Abstract
Background
Ovarian tissue cryopreservation is an experimental fertility preservation method and the transplantation techniques are still evolving.
Objective
We attempted to improve the technique with the utility of a human decellularized extracellular tissue matrix scaffold, robot-assisted minimally invasive surgery and peri-operative pharmacological support.
Study design
We prospectively studied 2 subjects with Hemophagocytic Lymphohistiocytosis (P-A) and Non-Hodgkin Lymphoma (P-B) who underwent ovarian tissue cryopreservation at the age of 23, before receiving preconditioning chemotherapy for hematopoietic stem cell transplantation. Both experienced ovarian failure post-chemotherapy and we transplanted ovarian cortical tissues to the contralateral menopausal ovary 7 and 12 years later, using a human extra cellular tissue matrix scaffold and robotic-assistance. The extra cellular tissue matrix scaffold-tissue compatibility was shown in pre-clinical studies. Patients also received estrogen supplementation and baby aspirin preoperatively to aid in the revascularization process.
Results
Ovarian follicle development was observed approximately ten (P-A) and eight (P-B) weeks after ovarian tissue transplantation. Following eight and seven cycles of in vitro fertilization, nine and ten day-3 embryos were cryopreserved (P-A and P-B, respectively). While the baseline FSH (range: 3.6–15.4 mIU/mL) levels near-normalized by seven months and remained steady post ovarian transplantation in P-A, P-B showed improved but elevated FSH levels throughout (range: 21–31 mIU/mL). Highest follicle yield was achieved 14 (8 follicles; P-A) and 11 months (6 follicles; P-B) post intervention. P-A experienced a chemical pregnancy after the third frozen embryo transfer attempt. She then conceived following her first fresh in vitro fertiliza tion-embryo transfer and the pregnancy is currently ongoing. P-B conceived after the first frozen embryo transfer attempt and delivered a healthy boy at term.
Conclusions
We reported the first pregnancies after the minimally-invasive transplantation of previously cryopreserved ovarian tissue with an extra-cellular tissue matrix scaffold. This approach seems to be associated with steady ovarian function after a follow up of up to 2 years.
Keywords: Fertility Preservation, Ovarian Cryopreservation and Transplantation, Alloderm®, Robotic Surgery, In Vitro Fertilization, Translational Research
INTRODUCTION
Ovarian cryopreservation is one of the key strategies in fertility preservation. Utilizing the previously accumulated knowledge from animal1 and human ovarian xenografting2 studies, we performed the first autologous ovarian tissue transplantation (OTT) case with frozen-thawed tissue in 1999.3,4 Though the patient did not desire pregnancy, she demonstrated ovarian follicle development 8 weeks after the transplant with documented function for up to 9 months.5 The livebirths were to follow several years later as reported by numerous investigators.6–9 In addition, we reported heterotopic OTT techniques where the tissues were grafted subcutaneously under the forearm or abdominal skin.10,11 The latter resulted in oocyte retrievals and embryo development.
With the current techniques, akin to skin grafting, one has to rely on the natural process of revascularization from the recipient site. Because it takes up to 10 days for new human ovarian micro-vessels to reach full maturity, the graft suffers an initial ischemic injury.12 This initial ischemic injury has been shown to result in the loss of nearly two thirds of all primordial follicles in ovarian xenograft models.13 This inefficiency largely explains the unpredictable longevity of ovarian transplants. The length of ovarian function has been reported to range from 1 to >7 years14, 15 with an average of 4–5 years in successful orthotopic and heterotopic ovarian transplants with frozen-banked ovarian tissue.15
In addition, issues with oocyte quality have been cited by some after in vitro fertilization (IVF); one possible explanation being the restricted blood flow.16 The baseline follicle-stimulating hormone (FSH) levels remain high and anti-müllerian hormone (AMH) levels tend to be low in majority of ovarian transplants with cryopreserved tissue, possibly reflecting the restriction in blood flow.17 Therefore, there is convincing evidence to support the view that vascularization issues curb OTT success both acutely in the form of follicle loss and chronically with limited microvascular flow.
AlloDerm® (LifeCell Corp., Branchburg, NJ, USA) is a decellularized human extracellular tissue matrix (ECTM) generated from cadaver skin. It has been used in cosmetic surgery, breast reconstruction and dentistry and other surgical reconstructive fields to augment tissue grafts and aid in revascularization.18 In this study we hypothesized that the cryopreservation of ovarian tissue may preserve fertility. We also hypothesized that the use of the ECTM with robotic surgical assistance may improve outcomes, presumably by aiding the revascularization process.
MATERIALS AND METHODS
This translational work represents the outcomes ovarian cryopreservation and transplantation research protocol after up to 14-year follow up of two subjects who consecutively underwent grafting. The study was approved by the Institutional Review Board and the pre-clinical animal studies were also approved by the Institutional Animal Care and Use Committee (IACUC) at New York Medical College.
