Abstract
Helicobacter pylori causes a persistent infection in the human stomach, which can result in chronic gastritis and peptic ulcer disease. Despite an intensive proinflammatory response, the immune system is not able to clear the organism. However, the immune escape mechanisms of this common bacterium are not well understood. We investigated the interaction between H. pylori and human dendritic cells. Dendritic cells (DCs) are potent antigen-presenting cells and important mediators between the innate and acquired immune system. Stimulation of DCs with different concentrations of H. pylori for 8, 24, 48, and 72 h resulted in dose-dependent interleukin-6 (IL-6), IL-8, IL-10 and IL-12 production. Lipopolysaccharide (LPS) from Escherichia coli, a known DC maturation agent, was used as a positive control. The cytokine release after stimulation with LPS was comparable to that induced by H. pylori except for IL-12. After LPS stimulation IL-12 was only moderately released compared to the large amounts of IL-12 induced by H. pylori. We further investigated the potential of H. pylori to induce maturation of DCs. Fluorescence-activated cell sorting analysis of cell surface expression of maturation marker molecules such as CD80, CD83, CD86, and HLA-DR revealed equal upregulation after stimulation with H. pylori or LPS. We found no significant differences between H. pylori seropositive and seronegative donors of DCs with regard to cytokine release and upregulation of surface molecules. These data clearly demonstrate that H. pylori induces a strong activation and maturation of human immature DCs.
Helicobacter pylori is a gastric pathogenic gram-negative bacterium that colonizes the gastric mucus layer but does not invade the mucosal epithelium. This bacterial colonization leads to a cellular infiltrate of polymorphonuclear leukocytes, an acute immune response, followed by the migration of macrophages, lymphocytes, and plasma cells in the gastric mucosa, resulting in chronic gastritis. This chronic inflammation does not necessarily produce symptoms but does increase the risk of developing peptic ulcer disease, adenocarcinoma of the distal stomach (antrum and fundus), and primary non-Hodgkin's lymphoma of the stomach (MALTomas) (8, 42, 43).
Although H. pylori induces an immune response involving both the innate and the acquired immune systems, the host is unable to clear the organism from the mucosa, resulting in lifelong infection. This inability to eliminate the bacterium may be due to immune-evasive strategies. Possible mechanisms were investigated, with emphasis on the acquired immune response. Several studies have shown inhibitory effects of H. pylori on cell proliferation (11, 24-26, 60), and the induction of H. pylori-specific regulatory T cells that actively suppress T-cell response have been described (31).
Recent studies have investigated possible impairment of antigen presentation. VacA, an H. pylori virulence factor, was reported to interfere with proteolytic processing of tetanus toxoid and was shown to inhibit the Ii-dependent pathway of antigen presentation (38). Other studies showed that H. pylori induced inhibition of phagocytosis by professional phagocytes involving cag-PAI (pathogenicity island), a type IV secretion system (44).
Although several studies investigated the interaction between H. pylori and the innate immunity (9, 17, 21, 24, 35, 56), little is known about the influence of H. pylori on dendritic cells (DCs), especially in the human immune system (14, 59). DCs are central mediators between the innate and adaptive immune system and play an important role in capturing, processing, and presenting antigens (5, 6). The process of differentiation from an immature DC into a mature professional antigen-presenting cell (APC) can be induced by whole bacteria or their components, pathogen-associated molecular patterns. This process is accompanied by upregulation of major histocompatibility complex (MHC) classes I and II, costimulatory molecules such as CD80, CD83, and CD86, and adhesion molecules such as CD54 (6, 13), together with cytokine production (10, 58).
DCs are found in almost all tissues, including the gastrointestinal mucosa. Here they are capable of opening the tight junctions that enable them to interact directly with bacteria on the mucosal layer (45, 49). Moreover, recent studies have shown the potential of H. pylori to disrupt the epithelial apical-junctional complex (1), which would increase the probability for DCs to get into direct contact with the bacteria. These findings make a direct interaction between H. pylori and mucosal DCs in vivo very likely, resulting in (i) activation, maturation, and differentiation of DCs and (ii) phagocytosis, processing, and presenting of H. pylori antigen to antigen-specific T lymphocytes.
Different scenarios may explain this interaction. H. pylori induces activation, maturation, and cytokine release via the Toll-like receptor 2 (TLR2), TLR4, and TLR5 (23, 51, 57), or H. pylori induces perturbation of DC function via inhibitory mechanisms described earlier (26). The latter would result in an impairment of clonal H. pylori-specific T-cell proliferation and the persistence of infection.
Thus, the purpose of the present study was to evaluate the effect of H. pylori on the activation and maturation of human DCs. The data presented demonstrate that H. pylori induces IL-10, IL-12, IL-6, and IL-8 secretion, as well as upregulation of costimulatory molecules such as CD80, CD83, CD86, and HLA-DR in human immature DCs independent of the H. pylori serostatus of the donor.
MATERIALS AND METHODS
H. pylori serology.
Serum samples from each healthy donor were obtained and screened for the presence of immunoglobulin G antibody titers to H. pylori by using a commercial enzyme-linked immunosorbent assay (ELISA; Behring, Marburg, Germany).
Cell culture.
