Abstract
Background
The last three decades have witnessed limited therapeutic advances in SCLC management. We evaluated real-world trends in use of systemic therapies and the impact on patient outcome in US.
Methods
We employed SEER-MEDICARE for SCLC patients diagnosed between 1985 and 2005. The 1985-1990 period served as baseline for temporal analysis conducted at 5-year intervals (1985-1990, 1991-1995, 1996-2000, 2001-2005). Cox proportional models were employed to estimate the effect of chemotherapy on survival. Results were validated using propensity-matched analysis.
Results
There were 47,351 eligible patients; male (52%); median age: 71 years; Whites-87%; Blacks-7%; Asians-1.4%. The proportion of patients treated with chemotherapy was low but increased over time (38, 55, 50, 53%; p<0.001). Race, diagnosis period, age, stage and location of residence significantly predicted chemotherapy use. Females (51%), Asians (53%) and rural residents (60%) were more likely to receive chemotherapy. The median overall survival with and without chemotherapy was 9.6 and 3.6 months. Linear trend analyses showed modest reduction in the impact of chemotherapy on survival in patients treated with chemotherapy over untreated patients (HRs: 0.59, 0.61, 0.64, 0.62; p<0.001) but an overall trend of improved survival within treated (HRs: 1.0, 1.03, 1.00, 0.96; p=0.005) and untreated (HRs: 0.99, 0.94, 0.92; p<0.001) patients. There was no survival difference between patients treated with carboplatin versus cisplatin (HR: 0.99 CI: 0.81-1.19; p=0.875). Additional therapy beyond platinum-based chemotherapy was associated with survival benefit (HR: 0.78 CI: 0.75-0.81; p<0.001).
Conclusions
Chemotherapy use was associated with survival benefit in MEDICARE SCLC patients treated in the real-world setting.
Keywords: SCLC, SEER MEDICARE, systemic therapy, trend analyses, survival, predictors
Introduction
Lung cancer is the leading cause of cancer related mortality1, 2 and more than 220,000 new cases of lung cancer were estimated to be diagnosed in the US in 2014.3 While the proportion of cases diagnosed as small cell lung cancer (SCLC) has declined from approximately 20% to 13%, this subset is still a major cause of disease burden with close to 30,000 new cases annually.4,5, 6 SCLC is associated with overall poor prognosis and median survival in untreated patients has been reported as 2-4 months.7, 8
Systemic chemotherapy remains a cornerstone of the management of SCLC.9 Platinum-based doublet chemotherapy, single agent topotecan and the multiagent chemotherapy regimen of cyclophosphamide, adriamycin and vincristine (CAV) are established regimens in the frontline and post frontline settings.9 Due to unsuccessful attempts of prospective clinical trials to establish the efficacy of newer treatment options for this disease in the last 3 decades, agents with promising efficacy in small phase II studies are commonly utilized based on the endorsement and guidelines enunciated by professional bodies.10 Such agents include paclitaxel, irinotecan, gemcitabine and docetaxel.10
The utilization of systemic agents in the real world is limited by their significant toxicity coupled with a high prevalence of other tobacco-related co-morbid illnesses in patients with SCLC. It is well known that a large proportion of patients with advanced lung cancer do not receive potentially beneficial treatment.6 Whether therapies recommended for treatment of SCLC based on evidence from clinical trials and or consensus guidelines are adopted in the real-world setting has not been carefully studied. Moreover, the clinical benefit of these therapeutic agents in the real world remains to be demonstrated. We therefore studied the predictors and clinical impact of systemic agents available for real-world management of SCLC in the last 3 decades. We also examined the pattern and trends in usage of chemotherapy agents in patients with SCLC and the clinical or socioeconomic factors that influence and predict the use of systemic therapy for SCLC. We also analyzed trends in the use of systemic therapy and the impact of these agents on patient survival.
In this analysis, we employed the linked Surveillance, Epidemiology, and End Results (SEER)-MEDICARE database 11 to evaluate trends in the real-world efficacy of available systemic therapies and the impact on outcomes in the US over two decades.
