Abstract
Background
Although hematogenous spread of osteosarcoma is well known, the imaging findings of cardiovascular involvement by osteosarcoma are seldom reported and can be difficult to recognize. The enhanced resolution of modern CT and MRI scanners may lead to better detection of cardiovascular involvement.
Objective
To describe the key imaging findings and clinical behavior of cardiovascular involvement by osteosarcoma.
Materials and methods
We retrospectively reviewed the imaging findings and clinical characteristics of 20 patients with cardiovascular involvement by osteosarcoma identified by two pediatric radiologists from a review of imaging studies at our institution from 2007 to 2013.
Results
At initial diagnosis, the median age of the patients was 15.1 years (range 4.8–24.6 years), and 7 (35%) patients had detectable metastases. Median time to detection of cardiovascular metastases was 1.8 years (range 0–7.3 years). Sixteen patients died of disease; 4 have survived a median of 7.4 years since initial diagnosis. The sites of cardiovascular involvement were the systemic veins draining the primary and metastatic osteosarcoma, pulmonary arteries, pulmonary veins draining the pulmonary metastases, and heart. A dilated and mineralized terminal pulmonary arteriole is an early sign of metastatic osteosarcoma in the lung. Unfamiliarity with the imaging features resulted in under-recognition and misinterpretation of intravascular tumor thrombus as bland thrombus.
Conclusion
Knowledge of imaging findings in the era of modern imaging modalities has enhanced our ability to detect cardiovascular involvement and lung metastases early and avoid misinterpreting tumor thrombus in draining systemic veins or pulmonary arteries as bland thrombus.
Keywords: Children, Computed tomography, Heart, Inferior vena cava, Magnetic resonance imaging, Osteosarcoma, Positron emission tomography-computed tomography, Superior vena cava, Tumor thrombus, Vascular metastasis
Introduction
Osteosarcoma is the most common primary bone malignancy in children, adolescents and young adults, and its most common metastatic sites are lung and bone [1]. Although osteosarcoma is known to spread hematogenously, few imaging findings of cardiovascular involvement by osteosarcoma have been reported, and only relatively few isolated case reports have been published. An extensive literature search yielded 73 case reports and other reports describing 76 cases. However, no detailed analysis of a series of patients with osteosarcoma and cardiovascular involvement has been reported. The most recent 34 articles describing 37 cases dating to 2000 are cited here [2–35].
Imaging findings of cardiovascular involvement by osteosarcoma can be subtle and thus are likely to be missed or misinterpreted, with potentially undesirable consequences. The incidence of cardiovascular metastasis and the impact of its early recognition are not clear. During our routine practice we observed cardiovascular involvement early in the course of the disease within the systemic vein draining the primary tumor and in small pulmonary arterial branches, and in advanced cases within the veins draining the lung and extrapulmonary metastases.
The purpose of this report is to describe the key imaging characteristics and clinical behavior of cardiovascular involvement by osteosarcoma. Recognition of these findings can improve staging accuracy, allowing better determination of appropriate cancer therapy and prognosis. Additionally, correct identification of systemic and pulmonary intravascular filling defects as tumor thrombus rather than bland thrombus can prevent unnecessary anticoagulation. The optimal use of advanced imaging techniques such as CT, MRI and PET-CT (positron emission tomography–computed tomography) and an increased index of suspicion for these imaging findings can increase the likelihood of detecting cardiovascular involvement earlier in the disease course.
Materials and methods
We obtained institutional review board approval, including waiver of informed consent, for this retrospective review. Approximately 80 patients with osteosarcoma are evaluated at our institution annually. Twenty patients with osteosarcoma and cardiovascular involvement were identified by two radiologists during their routine practice at our institution between 2007 and 2013. Imaging studies and medical records for these patients were retrospectively reviewed from the time of diagnosis through December 2014.
Clinicopathological data were collected on all patients, including age at diagnosis of osteosarcoma, primary tumor location and histological subtype, presence or absence of metastatic disease at presentation, time to detection of cardiovascular involvement from initial diagnosis, sites of cardiovascular involvement and other metastases, percentage of tumor necrosis at the time of definitive surgery, and survival period from time of primary tumor diagnosis. The patient ages mentioned in the figures of this manuscript are the age at the time of the first presented imaging study.
Imaging findings were reviewed by two radiologists with 2 years of post-training experience as a faculty member in pediatric radiology and abdominal imaging (S.Y. and A.M.) in consensus. A detailed description of all imaging techniques used is beyond the scope of this report because imaging studies from several outside hospitals were included. Standard institutional protocols were used for the CT, MR and PET-CT examinations performed at our institution. The available imaging studies were evaluated for location of metastases, with particular attention to the presence of cardiovascular involvement in the veins draining the primary and metastatic tumors, in the pulmonary arteries (including their temporal evolution to involve the adjacent pulmonary parenchyma and further extension into the pulmonary veins and left heart), and in the heart by direct involvement. We reviewed all available first and last CT, PET-CT and MRI studies in all patients. In addition, we reviewed interval imaging studies to determine the time of initial detection of cardiovascular involvement and its temporal evolution. Chest CTs were available in all 20 patients, at least one PET-CT in 18 patients and MRI of various sites in 19 patients (including 12 with MRI of primary tumor). Cardiovascular involvement was determined based on imaging findings of temporal evolution of abnormality (including interval growth or mineralization), direct intravascular extension with thrombus showing similar imaging characteristics to primary tumor, [F-18]2-fluoro-2-deoxyglucose (FDG) avidity, histological confirmation and combinations of these features.
Results
Clinical characteristics
We identified a total of 20 patients with cardiovascular involvement by osteosarcoma evaluated at our institution within the time period specified. Cardiovascular involvement was determined based on findings on imaging studies in all 20 patients; histological confirmation of cardiovascular involvement was available in 9 (45%) patients. Relevant clinicopathological and imaging data are summarized in Table 1. The median age at initial diagnosis was 15.1 years (range 4.8–24.6 years), and 55% were female. The median time to detection of cardiovascular involvement was 1.8 years (range 0–7.3 years).
Table 1.