Monocytes were isolated by leukapheresis of healthy donors, subsequent Ficoll-Hypaque density gradient centrifugation, and countercurrent elutriation in a J6 M-E centrifuge (Beckman, Munich, Germany) as previously described (27). Monocytes were >90% pure as determined by flow cytometry (data not shown). Immature monocyte-derived DCs were generated by culturing elutriated monocytes in complete RPMI 1640 (Biochrom KG, Berlin, Germany) containing 5% fetal calf serum, vitamins, pyruvate, and nonessential amino acids (all from Life Technologies, Karlsruhe, Germany), 5 × 10−8 M β-mercaptoethanol, 500 U of rhIL-4 (Schering-Plough, Bloomfield, N.J., or Promocell, Heidelberg, Germany)/ml, and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; Essex, Munich, Germany) as described previously (27). On day 3 additional 250 U each of rhIL-4 and rhGM-CSF/ml was added to the cell cultures.
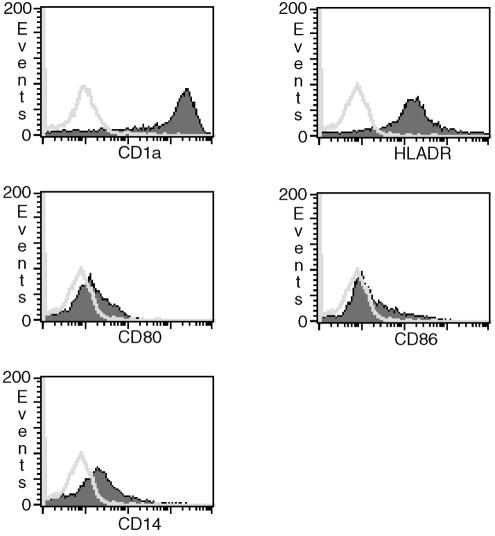
After 7 days, the cells were harvested and washed twice with 10 ml of phosphate-buffered saline (PBS). Cell purity and phenotype of immature DCs was routinely determined by flow cytometry. Immature DC expressed high levels of CD1a and HLA-DR, as well as low levels of CD80 and CD86. Expression of CD14 was low and markedly downregulated compared to freshly isolated monocytes (Fig. 1). Cells were >99% negative for CD3 and CD8. A total of 106 cells were cultured in 1 ml of complete RPMI 1640 in 24-well plates (Becton Dickinson, Heidelberg, Germany). Cells were stimulated with 10 μl of PBS, 100 ng of LPS (Sigma-Aldrich, Taufkirchen, Germany)/ml, or H. pylori at a multiplicity of infection (MOI) of 0.01 to 100.
FIG. 1.
Surface phenotype of immature DCs as determined by flow cytometry. Cells were stained with fluorescence-conjugated monoclonal antibodies prior to stimulation (open curve, isotype control; shaded curve, specific staining). Immature DCs expressed high levels of CD1a (isotype-corrected median [med] = 1,851), HLA-DR (med = 156), and low levels of CD80 (med = 6), and CD86 (med = 4). CD14 expression was markedly downregulated (med = 13) compared to freshly isolated monocytes (med = 510 [data not shown]).
Culture and preparation of H. pylori.
H. pylori was inoculated on Wilkins-Chalgren agar plates (WC) supplemented with 10% lysed horse blood and 25 mg of DENT (10 mg of vancomycin, 5 mg of trimethoprim, 5 mg of cefsulodin, 5 mg of amphotericin; Abtek Biologicals, Ltd., Liverpool, United Kingdom)/liter under microaerophilic conditions (11% O2, 9% CO2, and 80% N2) at 36°C. After 48 h H. pylori was harvested with a sterile cotton swab, suspended, and washed three times in ice-cold PBS. The solution's optical density at 600 nm (OD600) was measured and used to calculate the number of H. pylori per ml by using a factor determined earlier by serial dilutions (1 OD600 = 2,28 × 108 H. pylori/ml). The bacterial strain used for stimulation was H. pylori 2802, a clinical isolate, which induced IL-8 secretion in gastric epithelial cells (AGS cells). Strain 2802 was vacs1, vacm2, babA2, iceA1, iceA2, and cagA positive as determined by PCR (data not shown).
Quantification of cytokines by immunoassay.
Immature monocyte-derived DCs (106 cells/ml in 24-well plates) were incubated for 8, 24, 48, and 72 h with H. pylori or LPS. PBS-incubated cells served as a negative control. The culture supernatants were collected and stored at −80°C until assayed. IL-6, IL-8, IL-10, and IL-12 were determined from culture supernatants by ELISA with commercially available assay kits (Becton Dickinson) according to standard procedures. In these assays, the lower limits of detection were 4.7 pg/ml for IL-6, 3.1 pg/ml for IL-8, 7.8 pg/ml for IL-10, and 7.8 pg/ml for IL-12.
FACS analysis.
For fluorescence-activated cell sorting (FACS) analysis, 2.5 × 105 DCs were resuspended in 100 μl of PBS containing 1% fetal calf serum and 0.1% sodium azide (Merck, Darmstadt, Germany) (FACS buffer) and incubated with 4 μl of the appropriate fluorescein isothiocyanate- and/or phycoerythrin-labeled antibody (Becton Dickinson or Beckman-Coulter, Krefeld, Germany) for 20 min on ice in the dark. Cells were then washed twice with 2 ml of FACS buffer and resuspended in 500 μl of PBS supplemented with 5% of paraformaldehyde (Sigma-Aldrich). Cell death was determined by adding propidium iodide (Sigma-Aldrich) to cell suspensions at a final concentration of 0.5 μg/ml prior to flow cytometric analysis. Analysis was performed with Coulter Epics XL MCL flow cytometer (Beckman-Coulter).
Statistical analysis.