Materials and Methods
The MEDICARE database maintained by the Centers for Medicare and Medicaid Services for eligible US residents covers 97% of the US population aged 65 years or older.12 This database is purpose-linked to the cancer registry data maintained by the Surveillance Epidemiology and Endpoint Research (SEER) program of the National Cancer Institute (NCI). The SEER database is a quality-assured cancer data repository that collects data from 17 cancer registries across the entire US. SEER registries cover approximately 25% of the whole US population and contain a complete data set on treatment information for approximately 93% of all eligible patients.12 The linkage of the SEER and MEDICARE databases (SEER-MEDICARE) provides full treatment information from the MEDICARE insurance program along with individual patient level clinical and survival data from the population-based SEER cancer registry program.11 This database has been previously employed to interrogate the interplay between treatment intervention and patient characteristics and outcome. The SEER program managers and the Institutional Review Board of Emory University approved this study.
Data Extraction
Patients were identified using International Classification of Diseases for Oncology, third edition (ICD-O-3) codes 8041 to 8045. Chemotherapy information was obtained from the linked data using the chemotherapy procedure and administration data set from the MEDICARE claims file (MEDPAR, DME, HHA, HSPS, NCH, and OUTSAF) for every year from 1985 to 2005 using the following drug-specific codes: vinorelbine (J9390); pemetrexed (J9305); docetaxel (J9170); paclitaxel (J9265); cisplatin (J9060, J9062, C9418); carboplatin (J9045); gemcitabine (J9201); doxorubicin (J9000, J9001, Q2050, Q2048, J9002, Q2049, C9415); topotecan (J9350, J8705, J9351); etoposide (C9414, C9425, J8560, J9181, J9182), vincristine (J9370, J9375, J9380) and cyclophosphamide (C9420, C9421, J8530, J9070, J9080, J9090, J9091, J9092, J9093, J9094, J9095, J9096, J9097). The determination of whether a patient received chemotherapy (yes/no) was based on the data entry from the chemotherapy procedure or administration codes while the specific chemotherapy agent was identified by using the drug-specific codes in the claim files. Patients with missing information were excluded for specific analyses. In addition, we determined other treatment and supportive interventions such as radiation and palliative care by using the applicable codes in the procedure and claims file.
Patient Selection
All patients coded with a diagnosis of SCLC in the SEER-MEDICARE database between 1985 and 2005 were potentially eligible for inclusion in the analysis. Patients with additional cancer diagnosis beside SCLC were excluded in order to eliminate competing risk for the primary outcome and also to avoid potential confounding arising from possible use of chemotherapy agents for a different cancer indication. Also, patients with missing information were excluded for specific analyses where the missing data was required for analysis. Prior to 1991, the SEER-MEDICARE database collected general information on chemotherapy treatment but not the specific information on the type of chemotherapy administered. Therefore, patients diagnosed between 1985 and 1990 were excluded from analysis of survival impact of specific chemotherapy agents.
Statistical Analysis
Patient outcome associated with or without the receipt of chemotherapy was assessed across four different time intervals to explore any temporal trend in chemotherapy use and the effect of treatment on survival calculated from the time of initial diagnosis. The 1985-1990 interval period served as a baseline for a temporal survival analysis conducted at approximately 5-year intervals (1985-1990, 1991-1995, 1996-2000 & 2001-2005).
The association between survival and chemotherapy use overall (yes vs. no) or the use of specific chemotherapy agents commonly used as standard of care therapy for SCLC (cyclophosphamide, vincristine, etoposide, carboplatin, cisplatin, topotecan, doxorubicin, paclitaxel, docetaxel, gemcitabine and vinorelbine) was analyzed. Period of diagnosis (1985-1990, 1991-1995, 1996-2000, or 2001-2005), age at diagnosis, gender, race (White, Black, Asian, Hispanic, or other), stage (IV vs. others), MEDICARE qualifying event (aged vs. others), urban/rural residence (less urban/rural vs. urban/metro), radiation (yes vs. no), and surgery (yes vs. no) as predictors of clinical benefit was also analyzed.
