Abstract
Exposure to ionizing radiation (IR) increases the production of reactive oxygen species (ROS) not only by the radiolysis of water but also through IR-induced perturbation of the cellular metabolism and disturbance of the balance of reduction/oxidation reactions. Our recent studies showed that the increased production of intracellular ROS induced by IR contributes to IR-induced late effects, particularly in the hematopoietic system, because inhibition of ROS production with an antioxidant after IR exposure can mitigate IR-induced long-term bone marrow (BM) injury. Metformin is a widely used drug for the treatment of type 2 diabetes. Metformin also has the ability to regulate cellular metabolism and ROS production by activating AMP-activated protein kinase. Therefore, we examined whether metformin can ameliorate IR-induced long-term BM injury in a total-body irradiation (TBI) mouse model. Our results showed that the administration of metformin significantly attenuated TBI-induced increases in ROS production and DNA damage and upregulation of NADPH oxidase 4 expression in BM hematopoietic stem cells (HSCs). These changes were associated with a significant increase in BM HSC frequency, a considerable improvement in in vitro and in vivo HSC function, and complete inhibition of upregulation of p16Ink4a in HSCs after TBI. These findings demonstrate that metformin can attenuate TBI-induced long-term BM injury at least in part by inhibiting the induction of chronic oxidative stress in HSCs and HSC senescence. Therefore, metformin has the potential to be used as a novel radioprotectant to ameliorate TBI-induced long-term BM injury.
Keywords: Ionizing radiation, Oxidative stress, Hematopoietic stem cells, Radioprotection, Metformin, Free radicals
Bone marrow (BM) suppression is the most common side effect of conventional cancer therapy using ionizing radiation (IR) and chemotherapeutic agents and is the primary cause of death after accidental or intentional exposure to high doses of total-body irradiation (TBI) [1–4]. Acute myelosuppression is the result of the induction of apoptosis in the rapidly proliferating hematopoietic progenitor cells (HPCs) and to a lesser degree in the relatively quiescent hematopoietic stem cells (HSCs) after exposure to IR and chemotherapy [2,3]. Acute BM suppression can cause high mortality and morbidity but has been better managed by the use of various hematopoietic growth factors (HGFs), which have the ability to promote the recovery of BM hematopoietic function primarily by stimulating HSC and HPC proliferation and differentiation [4,5]. Although many patients recover rapidly from acute myelosuppression after IR and/or chemotherapy, a large percentage of the patients will develop residual BM injury manifested by a decrease in HSC reserves and impairment in HSC self-renewal [1,2]. Unlike acute myelosuppression, residual BM damage is latent. Patients and animals with residual BM injury usually have normal blood cell counts under normal homeostatic conditions despite a decrease in HSC reserves [4,5]. Because of this latency, the clinical implications of residual BM injury have been largely overlooked. Moreover, the importance of long-term BM damage is further obscured by the seemingly complete recovery of peripheral blood cell counts and BM cellularity, especially after the use of HGFs. In fact, the use of HGFs may worsen chemotherapy- and IR-induced residual BM damage by promoting HSC and HPC proliferation and differentiation at the expense of HSC self-renewal [6–8]. This could lead to an accelerated exhaustion of HSCs and further compromise the long-term recovery of BM hematopoietic function. Although residual BM damage is latent, it is long-lasting and shows a slight tendency for recovery [1]. Therefore, an effective treatment that can prevent and/or mitigate irradiation-induced long-term BM suppression is needed.
Evidence has emerged that IR induces residual BM injury primarily via the induction of HSC senescence [4,5], which is an irreversible loss of the proliferation capacity of HSCs. Our recent studies have demonstrated that exposure of mice to a sublethal dose of TBI induces persistent oxidative stress in HSCs in part via the upregulation of NADPH oxidase 4 (NOX4) [9]. Oxidative stress can induce HSC senescence by causing sustained DNA damage and/or inducing p16Ink4a (p16) expression through the p38 mitogen-activated protein kinase pathway [10–14]. Several recent studies have shown that the induction of oxidative stress is primarily responsible for the loss of HSC self-renewal and premature exhaustion of HSCs in mice with mutation of ataxia telangiectasia [11,15] and deletion of FoxO3(s) [12–14]. Furthermore, prolonged treatment of these mice with an antioxidant can rescue the defect [11–15].
Metformin (1,1-dimethylbiguanide hydrochloride), a biguanide derivate, is the most widely prescribed drug for the treatment of hyperglycemia in individuals with type 2 diabetes. It also has the ability to regulate cellular metabolism and reactive oxygen species (ROS) production by the activation of AMP-activated protein kinase (AMPK) [16–18]. Apart from its well-known antidiabetic effect, metformin also has antiaging [19–21] and anticancer activities [22–26]. Emerging evidence from epidemiological studies suggests that diabetic patients treated with metformin have a lower risk of cancer incidence and mortality in a broad range of neoplasms [27,28]. Its antioxidant effect may be one of the important molecular mechanisms of action of metformin because metformin can directly scavenge ROS or act indirectly to modulate intracellular production of superoxide anion, of which NOXs constitute a major source [29]. Additionally, metformin can increase the expression of the antioxidant thioredoxin, which mediates metformin’s effects on ROS reduction. Metformin increases thioredoxin expression through activation of the AMPK pathway [30]. Moreover, metformin can protect tissues or cells against DNA damage and mutations by inhibiting the formation of ROS [31–37]. In recent years, studies have found that metformin possesses radioprotective properties against radiation-induced tissue or cell damages in cochlear and splenic cells [38,39]. Because of the remarkable therapeutic potential of metformin, we examined its effects on TBI-induced long-term BM suppression in our well-established and characterized mouse model. The data presented in this study demonstrate that metformin treatment significantly inhibited the TBI-induced increases in the levels of ROS, DNA double-strand breaks (DSBs), and NOX4 expression in HSCs. The reduction in oxidative stress was associated with significant increases in HSC frequency and clonogenic function. These findings suggest that metformin treatment may inhibit IR-induced HSC senescence. This suggestion is supported by the finding that metformin treatment reduced the IR-induced expression of p16 mRNA in HSCs and improved the long-term and multilineage engraftment of irradiated HSCs after transplantation. Therefore, the results from this study demonstrated that metformin has the potential to be used as a novel radioprotectant to ameliorate TBI-induced residual BM injury.
Materials and methods
Reagents
Anti-mouse CD117 (c-kit)-APC (clone 2B8), anti-mouse Ly-6 A/EA (Sca-1)-PE/Cy7 (clone D7), biotin-conjugated anti-mouse CD5 (clone 53-7.3), biotin-conjugated anti-mouse CD4 (clone GK1.5), anti-mouse CD8 (clone 53-6.7), anti-mouse CD45R/B220 (clone RA3-6B2), anti-mouse Ly6G/Gr-1 (clone RB6-8C5), anti-mouse CD11b (clone M1/70), anti-mouse Ter-119 (clone Ter-119), and APC-Cy7-conjugated streptavidin were obtained from eBioscience (San Diego, CA, USA). Anti-mouse CD45.1-FITC (clone A20, Ly5.1), anti-mouse CD45.2-PE (clone104, Ly5.2), anti-mouse Ly6G/Gr-1-PE/Cy7 (cloneRB6-8C5), anti-mouse CD45R/B220-PerCP (cloneRA3-6B2), anti-mouse CD11b-PE/Cy7 (cloneM1/70), anti-mouse CD3-APC (clone145-2C11), and streptavidin-PerCP (405213) were obtained from Biolegend (San Diego, CA, USA). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFDA) and dichlorofluorescein (DCF) were obtained from Sigma (St. Louis, MO, USA). Dihydroethidium (DHE) was obtained from Vigorous (Beijing, China). MitoSOX red mitochondrial superoxide indicator was obtained from Life Technologies (Grand Island, NY, USA). Rabbit anti-γH2AX was obtained from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-NOX4 was obtained from Proteintech (Wuhan, China). FITC-conjugated goat anti-rabbit antibodies were obtained from Abcam Biotechnology (Cambridge, MA, USA). Cytofix/Cytoperm buffer (554722), Perm/Wash buffer (554723), and Cytoperm Permeabilization Buffer Plus (561651) were obtained from BD Pharmingen (San Diego, CA, USA). Metformin was obtained from Beyotime Institute of Biotechnology (CAS No. 1115-70-4; Shanghai, China).