Patient-A (P-A)
The patient was diagnosed with Familial Hemophagocytic Lymphohistiocytosis (FHLH) in April 2006, at the age of 23. Shortly before the preconditioning chemotherapy (Thiotepa and Fludarabine) and total body irradiation (TBI, 1375 cGY) for hematopoietic stem cell transplant (HSCT), we performed a laparoscopic oophorectomy. Ovarian cortical strips were cryopreserved with a slow freeze protocol.19 In April 2013, her blood work was consistent with menopausal state (FSH: 108.7 mIU/mL; luteinizing-hormone (LH): 61.4 mIU/mL; estradiol (E2): <6 pg/mL; AMH: <0.16 ng/mL) and a transvaginal ultrasound showed that the remaining ovary and the endometrium to be atrophic.
Patient-B (P-B)
The patient was diagnosed with Non-Hodgkin Lymphoma (NHL) at the age of 17 and completed a chemotherapy regimen including cyclophosphamide (600 mg/m2 × 8 courses every three weeks), prednisone, procarbazine, adriamycin, bleomycin and vinblastine in October 1996. She experienced mediastinal recurrence at the age of 23. We performed ovarian cryopreservation with a protocol identical to P-A, shortly before receiving the highly gonadotoxic DICE regimen (dexamethasone, ifosfamide, carboplatin and etoposide) prior to HSCT. In July 2013, her FSH and LH levels were found to be 54 and 70 mIU/mL, and 47 and 33 mIU/mL one month apart respectively. Her AMH was <0.16 ng/mL with ultrasound examination showing no obvious healthy follicles, confirming the diagnosis of ovarian failure.
Follicle Density Assessment
P-A
Because the diagnosis was non-cancer and an ovarian sample to rule out metastasis was not needed at the time of OTC, we could not assess pre-cryopreservation follicle density. However, one vial of tissue was thawed and the follicle density was assessed before the OTT. This revealed a mean follicle density of 1.66 ± 0.37 follicles/mm2. Based on these results, and after discussion with the cou ple, we empirically decided to thaw 5 of 10 vials, containing 10 pieces of ovarian tissue for transplantation.
P-B
Likewise, one vial of tissue was thawed prior to OTT, and revealed a mean follicle density of 0.62 ± 0.32 follicles/mm2. Though P-B was of similar age with P-A at the time of ovarian tissue cryopreservation, this density was significantly lower than that of with P-A (P<0.05). Furthermore, when compared to patient’s precryopreservation follicle density of 1.4 follicles/mm2, >50% of follicles appeared to have been lost during freezing and thawing. Based on these results and following a discussion with the couple, we empirically decided to thaw and transplant 6 of 12 vials, containing 12 pieces of ovarian tissue.
Preoperative Preparation
Before the procedure, both patients underwent a hysterosonogram and the partners were evaluated with a semen analysis. In addition, P-A also underwent a pelvic MRI to rule out any obvious TBI-induced uterine damage. Ten weeks prior to the procedures, both patients received transdermal 0.1 mg E2 (Climara®, Bayer Healthcare Pharmaceuticals Inc., Whippany, NJ, USA) weekly and vaginal progesterone 100 mg (Prometrium®, Schering-Plough, Kenilworth, NJ, USA) nightly with a 2-week-on / 2-week-off regimen. This regimen was continued after the transplant until a sign of ovarian function was seen. Hormone replacement was given as there is some evidence from animal studies that this may improve ovarian vascularization.20 Again, with the aim of enhancing revascularization, both patients were also given daily Baby Aspirin® 81 mg (Bayer Healthcare Pharmaceuticals Inc., Whippany, NJ, USA) for seven days which was discontinued two days before the surgery.