Results shown graphically are from a single representative experiment expressed as the mean ± the standard deviation of the mean, calculated by using aliquots from the same donor. In all, DCs from six seronegative donors and five seropositive donors were investigated in the present study. The data were analyzed by using the nonparametric Friedman test for multiple comparisons (PBS versus LPS and H. pylori). For analysis of seropositive and seronegative donors, the nonparametric Mann-Whitney test was used. P values of <0.05 were considered significant.
RESULTS
H. pylori induces dose-dependent cytokine secretion from immature DCs.
Immature DCs were generated and stimulated with H. pylori for 24 h at different MOIs ranging from 0.01 to 100. LPS at a concentration of 100 ng/ml was used as a known DC activation stimulus. After incubation, supernatants were collected and cytokine concentrations were determined by ELISA.
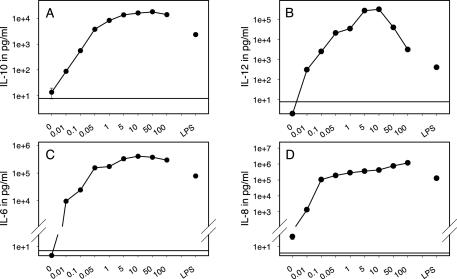
The cytokine levels were found to increase with rising MOIs. For IL-6, IL-10, and IL-12 the maximum cytokine release was obtained upon stimulation with an MOI of 10 to 50, whereas IL-8 production increased during the whole titration course (Fig. 2). FACS analysis showed cell death after stimulation with MOIs of >10 resulting in >50% of dead cells at an MOI of 100 (data not shown). These results strongly suggest that superphysiological high concentrations of H. pylori have a toxic effect. Increasing IL-8 levels in culture supernatants at superphysiological bacterial concentrations (Fig. 2D) could be explained with: (i) cell lyses and consequential cytokine release or (ii) cell death, which itself induces IL-8 release in the remaining viable cells (29). In contrast, IL-12, IL-10, and IL-6 release depends on cell vitality, as cytokine production decreases and cell death increases with MOIs of >50.
FIG. 2.
DCs stimulated with H. pylori show a dose-dependent release of IL-10, IL-12, IL-6, and IL-8. A total of 106 immature monocyte-derived DCs per ml were incubated with H. pylori at MOIs ranging from 0.01 to 100 for 24 h. LPS at 100 ng/ml served as a positive control. (A) IL-10 secretion, as determined by ELISA, was detectable at an MOI of 0.01 and reached a plateau at an MOI of 10. (B) IL-12 production peaked at an MOI of 10 and declined at higher H. pylori concentrations. (C) H. pylori at an MOI of 0.01 induced detectable amounts of IL-6, which reached saturation levels when stimulated with an MOI of >10. (D) IL-8 production increased slightly during the whole titration course, with the greatest leap occurring between an MOI of 0.01 and an MOI of 0.1. The horizontal lines indicate the cutoff levels of ELISAs. Mean values from one representative experiment are shown with logarithmic scales for the y axis (n = 4).
Cytokine production was detectable at an MOI of 0.01 (ca. 9,593 pg/ml for IL-6 and 1,333 pg/ml for IL-8). These results show that one bacterium per 100 cells is sufficient to induce DC activation and that activation is dose dependent. H. pylori and LPS were almost equally potent in inducing DC activation. Only for IL-12 did we find a significant difference in cytokine production between H. pylori and LPS. LPS induced moderate amounts of IL-12 (194 to 3,335 pg/ml) compared to H. pylori (8,224 to 319,000 pg/ml) (Table 1). The absolute amounts of cytokines produced from different donors in response to stimulation with H. pylori or LPS varied widely. For further experiments, we considered an MOI of 10 to be the optimal dose for DC stimulation.
TABLE 1.
DCs from seropositive and seronegative donors stimulated with H. pylori release comparable amounts of cytokines
| Cytokine and serostatus | Donor | Amt of cytokine produced (pg/ml) after treatment with:
|
||
|---|---|---|---|---|
| PBS | LPS | H. pylori | ||
| IL-6 | ||||
| Negative | 1 | <4.7 | 75,988 ± 4,987 | 168,000 ± 4,879 |
| 2 | <4.7 | 162,000 ± 27,450 | 75,200 ± 8,976 | |
| 3 | <4.7 | 202,000 ± 4,778 | 25,580 ± 157 | |
| 4 | <4.7 | 66,600 ± 17,670 | 114,500 ± 18,280 | |
| 5 | <4.7 | 69,720 ± 8,841 | 150,400 ± 9,068 | |
| 6 | <4.7 | 143,100 ± 3,968 | 274,300 ± 25,770 | |
| Positive | 1 | <4.7 | 212,967 ± 15,674 | 794,933 ± 15,974 |