Mice
Male C57BL/6-Ly-5.1 (Ly5.1) and C57BL/6-Ly-5.2 (Ly5.2) mice were purchased from Vital River (Beijing, China) and housed in the certified animal facility at the Institute of Radiation Medicine of the Chinese Academy of Medical Sciences (CAMS). C57BL/6-Ly-5.1/5.2 (Ly5.1/5.2) mice were bred at the certified animal care facility at the Institute of Radiation Medicine of CAMC. All of the mice were used at approximately 8–12 weeks of age. All of the experimental procedures were performed with the approval of the Animal Use Committee at the Institute of Radiation Medicine of CAMS (No. 1202).
TBI and metformin administration
Ly5.2 mice were divided randomly into three groups: (a) control, (b) vehicle + TBI, and (c) metformin + TBI. The mice in the metformin + TBI group were administered a dose of 250 mg/kg/day metformin by gavage 1 day before irradiation and 7 days after irradiation. As the control, the mice in the vehicle + TBI group received the same volume of distilled water at the same frequency and duration as the mice in the metformin + TBI group. The mice in the vehicle + TBI and metformin + TBI groups were exposed to 4.0- Gy TBI in an Exposure Instrument Gammacell-40 137Cs irradiator (Atomic Energy of Canada Ltd, Ottawa, ON, Canada) at a dose rate of 0.78 Gy/min. The control mice were sham-irradiated.
Isolation of BM mononuclear cells (BM-MNCs) and HSCs
BM-MNCs were isolated from BMNCs as described previously [4,5]. They were incubated with biotin-conjugated antibodies specific for murine CD5, CD11b, CD45R/B220, Ter-119, and Gr-1 and then stained with streptavidin-APC-Cy7, anti-Sca-1-PE/Cy7, and anti-c-kit-APC. HSCs (Lin−c-kit+ Sca-1+) were analyzed and sorted using a BD Aria FACSII cell sorter (BD Bioscience, San Jose, CA, USA).
Cobblestone area-forming cell (CAFC) assay
Competitive repopulation assay
Competitive repopulation assays were performed using the Ly5 congenic mice as described previously [9,40]. Specifically, donor BM cells (BMCs) were harvested from C57BL/6-Ly-5.2 mice after the various treatments. The cells (1 × 106 BMCs) were mixed with 2 × 105 competitive BMCs pooled from six Ly5.1 mice. The mixed cells were transplanted into lethally irradiated (9.0- Gy TBI) C57BL/6-Ly-5.1/5.2 hybrid recipient mice (nine mice/group) by lateral canthus vein injection. For analysis of engraftment, peripheral blood was obtained from the medial canthus using heparin-coated micropipettes (Drummond Scientific, Broomall, PA, USA) from all of the recipients 4 months after transplantation. After the red blood cells were lysed with 0.15 mol/L NH4Cl solution, the blood samples were stained with FITC-conjugated anti-CD45.1, PE-conjugated anti-CD45.2, PerCP-conjugated anti-B220, APC-conjugated anti-CD3, and PE/Cy7-conjugated anti-Gr-1 and CD11b and were analyzed using an LSR II flow cytometer (BD Bioscience).
Analysis of the levels of intracellular ROS
BM-MNCs were isolated from BMNCs as described previously [4,5]. They were incubated with biotin-conjugated antibodies specific for murine CD5, CD11b, CD45R/B220, Ter-119, and Gr-1 and then stained with streptavidin-APC-Cy7, anti-Sca-1-PE/Cy7, and anti-c-kit-APC. Then the cells were incubated with DCF (10 µM), DCFDA (10 µM), DHE (10 µM), and MitoSOX (5.0 µM), respectively, for 20 min at 37 °C. The levels of intracellular ROS in HSCs were analyzed by measuring the mean fluorescence intensity (MFI) of DCF, ethidium, and oxidized MitoSOX using a flow cytometer as described previously. The specificity of this assay to detect intracellular ROS in HSCs was validated in our recently reported studies [9,40]. For each sample, a minimum of 100,000 Lin− cells were acquired, and the data were analyzed using the FlowJo 7.6.1 software (Tree Star, Ashland, OR, USA). In all of the experiments, PE/Cy7 and APC isotype controls and other positive and negative controls were included as appropriate.
Analysis of γH2AX staining and NOX4 expression in HSCs
BM-MNCs were isolated from BMNCs as described previously [4,5]. They were incubated with biotin-conjugated antibodies specific for murine CD4, CD8, CD11b, CD45R/B220, Ter-119, and Gr-1 and then stained with streptavidin-PerCP, anti-Sca-1-PE/Cy7, and anti-c-kit-APC. The cells were fixed and permeabilized by BD Cytofix/Cytoperm buffer according to the manufacturer’s protocol and then stained with antibodies against γH2AX or NOX4 and FITC-conjugated second antibodies. γH2AX and NOX4 expression in HSCs was determined by analysis of the MFI of γH2AX and NOX4 staining in HSCs by flow cytometry.
Quantitative real-time PCR assays
Total RNA from 5000 sorted HSCs was extracted using the TRIzol reagent (Life Technologies) following the manufacturer’s protocol. The expression of p16, NOX4, superoxide dismutase 1 (SOD1), SOD2, catalase (CAT), and glutathione peroxidase 1 (GPX1) mRNA was determined by real-time RT-PCR as previously reported [41].
Apoptosis assay
The apoptosis assay was performed as we previously reported [3,5].
Analysis of enzymatic activity of SOD, CAT, and GPX1
SOD, CAT, and GPX1 enzymatic activities in BM-MNCs were analyzed using SOD, CAT, and GPX1 assay kits (Beyotime Institute of Biotechnology), respectively. The assays were done by following the manufacturer’s instructions.
Statistical analysis
The data were analyzed by analysis of variance. Differences were considered significant at p < 0.05. All of these analyses were performed using GraphPad Prism from GraphPad Software (San Diego, CA, USA).
