Abstract
Many clinical isolates of Streptococcus mutans produce peptide antibiotics called mutacins. Mutacin production may play an important role in the ecology of S. mutans in dental plaque. In this study, inactivation of a histidine kinase gene, ciaH, abolished mutacin production. Surprisingly, the same mutation also diminished competence development, stress tolerance, and sucrose-dependent biofilm formation.
Streptococcus mutans is considered the major etiologic agent in causing human dental caries (21, 22). In addition to the known virulence properties, such as biofilm formation and acid production and tolerance, most clinical isolates of S. mutans elaborate antimicrobial peptides called mutacins (5, 12). Mutacins exhibit antimicrobial activity against closely related streptococcal species and other gram-positive bacteria. The ability to produce mutacin may play an important role in the sustained existence of S. mutans in dental plaque (11, 14). So far, two types of mutacins have been characterized at the molecular level: the lantibiotics, represented by mutacins I, II, and III (25, 28, 29), mutacin JH1140 (15), and mutacin B-Ny266 (24); and the nonlantibiotic bacteriocins, represented by mutacin IV (27) and mutacin N (1). The lantibiotics are small peptides that are ribosomally synthesized and posttranslationally modified (31) and, in general, have a wider spectrum of activity than the nonlantibiotic bacteriocins.
The regulation of lantibiotic production has been shown to be mediated by a two-component signal transduction system (TCSTS) for nisin, subtilin, and related lantibiotics (9, 18). A TCSTS comprises two proteins, a histidine kinase sensor and its cognate response regulator (33). The histidine kinase sensor binds to a specific signal molecule, which triggers autophosphorylation. Signal transduction from the phosphorylated kinase sensor to the response regulator enables it to activate or repress transcription of its target genes. In previous studies with the lantibiotic mutacin II, Qi et al. demonstrated that a specific transcription activator, MutR, is required for transcription activation of the mutacin operon, and no other regulators appeared to be required (26). In contrast, regulation of mutacin I production appeared to be more complicated. In addition to a requirement for MutR, mutacin I production was also dependent on culture conditions that produced large bacterial aggregates (27; C. Bordador and F. Qi, unpublished data). In our efforts to find additional regulators for mutacin I production, we found a gene, ciaH, whose inactivation abolished mutacin I production. Surprisingly, the same mutation also affected other cellular functions.
Inactivation of ciaH abolished mutacin I production.
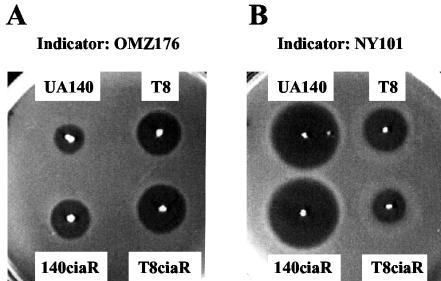
S. mutans strain UA140 was a clinical isolate from a severe caries lesion. Our investigators demonstrated earlier that UA140 produces two mutacins, the lantibiotic mutacin I and the nonlantibiotic mutacin IV (27). Previous studies have shown that production of mutacin I appears to be regulated by cell density, suggesting that signal transduction systems may be involved (27, 29). In order to find potential regulators, a BLAST search of the S. mutans UA159 genome sequence database was performed using known TCSTS sequences as queries. This search revealed 13 pairs of TCSTS sequences; 5 of these were chosen for further characterization based on their homology to other TCSTS sequences known to be involved in various stress responses. As an initial screen, only the histidine kinase gene from each pair was inactivated. Because these genes were the last gene of their respective operons, they could be disrupted by a single crossover integration without complications of polar effects. Typically, a ∼300-bp internal fragment close to the 5′ end of the gene was amplified by PCR using an upstream forward primer with an EcoRI site incorporated at its 5′ end and a downstream reverse primer with a BamHI site incorporated at its 5′ end. The fragment was digested with EcoRI and BamHI and cloned into the suicide vector pJY4164 digested with the same enzymes. The plasmid was transformed into UA140 using standard transformation procedures (32). Ten erythromycin-resistant transformants were randomly selected and tested for mutacin production on trypticase soy broth plus yeast extract (1%) plates by the deferred antagonism assay (5) using OMZ176 and NY101 as indicator strains. OMZ176 is a Streptococcus sobrinus strain which is sensitive only to the lantibiotic mutacins, and NY101 is a Streptococcus sanguis strain which is sensitive to both the lantibiotic mutacin I and the nonlantibiotic mutacin IV (F. Qi et al., unpublished data). Of the five histidine kinase genes (ciaH [SMu1031], spaK [Smu0602], phoR [Smu0946] (6), scnK [SMu1652] (17), and hk11 [SMu1407]) (3), only ciaH inactivation resulted in a total loss of mutacin I production (Fig. 1A). Since the same mutation did not affect mutacin IV production (Fig. 1B), this result suggested that ciaH may have specifically affected lantibiotic mutacin production. To test this, the same mutation was introduced into T8, a lantibiotic mutacin II-producing strain (25), to yield T8ciaH. As shown in Fig. 1, the ciaH mutation did not have a significant effect on mutacin II production. Taken together, these results suggest that the production of mutacin I and mutacin II is probably controlled by different regulatory mechanisms and that ciaH is required specifically for the production of mutacin I.