Differences in the characteristics (age, sex, race, stage, radiation, surgery, defined treatment period, rural/urban location and MEDICARE status) of patients treated and not treated with chemotherapy were tested with Wilcoxon rank-sum test, chi-square test or Fisher's exact test as appropriate. Multivariable analysis of chemotherapy versus no chemotherapy was conducted by including age, gender, race, stage, radiation, surgery, defined treatment period, geographic location and MEDICARE status in a logistic regression model and using a backward variable selection method with an alpha level set at 0.1 for removal criteria. For linear trend analysis, Cox proportional hazards models13 were employed to estimate the adjusted effect of chemotherapy on overall survival (OS) by period of diagnosis after adjusting for age, gender, race, stage, MEDICARE status, geographical location and radiation. To evaluate if the observed survival improvement over time was due to specific chemotherapy or to other factors, we calculated the relative ratio (RR) of the HR (each HR divided by the HR of the preceding time interval) followed by a p-trend analysis to test for statistical significance of the RRs. In order to better estimate the treatment effect on survival, a propensity score analysis was employed to adjust for any imbalances between the treated and non-treated groups. Multivariable logistic regression models were used to calculate the propensity score of chemotherapy use and of each specific chemotherapy agent after controlling for year of diagnosis, age, gender, race, stage, MEDICARE status, urban/rural location, and radiation. A Cox proportional hazards model was then employed to assess the effect of specific intervention and or chemotherapy agent using the propensity score as a covariate. Survival functions were estimated by the Kaplan- Meier method for patients with and without the treatment of interest along with a log-rank test14.
All analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina) and R package version 3.21 (The R Foundation for Statistical Computing) with a significance level of 0.05 set for all tests.
Results
Demographics
We identified 47,351 patients with a diagnosis of SCLC. Out of these, 23,535 (49.7 %) patients were treated with chemotherapy versus 23,816 patients (50.3 %) who were not treated with chemotherapy. The median age of the whole population was 71 years. The eligible patients were mostly White (87%), elderly with age ≥65 years (84%) and of male gender (52%). The clinical and demographic characteristics of patients treated and not treated with chemotherapy are shown in Table 1-a.
Table 1a. Clinical and demographic characteristics of patients by Chemotherapy administration.
| Variables | Level | Chemotherapy (N=23535) | No Chemotherapy (N=23816) | P-value* |
|---|---|---|---|---|
| Year of diagnosis | 1985-1990 | 3396 (38.02) | 5535 (61.98) | <.001 |
| 1991-1995 | 5121 (55.03) | 4184 (44.97) | ||
| 1996-2000 | 5371 (49.9) | 5392 (50.1) | ||
| 2001-2005 | 9647 (52.57) | 8705 (47.43) | ||
| Race | White | 20627 (50.2) | 20465 (49.8) | <.001 |
| Black | 1610 (47.48) | 1781 (52.52) | ||
| Other | 548 (40.65) | 800 (59.35) | ||
| Asian | 353 (53.4) | 308 (46.6) | ||
| Hispanic | 202 (48.79) | 212 (51.21) | ||
| Gender | Male | 11891 (48.63) | 12560 (51.37) | <.001 |
| Female | 11644 (50.85) | 11256 (49.15) | ||
| Medicare Status Code | Aged | 21120 (50.16) | 20988 (49.84) | <.001 |
| Others | 2413 (46.16) | 2815 (53.84) | ||
| Urban/Rural | Urban/Metro | 21143 (48.72) | 22250 (51.28) | <.001 |
| Less Urban/Rural | 2391 (60.42) | 1566 (39.58) | ||
| Radiation | Yes | 10798 (57.72) | 7908 (42.28) | <.001 |
| No | 12737 (44.47) | 15908 (55.53) | ||
| Radiation type | Brain and/or CNS | 1063 (54.23) | 897 (45.77) | <.001 |
| Others | 9735 (58.13) | 7011 (41.87) | ||
| Surgery | Yes | 1274 (49.96) | 1276 (50.04) | 0.965 |
| No | 21896 (49.92) | 21970 (50.08) | ||
| Stage | IV | 10629 (48.58) | 11249 (51.42) | <.001 |
| Others | 7201 (54.52) | 6008 (45.48) | ||
| Age at diagnosis | Median (Range) | 71 (27 - 101) | 72 (24 - 99) | <.001 |
Data are presented as number of patients (%) or median (range).