Results
Metformin inhibits TBI-induced chronic oxidative stress in HSCs
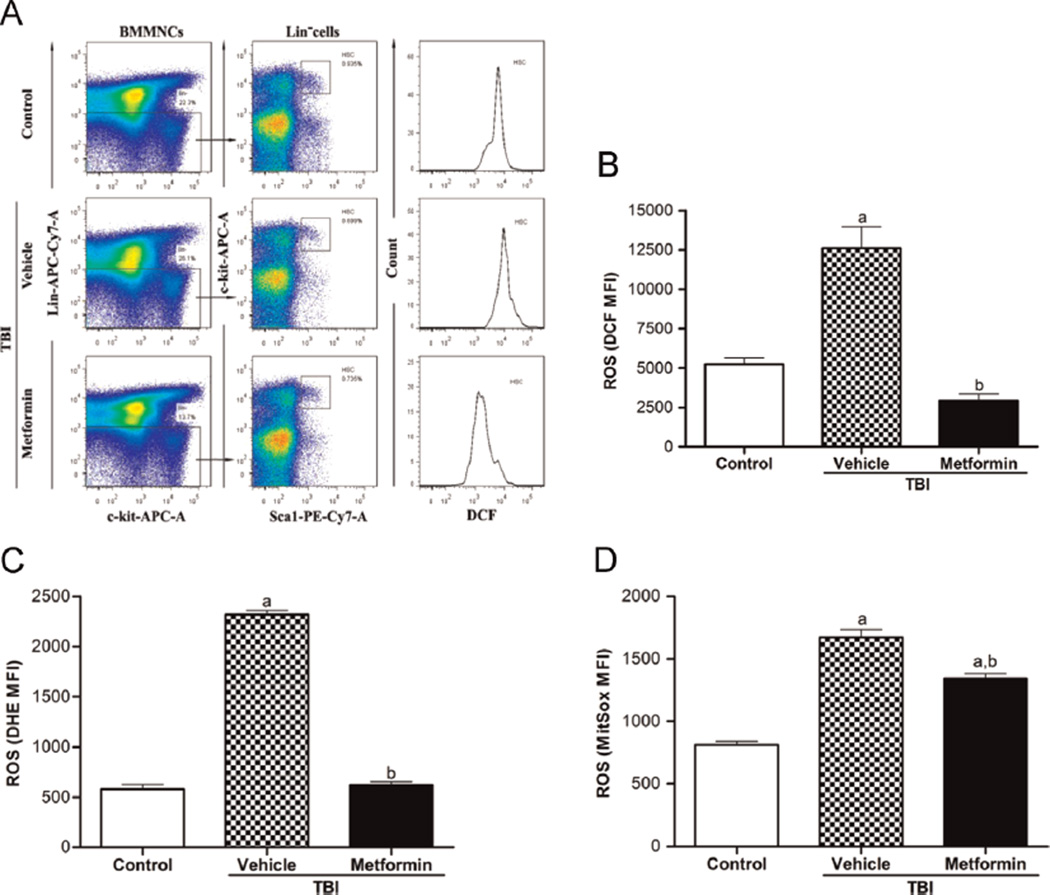
In our previous studies, we have demonstrated that the exposure of mice to a sublethal dose of TBI causes long-term BM suppression in part by induction of chronic oxidative stress and HSC senescence [9,40,41]. Metformin has the ability to reduce oxidative tissue damage via several different mechanisms in various pathological conditions [29]. Therefore, we examined whether metformin can ameliorate TBI-induced long-term BM suppression via inhibition of ROS production. ROS production in HSCs 4 weeks after TBI was analyzed initially by flow cytometry after DCFDA staining. As shown in Figs. 1A and 1B, the production of ROS in HSCs was significantly elevated after TBI according to the analysis of DCF MFI after the cells were incubated with DCFDA. The increases in DCF MFI in irradiated HSCs are unlikely to be attributable to the changes in the probe ester cleavage, uptake, or efflux, because HSCs from both control and irradiated mice showed similar DCF MFI after they were incubated with DCF (data not shown). Furthermore, analysis of ROS production by DHE and MitoSOX staining confirmed that HSCs from irradiated mice produced increased levels of ROS compared to HSCs from control unirradiated mice (Figs. 1C and 1D). In addition, our previous studies showed that the increase in ROS production in irradiated HSCs could be abrogated by preincubation of the cells with N-acetylcysteine or treatment with MnTE-2-PyP [9]. Collectively, these findings demonstrate that TBI can cause persistent oxidative stress in HSCs. Metformin treatment markedly attenuated the elevation of ROS production detected by DCFDA, DHE, and MitoSOX, demonstrating that metformin can effectively inhibit TBI-induced chronic oxidative stress in HSCs. However, the effect of metformin on ROS levels in irradiated HSCs was more pronounced when ROS were analyzed by DCFDA and DHE than when detected by MitoSOX, suggesting that the increased production of ROS in irradiated HSCs might not be derived only from mitochondria but also has a nonmitochondrial origin.
Fig. 1.
Metformin inhibits the TBI-induced ROS increases in HSCs. The mice were treated with vehicle or metformin (250 mg/kg) by gavage 1 day before exposure to 4.0- Gy TBI and then continuously for 7 days after TBI. A group of sham-irradiated mice was included as a control. The mice were euthanized 4 weeks after exposure to TBI to harvest BM-MNCs. The ROS levels in HSCs were analyzed and presented as the means ± SE from three independent experiments. ap < 0.05 vs control; bp < 0.05 vs vehicle + TBI. (A) Representative analysis of ROS levels in HSCs by flow cytometry. (B) The levels of intracellular ROS in HSCs detected by the DCF MFI. (C) The levels of intracellular ROS in HSCs detected by the DHE MFI. (D) The levels of intracellular ROS in mitochondria detected by the MitoSOX MFI.
Metformin inhibits TBI-induced increases in DNA DSBs in HSCs
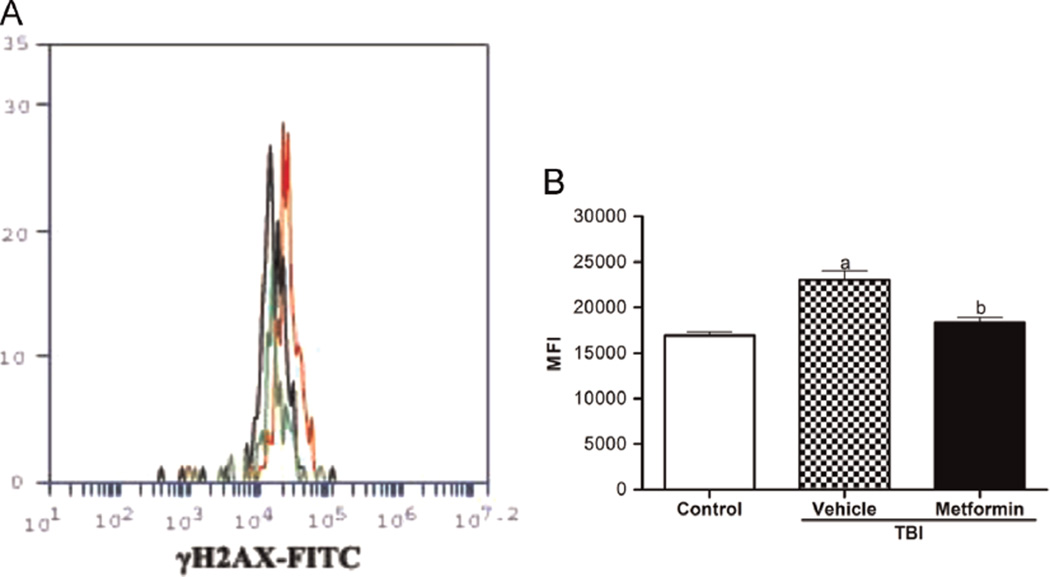
Because metformin can inhibit TBI-induced ROS production in HSCs, next we examined whether metformin could reduce TBI-induced persistent DNA damage in HSCs. This was accomplished by flow cytometric analysis of γH2AX staining in HSCs, because γH2AX staining has been widely used as a surrogate for DNA DSBs. As shown in Fig. 2, γH2AX staining in irradiated HSCs was significantly increased even 4 weeks after TBI, confirming our previous finding that exposure of mice to TBI induces persistent DNA damage in HSCs [9]. Treatment with metformin significantly reduced the increase in γH2AX staining in irradiated HSCs, indicating that metformin treatment can inhibit TBI-induced DSBs in HSCs.