Preclinical Evaluation of ECTM
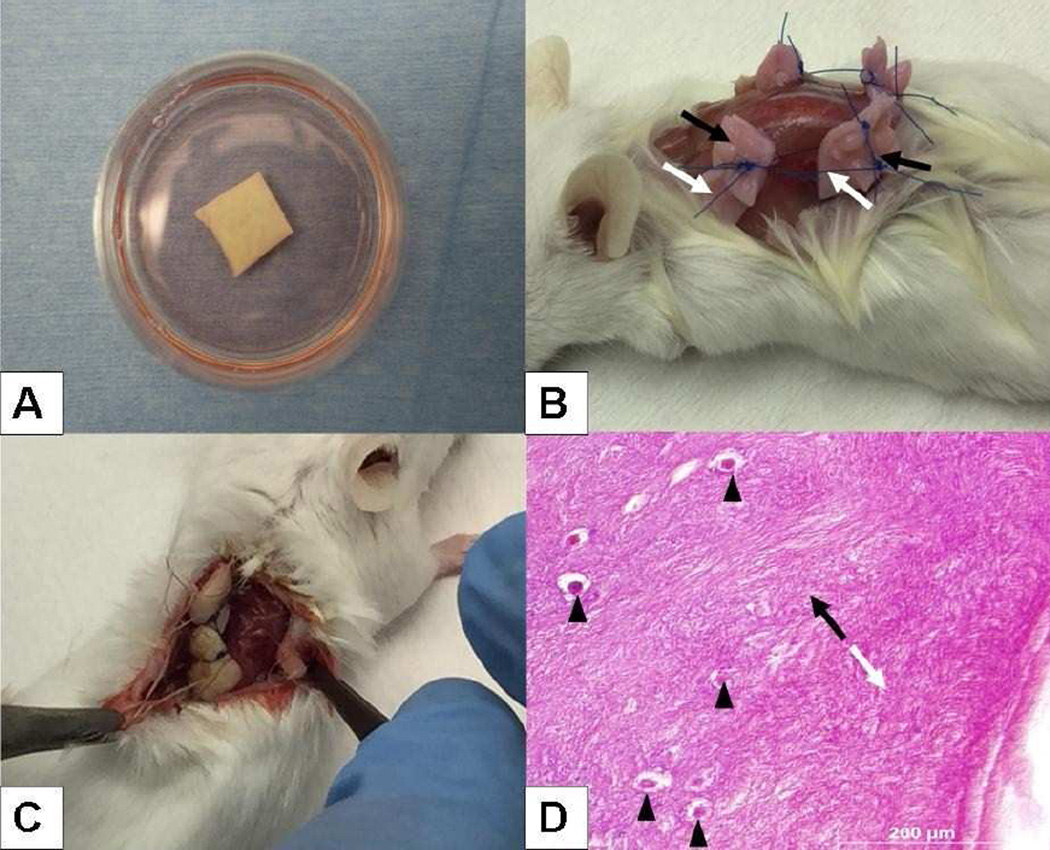
This ECTM has been used in the surgical field but there is no description of its use in reproductive surgery. To ensure its compatibility with ovarian tissues, we performed series of preclinical evaluations. First we evaluated various thicknesses (thin, medium, full-thickness) of the ECTM in thawing media (Fig. 1A) as well as simulating its use with ovarian cortical pieces from organ donor cadavers to determine the best thickness for handling. We found the medium thickness ECTM to be sturdy yet malleable and hence next tested it in a xenograft model. We subcutaneously xenografted 4×4-mm ovarian cortical pieces together with ECTM to immunodeficient mice (Fig. 1B), as we previously described.21 After 10 days of xenografting, the tissues were evaluated. We found that ovarian stroma has integrated into ECTM, without any pathological changes (Fig. 1C and D).
Fig 1.
Preoperative evaluation of extracellular matrix (ECTM) scaffold. A. Medium thickness ECTM during rehydration. B. Xenografting of ECTM (white arrows) with ovarian tissue (black arrows) to severe combined immunodeficiency (SCID) mouse. C. Gross, and D. histological evaluation showing proper integration of ECTM (white arrow) into ovarian tissue (black arrow).The transition zone begins where the tails of the two arrows oppose. Note the presence of normal appearing primordial follicles (arrowheads) near the transition zone.
Next we cultured mouse oocytes (N=30/group) with or without ECTM for 16 hours and assessed survival. We found that there was no difference in oocyte survival when ECTM was used, compared to controls (88.1% vs. 81.6%, p=0.39). These findings provided assurance that ECTM was compatible with ovarian tissues.
Ovarian Transplant Technique
Robotically assisted OTT procedures were performed in July 2013 on P-A at the age of 30 (7 years after ovarian cryopreservation), and on P-B in October 2013 at the age of 35 (12 years after cryopreservation). The OTT technique is described in Supplement 1 and illustrated in S1 Video.
Statistical Methods
Statistical analyses were performed with SPSS 15 for Windows (SPSS, Chicago, IL) using Student’s t-test (follicle density comparisons) or Fisher’s exact test (survival rates of mouse oocytes). A p value of ≤ 0.05 was considered statistically significant.