The p-value is calculated by Wilcoxon rank-sum test for age; and chi-square test or Fisher's exact test for categorical covariates, where appropriate.
Trends and predictors of chemotherapy use in SCLC patients
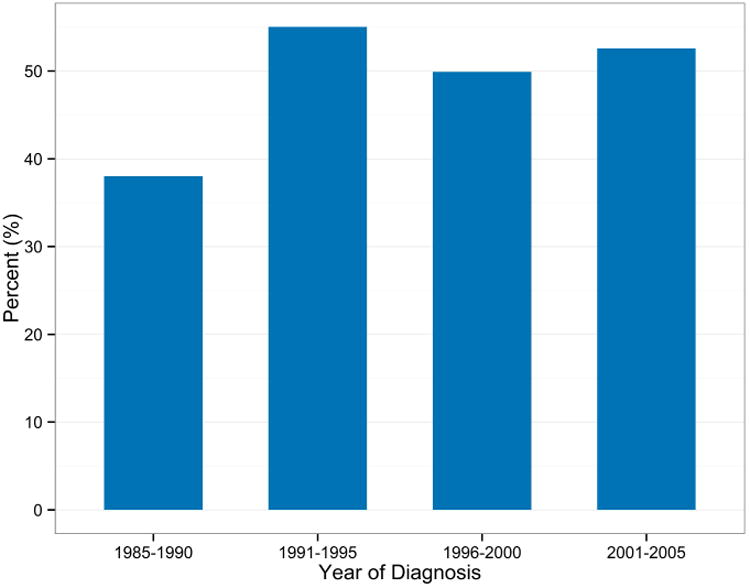
The proportion of patients treated with chemotherapy during the baseline period of 1985-1990 was very low, at only 38%. This proportion, however, increased significantly in the later time periods, reaching 53% in the 2001-2005 period (38%, 55%, 50%, 53%; p<0.001), Figure 1-a. There was variability in the rate of chemotherapy use across different racial groups with the highest rate noted in Asians (53.4%) compared to Blacks (47.5%), Whites (50.3%), and Hispanics (48.8%). Chemotherapy administration was higher in females compared to males (50.9% vs. 48.6%; p<0.001). Also, there was a significant difference in the proportion of treated and untreated patients based on MEDICARE qualifying status; 50% of patients who qualified for MEDICARE based on age received chemotherapy. Radiation use was higher in patients treated with chemotherapy compared to untreated group (57.7% vs. 42%; p<0.001). Radiation to the brain or CNS was given in approximately 10% of all patients that received radiation therapy. Patients treated with chemotherapy were more likely to receive radiation therapy both to the CNS and non CNS sites. Chemotherapy utilization was lower in stage IV patients as compared to earlier stages (48.6% vs. 54.5%; p<0.001).
Figure 1a. Proportion of treated patients by year of diagnosis.
Multivariable analysis identified years of diagnosis, white race relative to black or other races, female gender, MEDICARE qualifying status, early stages, rural residence, use of radiation, and younger age at diagnosis to be significantly associated with treatment with systemic chemotherapy agents (Table 1-b).