| 2 | <4.7 | 51,770 ± 6,601 | 551,800 ± 17,660 | |
| 3 | <4.7 | 55,495 ± 8,125 | 203,350 ± 4,738 | |
| 4 | <4.7 | 77,250 ± 7,442 | 399,600 ± 10,910 | |
| 5 | <4.7 | 35,780 ± 4,196 | 46,690 ± 14,460 | |
| IL-12 | ||||
| Negative | 1 | <7.8 | 1,595 ± 260 | 57,065 ± 219 |
| 2 | <7.8 | 2,004 ± 115 | 8,224 ± 1,024 | |
| 3 | <7.8 | 262 ± 2 | 155,900 ± 35,000 | |
| 4 | <7.8 | 194 ± 28 | 12,300 ± 2,303 | |
| 5 | <7.8 | 3,335 ± 161 | 18,682 ± 3,757 | |
| 6 | <7.8 | 2,281 ± 26 | 26,410 ± 4,127 | |
| Positive | 1 | 14 ± 8 | 877 ± 6 | 158,240 ± 8,126 |
| 2 | <7.8 | 1,310 ± 168 | 230,000 ± 82,110 | |
| 3 | <7.8 | 373 ± 19 | 13,180 ± 139 | |
| 4 | <7.8 | 416 ± 1 | 319,000 ± 40,110 | |
| 5 | <7.8 | 1,952 ± 114 | 28,522 ± 704 | |
| IL-10 | ||||
| Negative | 1 | 111 ± 6 | 3,293 ± 111 | 6,347 ± 472 |
| 2 | <7.8 | 3,790 ± 196 | 1,950 ± 267 | |
| 3 | <7.8 | 11,050 ± 2,172 | 6,082 ± 140 | |
| 4 | <7.8 | 2,695 ± 91 | 2,829 ± 131 | |
| 5 | <7.8 | 2,912 ± 310 | 14,605 ± 2,134 | |
| 6 | <7.8 | 904 ± 85 | 44,230 ± 883 | |
| Positive | 1 | ND | ND | ND |
| 2 | 34 ± 7 | ND | 2,890 ± 129 | |
| 3 | 15 ± 1 | 4,322 ± 506 | 15,990 ± 2,550 | |
| 4 | 14 ± 6 | 2,313 ± 158 | 15,860 ± 1,057 | |
| 5 | <7.8 | 1,902 ± 14 | 2,102 ± 132 | |
| IL-8 | ||||
| Negative | 1 | 1,066 ± 383 | 56,980 ± 2,897 | 117,000 ± 5,026 |
| 2 | ND | ND | ND | |
| 3 | 543 ± 100 | 122,000 ± 1,806 | 1218,000 ± 11,920 | |
| 4 | 44 ± 22 | 113,000 ± 21,650 | 161,400 ± 2,360 | |
| 5 | 107 ± 8 | 287,400 ± 19,740 | 333,400 ± 21,340 | |
| 6 | 33 ± 3 | 160,200 ± 18,520 | 929,300 ± 113,900 | |
| Positive | 1 | ND | ND | ND |
| 2 | 198 ± 13 | ND | 63,780 ± 15,520 | |
| 3 | 37 ± 13 | 55,495 ± 8,125 | 203,350 ± 4,738 | |
| 4 | 58 ± 24 | 128,400 ± 197 | 420,000 ± 12,060 | |
| 5 | 67 ± 9 | 39,280 ± 466 | 25,390 ± 2,561 | |
A total of 106 immature monocyte-derived DCs/ml were stimulated with H. pylori at an MOI of 10 or with LPS of 100 ng/ml for 24 h. DCs treated with 10 μl of PBS served as an unstimulated control. DCs from six seronegative and five seropositive donors were investigated. Cytokine levels (IL-6, IL-12, and IL-10) were below the cutoff for PBS stimulation in some experiments. For some donors, the IL-8 and IL-10 cytokine levels were not determined (ND). There was a significant difference between H. pylori and LPS in inducing IL-12 production (P < 0.5).
Kinetics of cytokine production in immature DCs after stimulation with H. pylori.
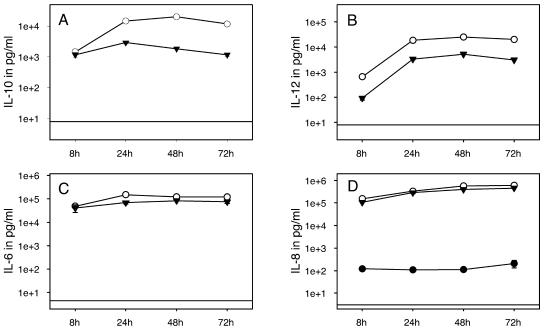
After determining the optimal bacterial dose for DC activation, we were interested in studying the kinetics of cytokine production by DCs stimulated with H. pylori. We used H. pylori at an MOI of 10 and LPS at a concentration of 100 ng/ml. IL-8 and IL-6 were detectable as soon as 4 h after stimulation with H. pylori and LPS (data not shown). Stimulation for 8 h induced high amounts of IL-6 and IL-8. IL-6 levels increased during the subsequent 24 h when they reached a plateau, whereas IL-8 production increased considerably during the complete stimulation period (Fig. 3C and 3D). IL-10 secretion started after 6 h and increased until 48 h, when it reached a maximum, and decreased slightly during the following 24 h of stimulation (Fig. 3A). The kinetics for IL-12 production were similar except that it was first detected after 8 to 10 h (Fig. 3B). Comparable kinetics of cytokine production were observed for stimulation with H. pylori and LPS.
FIG. 3.
Kinetics of cytokine production in DCs in response to H. pylori stimulation. A total of 106 immature monocyte-derived DCs per ml were incubated with PBS (•), with H. pylori at an MOI of 10 (○), or with LPS at 100 ng/ml (▾) for 8, 24, 48, and 72 h. (A) IL-10 production was detectable after 8 h. Cytokine levels reached a maximum at 24 to 48 h and slightly decreased for the following 24 h. (B) IL-12 accumulation started at 8 h, and its production was sustained until at least 72 h. (C) High amounts of IL-6 were detected after 8 h with a minor increase for the following stimulation period. (D) IL-8 production increased considerably for 72 h. The horizontal lines indicate the cutoff levels of ELISAs. Mean values from one representative experiment are shown with logarithmic scales for the y axis (n = 5).
