Introduction
In vivo metabolic spectroscopy provides the opportunity to probe mitochondrial dysfunction in vivo with the rigor previously limited to in vitro studies. In vivo studies are a critical step to understanding how mitochondria interact with the cell to contribute to tissue pathology. The translation of these new mechanistic insights to clinically relevant tools and interventions is facilitated by the ability to apply these in vivo tools to both animal models and human subjects.
Mitochondria at the center of disease
Mitochondrial dysfunction is a metabolic disorder that is pervasive in disease with profound clinical impact (total Pubmed citations as of 2014 for mitochondrial dysfunction and disease = >9,700). The integration of mitochondrial function with cell health places them at the heart of many diseases, as well as identifying them as an attractive target for interventions to improve health. Our ability to understand the mechanisms by which mitochondria contribute to pathology in conditions as diverse as diabetes, cancer, and aging and to translate basic research into the clinic will depend on our ability to study mitochondrial function in the context of the physiological environment. However, only recently have non-invasive approaches permitted study of this dysfunction in vivo.
Mitochondrial function in vivo with rigor of an in vitro assay
Traditionally, the study of mitochondrial function has only been possible in vitro in cells or isolated organelles(1). Here we describe non-invasive approaches involving cutting-edge innovations that permit measuring mitochondrial function and cell energy fluxes in vivo with the rigor previously only possible in vitro(2). This approach makes it possible to measure the key products of oxidation phosphorylation in vivo: mitochondrial O2 uptake and ATP generation, as well as the capacity of mitochondria to generate ATP (ATPmax) and the mitochondrial efficiency (ATP/O2 or P/O). In addition, these new tools permit measurement of ATP content, intracellular pH and glycolytic energy fluxes. Together these measures provide an integrated view of cell energetics that have identified roles for altered mitochondrial properties in dysfunctions associated with age and disease such as fatigue and disability, insulin resistance and oxidative stress. In this short review we focus on specific examples from aging muscle in both humans and mice to illustrate the application of these in vivo tools.
Natural indicators of cell and mitochondrial energetics in vivo
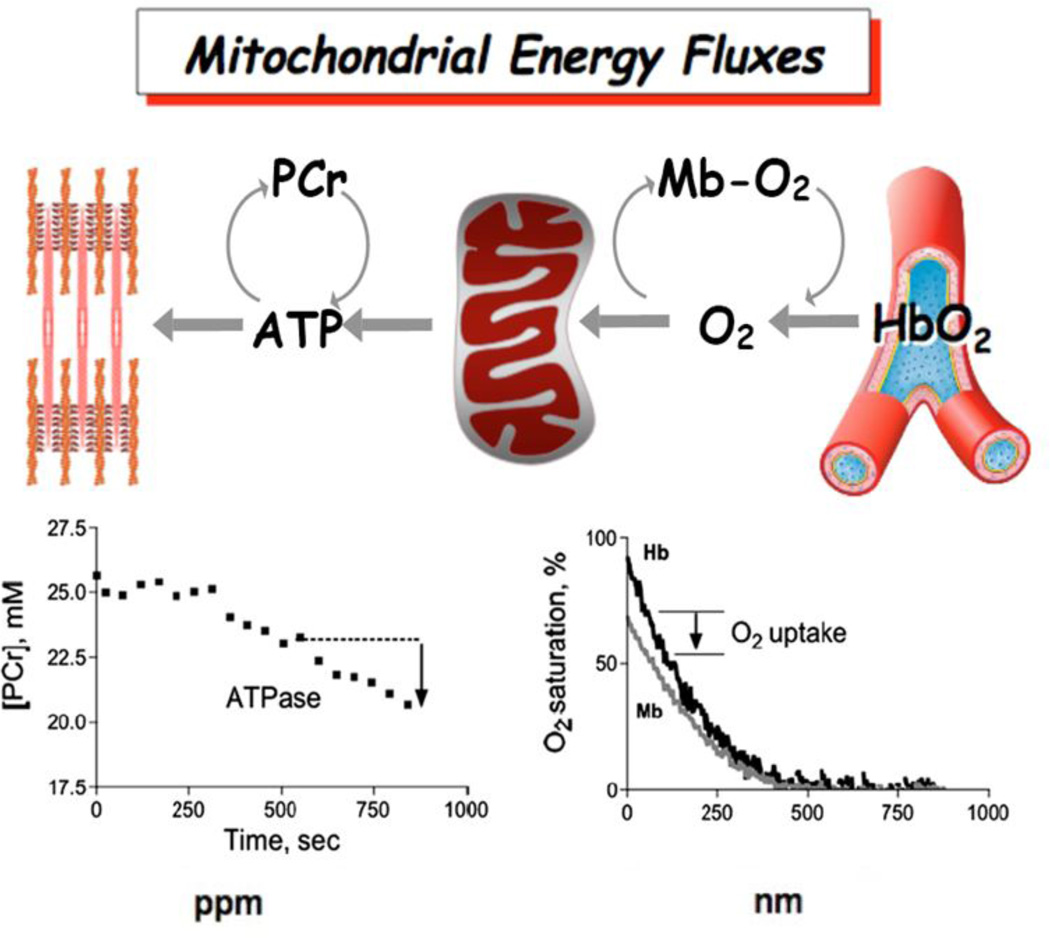
Figure 1 shows this metabolic spectroscopy approach for studying mitochondrial function in vivo(2). Highlighted are a series of metabolites that are used as natural indicators of metabolic flux in skeletal muscle by phosphorus magnetic resonance (31P MRS) and optical spectroscopy. The lower panels in this figure show how we follow changes in these metabolites during experiments designed to separate oxidation from phosphorylation to directly quantify ATP flux (left) and O2 uptake (right) in vivo. For example, a short