Summary of clinical and imaging findings of 20 patients with osteosarcoma and cardiovascular metastases
| Characteristic | Value (range or percentage) |
|---|---|
| Median age (range) in years | |
| At diagnosis | 15.1 years (4.8–24.6 years) |
| Age at time of CV metastasis | 17.3 years (4.9–25.7 years) |
| Male: Female | 9:11 |
| Race | |
| White | 14 (70%) |
| Hispanic | 5 (25%) |
| Asian | 1 (5%) |
| Metastasis at diagnosis | |
| Yes | 7 (35%) |
| No | 13 (65%) |
| Primary tumor site | |
| Femur | 13 (65%) |
| Humerus | 3 (15%) |
| Pelvis | 3 (15%) |
| Rib | 1 (5%) |
| Sites of cardiovascular involvementa | |
| Direct venous extension from primary tumor | 6 (30%) |
| Direct venous extension from extrapulmonary metastases | 3 (15%) |
| Pulmonary vessels | 16 (80%) |
| Direct venous extension from pulmonary metastases | 3 (15%) |
| Direct heart (LV and myocardium) involvement | 3 (15%) |
| Other metastatic sitesa | |
| Lungs | 19 (95%) |
| Bones | 12 (60%) |
| Soft tissues | 6 (30%) |
| Lymph nodes | 4 (20%) |
| Brain | 3 (15%) |
| Kidney | 2 (10%) |
| Adrenal gland | 1 (5%) |
| Histological subtype | |
| Not further specified | 7 (35%) |
| Osteoblastic | 6 (30%) |
| Chondroblastic | 5 (25%) |
| Osteoblastic and chondroblastic | 2 (10%) |
| Histological response to preoperative chemotherapy at initial surgery (n=15)b | |
| >90% tumor necrosis | 5 (33%) |
| <90% tumor necrosis | 10 (67%) |
| Patient outcome | |
| Dead | 16 (80%) |
| Alive | 4 (20%) |
CV cardiovascular, LV left ventricle
Some patients had metastases to more than one site
Three patients did not undergo resection of the primary tumor and two had no data on histological response
Seven patients (35%) had detectable metastases at the time of initial diagnosis. The distal femur was the most common location of the primary tumor. Tumors were high-grade osteosarcoma of the following subtypes: not further specified, osteoblastic, chondroblastic, and combined osteoblastic and chondroblastic. Sixteen patients died of disease at a median of 3.3 years (range 1.1–5.6 years) after diagnosis of the primary tumor. The other four patients have survived a median of 7.4 years (range 1.7–14.7 years) since diagnosis of the primary tumor. Two of the four survivors have no evidence of disease and two are alive with disease. Nearly all patients had lung metastases and the majority had bone metastases in addition to cardiovascular involvement by the conclusion of the review period. The vast majority of the patients had lung metastases before developing metastases in other sites such as bone and kidneys.
Imaging findings
The common locations of cardiovascular metastases were systemic veins draining the primary site (n=6), systemic veins draining metastatic sites (n=3), pulmonary arteries (n=16) including patients whose tumor further extended into pulmonary veins draining the pulmonary metastases and left atrium (n=3), and direct heart involvement (n=3). Ten patients had cardiovascular involvement in more than one of these sites.
Systemic veins draining the primary site
Six of the 20 patients showed tumor thrombus in the veins draining the primary tumor; histological confirmation of cardiovascular involvement was available in 5 of these 6 patients. All three patients with primary tumor in the pelvis (Figs. 1 and 2), 2 of 3 patients with primary tumor in the proximal humerus (Fig. 3), and 1 of 13 patients with primary tumor in the distal femur (Fig. 4) showed tumor thrombus in the systemic veins draining the primary tumor. On contrast-enhanced CT examination, tumor thrombus is seen as a filling defect in the draining vein. On noncontrast CT scan, the dilated vein with tumor thrombus shows progressive mineralization on follow-up examinations. On T2-weighted MR examinations, thrombus is recognized by absent vascular flow void when compared to the opposite side (Figs. 1 and 2). Tumor thrombus can be mistaken for nodal metastasis (Fig. 4). This misreading can be avoided by identifying the linear rather than ovoid appearance of the abnormality.
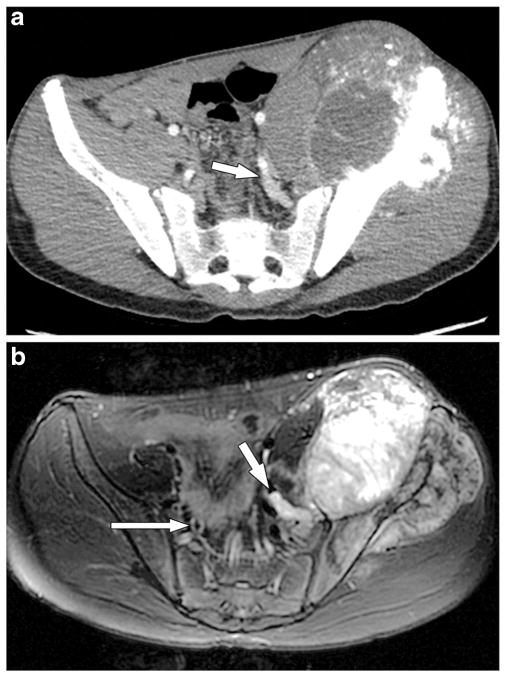
Fig. 1.
A 17-year-old boy with left iliac primary osteosarcoma and tumor thrombus in the left internal iliac vein that was confirmed by histology. a In this axial CT scan of the pelvis with contrast agent in the soft-tissue window, the high attenuation/enhancing tumor in the dilated left internal iliac vein (arrow) is not separately delineated from normal vascular enhancement. The CT image shows the primary osteosarcoma in the left ilium with its large soft-tissue component and osteoid matrix. b Axial T2-weighted MR image of the pelvis 4 months after the CT scan and just before surgical resection depicts absent flow void within the left internal iliac vein (short arrow), and this vein is filled with T2 hyperintense material/thrombus that is similar in signal intensity to the adjacent primary left iliac tumor. In addition, this thrombus showed enhancement on post-contrast imaging (not shown). This finding was not recognized preoperatively. The flow voids within the contralateral internal iliac vein (long arrow) are preserved
Fig. 2.
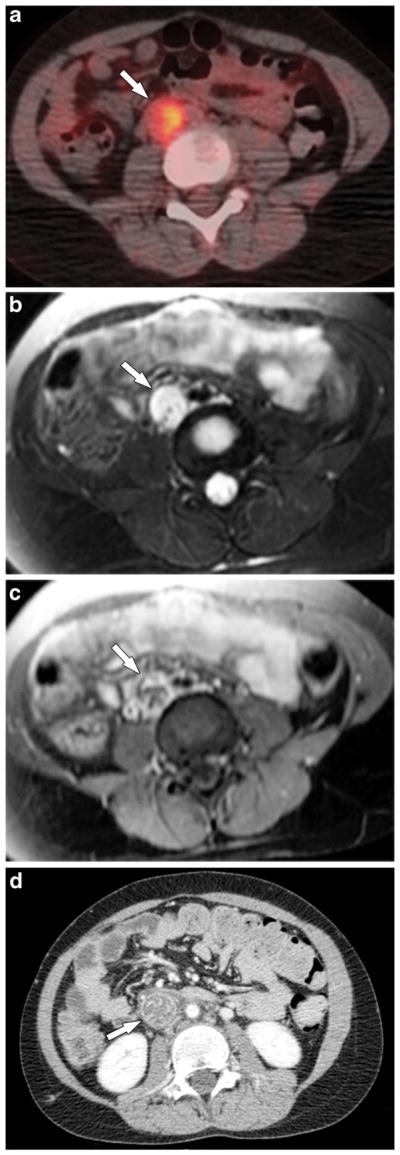
A 10-year-old girl with osteosarcoma of the right ilium and sacrum (not shown) and right iliac vein tumor thrombus. a Axial fused PET-CT image shows FDG-avid tumor thrombus in the right common iliac vein (arrow). b Axial fast spin-echo T2-W MR image with fat saturation and (c) post-contrast T1-W MR image with fat saturation confirm a dilatated and thrombosed right common iliac vein with absent flow void and enhancement most consistent with tumor thrombus. d Axial contrast-enhanced CT scan 3 months later shows further extension of thrombus into the inferior vena cava (arrow) that is now associated with mineralization on noncontrast CT. The inferior vena cava thrombus was proved to be metastatic osteosarcoma at surgery. FDG [F-18]2-fluoro-2-deoxyglucose, PET positron emission tomography
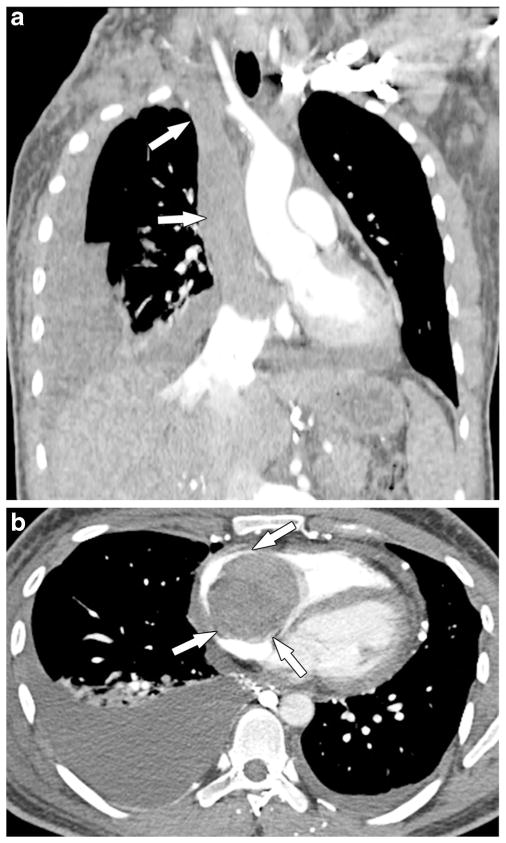
Fig. 3.
