identified proteins in all batches.
Functional characterization of proteins indentified within each NTHi lysate preparation.
Table lists the number of proteins identified per functional category in each of the lysate batches. Notably, the overall pattern of functional distribution was very similar among the batches.
| Molecular Function | Batch 1 | Batch 2 | Batch 3 |
|---|---|---|---|
| Sequence-specific DNA binding transcription factor activity | 1 | 12 | 4 |
| Catalytic activity | 107 | 431 | 165 |
| Receptor activity | 5 | 9 | 8 |
| Structural molecule activity | 10 | 29 | 25 |
| Transporter activity | 21 | 67 | 30 |
| Binding | 82 | 336 | 141 |
| Electron carrier activity | 6 | 13 | 6 |
| Antioxidant activity | 2 | 7 | |
| Enzyme regulator activity | 2 | 5 | 3 |
| Protein binding trascription factor activity | 3 | ||
| Potassium channel regulator activity | 1 | ||
| Molecular transducer activity | 2 | ||
| Superoxide dismutase activity | 1 | ||
| Sigma factor activity | 2 | ||
| Energy transducer activity | 1 |
The top 20 most abundant proteins in terms of peptide counts included many well studied and known NTHi proteins such as high molecular weight adhesins, outer membrane proteins P5 and P6 (OMP 5 and 6), IgA proteases, and heat shock proteins. Confirmatory Western blotting was performed for OMP6, with strong positive signal being found in each of the NTHi lysate preparations (Figure 3).
Figure 3. OMP6 protein assay in the 3 NTHi lysate batches by western blot analysis.
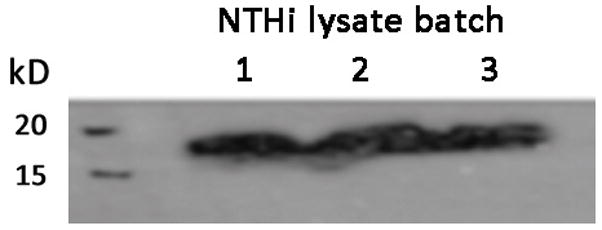
SDS-PAGE gel was ran for the 3 NTHi lysate batches (40 μg of total proteins), the proteins were transferred on a nitrocellulose membrane and incubated with an antibody anti-OMP6 and a secondary antibody coupled to HRP. Strong signal at 18 kD was noted in all samples confirming proteomics findings. Lane numbers corresponds to NTHi lysate batch.
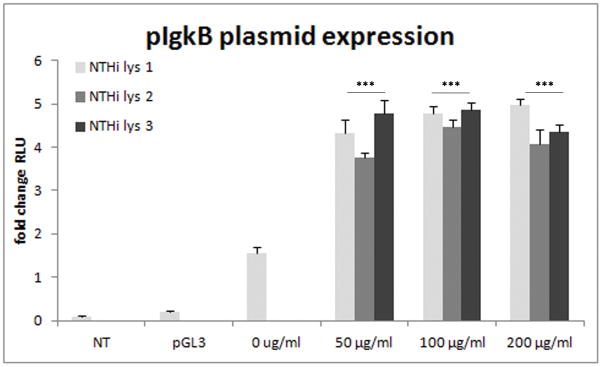
Given the identified protein variability per NTHi lysate batch we aimed to determine whether a differential pro-inflammatory response would be noted for each batch. Triplicate luciferase reporter assays for NF-kB activation were performed in mMEEC as described. Results indeed demonstrated significant NF-kB promoter response activation as expected with NTHi stimulation over control, but no significant difference in NF-kB activation for each NTHi lysate batch preparation. All batches showed a statistically significant 4–5 fold increase in NF-kB at al doses relative to control (Figure 4).
Figure 4. NF-kB reporter activity in response to NTHi lysate treatment.
mMEEC were cultured until 50 to 60% of confluence and transfected with the IgkB plasmid constructs containing 3 consensus NFkB responsive elements upstream of the luciferase gene and with the β-galactosidase as an internal control. After recovery and incubation in serum free medium, mMEEC were treated 16hrs with NTHi lysates at the noted doses to perform a luciferase assay. (NT-no transfection control; pGL3- empty vector without treatment). ***all p<0.0001 relative to 0ug/ml NTHi lysate.