Fig. 2.
Metformin reduces the TBI-induced DSBs in HSCs. The mice were sham-irradiated as a control or irradiated and then treated with vehicle or metformin as described in the text. BM-MNCs were isolated from the mice 4 weeks after TBI and then immunostained with antibodies against γH2AX to detect DSBs in HSCs. (A) Representative analysis of γH2AX expression in HSCs by flow cytometry. Black line depicts profiles of BM cells from control mice, red line depicts profiles of BM cells from vehicle + TBI mice, and green line depicts profiles of BM cells from metformin + TBI mice. (B) The MFI ± SE is shown. ap < 0.05 vs control; bp < 0.001 vs vehicle + TBI.
Metformin attenuates TBI-induced residual BM injury in part via inhibition of HSC senescence but not apoptosis
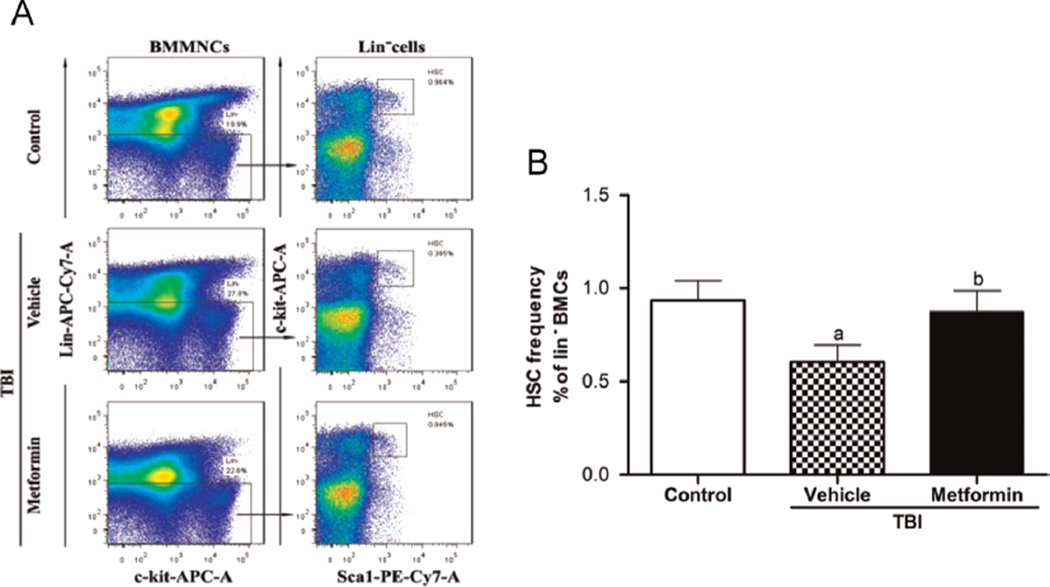
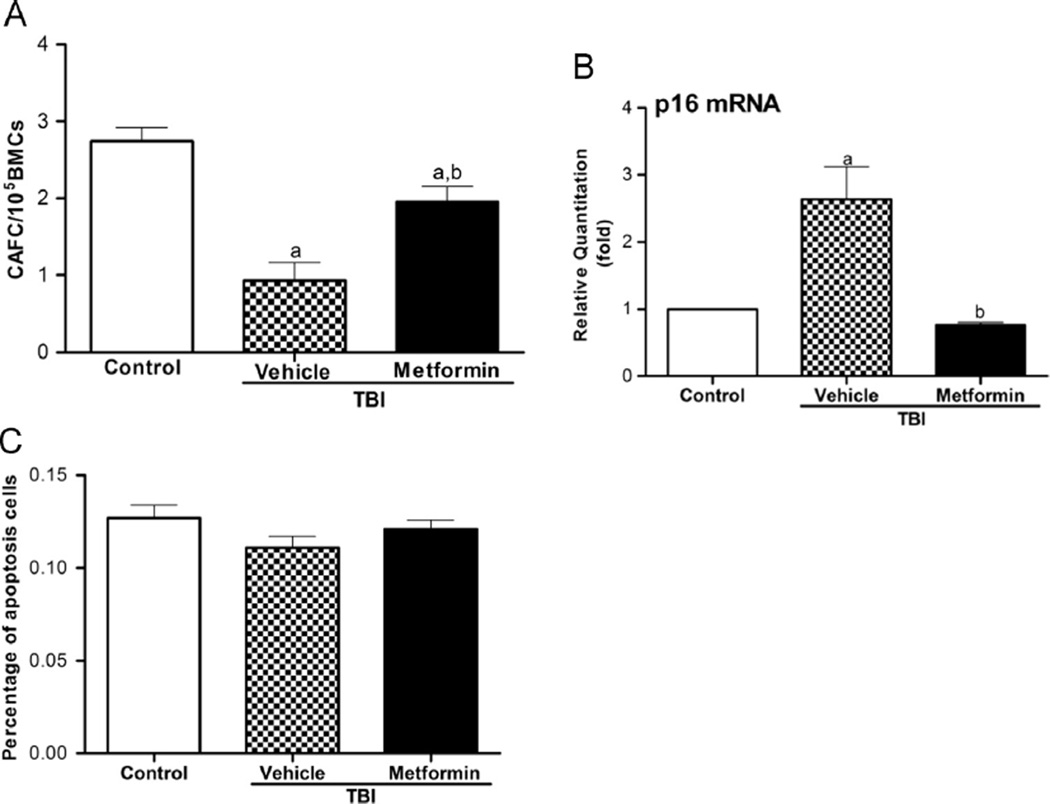
Our recent studies have shown that the exposure of mice to TBI induces persistent oxidative stress and DNA damage in HSCs, which leads to the induction of HSC senescence and long-term BM suppression [9,40,41]. These findings were confirmed in this study because the irradiated mice receiving vehicle treatment exhibited a substantial decrease in the frequency of HSCs (Fig. 3) and a significant reduction in 4-week CAFCs, which provides the measurement of HSC clonogenic function (Fig. 4A). The long-term BM suppression induced by TBI is probably attributable to the induction of HSC senescence as shown in our previous studies [4,5,9]. This suggestion is supported by the findings that HSCs from vehicle- treated TBI mice expressed elevated levels of p16 mRNA (Fig. 4B), a widely used senescence biomarker and an important mediator of cellular senescence induction [47,48]. However, after treatment with metformin, the irradiated mice showed a significant recovery in the frequency of HSCs (Fig. 3) and remarkable improvement in HSC clonogenic function (Fig. 4A), suggesting that metformin treatment inhibited the IR-induced HSC senescence. This suggestion is supported by the finding that metformin treatment also reduced the IR-induced expression of p16 mRNA in HSCs (Fig. 4B). In addition, no significant increase in apoptosis was detected in HSCs from mice that were exposed to TBI 4 weeks ago compared to HSCs from unirradiated control mice (Fig. 4C). These findings indicate that treatment with metformin can ameliorate TBI-induced residual BM injury at least in part via the inhibition of HSC senescence but not apoptosis.
Fig. 3.
Metformin attenuates the TBI-induced HSC reduction. The mice were sham-irradiated as a control or irradiated and then treated with vehicle or metformin as described in the text. The frequency of HSCs in Lin− BMCs was analyzed by flow cytometry after the mice were euthanized 4 weeks after exposure to TBI. (A) Representative results of the HSC flow cytometric analysis. (B) The frequencies of HSCs in Lin− BMCs are presented as the means ± SE of three independent experiments. ap < 0.05 vs control; bp < 0.05 vs vehicle + TBI.