FIG. 1.
Deferred antagonism assay to determine the effect of ciaH mutation on mutacin production. A single colony from a fresh TH plate was stabbed onto a trypticase soy broth plus yeast extract plate and grown anaerobically for 1 day. The plate was then heated at 80°C for 30 min to kill the producer cells and overlaid with 3 ml of soft agar mixed with 0.4 ml of overnight culture of the indicator strain, after being cooled to room temperature. The zone of inhibition was inspected after an overnight incubation at 37°C anaerobically. (A) S. sobrinus OMZ176 was used as the indicator. (B) S. sanguis NY101 was used as the indicator.
Inactivation of ciaR did not exert a significant effect on mutacin I production.
Given the fact that most (if not all) of the TCSTS were arranged as two-gene operons with the histidine kinase and the response regulator occurring as cognate pairs, we were interested in determining the function of ciaR, the putative response regulator of ciaH in the ciaRH operon. The ciaR gene was inactivated by using a terminatorless kanamycin resistance gene cassette insertion to prevent a polar effect on the downstream ciaH gene. A 1.38-kb DNA fragment encompassing regions upstream of ciaR and part of ciaH was generated by PCR and cloned into a TA cloning vector, pCRII (Invitrogen Co., San Diego, Calif.). The terminatorless kanamycin resistance gene cassette (aph III) (35) was inserted into the ciaR gene at a unique HincII site in the middle of the gene in the same orientation. The plasmid was linearized and transformed into UA140. Upon integration into the chromosome through homologous recombination at the ciaR locus, the recombinants were selected on Todd-Hewitt (TH) plates with 800 μg of kanamycin/ml and the insertion was confirmed by PCR. Reverse transcription-PCR was also performed to verify that the insertion had no polar effect on transcription of the downstream ciaH gene (data not shown). Ten isolates were then tested for mutacin production; one of them is shown in Fig. 2. It is apparent that the ciaR mutation did not exert much effect on production of any of the mutacins tested, although a slight increase in inhibition zone was observed for mutacin I (Fig. 2A). This result indicates that the ciaR gene is not required for mutacin biosynthesis.
FIG. 2.
Deferred antagonism assay to determine the effect of ciaR mutation on mutacin production. The experiment was performed as described in the legend for Fig. 1.
ciaH gene inactivation diminished competence development.
One of the major effects of ciaRH mutations in Streptococcus pneumoniae occurs with competence development (8, 10, 23). Insertional inactivation of ciaR and ciaH results in derepression of competence both in aerobic and microaerobic cultures (7). Since the CiaR and CiaH proteins in S. mutans share a high degree of similarity with the CiaR and CiaH proteins, respectively, in S. pneumoniae (89% identity and 93% similarity for CiaR; 55% identity and 72% similarity for CiaH), it was logical to test if the S. mutans ciaR and ciaH genes were also involved in competence development. We performed transformation assays using chromosomal DNA isolated from a UA140 derivative strain carrying a tetracycline resistance marker. Transformation assays were performed following standard procedures (26, 32) in competence development medium (TH broth [THB; 0.9% beef heart digest, 1.1% pancreatic digest of casein, 0.3% soybean peptone, 0.2% glucose, 0.25% sodium carbonate, 0.2% sodium chloride, and 0.05% monosodium phosphate] plus 0.2% bovine serum albumin). The experiments were repeated three times, and each time the transformation efficiency of the wild-type (wt) strain was arbitrarily assigned as 100%. The transformation efficiency of the mutants was calculated as the ratio of the number of transformants per milliliter of competent cells of the mutant versus that of the wt, times 100. The results showed a dramatic reduction in transformation efficiency for the ciaH mutant strain (∼0.1% ± 0.01% of the wt level). Surprisingly, the transformation efficiency for the mutR mutant strain did not show a significant reduction (73% ± 32% of the wt level).
ciaH gene inactivation altered sucrose-dependent biofilm formation.