RESULTS
P-A
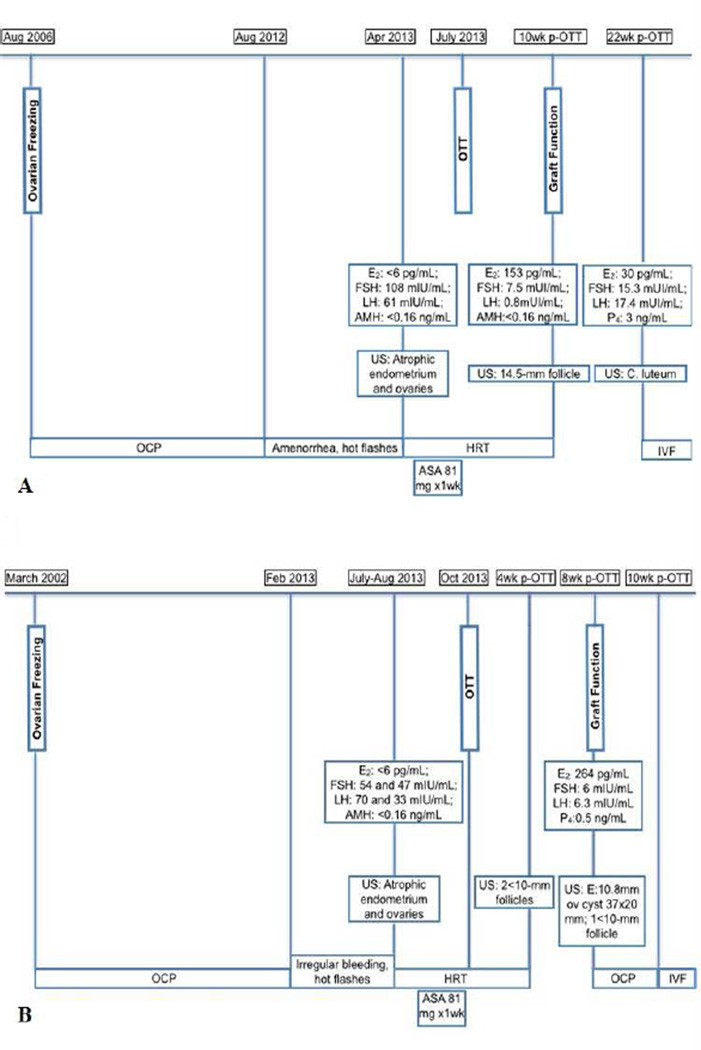
The first sign of ovarian activity was detected approximately ten weeks after OTT in October 2013, by the demonstration of a 14.5-mm follicle on a transvaginal ultrasound exam. The hormone replacement was then discontinued and the patient resumed cyclical menstruation (Fig 2A).
Fig 2.
Timeline from freezing to post-ovarian transplant.
(A) Patient A. (B) Patient B.
w: weeks; p-OTT: post-ovarian tissue transplant; OTT: ovarian tissue transplant; E2: estradiol; FSH: follicle stimulating hormone; LH: luteinizing hormone; AMH: antimüllerian hormone; P4 : progesterone; C. luteum: corpus luteum; US: ultrasound; OCP: oral contraceptive pill; HRT: hormone replacement therapy; ASA: acetylsalicylic acid; IVF: in vitro fertilization; E: endometrial thickness.
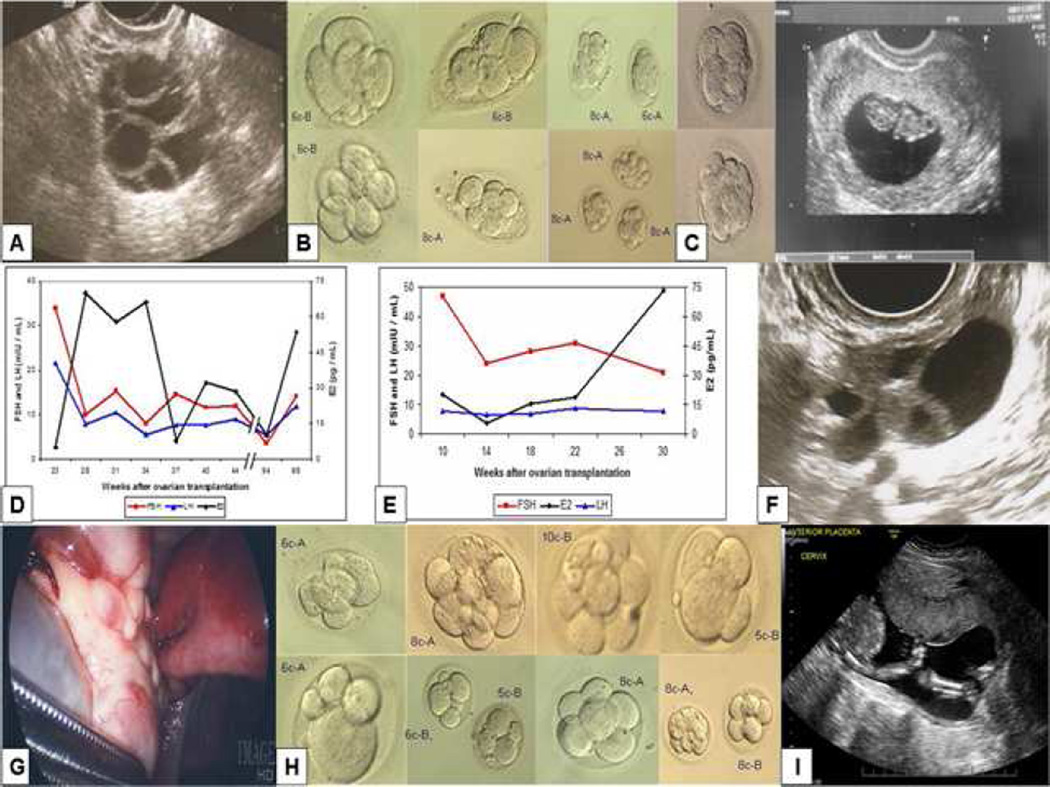
Subsequently, she underwent eight IVF cycles with peak E2 levels as high as 833 pg/mL (Table 1; see supplement-1 for monitoring and IVF protocol). The highest follicle yield (8 follicles) was obtained on her last stimulation cycle, 14 months post-OTT (Fig 3A), when six oocytes were retrieved. Thirty-one follicles developed in total during these eight cycles, of which ten were ≥ 17-mm on the trigger day. Of the twelve MII oocytes collected during these 8 cycles (table 1), ten fertilized after ICSI with a fertilization rate of 83.3%; nine D3-embryos were cryopreserved (Fig 3B). We proceeded with embryo cryopreservation rather than fresh IVF-ET because the ovarian graft longevity could not be predicted and the patients wished to preserve embryos for future pregnancy attempts.