Fig. 4.
Metformin mitigates TBI-induced residual BM injury in part by inhibition of HSC senescence. The mice were sham-irradiated as a control or irradiated and then treated with vehicle or metformin as described in the text. (A) The clonogenic function of HSCs in BMCs was measured by CAFC assay. The data are presented as the means ± SE of the frequency of CAFCs per 105 BMCs from three independent assays. ap < 0.05 vs control; bp < 0.05 vs vehicle + TBI. (B) The levels of p16 mRNA expression in sorted HSCs were analyzed by qRT-PCR and are expressed as the means ± SE of fold changes compared to the control (n = 3). ap < 0.01 vs control; bp < 0.01 vs vehicle + TBI. (C) HSC apoptosis was measured after the mice were euthanized 4 weeks after exposure to TBI. The percentage of apoptotic cells in the irradiated group was relatively unchanged compared to the unirradiated group.
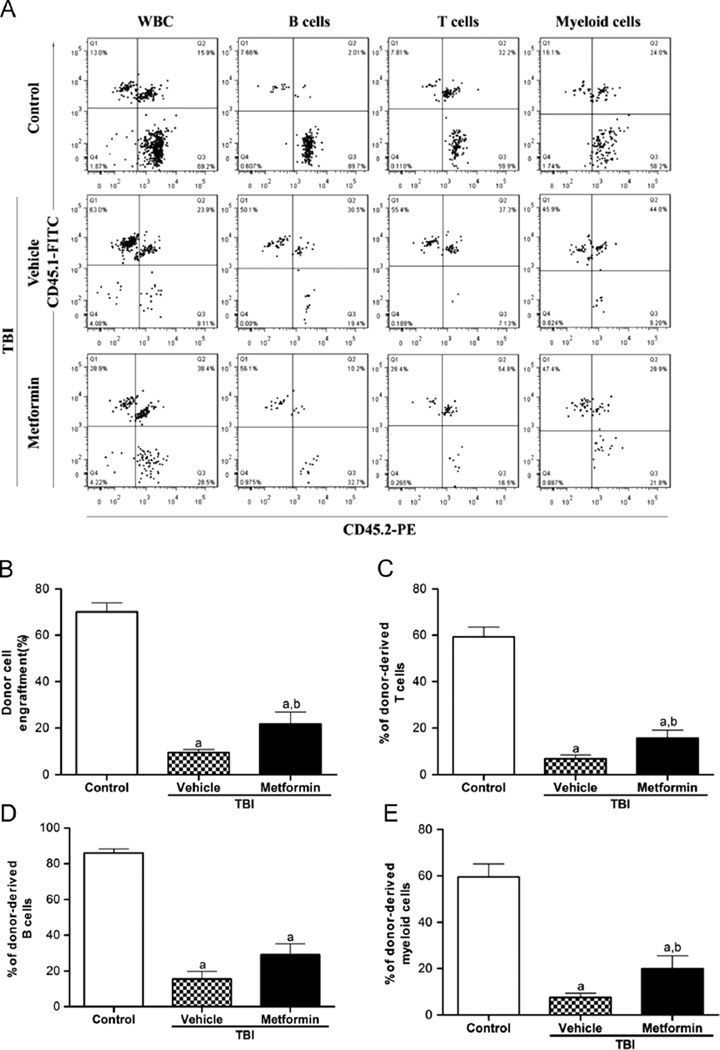
Metformin enhances long-term and multilineage engraftment of irradiated HSCs after BM transplantation
Because long-term and multilineage engraftment is the only gold standard for measuring HSC function [49], we performed a competitive repopulation assay to validate whether the inhibition of TBI-induced HSC senescence by metformin treatment can improve HSC function. The mice that received donor cells from unirradiated mice exhibited approximately 70% of the donor cell engraftment 4 months after transplantation (Fig. 5B), and 59.4% of their T cells, 85.9% of their B cells, and 59.5% of their myeloid cells were derived from the donor cells (Figs. 5C–5E). As shown in Figs. 5B–5E, the mice that received donor cells from irradiated mice with vehicle treatment showed a substantial decrease in donor cell engraftment in all of the lineages. This decrease was significantly reduced when the irradiated donor mice were treated with metformin (Figs. 5B–5E). These findings suggest that metformin treatment can preserve the function of HSCs after TBI, resulting in an enhanced long-term and multilineage engraftment after BM transplantation.
Fig. 5.
Metformin enhances long-term and multilineage engraftment of irradiated HSCs after BM transplantation. Donor cell engraftment was determined at 4 months in lethally irradiated recipients after transplantation of BM-MNCs from the control or TBI mice treated with vehicle or metformin as described in the text through a competitive repopulating assay. (A) Representative results of donor cell engraftment. The percentages of donor-derived (B) peripheral blood leukocytes (CD45.2+ cells), (C) T cells (CD45.2+ CD3+ cells), (D) B cells (CD45.2+ B220+ cells), and (E) myeloid cells (CD45.2+ CD11b+ and/or Gr-1+ cells granulocytes–monocytes–macrophages) are presented as the means ± SE (nine mice/group). ap < 0.01 vs control; bp < 0.05 vs vehicle + TBI.
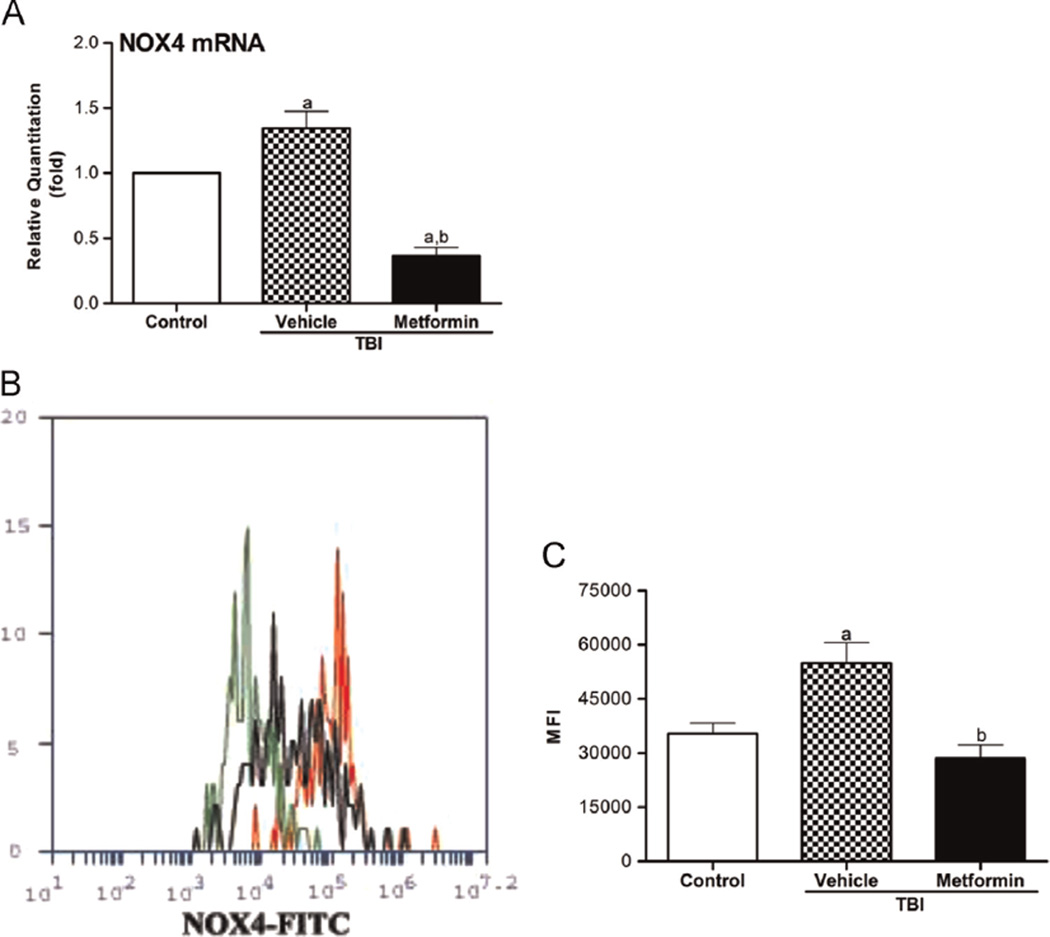
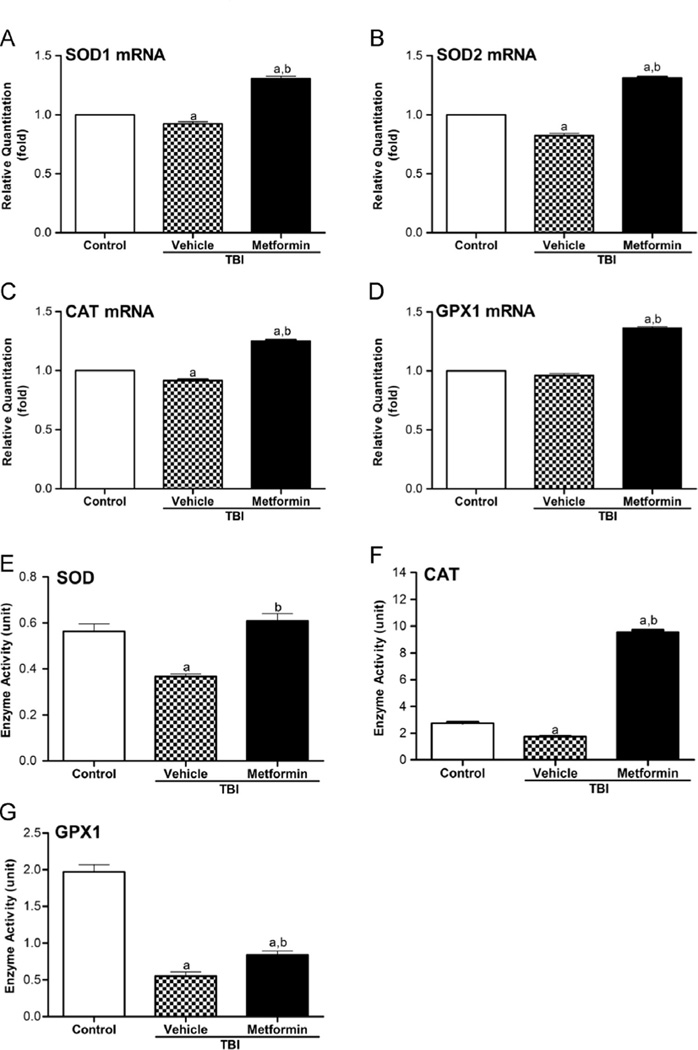
Metformin modulates the expression of NOX4 and antioxidant enzymes perturbed by TBI in HSCs