Discussion
Inflammation associated with acute bacterial infection is postulated to contribute to chronic otitis media (COM). Molecular mechanistic links of this are only recently beginning to be elucidated23,24. Work from our and other labs have shown that NTHi induction of the pro-inflammatory transcription factor NF-κB leads to potent induction of middle ear mucosal hyperplasia in vivo and upregulation of proinflammatory cytokines12,15. In this study, we employed an unbiased proteomics mass spectometry approach to better understand the global protein composition of NTHi lysates from clinical strain 12 in order to potentially elucidate and identify novel bacterial mediators of inflammatory regulation along with defining a list of abundant bacterial proteins.
It is noteworthy that despite stringent and consistent laboratory technique, a fair amount of variability was noted betwen the different preparations of NTHi lysates. Possible contributing factors include, environmental conditions such as light, temperature in the lab, the variability in the timing during the experiment (if the tube sat a bit more time on the bench), variability in sonication steps, exact phase of growth when bacteria where prepared for sonification among others, and variability among individual bacterial colonies picked for expansion. Moreover, there may be crossover in protein identification by mass spectometry. This information does however highlight how it should be expected that these preparations will not typically result in a uniform protein content and mix. Given this fact, experimental variablity may be observed when performing repeat conditional exposures of cells to different laboratory preparations of NTHi lysates. We sought to determine whether there would be an observable biological variability in pro-inflammatory effects, measured by NF-kappaB reporter assays, by lysate batch. Results showed that despite the noted lysate protein content variablity there was expected, robust and consistent NF-kappaB activation for each batch. As such, much of our subsequent protein analyses focused on the common proteins between the batches. A listing of all of the identified proteins is presented as supplemental data.
The initial interaction between NTHi and the host is the adherence of bacteria to the mucus or cells of the upper airway, primarily via the action or mediated by both pilus and nonpilus adhesin high molecular weight molecules (HMW) 4. As such it is notable that the top two most abundant proteins in our proteomics data included two HMW proteins, HMW 2A and HMW 1A. Importantly, these two proteins are known to be key mediators in biofilm formation25. After initial adhesion, the secretion of IgA protease by NTHi allows for avoidance of innate immunity and for further bacterial colonization. Not surprisingly, IgA proteases was also in the top 10 most abundant proteins identified by our methodology. In summary, results demonstrate that NTHi lysates are characterized by proteins critical for bacterial adhesion and immune evasion.
Outer membrane proteins were also noted to be highly prevalent in the identified protein lists. These OMPs are critically important not only because they mediate host pro-inflammatory activation via toll like receptors (TLR), such as the TLR-2 family, but because they provide targets for vaccine development against NTHi. Effective vaccine targets have included Outer Membrane Protein (OMP) D, which has been shown to effectively reduce up to one-third of AOM cases due to NTHi26. Despite these advances, additional antigen targets appear to be needed in order to more broadly cover against AOM cases due to NTHi27. OMP5, OMP1, OMP26, OMP6 and OMP2 were all among the most abundant proteins found in our lysate preparations. OMP6 is perhaps the most studied of these membrane proteins, yet previous proteomic analyses of NTHi lysates had failed to identify it as common using mass spectometry28. Undoubtedly, the sensitivity of mass spectometry, along with more robust bacterial protein databases, have increased our capability of comprehensively identifying proteins in biospecimens. OMP6 is not only an important mediator of bacterial-epithelial adhesion, it is also a known potent activator of MAP kinase pathways, NF-kB, and mucins in middle ear epithelium29,30. As such it represents an attractive target for modulation of potentially noxious NTHi middle ear effects.
Bacterial invasion into the middle ear often occurs via the action of lipoproteins which are able to interact with epithelial cell surface membrane and enter the epithelium into the submucosal spaces. This process typically occurs due to lipoprotein interaction with the platelet activated factor receptor (PAFR) through molecular mimicry, allowing for bacterial invasion in a PAFR-linked pinocytotic vacuole. PAFR is a G-protein coupled receptor targeting vacuolar contents to clathrin-coated pits or to the endocytic pathway, both which purportedly occur with NTHi25. Our findings demonstrated lipoproteins in all identified batches. It is also notable that these lipoproteins are also able to mediate anti-inflammatory effects via PAFR mediated activation of phosphoinositide 3-kinase (PI3K) an endogenous suppressor of TLR signaling31, thus limiting immune responses and favoring bacterial survival.