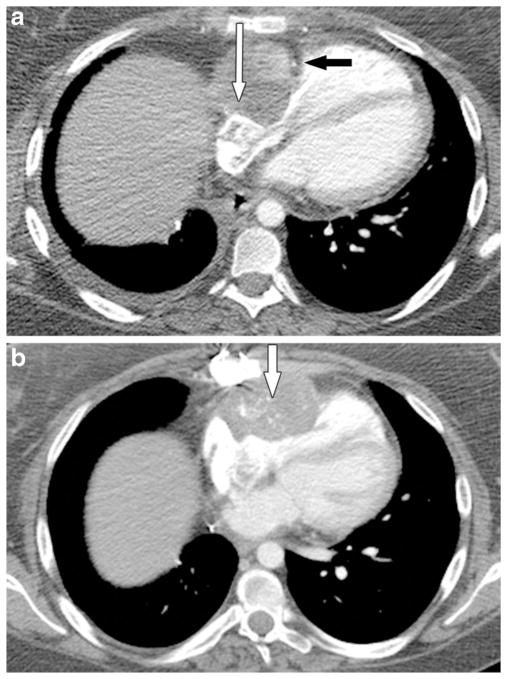
A 25-year-old man with osteosarcoma of the right proximal humerus and tumor thrombus in the innominate veins, superior vena cava and right heart. One year after resection of the primary osteosarcoma (right upper extremity forequarter amputation) the man presented with superior vena cava syndrome. a, b Routine coronal (a) and axial (b) post-contrast chest CT images show extensive tumor thrombus in the innominate veins, superior vena cava and right atrium that further extends into the right ventricle (arrows) across the tricuspid valve. The superior vena cava thrombus was biopsied at an outside facility and was proved to be osteosarcoma. The man died of the disease within a few days
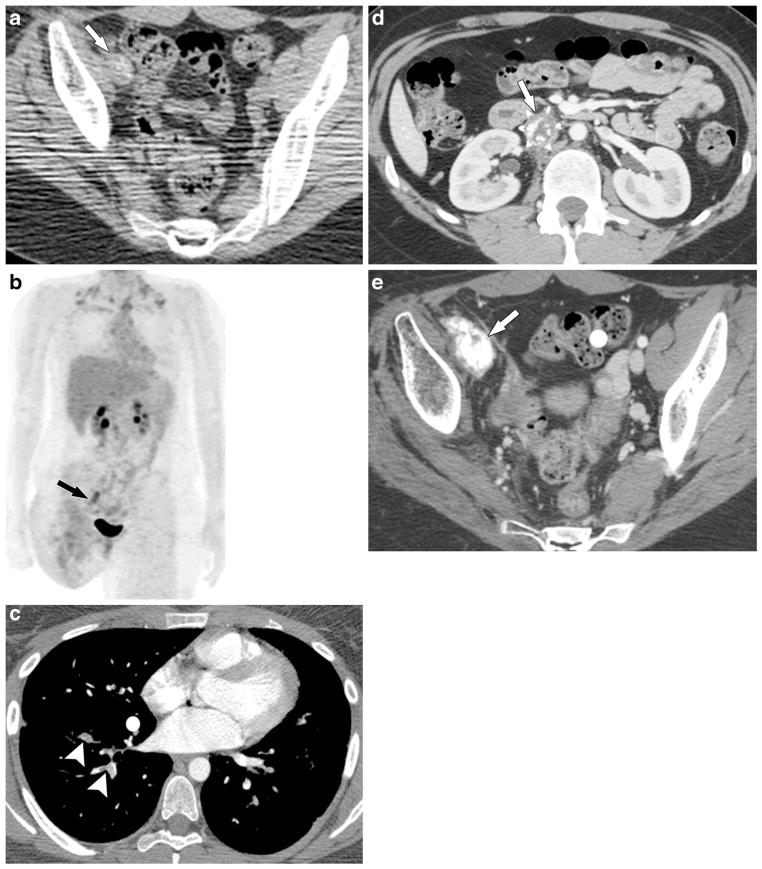
Fig. 4.
An 18-year-old woman with osteosarcoma of the right distal femur that recurred in the right proximal thigh/pelvis and was treated with right hip disarticulation at an outside institution; tumor thrombus was found in the right external iliac vein, inferior vena cava, and pulmonary arteries. Pathology review at the time of right hip disarticulation reported tumor in the right femoral vein at the resection margin. a Axial CT pelvis without contrast agent and (b) 3-D-weighted high-definition maximum-intensity projection PET images from the PET-CT scan show mineralized (arrow in a), FDG-avid (arrow in b) tumor thrombus in the right external iliac vein, which was misinterpreted as nodal metastasis. c A 3-month follow-up axial chest CT scan with contrast agent shows pulmonary arterial tumor emboli (arrowheads), confirmed by progression and mineralization on follow-up chest CT (not shown). d, e Axial contrast-enhanced CT images at the level of the kidneys (d) and pelvis (e) 7 months later show mineralized tumor thrombus within the inferior vena cava with a filter in place (arrow in d) and further mineralization of the right iliac vein thrombus (arrow in e). The inferior vena cava tumor thrombus was resected and proved to be metastatic osteosarcoma
Systemic veins draining metastatic sites
Three of the 20 patients showed tumor thrombus in the veins draining the metastatic osteosarcoma; histological confirmation of cardiovascular involvement was available in 1 of these 3 patients. In 1 patient with metastatic disease in the lumbar spine, tumor thrombus in the lumbar venous plexus further extended into the inferior vena cava (Fig. 5) and the right atrium. Two other patients showed tumor thrombi in the renal veins draining a kidney with a metastatic lesion.