Table 1.
Ovarian stimulation outcomes in ovarian transplantation patients.
| Cycles | Peak E2a (pg/mL) |
Folliclesb, n |
MII, n |
Embryos, n |
Embryo Grade (and Outcome) |
|---|---|---|---|---|---|
| P-A-1 | 208 | 2 (1) | 1 | 1 | 6c-B (RF) |
| P-A-2 | 352 | 3 (1) | 1 | 1 | 6c-B (RF) |
| P-A-3 | 808 | 6 (2) | 2c | 2 | 8c-A, 6c-B (both TT, NP) |
| P-A-4 | 430 | 4 (1) | 1 | 1 | 6c-B (RF) |
| P-A-5 | 602 | 3 (1) | 1 | 0 | 3c (degenerated) |
| P-A-6 | 833 | 3 (1) | 1 | 1 | 8c-A (TT, chemical pregnancy) |
| P-A-7 | 353 | 2 (2) | 0 | 0 | N/A |
| P-A-8 | 627 | 8 (1) | 5d | 3 | 3 × 8c-A (Thawed, 2 transferred as blastocysts, NP) |
| P-A-9e | 482 | 2 | 2 | 2 | Compacting-A, 8-A (fresh transfer, on-going pregnancy) |
| P-B-1 | 236 | 2 (1) | 1 | 1 | 6c-A (RF) |
| P-B-2 | 543 | 4 (1) | 3 | 3 | 8c-A, 10c-B, 5c-B (RF) |
| P-B-3 | 445 | 2 (1) | 0 | 0 | N/A |
| P-B-4 | 575 | 3 (1) | 1e | 1 | 6c-A (RF) |
| P-B-5 | 168 | 3 (2) | 2f | 2 | 6c-B, 5c-B (RF) |
| P-B-6 | 172 | 2 (1) | 1 | 1 | 8c-A (RF) |
| P-B-7 | 574 | 6 (0) | 2 | 2 | 8c-A, 8c-B (both TT, livebirth) |
n: number; MII: metaphase II oocyte; c: cell; A or B: correspond to the embryo grade on Day-3, A being the highest; IVM: in vitro maturation.; RF: remains frozen; TT: thawed and transferred; NP: no pregnancy; N/A: not available.
Peak E2 denotes highest measurement during the stimulation, including the post-trigger day.
Numbers in parenthesis denote those follicles ≥ 17-mm.
Only two oocytes could be retrieved due to a premature LH surge and ovulation.
Includes 2 MI (metaphase I) oocytes in vitro matured to MII.
Did not mature in vitro.
Includes an in vitro matured to MII from GV (germinal vesicle) stage.
Fig 3.
Ovarian transplantation outcomes. (A). Robust response to ovarian stimulation in P-A, 14 months after the ovarian tissue transplant (OTT). (B). Nine embryos were frozen from P-A after 8 IVF cycles; embryo grades are indicated adjacent to each embryo. (C). In addition, 2 embryos were transferred fresh in the last IVF cycle, which resulted in an ongoing pregnancy. (D-E). Respective early follicular phase FSH, LH and estradiol levels in P-A and P-B, indicating improvement in ovarian function after the OTT. (F). Multiple follicle development 11-months post-OTT in P-B. (G). Transplanted ovary at second-look laparoscopy. (H). Eight embryos were frozen from P-B after 7 IVF cycles; embryo grades are indicated adjacent to each embryo. (I). A 20-week fetal anatomical scan shows a normally developed fetus in P-B.
We initially attempted pregnancy with FETs; the third FET resulted in a biochemical pregnancy. We then proceed with a fresh cycle of ovarian stimulation. This resulted in the retrieval of 2 mature oocytes both of which were fertilized and developed in to grade A embryos on day-3 (Fig 3C, left panel). Considering these together with the frozen embryo cycles, the overall fertilization rate reached 85.7%. The transfer of these two embryos resulted in a pregnancy. Fetal heart activity was shown on the 7th week of gestation with appropriate crown rump length measurements (Fig 3C, right panel) and the pregnancy is currently ongoing at 14th week of gestation. A fetal free-DNA test (MaterniT21®1PLUS, Sequenom, San Diego, CA, USA) performed at 11th week of gestation showed normal complement for chromosomes 13, 18 and 21 and indicated a female gender.