In conclusion, we have described a comprehensive list of proteins identified in standard NTHi clinical strain 12 lysate preparations frequently used to interrogate pro-inflammatory activation both in vivo and in vitro. To date the full global protein complement of these lysates had not been described. A lengthy list of novel bacterial proteins were found in this study including extracellular membrane proteins, peptidases, chaperones, lipoproteins, heat shock proteins, among others. Although many of the proteins we identified are known NTHi virulence factors, a more extensive analysis of these proteins in order to determine their capability of activating host inflammation or an immunological response appears warranted. Despite variability of protein composition from different batch preparations from the same bacterial clinical strain, given abundant common proteins, a consistent biological effect of each batch in terms of NFκB activation is noted.
Supplementary Material
Acknowledgments
This work was supported by R01DC012377 from the NIDCD. We would like to acknowledge Dr. Mary Rose for scientific advice.
Footnotes
CONFLICT OF INTEREST STATEMENT
"The authors declare that they have no competing interests."
AUTHOR CONTRIBUTIONS: Dr. Preciado had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the report.
Study concept and design: Preciado, Val, Brown.
Acquisition of data, proteomic techniques: Poley, Val, Tsai, Tomney.
Lysate preparation: Tomney, Val
Western blots:Tomney, Poley
Analysis and interpretation of proteomic data: Poley, Tsai, Val, Preciado.
Drafting of the manuscript: Preciado.
Critical revision of the manuscript for important intellectual content: Val, Brown.
Statistical analysis: Brown, Preciado.
Study supervision: Val, Preciado.
All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marian Poley, Email: mpoley@cnmc.org.
Stephanie Tsai, Email: st8@princeton.edu.
Amarel Tomney, Email: amarel.saieg@gmail.com.
Kristy Brown, Email: kristy.brown@cnmc.org.
Stephanie Val, Email: sval@cnmc.org.
References
- 1.Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23:824–828. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 2.Klein JO. What's new in the diagnosis and management of otitis media? Pediatr Ann. 2002;31:777–778. doi: 10.3928/0090-4481-20021201-06. [DOI] [PubMed] [Google Scholar]
- 3.Brunton S. Current face of acute otitis media: microbiology and prevalence resulting from widespread use of heptavalent pneumococcal conjugate vaccine. Clin Ther. 2006;28:118–123. doi: 10.1016/j.clinthera.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Buscher AZ, Burmeister K, Barenkamp SJ, St Geme JW., 3rd Evolutionary and functional relationships among the nontypeable Haemophilus influenzae HMW family of adhesins. J Bacteriol. 2004;186:4209–4217. doi: 10.1128/JB.186.13.4209-4217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg D, Broides A, Blancovich I, Peled N, Givon-Lavi N, Dagan R. Relative importance of nasopharyngeal versus oropharyngeal sampling for isolation of Streptococcus pneumoniae and Haemophilus influenzae from healthy and sick individuals varies with age. J Clin Microbiol. 2004;42:4604–4609. doi: 10.1128/JCM.42.10.4604-4609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HY, Andalibi A, Webster P, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis. 2004;4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barenkamp SJ, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison A, Dyer DW, Gillaspy A, et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol. 2005;187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HY, Takeshita T, Shimada J, et al. Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC Infect Dis. 2008;8:87. doi: 10.1186/1471-2334-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, Jono H, Han J, Lim DJ, Li JD. Synergistic activation of NF-kappaB by nontypeable Haemophilus influenzae and tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 2004;101:3563–3568. doi: 10.1073/pnas.0400557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JH, Jono H, Koga T, et al. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice. PLoS One. 2007;2:e1032. doi: 10.1371/journal.pone.