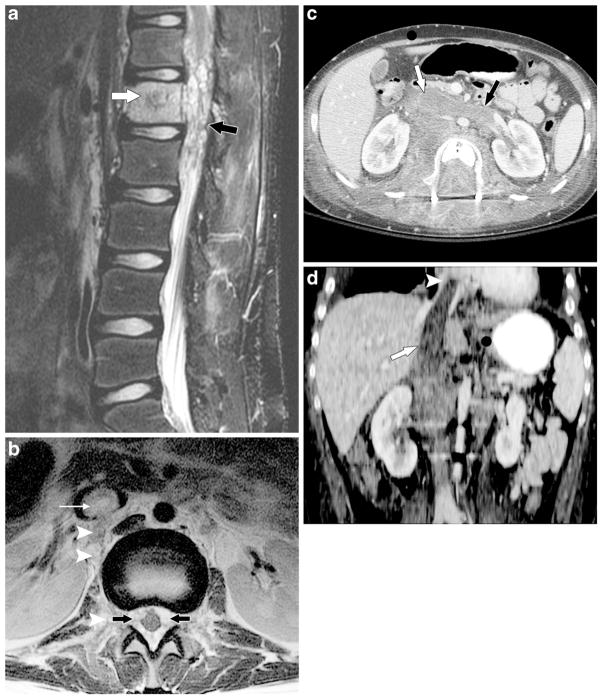
Fig. 5.
A 20-year-old man with osteosarcoma of the right distal femur with metastasis to L1 and tumor thrombus in the lumbar venous plexus and inferior vena cava. a Sagittal and (b) axial T2-weighted MR images of the lumbar spine show metastatic osteosarcoma at L1 (white arrow in a) with a large epidural component (black arrows in a, b). Tumor thrombus in the inferior vena cava (long white arrow in b) via the lumbar venous plexus (arrowheads in b) shows similar signal intensity to the metastatic osteosarcoma at L1 and was not initially recognized on MRI of the spine. c Axial and (d) coronal contrast-enhanced CT images 1 month later show progression of the vascular involvement with enhancing tumor thrombus in the inferior vena cava (white arrow in c and d) with additional involvement of the left renal vein (black arrow in c) and extension into the right atrium (arrowhead in d). The thrombus was presumed to be bland thrombus and the man was treated with an anticoagulant. This man died approximately 2 months later from progressive metastatic osteosarcoma
Pulmonary arteries
Sixteen of the 20 patients showed linear mineralized metastases in the distal pulmonary vessels, which we believe represent progressive tumor thrombi in the pulmonary arteries. One such lesion, which is very subtle in appearance (Fig. 6), was proved by histological examination to be metastatic osteosarcoma. The earliest imaging finding on this lesion is a dilated and non-tapering distal pulmonary artery, which on follow-up imaging shows progressive dilatation, mineralization and nodularity. Tumor thromboemboli are also seen in the proximal pulmonary arteries and can be misinterpreted as pulmonary thromboembolism (Fig. 4) if the radiologist is not aware of the possibility of tumor thromboembolism.
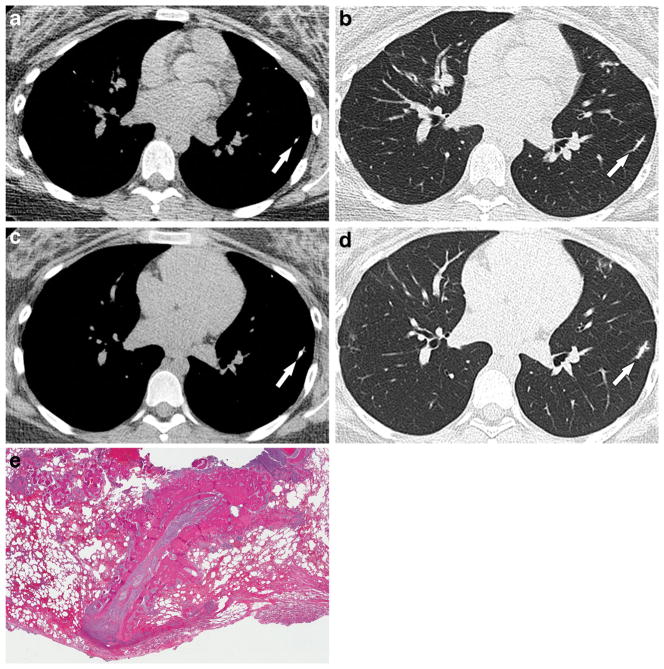
Fig. 6.
A 15-year-old girl with osteosarcoma of the left distal femur with metastasis to the lungs and cardiovascular involvement of the terminal pulmonary vessel. a, b Initial and (c, d) 14-week follow-up noncontrast chest CT images in mediastinal and lung windows show progressive mineralization, beaded appearance and dilatation of the distal pulmonary artery branch (arrows) consistent with early pulmonary vascular involvement. This was resected and proved to be metastatic osteosarcoma. e Pathology section of the resected left lower-lobe lesion confirms metastatic osteosarcoma in the lung parenchyma and depicts the peculiar linear pattern of this metastasis. Although it was difficult to confirm the intravascular location of the metastasis because it had grown out into the parenchyma, obliterating normal structures, the linear pattern correlates with the possibility of the lesion location within the pulmonary arteriole. The girl developed several similar recurrences
Pulmonary veins draining the pulmonary metastases with extension into left atrium
Three of the 16 patients who showed tumor in the distal pulmonary arteries demonstrated further extension of the pulmonary parenchymal metastases into draining pulmonary veins and left atrium. This finding is best seen on contrast-enhanced CT examinations, which show the tumor thrombus extended into the pulmonary veins and further into the left atrium. The serial imaging findings illustrated in Fig. 7 depict temporal evolution of tumor thrombus in the distal pulmonary arteries as it further infiltrates the pulmonary parenchyma and then shows clear extension into the draining pulmonary vein and left heart. Extension into the left atrium is generally considered a feature of advanced osteosarcoma, but one patient with unilateral lung involvement and left atrial involvement by tumor thrombus underwent radical resection and is now one of the long-term survivors in this series (Fig. 8). Histological confirmation of cardiovascular involvement was available in this patient.
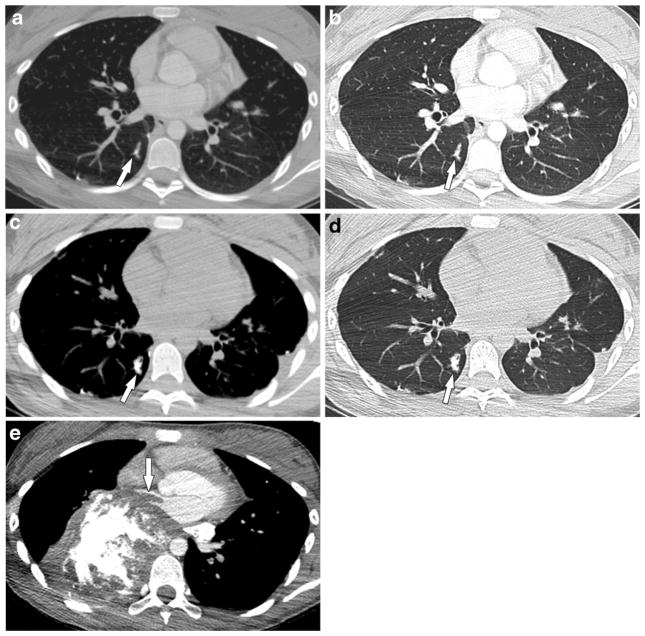
Fig. 7.