ROS are by-products of mitochondrial respiration, and thus, mitochondria have been frequently considered to be the main source of cellular-derived ROS, particularly after exposure to IR [42–44]. However, an increasing body of evidence demonstrates that cells can also produce ROS through the activation and/or induction of NOXs [45,46]. Our previous studies showed that the TBI-induced production of ROS in HSCs is probably attributable to the upregulation of NOX4 [9]. However, the effects of TBI on the expression of antioxidant enzymes in HSCs have not been studied. SOD, CAT, and GPX1 are three major cellular antioxidant enzymes. SOD converts superoxide radical into hydrogen peroxide (H2O2), whereas GPX1 along with CAT converts H2O2 into water. To elucidate the mechanisms of action through which metformin inhibits IR-induced chronic oxidative stress in HSCs after TBI, we measured the expression of NOX4, SOD1, SOD2, CAT, and GPX1 in HSCs from irradiated mice treated with vehicle or metformin. As shown in Figs. 6 and 7A–D, TBI upregulated the expression of NOX4 in HSCs but downregulated the expression of SOD1, SOD2, CAT, and GPX1 mRNA in HSCs to variable degrees compared with their respective controls. Metformin treatment not only reversed the effects of IR on the expression of NOX4, SOD1, SOD2, CAT, and GPX1 in HSCs but also significantly inhibited the expression of NOX4 in HSCs to a level that was even lower than that observed in the control nonirradiated HSCs while increasing the expression of SOD1, SOD2, CAT, and GPX1 mRNA (Figs. 6 and 7A–D). The modulation of antioxidant enzyme expression by TBI and metformin in BM hematopoietic cells was also confirmed by SOD, CAT, and GPX1 enzymatic assays shown in Fig. 7E–G. These results indicate that TBI may increase ROS production in HSCs not only by upregulation of NOX4 but also by downregulation of antioxidant enzymes, whereas metformin inhibits TBI-induced chronic oxidative stress in HSCs at least in part via downregulation of NOX4 expression and upregulation of SOD, CAT, and GPX1 expression.
Fig. 6.
Metformin inhibits the TBI-induced NOX4 expression in HSCs. The mice were sham-irradiated as a control or irradiated and then treated with vehicle or metformin as described in the text. (A) The levels of NOX4 mRNA expression in sorted HSCs were analyzed by qRT-PCR and are expressed as the means ± SE of fold changes compared to the control (n = 3). ap < 0.05 vs control; bp < 0.001 vs vehicle + TBI. (B) Representative analysis of NOX4 expression in HSCs by flow cytometry. Black line depicts profiles of BM cells from control mice, red line depicts profiles of BM cells from vehicle + TBI mice, and green line depicts profiles of BM cells from metformin + TBI mice. (C) BM-MNCs were isolated from the mice 4 weeks after TBI and then immunostained with antibodies against NOX4 to detect MFI ± SE in HSCs by flow cytometry. ap < 0.05 vs control; bp < 0.001 vs vehicle + TBI.
Fig. 7.
Metformin increases the expression of SOD1, SOD2, CAT, and GPX1 mRNA in HSCs and the enzyme activity of SOD, CAT, and GPX1 in BM cells. The mice were sham-irradiated as a control or irradiated and then treated with vehicle or metformin as described in the text. The mice were euthanized 4 weeks after exposure to TBI to harvest BM-MNCs. HSCs were isolated from these cells by cell sorting and analyzed for the expression of SOD1, SOD2, CAT, and GPX1 mRNA by qRT-PCR. Enzyme activity of SOD, CAT, and GPX1 in BM-MNCs was analyzed using a SOD assay kit, a CAT assay kit, and a cellular GPX1 assay kit, respectively. (A–D) Expression of SOD1, SOD2, CAT, and GPX1 mRNA in HSCs. (E–G) Enzyme activity of SOD, CAT, and GPX1 in BM-MNCs. The levels of SOD1, SOD2, CAT, and GPX1 mRNA expression are expressed as the means ± SE of fold changes compared to the control (n = 3). ap < 0.05 vs control; bp < 0.05 vs vehicle + TBI.