H. pylori induces expression of differentiation and maturation markers on immature DCs.
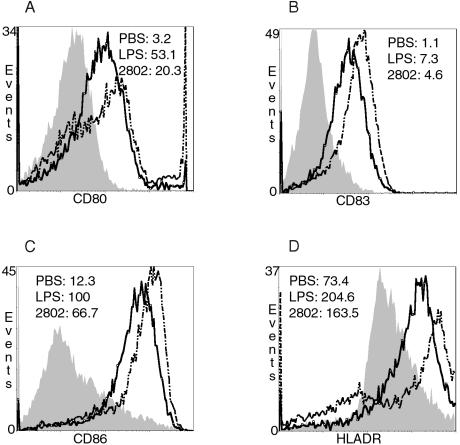
The potential of H. pylori to induce DC maturation was investigated by stimulating immature DCs with H. pylori at an MOI of 10 or with LPS (100 ng/ml). After 72 h, the expression of CD80, CD83, CD86, and HLA-DR was determined by FACS analysis.
The mean fluorescence intensity (MFI) for CD80, a costimulatory molecule for T-cell activation, was significantly increased by stimulation with H. pylori compared to basal expression (Fig. 4A). Stimulation with LPS, a known and well-described maturation agent for DCs, showed a significant increase in the MFI for CD80. In addition, the costimulatory molecules CD83 and CD86 were upregulated by stimulation with H. pylori, as well as with LPS, compared to the basal expression (Fig. 4B and C). MHC class II molecules are upregulated during the maturation process of immature DCs. Processed antigens are presented in the form of MHC class II peptide complexes on the cell surface and recognized by CD4 cells. Therefore, increased expression of MHC class II molecules is important for the interaction between innate and adaptive immunity. Stimulation with H. pylori showed a significant upregulation of MHC class II molecules (HLA-DR) on DC surfaces, as did LPS (Fig. 4D).
FIG. 4.
H. pylori and LPS induce similar phenotypic maturation of human DCs. Immature DCs were stimulated with PBS, H. pylori (MOI = 10) or LPS (100 ng/ml) for 72 h. Staining of surface markers was analyzed by FACScan flow cytometry. Filled histograms represent PBS treatment, straight lines represent stimulation with H. pylori, and scattered lines show LPS stimulation. (A) H. pylori and LPS increased CD80 expression significantly. (B and C) Comparable upregulation of CD83 (B) and CD86 (C) was observed after treatment with H. pylori and LPS. (D) HLA-DR expression was equally upregulated after H. pylori stimulation and LPS stimulation. Histograms from one representative experiment are presented (n = 7).
H. pylori-induced activation and maturation of immature DCs is independent of the serostatus.
We found cytokine response to be independent of the serostatus when we examined DCs from various seropositive and negative donors (Table 1). The absolute amounts of cytokine production and the susceptibility for LPS and H. pylori varied considerably between different donors. We did not detect any significant differences in cytokine responses for IL-6, IL-8, and IL-10 between DCs stimulated with H. pylori or LPS. In contrast to the observed low to moderate amounts of IL-12 induced by LPS, H. pylori always stimulated high levels of IL-12 release (P = 0.001). These data are in accordance with results presented above (Fig. 2B and 3B).
As for the cytokines, seropositive and seronegative donors did not show any significant differences regarding the maturation-induced expression of surface molecules (Table 2).
TABLE 2.
H. pylori induces upregulation of surface molecules on DCs independently of the serostatusa
| Treatment | MFI
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD80
|
CD83
|
CD86
|
HLA-DR
|
|||||
| − | + | − | + | − | + | − | + | |
| PBS | 3.2 | 5.1 | 1.1 | 1.0 | 12.3 | 12.4 | 78.4 | 42.1 |
| LPS | 53.1 | 23.0 | 7.3 | 3.0 | 100.0 | 55.2 | 204.6 | 127.6 |
| H. pylori | 20.3 | 9.3 | 4.6 | 2.7 | 66.7 | 21.5 | 163.5 | 72.4 |
Immature DCs were incubated with PBS, H. pylori (MOI = 10), or LPS (100 ng/ml) for 72 h. The MFI values for different surface molecules are shown from two representative experiments (n = 7).
DISCUSSION
We observed IL-6, IL-10, and IL-12p70 production, as well as IL-8 release, after the stimulation of DCs with H. pylori. The cytokine secretion showed distinct kinetics and was dose dependent. These findings conflict with a recent study (14), which reported little IL-6 release, no IL-10 production, and only small amounts of IL-12p70 production (∼100 pg/ml) after stimulation with H. pylori. Guiney et al. (14) used Salmonella enterica containing a highly stimulatory LPS as a positive control. DCs stimulated with S. enterica produced large amounts of IL-6 (∼100,000 pg/ml). These data are in accordance with the IL-6 production we observed after LPS stimulation. Others have shown that stimulation of DCs with LPS induced IL-10 (3,000 to 36,000 pg/ml), IL-6 (193,000 to 500,000 pg/ml), and IL-12p70 (7,000 to 13,000 pg/ml) at levels comparable to our results (28). These conflicting results regarding cytokine production after DC stimulation might be explained by different DC preparation and stimulation protocols. Guiney et al. (14) isolated monocytes by plastic adherence and differentiated the cells by culturing them for 5 days with 1,000 U of IL-4 and GM-CSF/ml, whereas we used leukapheresis, Ficoll-Hypaque density gradient centrifugation, and countercurrent elutriation for cell isolation and generated DCs by culturing them for 7 days with 500 U of IL-4 and GM-CSF/ml. We stimulated DCs in 24-well plates at a concentration of 106 DCs/ml compared to Guiney et al., who used only 2 × 105 DCs/well in 24-well plates, which might have resulted in reduced cell-cell interaction and therefore in decreased autocrine stimulation.