A 12-year-old girl with osteosarcoma of the right proximal humerus with metastasis to the lungs and tumor thrombus in the pulmonary artery, pulmonary vein and left atrium. a, b Initial and (c, d) 4-month follow-up axial post-contrast chest CT scans in mediastinal and lung windows show a progressive increase in mineralization and beaded appearance of the right lower lobe distal pulmonary artery (arrows). Surgery was refused and the tumor progressed on chemotherapy. e Chest CT scan with contrast agent in mediastinal windows 22 months later shows significant progression of metastatic disease with extension into the right inferior pulmonary vein and left atrium (arrow)
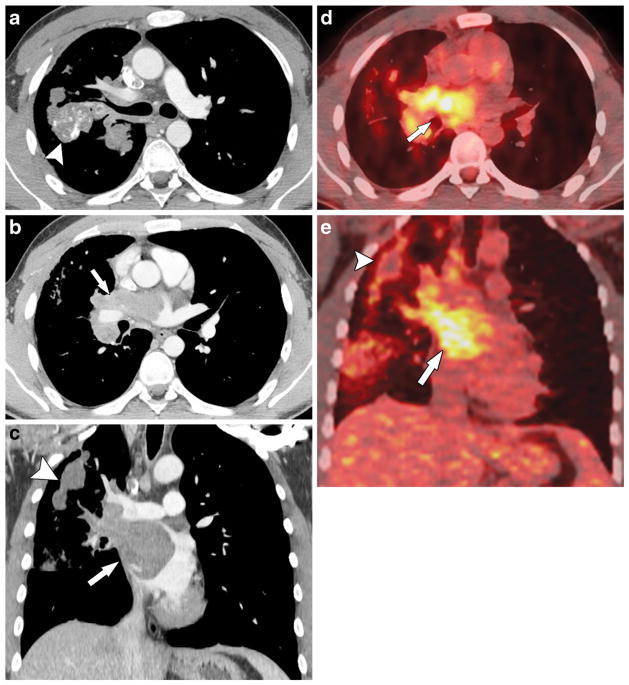
Fig. 8.
A 22-year-old man with osteosarcoma of the right distal femur metastatic to the lungs and heart and with tumor thrombus in the right pulmonary veins and left atrium. a, b Axial and (c) coronal post-contrast chest CT scan and (d) axial and (e) coronal fused PET-CT images demonstrate FDG-avid tumor thrombus in the right superior pulmonary vein and left atrium (arrows) and right lung metastases (arrowheads). The filling defect in the superior vena cava is a flow-related artefact. After radical resection and chemotherapy the man was disease-free for 6.8 years at the time of this report. Tumor thrombus in the pulmonary veins and left atrium was confirmed by histology at the time of surgery. FDG [F-18]2-fluoro-2-deoxyglucose, PET positron emission tomography
Direct heart involvement
Three of the 20 patients showed noncontiguous involvement of the heart; histological confirmation of cardiac involvement was available in 2 of these 3 patients. One patient showed subtle left ventricular metastases that were suspected because of presence of widely disseminated systemic metastatic disease (Fig. 9). Small intracardiac foci were difficult to delineate on initial cross-sectional imaging studies and necessitated further workup by echocardiogram and cardiac MRI. The second patient showed progressive metastasis in the atrioventricular groove (Fig. 10). The third patient, not illustrated in this article, showed subtle calcification in the right ventricular outflow tract.
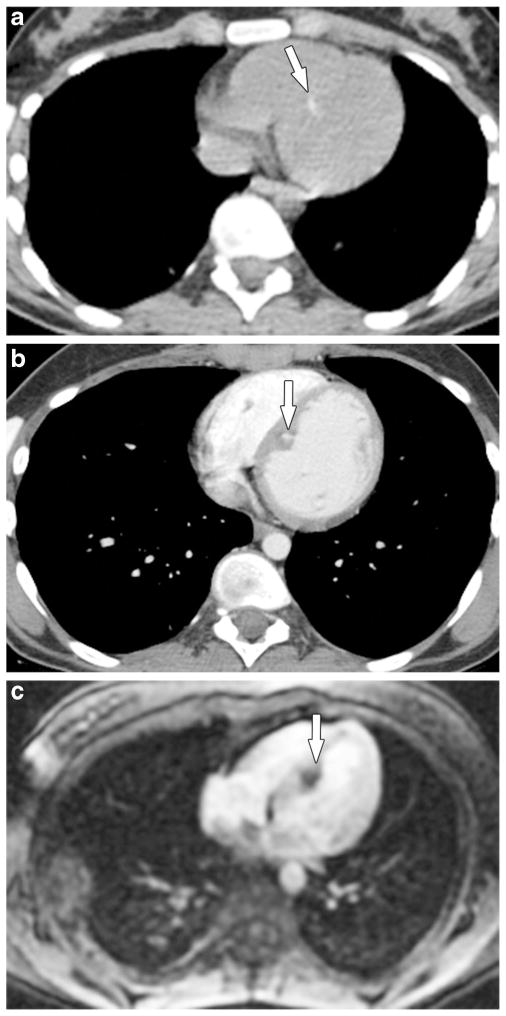
Fig. 9.
A 15-year-old girl with osteosarcoma of the right distal femur presented with new widespread metastases to bones, soft tissues and lungs, prompting a search for an intracardiac source. a Axial noncontrast and (b) contrast-enhanced chest CT images show subtle mineralized myocardial metastasis (arrows) within the interventricular septum. c Myocardial enhancement phase image from an fast gradient recalled echo first pass perfusion sequence in the four-chamber view shows enhancement of the metastasis around the calcium-related signal void (arrow). The girl had similar metastasis along the left ventricular free wall (not shown). The two intracardiac metastases were resected and proved to be metastatic osteosarcoma. The girl died 7 months after cardiac surgery
Fig. 10.
A 19-year-old woman with osteosarcoma of the distal femur metastatic to the heart. a Axial contrast-enhanced CT scan after initial partial resection of cardiac metastasis involving right atrium (not shown) shows residual tumor in the atrioventricular groove (white arrow) closely abutting the right coronary artery (black arrow). b Axial contrast-enhanced CT scan 2 years later shows slow progression of the metastasis (arrow) to involve the right ventricle. Radical resection and reconstruction was performed about 2 years after initial incomplete cardiac resection. The woman developed recurrent metastatic disease and died 7 months after the second surgery. This cardiac metastasis was proved by surgery and pathology to be osteosarcoma
Summary of the imaging findings
Tumor thrombus in the systemic veins draining the primary tumor and systemic and pulmonary metastases in osteosarcoma is seen as a filling defect in the draining veins and usually shows progressive mineralization on follow-up imaging. The typical appearance of lung metastasis is a round or oval, sharply marginated, mineralized nodule or mass. A dilated, beaded and mineralized or nonmineralized pulmonary vessel is one of the earliest signs of pulmonary vascular metastasis. In our experience, it is best seen on noncontrast CT images. Follow-up imaging shows progressive dilatation and mineralization of the vessel, followed by possible infiltration into lung parenchyma and then extension into pulmonary veins and left heart.