The patient’s baseline E2 and FSH levels normalized by 7 months post- OTT and remained at or near normal early follicular levels up until the time of FETs. However, on intermittent evaluations, the AMH measurements remained <0.16 ng/mL. After a pause for FETs, she underwent 2 additional baseline evaluations at approximately 22 and 23 months post-OTT. These showed baseline E2 and FSH levels of 10 pg/mL and 3.6 mIU/mL and 53.5 and 14.2, respectively, with two antral follicles on each transvaginal ultrasound. The patient conceived following the last baseline hormonal evaluation. This indicated the persistence of ovarian function for up to 2 years after the transplant (Fig 3D).
P-B
Four weeks after the OTT, small follicles were suspected on a transvaginal ultrasound which prompted the discontinuation of hormone replacement therapy. Four weeks later her laboratory evaluation showed an E2 of 264 pg/mL, FSH of 6 mIU/mL, LH of 6.3mIU/mL and a P4 value of 0.5 ng/mL (Fig 2B).
Baseline FSH levels showed continual improvement (Fig 3E) after the OTT but remained above normal early follicular phase levels. Intermittent serum measurements showed that the AMH levels remained <0.16 ng/mL though the peak E2 levels reached as high as 650 pg/mL. The highest number of follicles (6 follicles) developed 11 months post-OTT (Fig 3F).
Eight months post-OTT, we performed a laparoscopic salpingectomy to remove a contralateral hydrosalpinx. Laparoscopic evaluation showed that the ECTM graft had completely integrated with the patient’s native ovarian tissue and could not be discerned (see video in S1 video and Fig 3G), while confirming unilateral tubal patency.
A total of 22 follicles developed in response to 7 cycles of ovarian stimulation, of which 7 were ≥ 17-mm. These yielded 10 M-II oocytes (Table 1); 100% were fertilized and cryopreserved on day-3 (Fig 3H). We then performed a FET with two D3-embryos graded as 8-cell-A and 10-cell-B, which resulted in a positive pregnancy test. A fetal free-DNA test (MaterniT21®1PLUS, Sequenom, San Diego, CA, USA) performed at 11th week of gestation showed normal complement for chromosomes 13, 18 and 21 and indicated a female gender. A fetal anatomical scan on the 20th week of gestation also revealed a healthy fetus (Fig. 3I). The pregnancy has progressed to term without any complications. The patient had a normal spontaneous vaginal delivery of a healthy female child weighing 3,617 grams with Apgar scores of 9/9 at 39 weeks 5 days of gestation.
COMMENTS
Ovarian cryopreservation is a highly important fertility preservation strategy, which has several advantages over gamete and embryo freezing. It can be performed in prepubertal girls and children,22,23 does not require ovarian stimulation and delay in chemotherapy, restores ovarian endocrine function and provides the possibility of natural conception. However, despite the mounting number of live-births, the technique still remains in the experimental realm. Those who defend the experimental status for ovarian freezing and transplantation generally cite the relatively brief experience, limited number of pregnancies and the brevity of ovarian function.
In this translational study we presented two successful cases of OTT with a new approach, which resulted in robust ovarian function and pregnancies, with one progressing to term. Based on a literature review in all available languages including English in Pubmed, as well as unpublished reports identified from review articles and abstract presentations, this is the first report of livebirth and pregnancies after the use of this multipronged technique. In addition, our study has several other unique aspects. First, we for the first time reported outcomes of consecutive ovarian stimulation cycles and longitudinal follow up for the response to such stimulation. Follow-up of baseline hormone measurements and response to ovarian stimulation for up to two years gave us a unique perspective as to how the function of OT may improve over time.
There is scant information on IVF cycles after OTT and the few reports indicate relatively limited response.24 We observed a relatively high follicle yield (2–8 follicles/cycle, an average of 3.87 follicles/cycle in P-A and 2–6 follicles/cycle, an average of 3.14 in P-B). Interestingly, the highest number of follicles developed during the last stimulation cycles for both patients, 14 and 11 months after OTT, indicating a continuous improvement in ovarian function and no obvious sign of graft exhaustion.
We have also observed high fertilization rates (85.7–100%); the reported fertilization rates from previous studies with oocyte originating from orthotopic transplants range from 0–100% with a mean of 43%.24 The embryos from both patients were in general of high quality and the P-B conceived after the first transfer. Interestingly, the same patient had received a chemotherapy regimen containing an alkylating agent prior to ovarian cryopreservation. The relatively higher baseline FSH levels in P-B compared to P-A may be explained by the reduction of primordial follicle density due to exposure to alkylating agents prior to cryopreservation. While the P-A failed had a chemical pregnancy with the third FET, she conceived when two fresh embryos were transferred. Despite the initial setbacks with P-A, high fertilization rates and embryo grades, as well as ongoing and delivered pregnancies point to high oocyte quality following the OTT with ECTM.