In the oral cavity, S. mutans mostly exists in biofilms known as dental plaque. Biofilm formation by S. mutans involves two processes: sucrose-independent initial attachment mediated by surface binding proteins, and sucrose-dependent biofilm formation mediated by glucans synthesized by the glucosyltransferases (Gtfs) from sucrose (36, 37). In vitro, especially on glass surfaces, the latter process appears to play a more important role; without sucrose, the biofilm remains weakly associated with the surface and is very sensitive to shear force. Since biofilm formation was found to be regulated by the competence genes comCDE (20), we performed assays to test if the ciaH gene also was involved in biofilm formation due to its role in competence. UA140 and the mutant derivatives were grown overnight in THB (Difco Laboratories, Detroit, Mich.) at 37°C anaerobically. The culture was diluted 1:20 into fresh THB and further incubated until the culture reached an optical density at 600 nm of 0.5. The culture was then diluted 1:1,000 into THB containing 1% sucrose, and 0.4 ml of this cell suspension was added to each well of an eight-well Lab-Tek II chamber slide system (Nalge Nunc International, Naperville, Ill.). The chamber was incubated at 37°C for 20 h as a static culture to allow for biofilm formation. The supernatant was removed, and a labeling solution containing TH plus 0.2 mM CellTracker Orange (Molecular Probes) was added to the biofilm. The labeling proceeded for 2 h to allow live cells to metabolize the dye and develop fluorescence.
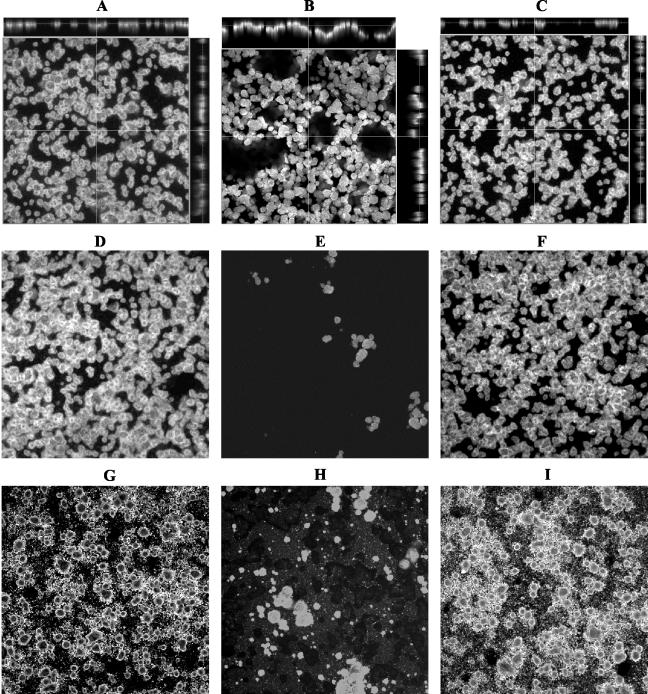
Biofilms were analyzed by confocal laser scanning microscopy. In our initial studies, we noticed that without disturbance the biofilm formed by 140ciaH appeared thicker than the one observed for the wt. However, this mutant biofilm did not attach tightly to the surface and was easily disrupted when rinsed. To get a clear picture of the apparent sensitivity of this particular biofilm to shear forces, we imaged the biofilm in two steps. First, biofilms were rinsed by gently adding and then removing 0.5 ml of phosphate-buffered saline, using a pipettor. After the image was taken (Fig. 3A to C), the biofilm was rinsed again with a squirt bottle to wash off loosely attached cells and a second image was taken (Fig. 3D to F). To minimize possible inconsistencies resulting from washing, biofilms were also washed with running water at a constant flow rate, and similar results were obtained (data not shown). As shown in Fig. 3, both the wt and the mutant strains formed similar dome-like microcolonies. However, despite a similar microcolony architecture, the gently rinsed biofilm of 140ciaH was much thicker than that of the wt (Fig. 3A to C). A closer inspection of the cross-sections of 140ciaH revealed that the majority of the dome-like microcolonies were not attached to the glass surface (Fig. 3B, side panels). Instead, they attached to each other to form a multilayered sheet that was loosely attached to the surface. In contrast, the biofilms of the wt and the ciaR mutant consisted of a single layer of microcolonies that were all attached to the surface (Fig. 3A and C). When the wash was applied more forcefully by using a squirt bottle or running water, the biofilm formed by 140ciaH came off with only a few microcolonies remaining attached (Fig. 3E). In contrast, the biofilm formed by the wt and the ciaR mutant remained intact even after such a vigorous wash (Fig. 3D and F). To see if this phenotype was caused by the artificial growth medium, we tested biofilm formation in sterile saliva supplemented with 20% THB and 1% sucrose. As shown in Fig. 3G to I, the same results were obtained. More interestingly, on the saliva-coated surface, clear marks were shown on the slide surface of the 140ciaH biofilm (Fig. 3H). These marks were probably left by detached microcolonies taking with them saliva deposits on the glass surface. Taken together, these results indicate that the ciaH mutation diminishes sucrose-dependent surface attachment for biofilm formation.