Table 1b. Multivariable logistic regression model of chemotherapy versus no chemotherapy.
| Covariate | Level | Odds Ratio (95% CI) | P-value |
|---|---|---|---|
| Year of diagnosis | 2001-2005 | 1.54 (1.42-1.66) | <.001 |
| 1996-2000 | 1.34 (1.24-1.46) | <.001 | |
| 1991-1995 | 1.69 (1.55-1.84) | <.001 | |
| 1985-1990 | 1 (Ref) | ||
| Race | Black | 0.88 (0.81-0.95) | 0.002 |
| Other | 0.64 (0.56-0.73) | <.001 | |
| Asian | 1.13 (0.96-1.34) | 0.147 | |
| Hispanic | 0.95 (0.76-1.17) | 0.611 | |
| White | 1 (Ref) | ||
| Gender | Male | 0.94 (0.90-0.98) | 0.005 |
| Female | 1 (Ref) | ||
| Medicare Status Code | Aged | 1.71 (1.56-1.86) | <.001 |
| Others | 1 (Ref) | ||
| Stage | IV | 0.85 (0.81-0.89) | <.001 |
| Others | 1 (Ref) | ||
| Urban/Rural | Urban/Metro | 0.62 (0.57-0.67) | <.001 |
| Less Urban/Rural | 1 (Ref) | ||
| Radiation | Yes | 1.68 (1.60-1.75) | <.001 |
| No | 1 (Ref) | ||
| Age at diagnosis | 0.98 (0.98-0.98) | <.001 |
Survival analysis
The median overall survival for all patients was 7.2 months with a significantly better overall survival in patients treated with chemotherapy over untreated patients (9.6 vs. 3.6 months, p<0.001, Figure 1-b). Analysis limited to treated patients showed no significant difference in survival between patients treated with carboplatin versus cisplatin regimens (9.6 vs. 8.4 months, p=0.775 Figure 2-a) or between those treated with platinum (carboplatin or cisplatin) chemotherapy versus non-platinum containing regimen (10.8 vs. 10.8 months, p= 0.237, Figure 2-b). In the chemotherapy treated group, patients treated with brain or CNS radiation had inferior survival compared to those receiving radiation to non CNS sites (HR: 1.21 CI: 1.13-1.29; p<0.001).
Figure 1b. Kaplan-Meier survival curves for those treated and untreated with chemotherapy.
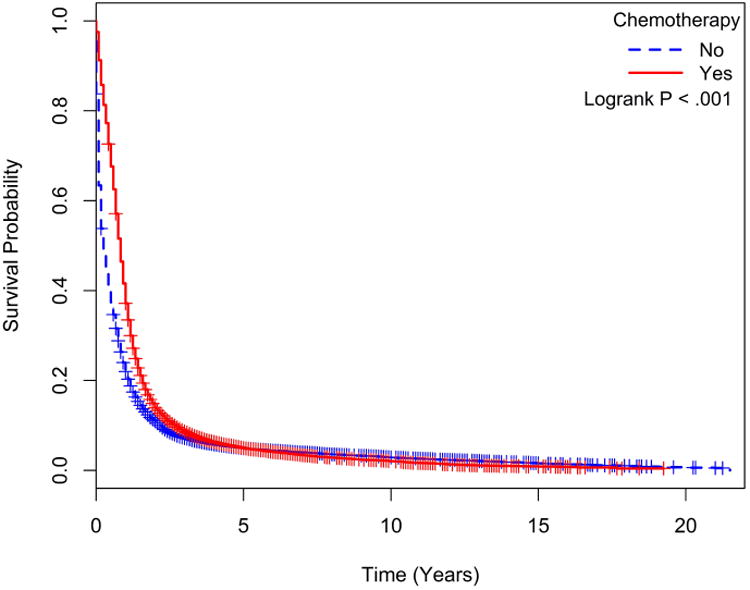
Figure 2a. Kaplan-Meier survival curves for those treated with Carboplatin versus Cisplatin.