However, even in cycles with a relatively large number of follicles and multiple oocyte recovery, and high quality embryos, estradiol levels were not proportionately high. Furthermore, intermittent AMH measurements remained <0.1 ng/mL, lower than expected given the number of follicles developed in response to ovarian stimulation. This may indicate that especially the venous vasculature in frozen-thawed ovarian tissue transplants may be at variance from that of in a normal ovary. This could be in the form of a weakly formed venous microcirculation25, which is unable to reflect AMH production from preantral follicles.
There is also very limited information on pregnancies with frozen embryos originating from transplanted ovaries. While Sanchez et al.26 cryopreserved M-II oocytes from one patient, which resulted in a pregnancy, only Suzuki et. al.7 reported embryo vitrification in primary ovarian insufficiency patients undergoing OTT after follicle activation. These reports all together indicate that further cryopreservation of oocytes or embryos originating from previously frozen-thawed and transplanted ovarian tissue may not necessarily be detrimental.
P-B had received cyclophosphamide at doses known to cause significant ovarian damage.27 The success with P-B also indicates that previous exposure to alkylating agents is not a contraindication for ovarian cryopreservation as some suggested.28 Because young patients have larger ovarian reserve, they may be able to tolerate partial losses and still be able to succeed with ovarian transplantation, even when a fraction of their tissues are thawed and transplanted as was the case in this report. In fact, three other studies reported pregnancies with ovarian tissues frozen after exposure to alkylating agents.29–31
From the current design, we cannot determine the relative contributions of peri-transplant pharmacological management, the surgical site, use of ECTM or minimally invasive surgery with robotic assistance to the success of our OTT technique. To prevent any damage to vascular bed, we refrain from cauterization during the procedure and the field of surgery may be too obscured for standard minimally invasive surgery. Robotic assistance may have advantages over standard laparoscopy because of the improved acuity of visualization with 3-dimensional view, increased dexterity with finely controlled instruments, ability to gently manipulate friable menopausal ovary as the recipient site and more precise placement of sutures at desired locations. We also feel that, it decreases the time from the removal of the tissue from the culture media to its transplantation to the vascular recipient site, thereby minimizing the time spent without sustenance. It is possible that, the robust and sustained ovarian function observed with our OTT approach is the result of interaction of many factors mentioned above.
Considering the current potential livebirth and unpublished reports,6–9 the total number of children born after autologous transplantation of cryopreserved ovarian tissue for fertility preservation appears to be approximately 60.32 However, efforts to determine success rates have been hampered by the limitation of the knowledge on the total number of attempts (denominator) as the studies tend to report only the successful attempts. When considering the few reports that included the number of attempts together with the pregnancies reported here6,15, the clinical pregnancy and ongoing plus livebirth rates for OTT with cryopreserved tissue is 36.3% and 27.2% per patient, respectively (Table 2). As a unique feature of ovarian cryopreservation, which sets it apart from the other fertility preservation procedures, approximately 50% of the pregnancies were achieved without a need for assisted reproduction. Furthermore, 93.9% of the procedures resulted in the restoration of ovarian endocrine function, including those that were performed with heterotopic transplantation techniques (Table 2).3,5,6,10,11,14,15
Table 2.
Current Outcomes and Success Rates with Transplantation of Cryopreserved Ovarian Tissue
| Age at Cryopreservation (range) | 23.5 ± 4.7 years (17–36) |
| Age at Transplantationa (range) | 30 ± 4.2 years (21–38) |
| Maternal Age at Delivery | 35.6 ± 1.9 years (25–39) |
| Gestational Age at Deliveryb | 38.1 ± 1.6 weeks (33–41) |
| Clinical Pregnancy/Patient (%) | 24/66c (36.3%) |
| Live + Ongoing Pregnancy/Patient (%) | 18/66c (27.2%) |
| Endocrine Function/Patient | 62/66d, (93.9%) |
In summation, in this translational study we reported the first livebirth and an ongoing pregnancy with an OTT technique utilizing a human ECTM as a scaffold, with robotassisted minimally-invasive surgery. Ovarian tissue cryopreservation is evolving as an effective fertility preservation approach. Overall, the quest for improving ovarian transplant techniques and outcomes is continuing. This includes pharmacological agents than can accelerate the revascularization of ovarian transplants.12 The currently reported OTT approach appears to provide robust and reproducible results and we will continue to assess its efficacy in larger trials.
Supplementary Material
Robot-Assisted Minimally Invasive Transplantation of Frozen-Banked Ovarian Tissue with Human Extra Cellular Tissue Matrix. This video depicts the thawing and transplantation of previously cryopreserved ovarian tissue with robotic assistance and the use of a human extracellular matrix scaffold. Author: Kutluk Oktay, MD. Videographer: Giuliano Bedoschi, MD. Participants: Kutluk Oktay, MD, Giuliano Bedoschi, MD, Volkan Turan, MD, Fernanda Pacheco, MD. Video Length: 3:49. File Size: 18.1 MB
Acknowledgments
Funding: KO is supported by RO1 HD053112 by NICHD and NCI; In addition, the laboratory work was supported by R21 HD061259 by NICHD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Paper presentation information: A preliminary version of this work was orally presented at the Annual Meeting of American Society of Reproductive Medicine in Hawaii, October 18–22, 2014.