Discussion
The exact incidence of venous extension of osteosarcoma and its prognostic significance are not known. Vascular invasion confirmed by histological examination has been reported in 2% of patients with resected extremity soft-tissue or bone sarcoma, including osteosarcoma, and has been associated with very poor prognosis [36]. Autopsy studies have shown a 20% incidence rate of cardiac metastases [23, 37]. Insight into tumor behavior illustrated by these cases has enhanced our ability to correctly identify cardiovascular metastases, particularly the metastases in the distal pulmonary vessels and also in the large draining veins from the primary and metastatic osteosarcoma. All but 1 of the 6 patients with cardiac involvement (either by direct heart involvement or extension of tumor thrombus into left atrium from the pulmonary veins) in our series died of progressive metastatic disease. The one surviving patient’s tumor recurred 7.3 years after diagnosis, with unilateral lung metastases and left atrial involvement, but after chemotherapy and radical resection this patient had been disease-free 6.8 years at the time of this report (Fig. 8).
Our series supports the findings described in several case reports. Vascular spread of osteosarcoma is increasingly recognized on imaging because of advances in cross-sectional imaging techniques. Although our study could not assess the incidence of vascular thrombi in osteosarcoma, we believe that our report will increase awareness about their imaging characteristics and that, once radiologists become accustomed to looking for these characteristics, tumor thrombi will be identified more frequently. In previous reports, cardiovascular involvement has been described in 37 patients, ages 8–48 years, with osteosarcoma. Similar to our findings, cardiovascular involvement was described in the veins draining the primary tumor in 14 patients (including 12 with pelvic tumors), in the renal vein draining a renal metastasis in 1 patient, in the pulmonary arteries in 12 patients, and in the pulmonary veins in 5 patients, and as direct heart involvement in 7 patients [2–35]. Histological confirmation of cardiovascular involvement was documented in 25 patients. Additionally, arterial tumor emboli in the superior mesenteric artery were described in one case report [7]. The most common primary tumor site in the reported cases was the pelvis, followed by femur and tibia; in our series the most common site was the femur followed by humerus and pelvis. The time to detection of cardiovascular involvement in our series ranged 0–7.3 years from diagnosis. This is similar to the range (0–7 years) described in the case reports for high-grade osteosarcoma.
Previous reports describe anticoagulant treatment for deep venous thrombosis and pulmonary thromboembolism before tumor thrombus was correctly diagnosed [6, 9, 30, 31]. This also occurred in our patient in Fig. 5. Although it is difficult to differentiate between tumor thrombi and bland thrombi on initial imaging studies, bland thrombi should either improve or resolve on follow-up imaging studies; even if the thrombus is persistent, the luminal caliber of the vein involved by a chronic bland thrombus should be relatively small. In our experience, the tumor thrombus is depicted by heterogeneous enhancement on contrast-enhanced CT or MR imaging and loss of intravascular flow voids on T2-weighted images, particularly when large vessels are involved. In areas accessible to US, the finding of pulsatile waveforms within the tumor thrombus on spectral Doppler interrogation favors a malignant lesion [38, 39]. In our experience a tumor thrombus is further characterized by increased metabolism on PET-CT in addition to enhancement on contrast-enhanced CT or MRI. Dedicated cardiac MRI helps to delineate the extent of cardiac involvement.
In light of the described imaging findings and because deep venous thrombosis is rare in young patients (incidence of <5 per 100,000 per year among children younger than 15 years [40]), a thrombus seen within the systemic vein draining the primary or metastatic osteosarcoma should be considered tumor thrombus until proved otherwise. Venous thromboembolism has been reported to occur in 10–13% of children and young adults with osteosarcoma; these events are most commonly related to venous catheters or tumor compression [41, 42]. Dual-energy spectral CT with quantification of thrombus iodine density has been shown to differentiate tumor thrombi from bland thrombi in portal veins in a series of patients with hepatocellular carcinoma, cirrhosis of the liver, viral hepatitis and alcoholic hepatitis, and colorectal metastasis [43]. However, the potential utility of this technique over standard contrast-enhanced CT techniques in osteosarcoma patients with cardiovascular involvement needs further study.
On the basis of our findings and a review of the literature, we have connected some dots with respect to the pattern of spread of osteosarcoma metastases. Osteosarcoma spreads predominantly by vascular invasion and rarely by lymphatic invasion. Mineralization in osteosarcoma aids in ready recognition of the tumor thrombus. Our findings illustrate the pattern of spread of osteosarcoma from the primary tumor to the systemic draining veins to the inferior or superior vena cava, to the right heart, to the pulmonary arteries, to the pulmonary parenchyma, to the draining pulmonary veins, to the left heart, and then to systemic sites (e.g., bone, kidney, adrenal gland, brain). This explains why, in our study and in other series, the incidence of lung metastasis is higher than that of any other site of metastasis [44, 45]. We have also illustrated that extrapulmonary metastases extend into the draining veins and then into the inferior or superior vena cava and right heart.
All three patients with pelvic osteosarcoma and two of the three patients with proximal humeral osteosarcoma showed tumor thrombus in draining veins, whereas only 1 of 13 patients with distal femoral osteosarcoma had this feature. We believe that this finding is most readily identified in patients with pelvic osteosarcoma because of the large size of the draining veins and availability of opposite-side imaging for comparison on the majority of pelvic CT and MR imaging studies. Detection of thrombus in the smaller extremity veins is difficult because of the small size of the veins and the lack of comparison with the other extremity on most MR imaging studies. Although our study was not designed to examine the outcomes of patients with humeral or pelvic osteosarcoma, others have reported poor prognosis of these patients [46, 47]. We speculate that one of the explanations for the relatively poor prognosis of humeral and pelvic osteosarcoma compared to femoral osteosarcoma is the relatively short distance the tumor thrombus needs to travel to reach the right heart.
The management of cardiovascular metastases poses a challenge to the multidisciplinary team involved in the treatment of osteosarcoma. The survival benefit from pulmonary metastatectomy has been reported in several studies [48–50]. In carefully selected patients, multimodality treatment may provide palliation and delay progression. Understanding the pattern of dilated and mineralized pulmonary vessels may result in early detection of pulmonary metastases (Fig. 6). The finding of dilated and beaded terminal pulmonary arterial branches as a sign of pulmonary vascular metastatic disease has also been reported in a series of four patients, including one case of metastatic osteosarcoma [51]. Although dilated and beaded vessels can be mistaken for mucus plugging in the distal bronchi, beaded arteries are always accompanied by air-filled bronchi on lung windows [17, 51].
More diligent assessment for venous thrombus in the draining veins of the primary tumor on presurgical imaging studies is needed. The presence of tumor thrombus in the draining veins of the primary osteosarcoma warrants a careful search for lung metastasis. Early recognition of pulmonary vascular metastasis may provide important information about prognosis and an opportunity for early treatment and help to prevent unnecessary anticoagulation. Further studies are required to answer the following: (1) What is the incidence and prognostic significance of finding macroscopic tumor thrombus in draining veins from primary osteosarcoma? and (2) Is the incidence of lung metastases increased in patients with these findings?
Another issue in early recognition of cardiovascular metastasis is the optimal chest CT imaging method for follow-up monitoring of patients with osteosarcoma. Mineralization is obscured by contrast agent, and nonmineralized tumor thrombi in large pulmonary vessels and heart may be obscured without contrast agent. Some institutions prefer contrast-enhanced chest CT imaging, while others perform chest CTs without contrast agent; however intermittent use of contrast agent during follow-up CT chest examinations would help to improve detection of cardiovascular metastases.