Previous studies showed locally increased production of cytokines such as IL-8 and IL-6 in the gastric mucosa (2, 32). IL-8 is a chemokine known to attract neutrophils. IL-6, a proinflammatory cytokine, is an important intermediary in the resolution of inflammation. It supports transition between the early, predominantly neutrophilic stage of an infection and the more sustained mononuclear cell influx. The production of these cytokines in the gastric mucosa correlates with the histological picture of H. pylori gastritis, which is a severe inflammation with polymorphonuclear infiltrations (47, 53). Several studies identified gastric epithelial cells as a source of the IL-8 production (18, 39, 41). We showed that DCs secreted IL-8 and IL-6 in response to stimulation with H. pylori. Our results suggest that the innate immune system contributes to the production of these cytokines, triggering and modulating the local inflammatory response.
It has been reported that H. pylori induces a Th1 response (4, 30, 36). Some studies suggest that a Th2 response to H. pylori infection leads to a less severe inflammation or even protects against a persistent infection (37, 52, 54). In considering the Th1 response induced by H. pylori, the simultaneous production of IL-12 and IL-10 seems surprising. DCs are divided into DC1 and DC2 according to their ability to induce a Th1 or Th2 response (22, 46). There is evidence that functional differences between APC lineages might contribute to the polarization of Th-cell response (17, 50). Another concept is based on the (tissue- and pathogen-type) context of DC activation, which leads either to a Th1- or Th2-promoting effector function (21). Recently, it was shown that the kinetics of DC activation and migration can influence the type of effector and memory T cells generated (28). That study showed distinct kinetics of cytokine and chemokine production by DCs stimulated with LPS and exhaustion of cytokine production. DC stimulation for 48 h led to exhaustion of cytokine production and switch from Th1- to Th2-inducing mode. These results indicate a flexible and dynamically regulated model of the Th1-Th2 polarizing capacity of DCs.
Our data are in accordance with those in the study by Langenkamp et al. (28). IL-6, IL-8, IL-10, and IL-12 were all present after 8 h of stimulation. IL-6 and IL-8 were detected as soon as 4 h after stimulation. In the case of IL-6 the production reached a plateau after 24 h, whereas IL-8 accumulation increased during the whole time course. The proinflammatory cytokine IL-6 and the chemokine IL-8 released in the early phase of an infection in the peripheral tissue lead to a recruitment of APCs and neutrophils. The kinetics for IL-12 were different. IL-12 was detected after 8 to 10 h and reached a maximum level after 48 h, decreasing slightly during the following 24 h, whereas measurable IL-10 production started after 6 h of stimulation, with a maximum level reached at the 48-h time point. The delayed IL-12 production, however, may coincide with the DC homing in lymph nodes, where IL-12 can influence the DC T-cell interaction. These results, together with the previously described model of the Th1-Th2-inducing capacity of DCs, make it very unlikely that H. pylori induces exclusively or even dominantly a Th1 response.
In several studies, however, there is evidence that IL-10 may play a relevant role in the H. pylori-induced immune response. IL-10 is important for the generation of type 1 regulatory T cells (Tr-1 cells) (7, 20, 61). Tr-1 cells are defined by their ability to produce high levels of IL-10 and transforming growth factor β. They have a low proliferative capacity and are able to suppress pathological immune responses in the setting of transplantation, allergy, or autoimmune disease. Their suppressive capacity is not always beneficial, since they can also suppress immune response to antigens of tumors or pathogens (12). H. pylori-specific regulatory T cells were previously described as suppressing the memory T-cell response to H. pylori in infected individuals (31). This suppression may contribute to the inability of the immune system to clear this bacterial infection. Pathogens which interact with DC-SIGN, the human immunodeficiency virus type 1 receptor on DCs, cause chronic infections and are reported to enhance IL-10 production (40). Binding of DC-SIGN to LeX-positive H. pylori lysate was recently demonstrated (3). Other studies suggest that IL-10 production is TLR4 mediated (15). These results are of particular interest since there is evidence that H. pylori activation is mediated by TLR2 and TLR5 but not by TLR4 (51). The receptor-organism interaction that results in IL-10 release needs further investigation. Whether the IL-10 production triggers the immune escape mechanisms of H. pylori by generating Tr-1 cells or whether the moderate IL-10 release in presence of huge amounts of IL-12 is not sufficient to generate a beneficial Th2 response is yet unclear and remains to be analyzed in more detail.
We have shown that H. pylori is a maturation stimulus for human monocyte-derived DCs. Incubation of DCs for 72 h with H. pylori or LPS generated phenotypically mature DCs with high levels of expression of costimulatory molecules (CD80, CD83, and CD86) and of MHC class II. Thus far, these results suggest no inhibitory potential of H. pylori on the innate immune system which would eventually explain one of the immune escape mechanisms of this persistent pathogen. Thus, further studies are required to investigate the biologic activity of H. pylori-induced mature DCs in the context of DC-T-cell interaction. Immature DCs are very efficient in antigen capture. They use different pathways to internalize antigens: phagocytosis (17, 33), macropinocytosis (48), and adsorptive endocytosis (19, 55). Previous studies on monocytes described the inhibition of phagocytosis by H. pylori involving cagA secretion components (44). It would be of great interest to investigate the antiphagocytic activity on immature DCs. Inhibiting the uptake of bacteria and their components on the one hand and simultaneously inducing DC maturation on the other hand would suggest that the maturation process is initiated by secreted bacterial components and mediated via receptors on the cell surface. It is very likely that TLRs play an important role in this interaction. DCs express all known TLRs (16, 32), and TLR ligands can induce DC maturation (34). Furthermore, H. pylori has been shown to induce NF-κB activity via TLR2 and TLR5 in human epithelial cells (51) and via TLR4 in gastric pit cells (23).