REFERENCES
- 1.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 2.Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13:1133–1138. doi: 10.1093/humrep/13.5.1133. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342:1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 4.Oktay K, Aydin BA, Karlikaya G. A technique for laparoscopic transplantation of frozen-banked ovarian tissue. Fertil Steril. 2001;75:1212–1216. doi: 10.1016/s0015-0282(01)01776-9. [DOI] [PubMed] [Google Scholar]
- 5.Akar M, Oktay K. Restoration of ovarian endocrine function by ovarian transplantation. Trends Endocrinol Metab. 2005;16:374–380. doi: 10.1016/j.tem.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31:1557–1564. doi: 10.1007/s10815-014-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Wallberg KA, Karlström PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, Sheikhi M, Ouvrier B, Bozóky B, Olofsson JI, Lundqvist M, Hovatta O. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94:324–328. doi: 10.1111/aogs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demeestere I, Simon P, Dedeken L, Moffa F, Tsépélidis S, Brachet C, Delbaere A, Devreker F, Ferster A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30(9):2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 10.Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- 11.Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryo-preserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 12.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6:e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140:462–471. doi: 10.1210/endo.140.1.6453. [DOI] [PubMed] [Google Scholar]
- 14.Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93:762–768. doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 16.van Eyck AS, Jordan B, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–381. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 17.David A, Van Langendonckt A, Gilliaux S, Dolmans MM, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of Kit ligand and anti-mullerian hormone in human ovarian tissue. Hum Reprod. 2012;27:1088–1095. doi: 10.1093/humrep/des013. [DOI] [PubMed] [Google Scholar]
- 18.Jansen LA, De Caigny P, Guay NA, Lineaweaver WC, Shokrollahi K. The evidence base for the acellular dermal matrix AlloDerm: a systematic review. Ann Plast Surg. 2013;70:587–594. doi: 10.1097/SAP.0b013e31827a2d23. [DOI] [PubMed] [Google Scholar]
- 19.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 20.Arrau J, Roblero L, Cury M, González R. Effect of exogenous sex steroids upon the number of germ cells and the growth of foetal ovaries grafted under the kidney capsule of adult ovariectomized hamsters. J Embryol Exp Morphol. 1983;78:33–42. [PubMed] [Google Scholar]
- 21.Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod. 2014;29:107–113. doi: 10.1093/humrep/det391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, Dechene J, Ferster A, Veys I, Fastrez M, Englert Y, Demeestere I. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29:1931–1940. doi: 10.1093/humrep/deu158. [DOI] [PubMed] [Google Scholar]
- 23.Oktay K. Fertility preservation: we are in this for a long haul. Am J Obstet Gynecol. 2013;209:77–79. doi: 10.1016/j.ajog.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 24.Greve T, Schmidt KT, Kristensen SG, Ernst E, Andersen CY. Evaluation of the ovarian reserve in women transplanted with frozen and thawed ovarian cortical tissue. Fertil Steril. 2012;97:1394–1398. doi: 10.1016/j.fertnstert.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Crandall CG, Davis SL. Cutaneous vascular and sudomotor responses in human skin grafts. J Appl Physiol (1985) 2010;109(5):1524–1530. doi: 10.1152/japplphysiol.00466.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez-Serrano M, Crespo J, Mirabet V, Cobo AC, EscribácMJ, Simón C, Pellicer A. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93:268.e11–268.e13. doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110(10):2222–2229. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- 28.Wallace WH, Smith AG, Kelsey TW, Edgar AE, Anderson RA. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 2014;15:1129–1136. doi: 10.1016/S1470-2045(14)70334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 30.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 31.Tanbo T, Greggains G, Storeng R, Busund B, Langebrekke A, Fedorcsak P. Autotransplantation of cryopreserved ovarian tissue after treatment for malignant disease - the first Norwegian results. Acta Obstet Gynecol Scand. 2015;94(9):937–941. doi: 10.1111/aogs.12700. [DOI] [PubMed] [Google Scholar]
- 32.Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015 Jul 26; doi: 10.1007/s10815-015-0544-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Robot-Assisted Minimally Invasive Transplantation of Frozen-Banked Ovarian Tissue with Human Extra Cellular Tissue Matrix. This video depicts the thawing and transplantation of previously cryopreserved ovarian tissue with robotic assistance and the use of a human extracellular matrix scaffold. Author: Kutluk Oktay, MD. Videographer: Giuliano Bedoschi, MD. Participants: Kutluk Oktay, MD, Giuliano Bedoschi, MD, Volkan Turan, MD, Fernanda Pacheco, MD. Video Length: 3:49. File Size: 18.1 MB