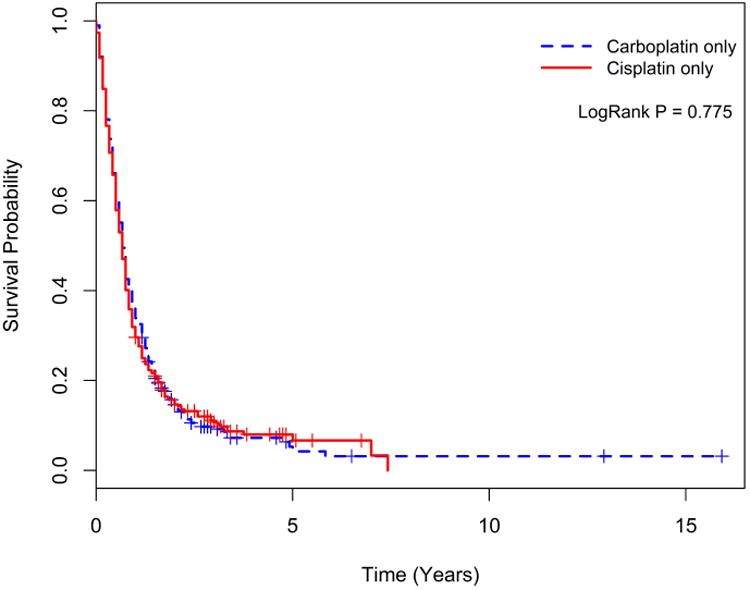
Figure 2b. Kaplan-Meier survival curves for those treated with Carboplatin or Cisplatin versus Cyclophosphamide/Doxorubicin/Vincristine.
Linear trend analyses across the defined 5-year intervals showed that survival in patients treated with chemotherapy was superior to untreated patients (HRs: 0.59; 0.61, 0.64, and 0.62 for 1985-1990, 1991-1995, 1996-2000 and 2001-2005 respectively; p<0.001) after adjusting for significant predictors of chemotherapy use (Table 2). When comparing survival trends within the patient subgroup treated with chemotherapy, we observed a significant improvement in survival over time (HRs: 1.0, 1.03, 1.00, 0.96; p=0.005; Table 3-a). Similar improvement was observed in untreated patient subgroup (HRs: 1.0, 0.99, 0.94, 0.92; p<0.001; Table 3-a).
Table 2. Relative adjusted hazard ratio of chemotherapy versus no chemotherapy across years of diagnosis.
| Period (years) | HR *(95% CI) | P-value | Ratio a (95% CI) | P-value | Ratio b (95% CI) | P-value | Ratio c (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| 1985-1990 (N=3191) | 0.587 (0.546 - 0.631) | <.001 | 1 (Ref) | - | - | - | - | - |
| 1991-1995 (N=6741) | 0.609 (0.579 - 0.640) | <.001 | 1.037 (0.995-1.080) | 0.086 | 1 (Ref) | - | - | - |
| 1996-2000 (N=8830) | 0.638 (0.611 - 0.666) | <.001 | 1.086 (1.046-1.127) | <.001 | 1.047 (1.013-1.082) | 0.005 | 1 (Ref) | - |
| 2001-2005 (N=16057) | 0.619 (0.598 - 0.640) | <.001 | 1.053 (1.022-1.086) | <.001 | 1.016 (0.988-1.045) | 0.256 | 0.970 (0.945-0.996) | 0.024 |
HR (Hazard Ratio) is calculated by the multivariable Cox proportional hazards model with the chemotherapy (compared to no chemotherapy) after adjusting for age, sex, race, stage, Medicare status, urban/rural, and radiation.
Ratio is the ratio of the adjusted HR for chemotherapy (compared to no chemotherapy) for the diagnosis year of 1991-1995, 1996-2000or 2001-2005 relative to the adjusted HR for chemotherapy (compared to no chemotherapy) for the diagnosis year of 1985-1990.
Ratio is the ratio of the adjusted HR for chemotherapy (compared to no chemotherapy) for the diagnosis year of 1996-2000or 2001-2005 relative to the adjusted HR for chemotherapy (compared to no chemotherapy) for the diagnosis year of 1991-1995.
Ratio is the ratio of the adjusted HR for chemotherapy (compared to no chemotherapy) for the diagnosis year of 2001-2005 relative to the adjusted HR for chemotherapy (compared to no chemotherapy) for the diagnosis year of 1996-2000.