This study has some limitations. Several of the cases included in our study cohort were diagnosed and treated at outside facilities before referral to our center. The information presented is based on available clinical records and imaging studies. In addition, our retrospective study was designed to illustrate the imaging findings of cardiovascular involvement by osteosarcoma. The cases were collected by two radiologists over a period of 6 years during their routine clinical work and not through a systematic search for all cases seen during this time period. Hence we could not determine the actual prevalence of cardiovascular involvement by osteosarcoma, although we can roughly estimate that in our patient population it was at least 4.2% (20 of 480 patients seen over 6 years). Furthermore, because our center is a tertiary referral center, some of the patients were referred to our center when their disease was at an advanced stage, leading to a selection bias.
Conclusion
Conventional CT scanning plays a major role in the diagnosis of cardiovascular metastases of osteosarcoma. Local venous extension of the primary tumor may be seen early in the course of the disease, and thrombus in local draining veins should be considered tumor thrombus until proved otherwise. Dilatation and mineralization of terminal pulmonary arterioles is an important early sign of metastatic osteosarcoma. Left heart involvement should be considered when a patient presents with extrapulmonary metastasis. Cardiovascular involvement is seen mostly in advanced osteosarcoma, but we have observed and illustrated involvement of the draining veins from the primary osteosarcoma and pulmonary arteries early in the course of the disease. Familiarity with the imaging findings of cardiovascular involvement by osteosarcoma described in this article should lead to accurate staging of osteosarcoma that will aid in better patient management decisions.
Acknowledgments
We are grateful to Nancy E. Fitzgerald, MD, a pediatric radiologist formerly at MD Anderson Cancer Center, for generously contributing the vast majority of the cases reviewed in this study. Most of the imaging findings illustrated in this manuscript are her observations.
This study is supported by the NIH/NCI under award number P30CA016672.
Footnotes
This study was presented in part as a scientific informal poster at the Radiological Society of North America annual meeting in December 2013.
Conflicts of interest None
References
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navalkele P, Jones SM, Jones JK, et al. Osteosarcoma tumor thrombus: a case report with a review of the literature. Tex Heart Inst J. 2013;40:75–78. [PMC free article] [PubMed] [Google Scholar]
- 3.Lin WC, Lin CH, Chao YH, et al. Simultaneous pulmonary and inferior vena cava thromboembolism secondary to pelvic osteosarcoma. J Pediatr Hematol Oncol. 2013;35:e320–e322. doi: 10.1097/MPH.0b013e3182707a1a. [DOI] [PubMed] [Google Scholar]
- 4.Elasfar A, Khalifa A, AlGhamdi A, et al. Asymptomatic metastatic osteosarcoma to the right ventricle: case report and review of the literature. J Saudi Heart Assoc. 2013;25:39–42. doi: 10.1016/j.jsha.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buderi S, Theologou T, Gosney J, et al. Pulmonary artery tumor embolism in a patient with previous fibroblastic osteosarcoma. Ann Thorac Surg. 2013;95:2155–2157. doi: 10.1016/j.athoracsur.2012.10.062. [DOI] [PubMed] [Google Scholar]
- 6.Mavrogenis AF, Angelini A, Sakellariou VI, et al. Osteosarcoma invasion of the inferior vena cava and right atrium. J Surg Orthop Adv. 2012;21:107–112. doi: 10.3113/jsoa.2012.0107. [DOI] [PubMed] [Google Scholar]
- 7.Dahle E, Gogenur I, Norgaard P. Intestinal necrosis in young patient due to arterial tumour embolism. BMJ Case Rep. 2012 doi: 10.1136/bcr.01.2012.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennani A, Oualla K, Badioui I, et al. Symptomatic renal metastasis of osteosarcoma occurring after 7 years of complete remission: a case report. J Cancer Sci Ther. 2012;4:4–5. [Google Scholar]
- 9.Shapiro M, Wistinghausen B, Midulla P, et al. Metastatic osteosarcoma presenting as a single pulmonary microembolus. J Pediatr Surg. 2011;46:574–576. doi: 10.1016/j.jpedsurg.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Tekbas E, Tekbas G, Islamoglu Y, et al. Unusual metastatic localization of osteosarcoma in a teenager with ventricular tachycardia. Am J Emerg Med. 2010;28:1059.e1–1059.e3. doi: 10.1016/j.ajem.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Sbai MA, Ben Hmida N, Daas S, et al. Cardiac metastasis of a femoral osteosarcoma. Tunis Med. 2010;88:860–861. [PubMed] [Google Scholar]
- 12.Sahin H, Ceylan N, Bayraktaroglu S, et al. Cardiac metastasis of osteosarcoma. Cent Eur J Med. 2010;5:551–555. [Google Scholar]
- 13.Iyigun T, Ciloglu U, Ariturk C, et al. Recurrent cardiac metastasis of primary femoral osteosarcoma: a case report. Heart Surg Forum. 2010;13:E333–E335. doi: 10.1532/HSF98.20101006. [DOI] [PubMed] [Google Scholar]
- 14.De Sousa DWL, Constancio AP, Melo FM, et al. Cardiac metastasis from osteosarcoma: a case report. Pediatr Blood Cancer. 2009;53:799. [Google Scholar]
- 15.De Santi CS, dos Santos MVC, Macedo CRD, et al. Left-sided cardiac metastasis of osteosarcoma — case report. Pediatr Blood Cancer. 2009;53:800–801. [Google Scholar]
- 16.Shao L, Willard MJ, Lowe LH, et al. Fatal pulmonary tumor embolism in a child with chondroblastic osteosarcoma. Pediatr Dev Pathol. 2008;11:156–159. doi: 10.2350/07-02-0241.1. [DOI] [PubMed] [Google Scholar]
- 17.Rastogi R, Garg R, Thulkar S, et al. Unusual thoracic CT manifestations of osteosarcoma: review of 16 cases. Pediatr Radiol. 2008;38:551–558. doi: 10.1007/s00247-007-0735-3. [DOI] [PubMed] [Google Scholar]
- 18.Arkader A, Morris CD. Inferior vena cava extension of pelvic osteogenic sarcoma. Cancer Imaging. 2008;8:6–7. doi: 10.1102/1470-7330.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sales Badia JG, Galbis Caravajal JM, Vinals Larruga B, et al. Pneumonectomy with extracorporeal circulation to treat pulmonary metastasis. Arch Bronconeumol. 2007;43:180–182. [PubMed] [Google Scholar]
- 20.Hines N, Lantos G, Hochzstein J, et al. Osteosarcoma of the lumbosacral spine invading the central venous pathways, right-sided cardiac chambers, and pulmonary artery. Skeletal Radiol. 2007;36:1091–1096. doi: 10.1007/s00256-007-0338-y. [DOI] [PubMed] [Google Scholar]