Discussion
Recent clinical trials suggest that metformin, in addition to its efficacy in treating type 2 diabetes, may also have therapeutic potential in other conditions, including diabetic nephropathy, cardiovascular diseases, and polycystic ovary disease, and for prevention or treatment of cancer [26,50,51]. However, the therapeutic potential of metformin as a radiation protectant against IR-induced long-term BM injury has not been investigated.
Our previous studies showed that exposure of mice to a sublethal dose (6.0 or 6.5 Gy) of TBI can induce HSC senescence and long-term BM suppression [9,40,41]. However, whether exposure of mice to a lower dose (such as 4.0 Gy) of TBI can also induce HSC injury has not been examined. The results from the present study showed that exposure of mice to 4.0- Gy TBI also caused chronic BM injury primarily by the induction of chronic oxidative stress in HSCs, which led to persistent increases in DNA damage in HSCs and induction of HSC senescence but not apoptosis. In addition, we found that exposure to 4.0- Gy TBI upregulates NOX4 expression and increases mitochondrial ROS production in HSCs, suggesting that increased production of ROS in irradiated HSCs may be attributable to NOX4 and mitochondria. It has been shown that metformin treatment can not only directly act on mitochondria to inhibit respiration and ROS production [52] but also decrease the production of ROS in podocytes and aortic endothelial cells through reduction in NOX activity [53–55]. Our recent studies also showed that resveratrol can ameliorate IR-induced long-term HSC injury by decreasing ROS production in association with downregulation of NOX4 expression [41]. Therefore, we examined whether metformin can inhibit IR-induced long-term BM injury in part via downregulation of NOX4 and inhibition of mitochondrial ROS production and senescence in HSCs. As shown in our study, we found that treatment with metformin effectively inhibited TBI-induced NOX4 expression and mitochondrial ROS production in HSCs, which led to a significant improvement in HSC clonogenic function and long-term repopulating activity after transplantation. Furthermore, we measured the expression of SOD1, SOD2, CAT, and GPX1 mRNA in HSCs and the enzymatic activity of SOD, CAT, and GPX1 in BM cells and found that TBI reduced their expression and activity in hematopoietic cells. The reduction was attenuated by metformin treatment. This finding suggests that metformin may also inhibit TBI-induced chronic oxidative stress in HSCs in part by modulating the expression of antioxidant enzymes.
Previous studies have found that oxidative stress can lead to DNA damage such as DSBs [11,15]. DSBs can initiate DNA damage response via sequential activation of ATM, Chk2, and p53 [56]. Activation of p53 induces p53 downstream targets, including the cell cycle inhibitor p21Cip1/Waf1, to induce cell cycle arrest. It has been suggested that the induction of DNA damage is also likely to be responsible for the induction of hematopoietic genetic instability by IR [57,58]. The induction of hematopoietic genetic instability can lead to the development of leukemia in the victims of nuclear events and cancer patients after radiation therapy. Previous studies have shown that metformin is a nongenotoxic and noncytotoxic compound and may protect against genomic instability induced by hyperglycemia [59]. In this study, we found that metformin could reduce the increases in γH2AX expression in HSCs after TBI, which confirmed the effects of metformin on DNA damage.
However, the mechanism by which metformin regulates NOX4 and SOD1, SOD2, CAT, and GPX1 expression has yet to be elucidated. It has been well established that activation of AMPK is closely related to the diversity function of metformin [16–18]. Activated AMPK switches cells from an anabolic to a catabolic state, shutting down the ATP-consuming synthetic pathways and restoring energy balance. This regulation involves the phosphorylation by AMPK of key metabolic enzymes and transcription factors that regulate gene expression [60]. As a result, glucose, lipid, and protein synthesis are inhibited, whereas fatty acid oxidation and glucose uptake are stimulated. In addition, a growing body of evidence from clinical studies and animal models suggests that the primary function of metformin is to decrease hepatic glucose production [61], mainly by inhibiting gluconeogenesis [62,63]. Several mechanisms have been proposed to explain this inhibitory action on hepatic gluconeogenesis, including changes in enzyme activities [64–66] and a reduction in the hepatic uptake of gluconeogenic substrates [67]. It remains to be determined whether the protection of metformin against IR-induced BM damage is related to activation of AMPK and modulation of these metabolic pathways.
Our studies indicate that metformin can be used as an efficacious medical radiation countermeasure, particularly because metformin is a safe drug that has been used extensively in the clinic. The dose of metformin used in our study is safely achievable in humans because the dose of 250 mg/kg/day of metformin in mice is equivalent to 25 mg/kg/day in humans and a clinical study has shown that metformin does not cause any toxicity or side effects in humans administered 1.0 to 2.5 g of metformin every day [68]. An increasing body of evidence shows that metformin is potent in cancer prevention and treatment, including breast cancer, prostate cancer, endometrial cancer, nasopharyngeal carcinoma, and esophageal squamous cell carcinoma [23–26,69]. Moreover, metformin can function as a radiosensitizer to increase the radiosensitivity of cancer cells and significantly enhance the therapeutic efficacy of radiation therapy for cancer [70–74]. Therefore, metformin not only has a radioprotection effect on HSCs but also can act synergistically with IR in the treatment of cancer.
Acknowledgments
This study was supported by the National Program on Key Basic Research Project (973 Program, 2011CB964800-G) and grants from the National Natural Science Foundation of China (Nos. 81129020 and 81372928), the Natural Science Foundation of Tianjin (15JCZDJC35200), and the U.S. National Institutes of Health (CA122023).