FIG. 3.
Effect of ciaH gene inactivation on biofilm formation. UA140 wt (A, D, and G), ciaH mutant (B, E, and H), and ciaR mutant (C, F, and I) biofilms were grown in THB plus 1% sucrose (A to F) or in sterile saliva supplemented with 20% THB and 1% sucrose (G to I). The biofilms were gently rinsed first (A to C) and then vigorously washed (D to I). Side frames in panels A to C are the z plane of the confocal image, which shows the thickness of the biofilm and the architecture as viewed from the cross-section. The thin line on the z plane indicates the level at which the photograph of the x-y plane was taken. (H) To show the marks left by the detached microcolonies of the ciaH mutant grown in saliva, the photograph was deliberately overexposed. Photographs were taken at a magnification of ×100.
Sucrose-dependent biofilm formation plays a pivotal role in the cariogenicity of S. mutans (36). In addition to the gtf genes that are required for glucan synthesis and biofilm formation (4, 16, 36), a number of other genes have been reported recently which affect sucrose-dependent biofilm formation (13, 30, 34). Some of these genes encode proteins associated with the cell wall (WapA) (30), and others may be involved in cell membrane synthesis or cell surface structures (13, 34). While these genes can be called terminal or effector genes, no regulatory gene has been reported to be involved in regulation of this importance function. Of the three TCSTS that have been reported to be involved in biofilm formation, all were studied in the absence of sucrose (2, 19, 20, 37). To our knowledge, ciaH is the first regulatory gene reported to affect sucrose-dependent biofilm formation.
The ciaH gene is involved in acid tolerance.
The results presented above suggest that the ciaH gene may act as a global regulator controlling multiple cellular functions. Previous studies have shown that some of the genes regulating competence development or biofilm formation in S. mutans were also involved in regulating acid tolerance (19). Therefore, we were interested in determining if the ciaH gene was also involved in acid tolerance. To measure the acid sensitivity of the ciaH mutant, we grew UA140, 140ciaH, and 140ciaR in THB overnight. The cultures were then diluted 1:40 into THB and THB pH 6.4 and incubated at 37°C as static cultures. Samples were taken at designated time points, and the cell density was measured as the optical density at 600 nm. As shown in Table 1, the ciaH mutant exhibited a 60% reduction in growth rate when grown at pH 6.4; in comparison, the wt and the ciaR mutant showed only 20% reduction in growth rate under the same conditions. This difference in growth rate under acidic conditions between the wt and the ciaH mutant was statistically significant (P = 0.0006). These results suggest that the ciaH mutation greatly reduced acid tolerance in strain UA140.
TABLE 1.
Effect of low pH on growth of S. mutans UA140 wt and ciaH and ciaR mutants
| Strain | Doubling time (min)a in:
|
% Reduction in growth rate (TH pH 6.4 vs TH) | |
|---|---|---|---|
| TH | TH pH 6.4 | ||
| UA140 | 69.4 ± 5.1 | 86.7 ± 4.5 | 20 |
| 140ciaH | 74.5 ± 7.5 | 119.5 ± 3.5 | 60 |
| 140ciaR | 72.9 ± 6.9 | 88.5 ± 7.6 | 20 |
Doubling time was calculated based on the formulas ln Z − ln Z0 = k(t − t0), where k is the growth rate, and g = 0.693/k, where g is the doubling time. Values are the mean ± standard deviation obtained from three independent experiments.