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon SK, Lee HY, Pan H, et al. Synergistic effect of interleukin 1 alpha on nontypeable Haemophilus influenzae-induced up-regulation of human beta-defensin 2 in middle ear epithelial cells. BMC Infect Dis. 2006;6:12. doi: 10.1186/1471-2334-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komatsu K, Jono H, Lim JH, et al. Glucocorticoids inhibit nontypeable Haemophilus influenzae-induced MUC5AC mucin expression via MAPK phosphatase-1-dependent inhibition of p38 MAPK. Biochem Biophys Res Commun. 2008;377:763–768. doi: 10.1016/j.bbrc.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 15.Preciado DBK, Ghimbovschi S, Rose M. NTHi Induction of Cxcl2 and Middle Ear Mucosal Metaplasia in Mice. Laryngoscope. 2013 doi: 10.1002/lary.24097. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preciado D, Goyal S, Rahimi M, et al. MUC5B Is the Predominant Mucin Glycoprotein in Chronic Otitis Media Fluid. Pediatr Res. 2010;68:231–236. doi: 10.1203/PDR.0b013e3181eb2ecc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saieg A, Brown KJ, Pena MT, Rose MC, Preciado D. Proteomic analysis of pediatric sinonasal secretions shows increased MUC5B mucin in CRS. Pediatr Res. 77:356–362. doi: 10.1038/pr.2014.187. [DOI] [PubMed] [Google Scholar]
- 18.Hathout Y. Approaches to the study of the cell secretome. Expert Rev Proteomics. 2007;4:239–248. doi: 10.1586/14789450.4.2.239. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya K, Kim Y, Ondrey FG, Lin J. Characterization of a temperature-sensitive mouse middle ear epithelial cell line. Acta Otolaryngol. 2005;125:823–829. doi: 10.1080/00016480510031533. [DOI] [PubMed] [Google Scholar]
- 20.Preciado D, Lin J, Wuertz B, Rose M. Cigarette smoke activates NF kappa B and induces Muc5b expression in mouse middle ear cells. Laryngoscope. 2008;118:464–471. doi: 10.1097/MLG.0b013e3185aedc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanno T, Brown K, Siebenlist U. Evidence in support of a role for human T-cell leukemia virus type I Tax in activating NF-kappa B via stimulation of signaling pathways. J Biol Chem. 1995;270:11745–11748. doi: 10.1074/jbc.270.20.11745. [DOI] [PubMed] [Google Scholar]
- 22.Berger JT, Voynow JA, Peters KW, Rose MC. Respiratory carcinoma cell lines. MUC genes and glycoconjugates. Am J Respir Cell Mol Biol. 1999;20:500–510. doi: 10.1165/ajrcmb.20.3.3383. [DOI] [PubMed] [Google Scholar]
- 23.Lim DJ, Hermansson A, Hellstrom SO, et al. Recent advances in otitis media. 3. Animal models; anatomy and pathology; pathogenesis; cell biology and genetics. Ann Otol Rhinol Laryngol Suppl. 2005;194:31–41. [PubMed] [Google Scholar]
- 24.Mikami F, Lim JH, Ishinaga H, et al. The transforming growth factor-beta-Smad3/4 signaling pathway acts as a positive regulator for TLR2 induction by bacteria via a dual mechanism involving functional cooperation with NF-kappaB and MAPK phosphatase 1-dependent negative cross-talk with p38 MAPK. J Biol Chem. 2006;281:22397–22408. doi: 10.1074/jbc.M602124200. [DOI] [PubMed] [Google Scholar]
- 25.Erwin AL, Smith AL. Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol. 2007;15:355–362. doi: 10.1016/j.tim.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 27.Murphy TF, Bakaletz LO, Kyd JM, Watson B, Klein DL. Vaccines for otitis media: proposals for overcoming obstacles to progress. Vaccine. 2005;23:2696–2702. doi: 10.1016/j.vaccine.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Thoren K, Gustafsson E, Clevnert A, Larsson T, Bergstrom J, Nilsson CL. Proteomic study of non-typable Haemophilus influenzae. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;782:219–226. doi: 10.1016/s1570-0232(02)00560-3. [DOI] [PubMed] [Google Scholar]
- 29.Chen R, Lim JH, Jono H, et al. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun. 2004;324:1087–1094. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 30.Shuto T, Xu H, Wang B, et al. Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha /beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci U S A. 2001;98:8774–8779. doi: 10.1073/pnas.151236098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.