References
- 1.Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985;5:101–110. [PubMed] [Google Scholar]
- 2.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 3.Meng A, Wang Y, Brown SA, Van Zant G, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp. Hematol. 2003;31:1348–1356. doi: 10.1016/j.exphem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–5419. [PubMed] [Google Scholar]
- 6.Gardner RV, Begue R, McKinnon E. The effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on primitive hematopoietic stem cell (PHSC) function and numbers, after chemotherapy. Exp. Hematol. 2001;29:1053–1059. doi: 10.1016/s0301-472x(01)00685-3. [DOI] [PubMed] [Google Scholar]
- 7.van Os R, Robinson S, Sheridan T, Mislow JM, Dawes D, Mauch PM. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood. 1998;92:1950–1956. [PubMed] [Google Scholar]
- 8.van Os R, Robinson S, Sheridan T, Mauch PM. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000;18:120–127. doi: 10.1634/stemcells.18-2-120. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic. Biol. Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid. Redox Signaling. 2008;10:1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegué E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, Ghaffari S. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J. Biol. Chem. 2008;283:25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 18.Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem. Biophys. Res. Commun. 2010;396:199–205. doi: 10.1016/j.bbrc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Bulterijs S. Metformin as a geroprotector. Rejuvenation Res. 2011;14:469–482. doi: 10.1089/rej.2011.1153. [DOI] [PubMed] [Google Scholar]
- 20.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 23.Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SC, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Park HJ, Park CS, Oh ET, Choi BH, Williams B, Lee CK, Song CW. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One. 2014;9:e87979. doi: 10.1371/journal.pone.0087979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Frimer M, Conroy E, Goldberg GL, Einstein MH. Metformin use and endometrial cancer survival. Gynecol. Oncol. 2014;132:236–240. doi: 10.1016/j.ygyno.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnefont-Rousselot D, Raji B, Walrand S, Gardès-Albert M, Jore D, Legrand A, Peynet J, Vasson MP. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism. 2003;52:586–589. doi: 10.1053/meta.2003.50093. [DOI] [PubMed] [Google Scholar]
- 30.Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem. Biophys. Res. Commun. 2010;396:199–205. doi: 10.1016/j.bbrc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Chang J, Jung HH, Yang JY, Lee S, Choi J, Im GJ, Chae SW. Protective effect of metformin against cisplatin-induced ototoxicity in an auditory cell line. J. Assoc. Res. Otolaryngol. 2014;15:149–158. doi: 10.1007/s10162-013-0431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm. Metab. Res. 2012;44:891–895. doi: 10.1055/s-0032-1321878. [DOI] [PubMed] [Google Scholar]
- 33.Piro S, Rabuazzo AM, Renis M, Purrello F. Effects of metformin on oxidative stress, adenine nucleotides balance, and glucose-induced insulin release impaired by chronic free fatty acids exposure in rat pancreatic islets. J. Endocrinol. Invest. 2012;35:504–510. doi: 10.3275/7866. [DOI] [PubMed] [Google Scholar]
- 34.Ota K, Nakamura J, Li W, Kozakae M, Watarai A, Nakamura N, Yasuda Y, Nakashima E, Naruse K, Watabe K, Kato K, Oiso Y, Hamada Y. Metformin prevents methylglyoxal-induced apoptosis of mouse Schwann cells. Biochem. Biophys. Res. Commun. 2007;357:270–275. doi: 10.1016/j.bbrc.2007.03.140. [DOI] [PubMed] [Google Scholar]
- 35.Halicka HD, Zhao H, Li J, Traganos F, Zhang S, Lee M, Darzynkiewicz Z. Genome protective effect of metformin as revealed by reduced level of constitutive DNA damage signaling. Aging. 2011;3:1028–1038. doi: 10.18632/aging.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Algire C, Moiseeva O, Deschênes-Simard X, Amrein L, Petruccelli L, Birman E, Viollet B, Ferbeyre G, Pollak MN. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev. Res (Philadelphia) 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 37.Na HJ, Park JS, Pyo JH, Lee SH, Jeon HJ, Kim YS, Yoo MA. Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech. Ageing Dev. 2013;134:381–390. doi: 10.1016/j.mad.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Mujica-Mota MA, Salehi P, Devic S, Daniel SJ. Safety and otoprotection of metformin in radiation-induced sensorineural hearing loss in the guinea pig. Otolaryngol. Head Neck Surg. 2014;150:859–865. doi: 10.1177/0194599814521013. [DOI] [PubMed] [Google Scholar]
- 39.Miller RC, Murley JS, Grdina DJ. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat. Res. 2014;181:464–470. doi: 10.1667/RR13672.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Wang Y, Pazhanisamy SK, Shao L, Batinic-Haberle I, Meng A, Zhou D. Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic. Biol. Med. 2011;51:30–37. doi: 10.1016/j.freeradbiomed.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Zhai Z, Wang Y, Zhang J, Wu H, Wang Y, Li C, Li D, Lu L, Wang X, Chang J, Hou Q, Ju Z, Zhou D, Meng A. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013;54:40–50. doi: 10.1016/j.freeradbiomed.2012.10.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: a review. Mutagenesis. 2006;21:361–367. doi: 10.1093/mutage/gel048. [DOI] [PubMed] [Google Scholar]
- 43.Gius D, Spitz DR. Redox signaling in cancer biology. Antioxid. Redox Signaling. 2006;8:1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 44.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 45.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 46.Lambeth JD. Nox enzymes ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpless NE. Ink4a/Arf links senescence and aging. Exp. Gerontol. 2004;39:1751–1759. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Kellner J, Liu L, Zhou D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011;20:1143–1152. doi: 10.1089/scd.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klachko D, Whaley-Connell A. Use of metformin in patients with kidney and cardiovascular diseases. Cardiorenal Med. 2011;1:87–95. doi: 10.1159/000327151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drzewoski J, Drozdowska A, Sliwińska A. Do we have enough data to confirm the link between antidiabetic drug use and cancer development? Pol. Arch. Med. Wewn. 2011;121:81–87. [PubMed] [Google Scholar]
- 52.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepiński JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem. Biophys. Res. Commun. 2010;393:268–273. doi: 10.1016/j.bbrc.2010.01.119. [DOI] [PubMed] [Google Scholar]
- 54.Ouslimani N, Peynet J, Bonnefont-Rousselot D, Thérond P, Legrand A, Beaudeux JL. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54:829–834. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, Miura D, Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232:156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 56.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 57.Huang L, Snyder AR, Morgan WF. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene. 2003;22:5848–5854. doi: 10.1038/sj.onc.1206697. [DOI] [PubMed] [Google Scholar]
- 58.Wright EG. Radiation-induced genomic instability in haemopoietic cells. Int. J. Radiat. Biol. 1998;74:681–687. doi: 10.1080/095530098140943. [DOI] [PubMed] [Google Scholar]
- 59.Attia SM, Helal GK, Alhaider AA. Assessment of genomic instability in normal and diabetic rats treated with metformin. Chem. Biol. Interact. 2009;180:296–304. doi: 10.1016/j.cbi.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol. (Oxford) 2009;196:81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1996;81:4059–4067. doi: 10.1210/jcem.81.11.8923861. [DOI] [PubMed] [Google Scholar]
- 62.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49:434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- 64.Argaud D, Roth H, Wiernsperger N, Leverve XM. Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur. J. Biochem. 1993;213:1341–1348. doi: 10.1111/j.1432-1033.1993.tb17886.x. [DOI] [PubMed] [Google Scholar]
- 65.Large V, Beylot M. Modifications of citric acid cycle activity and gluconeogenesis in streptozotocin-induced diabetes and effects of metformin. Diabetes. 1999;48:1251–1257. doi: 10.2337/diabetes.48.6.1251. [DOI] [PubMed] [Google Scholar]
- 66.Mithieux G, Guignot L, Bordet JC, Wiernsperger N. Intrahepatic mechanisms underlying the effect of metformin in decreasing basal glucose production in rats fed a high-fat diet. Diabetes. 2002;51:139–143. doi: 10.2337/diabetes.51.1.139. [DOI] [PubMed] [Google Scholar]
- 67.Radziuk J, Zhang Z, Wiernsperger N, Pye S. Effects of metformin on lactate uptake and gluconeogenesis in the perfused rat liver. Diabetes. 1997;46:1406–1413. doi: 10.2337/diab.46.9.1406. [DOI] [PubMed] [Google Scholar]
- 68.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski HJ, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 69.Hahn SS, Tang Q, Zheng F, Zhao S, Wu J, Chen J. Repression of integrin-linked kinase by antidiabetes drugs through cross-talk of PPARγ- and AMPKα-dependent signaling: role of AP-2α and Sp1. Cell Signaling. 2014;26:639–647. doi: 10.1016/j.cellsig.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat. Res. 2014;182:50–59. doi: 10.1667/RR13568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang T, Zhang L, Zhang T, Fan J, Wu K, Guan Z, Wang X, Li L, Hsieh JT, He D, Guo P. Metformin sensitizes prostate cancer cells to radiation through EGFR/p-DNA-PKCS in vitro and in vivo. Radiat. Res. 2014;181:641–649. doi: 10.1667/RR13561.1. [DOI] [PubMed] [Google Scholar]
- 72.Zannella VE, Dal Pra A, Muaddi H, McKee TD, Stapleton S, Sykes J, Glicksman R, Chaib S, Zamiara P, Milosevic M, Wouters BG, Bristow RG, Koritzinsky M. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin. Cancer Res. 2013;19:6741–6750. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- 73.Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G, Tsakiridis T. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br. J. Cancer. 2013;108:2021–2032. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song CW, Lee H, Dings RP, Williams B, Powers J, Santos TD, Choi BH, Park HJ. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci. Rep. 2012;2:362. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]