ischemic bout is used to elicit declines in the oxygen carriers, hemoglobin (ΔHb-O2) and myoglobin (ΔMb-O2), that are used to quantify O2 uptake in resting muscle. We allow O2 to be depleted in the cell, which requires phosphocreatine (PCr) to provide the ATP used by the cell. This PCr change directly measures resting mitochondrial ATP flux. The ratio of ATP flux to O2 uptake provides the efficiency of oxidative phosphorylation (ATP produced per O2 consumed or P/O), which has been found to decline with age. Simultaneous with these flux measures is measurement of the change in intracellular pH based on the position of the inorganic phosphate (Pi) MRS peak, which is used to determine the rate of glycolytic H+ and ATP production. A second simple experiment provides the mitochondrial ATP synthesis capacity (ATPmax) based on the rate of PCr recovery after an exercise or ischemic perturbation. The end result is determination of 3 key functional properties of mitochondria in vivo: oxidative phosphorylation (O2 uptake and ATP flux), mitochondrial coupling or efficiency (ATP produced per O2 consumed or P/O) and mitochondrial capacity (ATPmax). Thus monitoring natural indicators in the cell during simple experiments in human and animal muscle provides measures of the key energetic fluxes and the efficiency of generating those fluxes as well as the capacity of mitochondria to meet energetic needs.
Figure 1. Measuring metabolic flux in vivo.
ATP and O2 fluxes (top diagram) are measured by magnetic resonance (MRS, lower left) and optical spectroscopic methods (OS, lower right). ATP fluxes are determined from changes in phosphocreatine (PCr) and inorganic phosphate (Pi)(lower left panel). O2 flux is determined from Mb-O2 (myoglobin) and Hb-O2 (hemoglobin) % saturation (lower right panel).
Mitochondrial dysfunction in humans in vivo
An early example of the insights made possible by these tools was the first demonstration of mitochondrial dysfunction with age in human muscle in vivo (see references in (3)). Both the quality and quantity of mitochondria were found to decline in elderly muscle. Reduced mitochondrial capacity was evident by a 50% decline in maximum ATP generation in the elderly and was reflected in a reduced exercise capacity. Half of the decline in energetic capacity was due to fewer mitochondria but the other half was reduced function of the mitochondria themselves. A lower ATP production per mitochondria pointed to uncoupling of oxidative phosphorylation that was directly linked to reduced exercise efficiency of the elderly. Remarkably, both age-related changes were strikingly improved by exercise training indicating that age-related changes in both mitochondrial capacity and quality are reversible(3). These early findings have since been confirmed in both elderly human and mouse muscle by new tools that permit direct measurement of mitochondrial coupling (P/O)(2). Mostly importantly, these new tools now allow us to directly test the mechanisms responsible for these dysfunctions and to evaluate the impact of interventions to reverse these deficits.