In summary, we characterized a putative histidine kinase gene, ciaH, in S. mutans. Inactivation of ciaH abolished mutacin production, diminished competence development, altered sucrose-dependent biofilm formation, and significantly reduced acid tolerance. Although ciaH and ciaR are in the same genomic organization as the ciaRH operon in S. pneumoniae, unlike the ciaRH system in S. pneumoniae, inactivation of the putative cognate regulator ciaR did not reveal any conspicuous phenotype. A possible explanation is that CiaH may have a second cognate regulator located at a different location which is responsible for regulation of the above cellular functions. Assays for protein-protein interactions, such as the yeast two-hybrid system or in vitro biochemical analysis with purified proteins, will be required to resolve this question.
Acknowledgments
We thank J. Yother for providing the plasmid pJY4164 and F. Gu for assistance in statistical analysis. We greatly appreciate the public release of the S. mutans sequence data from the Streptococcal mutans Genome Sequencing Project funded by a U.S. Public Health Service, National Institutes of Health (NIH) grant from the Dental Institute and B. A. Roe, R. Y. Tian, H. G. Jia, Y. D. Qian, S. P. Lin, S. Li, S. Kenton, H. Lai, J. D. White, R. E. McLaughlin, M. McShan, D. Ajdic, and J. Ferretti from the University of Oklahoma.
This work was supported in part by NIH grant R01 DE 014757 to F. Qi, NIH MPTG Training Grant T32-AI07323 to J. Merritt, and a BioStar/C3 Scientific Corporation grant and a Washington Dental Service Grant to W. Shi.
Editor: V. J. DiRita
REFERENCES
- 1.Balakrishnan, M., R. S. Simmonds, A. Carne, and J. R. Tagg. 2000. Streptococcus mutans strain N produces a novel low molecular mass non-lantibiotic bacteriocin. FEMS Microbiol Lett. 183:165-169. [DOI] [PubMed] [Google Scholar]
- 2.Biswas, I., and J. R. Scott. 2003. Identification of rocA, a positive regulator of covR expression in the group A streptococcus. J. Bacteriol. 185:3081-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brian, P., P. J. Riggle, R. A. Santos, and W. C. Champness. 1996. Global negative regulation of Streptomyces coelicolor antibiotic synthesis mediated by an absA-encoded putative signal transduction system. J. Bacteriol. 178:3221-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burne, R. A., Y. Y. Chen, and J. E. Penders. 1997. Analysis of gene expression in Streptococcus mutans in biofilms in vitro. Adv. Dent. Res. 11:100-109. [DOI] [PubMed] [Google Scholar]
- 5.Caufield, P. W., N. K. Childers, D. N. Allen, and J. B. Hansen. 1985. Distinct bacteriocin groups correlate with different groups of Streptococcus mutans plasmids. Infect. Immun. 48:51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cvitkovitch, D. G., J. A. Gutierrez, J. Behari, P. J. Youngman, J. E. Wetz, P. J. Crowley, J. D. Hillman, L. J. Brady, and A. S. Bleiweis. 2000. Tn917-lac mutagenesis of Streptococcus mutans to identify environmentally regulated genes. FEMS Microbiol. Lett. 182:149-154. [DOI] [PubMed] [Google Scholar]
- 7.Echenique, J. R., S. Chapuy-Regaud, and M. C. Trombe. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol. Microbiol. 36:688-696. [DOI] [PubMed] [Google Scholar]
- 8.Echenique, J. R., and M. C. Trombe. 2001. Competence repression under oxygen limitation through the two-component MicAB signal-transducing system in Streptococcus pneumoniae and involvement of the PAS domain of MicB. J. Bacteriol. 183:4599-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelke, G., Z. Gutowski-Eckel, P. Kiesau, K. Siegers, M. Hammelmann, and K. D. Entian. 1994. Regulation of nisin biosynthesis and immunity in Lactococcus lactis 6F3. Appl. Environ. Microbiol. 60:814-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giammarinaro, P., M. Sicard, and A. M. Gasc. 1999. Genetic and physiological studies of the CiaH-CiaR two-component signal-transducing system involved in cefotaxime resistance and competence of Streptococcus pneumoniae. Microbiology 145:1859-1869. [DOI] [PubMed] [Google Scholar]
- 11.Gronroos, L., M. Saarela, J. Matto, U. Tanner-Salo, A. Vuorela, and S. Alaluusua. 1998. Mutacin production by Streptococcus mutans may promote transmission of bacteria from mother to child. Infect. Immun. 66:2595-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada, S., and T. Ooshima. 1975. Production and properties of bacteriocins (mutacins) from Streptococcus mutans. Arch. Oral Biol. 20:641-648. [DOI] [PubMed] [Google Scholar]