Our findings that H. pylori stimulates DC maturation despite its ability to inhibit phagocytosis and induces IL-10 and IL-12 production simultaneously are very interesting phenomena. Further investigation is needed to elucidate the interaction between DC and H. pylori in detail and to investigate the activation cascade downstream of cell surface receptors.
Acknowledgments
We thank Marina Kreutz (Department Hematology, University Hospital Regensburg) for providing the flow cytometry profile of immature dendritic cells and Jörg Marienhagen for reviewing the statistical analysis.
This study was supported by the DFG Sonderforschungsbereich 585 TP B3/B4 to W.S.-B., N.L., and L.D.
Editor: J. B. Bliska
REFERENCES
- 1.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando, T., G. I. Perez-Perez, K. Kusugami, M. Ohsuga, K. C. Bloch, and M. J. Blaser. 2000. Anti-CagA immunoglobulin G responses correlate with interleukin-8 induction in human gastric mucosal biopsy culture. Clin. Diagn. Lab. Immunol. 7:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelmelk, B. J., D. van, I., S. J. van Vliet, C. M. Vandenbroucke-Grauls, T. B. Geijtenbeek, and Y. van Kooyk. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. [DOI] [PubMed] [Google Scholar]
- 4.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 19: 392:245-252. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Z. M., M. J. O'Shaughnessy, I. Gramaglia, A. Panoskaltsis-Mortari, W. J. Murphy, S. Narula, M. G. Roncarolo, and B. R. Blazar. 2003. IL-10 and TGF-β induce alloreactive CD4+ CD25− T cells to acquire regulatory cell function. Blood 101:5076-5083. [DOI] [PubMed] [Google Scholar]
- 8.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge, R., J. G. Kusters, M. S. Timmer, V. Gimmel, B. J. Appelmelk, S. Bereswill, A. H. van Vliet, S. G. Meuwissen, M. Kist, C. M. Vandenbroucke-Grauls, and E. J. Kuipers. 2001. The role of Helicobacter pylori virulence factors in interleukin production by monocytic cells. FEMS Microbiol. Lett. 196:235-238. [DOI] [PubMed] [Google Scholar]
- 10.Dixon, G. L., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. van der Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 69:4351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 12.Groux, H. 2003. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation 75:8S-12S. [DOI] [PubMed]
- 13.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T-cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 14.Guiney, D. G., P. Hasegawa, and S. P. Cole. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 71:4163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins, S. C., E. C. Lavelle, C. McCann, B. Keogh, E. McNeela, P. Byrne, B. O'Gorman, A. Jarnicki, P. McGuirk, and K. H. Mills. 2003. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 171:3119-3127. [DOI] [PubMed] [Google Scholar]
- 16.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 17.Innocenti, M., A. M. Svennerholm, and M. Quiding-Jarbrink. 2001. Helicobacter pylori lipopolysaccharides preferentially induce CXC chemokine production in human monocytes. Infect. Immun. 69:3800-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail, S., M. B. Hampton, and J. I. Keenan. 2003. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect. Immun. 71:5670-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, W., W. J. Swiggard, C. Heufler, M. Peng, A. Mirza, R. M. Steinman, and M. C. Nussenzweig. 1995. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375:151-155. [DOI] [PubMed] [Google Scholar]
- 20.Jutel, M., M. Akdis, F. Budak, C. Aebischer-Casaulta, M. Wrzyszcz, K. Blaser, and C. A. Akdis. 2003. IL-10 and TGF-β cooperate in the regulatory T-cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 33:1205-1214. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski, P., C. M. Hilkens, E. A. Wierenga, and M. L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20:561-567. [DOI] [PubMed] [Google Scholar]
- 22.Kapsenberg, M. L., C. M. Hilkens, E. A. Wierenga, and P. Kalinski. 1999. The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin. Exp. Allergy 29(Suppl. 2):33-36. [PubMed] [Google Scholar]
- 23.Kawahara, T., Y. Kuwano, S. Teshima-Kondo, T. Kawai, T. Nikawa, K. Kishi, and K. Rokutan. 2001. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J. Med. Investig. 48:190-197. [PubMed] [Google Scholar]
- 24.Knipp, U., S. Birkholz, W. Kaup, K. Mahnke, and W. Opferkuch. 1994. Suppression of human mononuclear cell response by Helicobacter pylori: effects on isolated monocytes and lymphocytes. FEMS Immunol. Med. Microbiol. 8:157-166. [DOI] [PubMed] [Google Scholar]
- 25.Knipp, U., S. Birkholz, W. Kaup, and W. Opferkuch. 1993. Immune suppressive effects of Helicobacter pylori on human peripheral blood mononuclear cells. Med. Microbiol. Immunol. 182:63-76. [DOI] [PubMed] [Google Scholar]
- 26.Knipp, U., S. Birkholz, W. Kaup, and W. Opferkuch. 1996. Partial characterization of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect. Immun. 64:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause, S. W., M. Rehli, M. Kreutz, L. Schwarzfischer, J. D. Paulauskis, and R. Andreesen. 1996. Differential screening identifies genetic markers of monocyte to macrophage maturation. J. Leukoc. Biol. 60:540-545. [DOI] [PubMed] [Google Scholar]
- 28.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311-316. [DOI] [PubMed] [Google Scholar]
- 29.Li, M., D. F. Carpio, Y. Zheng, P. Bruzzo, V. Singh, F. Ouaaz, R. M. Medzhitov, and A. A. Beg. 2001. An essential role of the NF-κB/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 166:7128-7135. [DOI] [PubMed] [Google Scholar]