Innovative tools permit studying mechanisms of dysfunction
One example of new mechanistic tests made possible by these in vivo metabolic spectroscopy tools is the role of the redox environment on mitochondrial deficits in aging skeletal muscle. Several studies have demonstrated that the redox environment in aged tissues is more oxidized in part due to the greater H2O2 production from aged mitochondria. Both mitochondrial quality (P/O) and capacity (ATPmax) can be manipulated in aging mouse skeletal muscle by acutely modifying the mitochondrial redox environment (see references in (4)). Induction of a mild oxidative stress with paraquat treatment reproduced age-related changes to in vivo mitochondrial function. These changes occurred within 24 hours of a single low paraquat dose and returned to normal after three days. Interestingly, mitochondrial energetics in skeletal muscle from old mice was more sensitive to this mild oxidative stress. Conversely, reducing mitochondrial H2O2 production and the GSH redox couple in aged mouse skeletal muscle by treating with the mitochondrial targeted peptide SS-31 reversed age-related mitochondrial deficits(4). These improvements occurred approximately one hour after treatment and included changes in both mitochondrial quality and capacity in the aged muscles, while there was no effect on the mitochondrial energetics in young skeletal muscle. The improved energetics were accompanied by reduced muscle fatigue and one week of SS-31 treatment led to increased exercise tolerance in the old mice. The rapid reversal of in vivo energy deficits supports the dynamic nature of mitochondrial function and suggests that reversible redox control may contribute to mitochondrial deficits in aged muscle.
Natural indicators of mitochondrial (dys)function in vivo
Two new non-invasive measures reflect key players in oxidative phosphorylation and hold promise as monitors of the inner workings of mitochondria in vivo. Figure 1 shows the approach to measure what mitochondria do: consume O2 and make ATP. New metabolite markers provide insight into how this is accomplished using natural indicators of the mechanisms underlying oxidation and phosphorylation. A well-known indicator from optical studies in cells and isolated organs is nicotinamide adenine dinucleotide (NAD), which is a co-enzyme that is the substrate for oxidation, is integral to cell metabolism and signals mitochondrial biogenesis. Recent advances in MR spectroscopic measures have demonstrated that NAD redox state can be determined in vivo, thereby opening a window on the inner workings of mitochondria across the wide range of tissues accessible by MRS.
A second natural, non-invasive indicator linked to oxidative phosphorylation that is detectable in vivo by MRS is a pair of Pi peaks. Their spectral position sensitively measures pH and together they reflect “…a direct measurement of the pH inside and outside of an organelle…[i.e., mitochondria]” (5). The resulting difference in pH provides a measure of the pH gradient (ΔpH) that parallels membrane potential (ΔΨ) reflecting the proton motive force generated by oxidative phosphorylation. Advances in human MRI systems allow us to build on >30 years of animal studies that have used this 31P MRS based ΔpH measure to study mitochondrial function both in vivo and in vitro. The end result is two natural indicators, one representing the mechanisms governing oxidation (NAD redox state) and the other providing a measure of the proton motive force. Together these two natural indicators of the mechanisms underlying oxidative phosphorylation hold great potential as probes of the inner workings of mitochondria and how these inner workings change with age, disease and interventions to improve dysfunction.
Future directions and applications
The approaches and new directions described here now allow us to not only study mitochondrial changes with age and disease and their response to treatment but also the mechanisms responsible for these changes in vivo. Combining these in vivo approaches with genetic and pharmacological manipulation in animal models provides a powerful strategy for bridging the gap between novel discoveries and mechanistic insights. The ability to study human subjects with the same cutting-edge in vivo spectroscopic tools facilitates the translation of these insights into clinically relevant tools for improving human health.
Acknowledgments
Funding: The studies described in this commentary were supported over a 20 year period by NIH grants AG001751, AG042637, AG028455, RC2 036606, RC2AG036594, AR41928, AR45184 and AGAR10853.
Footnotes
Disclosure: DJM serves on the scientific advisory board for Stealth Peptides, Inc.
References cited
- 1.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LS, Conley KE. Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods. 2008;46(4):312–318. doi: 10.1016/j.ymeth.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conley KE, Jubrias SA, Cress ME, Esselman PC. Elevated energy coupling and aerobic capacity improves exercise performance in endurance-trained elderly subjects. Exp Physiol. 2013;98(4):899–907. doi: 10.1113/expphysiol.2012.069633. [DOI] [PubMed] [Google Scholar]
- 4.Marcinek DJ, Siegel MP. Targeting redox biology to reverse mitochondrial dysfunction. Aging (Albany NY) 2013;5(8):588–599. doi: 10.18632/aging.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholls DG, Ferguson SJ. Bioenergetics 3. London, UK: Academic Press; 2002. [Google Scholar]