- 13.Hazlett, K. R., J. E. Mazurkiewicz, and J. A. Banas. 1999. Inactivation of the gbpA gene of Streptococcus mutans alters structural and functional aspects of plaque biofilm which are compensated by recombination of the gtfB and gtfC genes. Infect. Immun. 67:3909-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman, J. D., A. L. Dzuback, and S. W. Andrews. 1987. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J. Dent. Res. 66:1092-1094. [DOI] [PubMed] [Google Scholar]
- 15.Hillman, J. D., J. Novak, E. Sagura, J. A. Gutierrez, T. A. Brooks, P. J. Crowley, M. Hess, A. Azizi, K. Leung, D. Cvitkovitch, and A. S. Bleiweis. 1998. Genetic and biochemical analysis of mutacin 1140, a lantibiotic from Streptococcus mutans. Infect. Immun. 66:2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson, M. C., and R. Curtiss III. 1990. Regulation of expression of Streptococcus mutans genes important to virulence. Infect. Immun. 58:464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes, W. L., J. J. Ferretti, and J. R. Tagg. 1993. Cloning of the gene encoding streptococcin A-FF22, a novel lantibiotic produced by Streptococcus pyogenes, and determination of its nucleotide sequence. Appl. Environ. Microbiol. 59:1969-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein, C., C. Kaletta, and K. D. Entian. 1993. Biosynthesis of the lantibiotic subtilin is regulated by a histidine kinase/response regulator system. Appl. Environ. Microbiol. 59:296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y. H., P. C. Lau, N. Tang, G. Svensater, R. P. Ellen, and D. G. Cvitkovitch. 2002. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 184:6333-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Y. H., N. Tang, M. B. Aspiras, P. C. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 184:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh, P. D. 1994. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 8:263-271. [DOI] [PubMed] [Google Scholar]
- 23.Martin, B., M. Prudhomme, G. Alloing, C. Granadel, and J. P. Claverys. 2000. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38:867-878. [DOI] [PubMed] [Google Scholar]
- 24.Mota-Meira, M., C. Lacroix, G. LaPointe, and M. C. Lavoie. 1997. Purification and structure of mutacin B-Ny266: a new lantibiotic produced by Streptococcus mutans. FEBS Lett. 410:275-279. [DOI] [PubMed] [Google Scholar]
- 25.Novak, J., P. W. Caufield, and E. J. Miller. 1994. Isolation and biochemical characterization of a novel lantibiotic mutacin from Streptococcus mutans. J. Bacteriol. 176:4316-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi, F., P. Chen, and P. W. Caufield. 1999. Functional analyses of the promoters in the lantibiotic mutacin II biosynthetic locus in Streptococcus mutans. Appl. Environ. Microbiol. 65:652-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi, F., P. Chen, and P. W. Caufield. 2001. The group I strain of Streptococcus mutans, UA140, produces both the lantibiotic mutacin I and a nonlantibiotic bacteriocin, mutacin IV. Appl. Environ. Microbiol. 67:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi, F., P. Chen, and P. W. Caufield. 2000. Purification and biochemical characterization of mutacin I from the group I strain of Streptococcus mutans, CH43, and genetic analysis of mutacin I biosynthesis genes. Appl. Environ. Microbiol. 66:3221-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi, F., P. Chen, and P. W. Caufield. 1999. Purification of mutacin III from group III Streptococcus mutans UA787 and genetic analyses of mutacin III biosynthesis genes. Appl. Environ. Microbiol. 65:3880-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian, H., and M. L. Dao. 1993. Inactivation of the Streptococcus mutans wall-associated protein A gene (wapA) results in a decrease in sucrose-dependent adherence and aggregation. Infect. Immun. 61:5021-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahl, H. G., and G. Bierbaum. 1998. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 32.Shah, G. R., and P. W. Caufield. 1993. Enhanced transformation of Streptococcus mutans by modifications in culture conditions. Anal. Biochem. 214:343-346. [DOI] [PubMed] [Google Scholar]
- 33.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao, L., and J. M. Tanzer. 2002. Novel sucrose-dependent adhesion co-factors in Streptococcus mutans. J. Dent. Res. 81:505-510. [DOI] [PubMed] [Google Scholar]
- 35.Trieu-Cuot, P., and P. Courvalin. 1983. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′ 5"-aminoglycoside phosphotransferase type III. Gene 23:331-341. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita, Y., W. H. Bowen, R. A. Burne, and H. K. Kuramitsu. 1993. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 61:3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida, A., and H. K. Kuramitsu. 2002. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 68:6283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]