- 30.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundgren, A., E. Suri-Payer, K. Enarsson, A. M. Svennerholm, and B. S. Lundin. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luzza, F., T. Parrello, G. Monteleone, L. Sebkova, M. Romano, R. Zarrilli, M. Imeneo, and F. Pallone. 2000. Upregulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J. Immunol. 165:5332-5337. [DOI] [PubMed] [Google Scholar]
- 33.Matsuno, K., T. Ezaki, S. Kudo, and Y. Uehara. 1996. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. J. Exp. Med. 183:1865-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Means, T. K., F. Hayashi, K. D. Smith, A. Aderem, and A. D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 170:5165-5175. [DOI] [PubMed] [Google Scholar]
- 35.Meyer, F., K. T. Wilson, and S. P. James. 2000. Modulation of innate cytokine responses by products of Helicobacter pylori. Infect. Immun. 68:6265-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 37.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. J. Czinn. 1997. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 38.Molinari, M., M. Salio, C. Galli, N. Norais, R. Rappuoli, A. Lanzavecchia, and C. Montecucco. 1998. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 187:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakachi, N., T. W. Klein, H. Friedman, and Y. Yamamoto. 2000. Helicobacter pylori infection of human gastric epithelial cells induces IL-8 and TNFα, but not TGFβ1 mRNA. FEMS Immunol. Med. Microbiol. 29:23-26. [DOI] [PubMed] [Google Scholar]
- 40.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477-7485. [DOI] [PubMed] [Google Scholar]
- 41.Owen, R. J., S. Sharp, A. J. Lawson, Z. Durrani, S. Rijpkema, and M. Kidd. 2003. Investigation of the biological relevance of Helicobacter pylori cagE locus diversity, presence of CagA tyrosine phosphorylation motifs and vacuolating cytotoxin genotype on IL-8 induction in gastric epithelial cells. FEMS Immunol. Med. Microbiol. 36:135-140. [DOI] [PubMed] [Google Scholar]
- 42.Parsonnet, J., G. D. Friedman, N. Orentreich, and H. Vogelman. 1997. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsonnet, J., S. Hansen, L. Rodriguez, A. B. Gelb, R. A. Warnke, E. Jellum, N. Orentreich, J. H. Vogelman, and G. D. Friedman. 1994. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 330:1267-1271. [DOI] [PubMed] [Google Scholar]
- 44.Ramarao, N., S. D. Gray-Owen, S. Backert, and T. F. Meyer. 2000. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol. Microbiol. 37:1389-1404. [DOI] [PubMed] [Google Scholar]
- 45.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 46.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, M. R. de Waal, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 47.Rocha, G. A., D. M. Queiroz, E. N. Mendes, A. J. Barbosa, G. F. Lima Junior, and C. A. Oliveira. 1991. Helicobacter pylori acute gastritis: histological, endoscopical, clinical, and therapeutic features. Am. J. Gastroenterol. 86:1592-1595. [PubMed] [Google Scholar]
- 48.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheinecker, C., R. McHugh, E. M. Shevach, and R. N. Germain. 2002. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196:1079-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 51.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-κB activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552-32560. [DOI] [PubMed] [Google Scholar]
- 52.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-γ, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 53.Sobala, G. M., J. E. Crabtree, M. F. Dixon, C. J. Schorah, J. D. Taylor, B. J. Rathbone, R. V. Heatley, and A. T. Axon. 1991. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32:1415-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sommer, F., G. Faller, M. Rollinghoff, T. Kirchner, T. W. Mak, and M. Lohoff. 2001. Lack of gastritis and of an adaptive immune response in interferon regulatory factor-1-deficient mice infected with Helicobacter pylori. Eur. J. Immunol. 31:396-402. [DOI] [PubMed] [Google Scholar]
- 55.Sousa, C., P. D. Stahl, and J. M. Austyn. 1993. Phagocytosis of antigens by Langerhans cells in vitro. J. Exp. Med. 178:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stassi, G., A. Arena, A. Speranza, D. Iannello, and P. Mastroeni. 2002. Different modulation by live or killed Helicobacter pylori on cytokine production from peripheral blood mononuclear cells. New Microbiol. 25:247-252. [PubMed] [Google Scholar]
- 57.Su, B., P. J. Ceponis, S. Lebel, H. Huynh, and P. M. Sherman. 2003. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect. Immun. 71:3496-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verhasselt, V., C. Buelens, F. Willems, D. De Groote, N. Haeffner-Cavaillon, and M. Goldman. 1997. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 158:2919-2925. [PubMed] [Google Scholar]
- 59.Voland, P., N. Hafsi, M. Zeitner, S. Laforsch, H. Wagner, and C. Prinz. 2003. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect. Immun. 71:3837-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, J., E. G. Brooks, K. B. Bamford, T. L. Denning, J. Pappo, and P. B. Ernst. 2001. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 167:926-934. [DOI] [PubMed] [Google Scholar]
- 61.Zeller, J. C., A. Panoskaltsis-Mortari, W. J. Murphy, F. W. Ruscetti, S. Narula, M. G. Roncarolo, and B. R. Blazar. 1999. Induction of CD4+ T-cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-β. J. Immunol. 163:3684-3691. [PubMed] [Google Scholar]