- 21.Ting PT, Burrowes PW, Gray RR. Intravascular pulmonary metastases from sarcoma: appearance on computed tomography in 3 cases. Can Assoc Radiol J. 2005;56:214–218. [PubMed] [Google Scholar]
- 22.Tateishi U, Yamaguchi U, Terauchi T, et al. Extraskeletal osteosarcoma: extensive tumor thrombus on fused PET-CT images. Ann Nucl Med. 2005;19:729–732. doi: 10.1007/BF02985124. [DOI] [PubMed] [Google Scholar]
- 23.Platonov MA, Turner AR, Mullen JC, et al. Tumour on the tricuspid valve: metastatic osteosarcoma and the heart. Can J Cardiol. 2005;21:63–67. [PubMed] [Google Scholar]
- 24.Jani JC, Massad M, Kpodonu J, et al. High-grade pelvic osteosarcoma with intravascular extension to the right side of the heart: a case report and review of the literature. Arch Pathol Lab Med. 2005;129:241–243. doi: 10.5858/2005-129-241-HPOWIE. [DOI] [PubMed] [Google Scholar]
- 25.Chrouser KL, Sim FH, Lieber MM. Intravascular extension of an osteosarcoma of the pubic bone into periprostatic venous plexus. Urology. 2005;65:1001. doi: 10.1016/j.urology.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Yin WH, Young MS, Lan GY, et al. Osteogenic sarcoma metastatic to the heart via infradiaphragmatic venous extension —a case report. Acta Cardiol Sin. 2004;20:52–55. [Google Scholar]
- 27.Shafique QS, Rajesh CM, Rakesh SN. Cardiac metastases of osteosarcoma. J Coll Physicians Surg Pak. 2004;14:430–432. [PubMed] [Google Scholar]
- 28.Shah AP, Parmar S, O’Regan R. Right atrial and ventricular thrombus infiltrated with osteoblastic osteosarcoma. J Cardiovasc Pharmacol Ther. 2003;8:307–311. doi: 10.1177/107424840300800408. [DOI] [PubMed] [Google Scholar]
- 29.Santos-Machado TM, Mendes Oliveira CR, Croci AT, et al. Parosteal osteosarcoma with myocardial metastasis 13 years after follow-up. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:113–118. doi: 10.1590/s0041-87812003000200010. [DOI] [PubMed] [Google Scholar]
- 30.Newkirk L, Vater Y, Oxorn D, et al. Intraoperative TEE for the management of pulmonary tumour embolism during chondroblastic osteosarcoma resection. Can J Anaesth. 2003;50:886–890. doi: 10.1007/BF03018733. [DOI] [PubMed] [Google Scholar]
- 31.Garcia ND, Morasch MD, Sam AD, 2nd, et al. Inferior vena cava thrombus removal using hypothermic circulatory arrest in two patients with osteosarcoma. Ann Vasc Surg. 2003;17:686–689. doi: 10.1007/s10016-003-0077-z. [DOI] [PubMed] [Google Scholar]
- 32.Chai Y, Shen G. Successful resection of osteosarcoma pulmonary metastasis extending into left side of heart under cardiopulmonary bypass: a case report. Chin Med J (Engl) 2002;115:1743–1744. [PubMed] [Google Scholar]
- 33.Yilmaz M, Farsak B, Altundag MK, et al. Isolated cardiac metastasis of osteosarcoma. J Exp Clin Cancer Res. 2000;19:395–397. [PubMed] [Google Scholar]
- 34.Nelson E, Klein JS. Pulmonary infarction resulting from metastatic osteogenic sarcoma with pulmonary venous tumor thrombus. AJR Am J Roentgenol. 2000;174:531–533. doi: 10.2214/ajr.174.2.1740531. [DOI] [PubMed] [Google Scholar]
- 35.Hauret L, Minvielle F, Ehre P, et al. Metastasis in pulmonary arteries. J Radiol. 2000;81:807–809. [PubMed] [Google Scholar]
- 36.Merimsky O, Isakov J, Kollender Y, et al. Vascular invasion in high grade sarcoma of the extremity is associated with short overall survival. Oncol Rep. 1998;5:985–989. doi: 10.3892/or.5.4.985. [DOI] [PubMed] [Google Scholar]
- 37.Seibert KA, Rettenmier CW, Waller BF, et al. Osteogenic sarcoma metastatic to the heart. Am J Med. 1982;73:136–141. doi: 10.1016/0002-9343(82)90940-8. [DOI] [PubMed] [Google Scholar]
- 38.Tublin ME, Dodd GD, 3rd, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. 1997;168:719–723. doi: 10.2214/ajr.168.3.9057522. [DOI] [PubMed] [Google Scholar]
- 39.Dodd GD, 3rd, Memel DS, Baron RL, et al. Portal vein thrombosis in patients with cirrhosis: does sonographic detection of intrathrombus flow allow differentiation of benign and malignant thrombus? AJR Am J Roentgenol. 1995;165:573–577. doi: 10.2214/ajr.165.3.7645473. [DOI] [PubMed] [Google Scholar]
- 40.White RH. Identifying risk factors for venous thromboembolism. Circulation. 2003;107:4–8. doi: 10.1161/CIRCULATIONAHA.112.102814. [DOI] [PubMed] [Google Scholar]
- 41.Athale U, Cox S, Siciliano, et al. Thromboembolism in children with sarcoma. Pediatr Blood Cancer. 2007;49:171–176. doi: 10.1002/pbc.21047. [DOI] [PubMed] [Google Scholar]
- 42.Paz-Priel I, Long L, Helman LJ, et al. Thromboembolic events in children and young adults with pediatric sarcoma. J Clin Oncol. 2007;25:1519–1524. doi: 10.1200/JCO.2006.06.9930. [DOI] [PubMed] [Google Scholar]
- 43.Qian LJ, Zhu J, Zhuang ZJ, et al. Differentiation of neoplastic from bland macroscopic portal vein thrombi using dual-energy spectral CT imaging: a pilot study. Eur Radiol. 2012;22:2178–2185. doi: 10.1007/s00330-012-2477-3. [DOI] [PubMed] [Google Scholar]
- 44.Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45:2367–2375. doi: 10.1016/j.ejca.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 46.Cho WH, Song SW, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17:702–708. doi: 10.1245/s10434-009-0825-6. [DOI] [PubMed] [Google Scholar]
- 47.Isakoff MS, Barkauskas DA, Ebb D, et al. Poor survival for osteosarcoma of the pelvis: a report from the Children’s Oncology Group. Clin Orthop Relat Res. 2012;470:2007–2013. doi: 10.1007/s11999-012-2284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizuno T, Taniguchi T, Ishikawa Y, et al. Pulmonary metastasectomy for osteogenic and soft tissue sarcoma: who really benefits from surgical treatment? Eur J Cardiothorac Surg. 2013;43:795–799. doi: 10.1093/ejcts/ezs419. [DOI] [PubMed] [Google Scholar]
- 49.Salah S, Fayoumi S, Alibraheem A, et al. The influence of pulmonary metastasectomy on survival in osteosarcoma and soft-tissue sarcomas: a retrospective analysis of survival outcomes, hospitalizations and requirements of home oxygen therapy. Interact Cardiovasc Thorac Surg. 2013;17:296–302. doi: 10.1093/icvts/ivt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briccoli A, Rocca M, Salone M, et al. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985–2005. Surg Oncol. 2010;19:193–199. doi: 10.1016/j.suronc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Shepard JA, Moore EH, Templeton PA, et al. Pulmonary intravascular tumor emboli: dilated and beaded peripheral pulmonary arteries at CT. Radiology. 1993;187:797–801. doi: 10.1148/radiology.187.3.8497633. [DOI] [PubMed] [Google Scholar]