Table 3a. Overall survival analysis of year of diagnosis for patients treated and not treated with chemotherapy.
| Period (years) | N | Hazard Ratio (95% CI) | P-value | Overall P-value |
|---|---|---|---|---|
| Overall population treated with chemotherapy | 0.005 | |||
|
| ||||
| 1985-1990 | 1331 | 1 (Ref) | - | |
| 1991-1995 | 3666 | 1.027 (0.964 - 1.094) | 0.404 | |
| 1996-2000 | 4327 | 1.002 (0.941 - 1.066) | 0.956 | |
| 2001-2005 | 8393 | 0.959 (0.904 - 1.017) | 0.160 | |
|
| ||||
| Overall population not treated with chemotherapy | <.001 | |||
|
| ||||
| 1985-1990 | 1860 | 1 (Ref) | - | |
| 1991-1995 | 3075 | 0.992 (0.936 - 1.052) | 0.798 | |
| 1996-2000 | 4503 | 0.944 (0.894 - 0.997) | 0.039 | |
| 2001-2005 | 7664 | 0.918 (0.872 - 0.967) | 0.001 | |
Hazard Ratio is calculated by the multivariable Cox proportional hazards model with year of diagnosis after adjusting for age, sex, race, stage, Medicare status, urban/rural, and radiation.
Survival within racial subgroups showed that the survival for Blacks during each of the four defined time periods was modestly inferior to that of Whites, while the survival for Hispanics and Asians were modestly better than for Whites (Table 3-b). Linear trend analyses of survival by race across the 5-year intervals using White patients as the reference group showed modestly improved survival for Blacks (HRs: 1.17, 1.10, 1.10, 1.05; p<0.001; Table 3-b).
Table 3b. Relative adjusted hazard ratio of raceacross years of diagnosis.
| Period (years) | Race (White as a reference) | HR *(95% CI) | P-value | Ratio a (95% CI) | P-value | Ratio b (95% CI) | P-value | Ratio c (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| 1985-1990 (N=3191) | Black | 1.172 (1.02 - 1.347) | 0.025 | 1 (Ref) | - | - | - | - | - |
| Other | 1.068 (0.883 - 1.292) | 0.498 | 1 (Ref) | - | - | - | - | - | |
| Asian | 0.363 (0.091 - 1.458) | 0.153 | 1 (Ref) | - | - | - | - | - | |
| Hispanic † | NA | NA | 1 (Ref) | - | - | - | - | - | |
|
| |||||||||
| 1991-1995 (N=6741) | Black | 1.096 (1.001 - 1.199) | 0.047 | 0.935 (0.866 - 1.008) | 0.081 | 1 (Ref) | - | - | - |
| Other | 1.252 (1.107 - 1.415) | <.001 | 1.172 (1.057 - 1.3) | 0.003 | 1 (Ref) | - | - | - | |
| Asian | 0.544 (0.402 - 0.734) | <.001 | 1.496 (0.916 - 2.444) | 0.108 | 1 (Ref) | - | - | - | |
| Hispanic | 0.946 (0.682 - 1.314) | 0.742 | NA | NA | 1 (Ref) | - | - | - | |
|
| |||||||||
| 1996-2000 (N=8830) | Black | 1.102 (1.016 - 1.194) | 0.018 | 0.94 (0.877 - 1.008) | 0.080 | 1.005 (0.947 - 1.068) | 0.858 | 1 (Ref) | - |
| Other | 0.992 (0.866 - 1.137) | 0.910 | 0.929 (0.83 - 1.039) | 0.198 | 0.793 (0.722 - 0.87) | <.001 | 1 (Ref) | - | |
| Asian | 0.816 (0.711 - 0.937) | 0.004 | 2.247 (1.533 - 3.294) | <.001 | 1.502 (1.29 - 1.748) | <.001 | 1 (Ref) | - | |
| Hispanic | 0.963 (0.804 - 1.154) | 0.684 | NA | NA | 1.018 (0.854 - 1.212) | 0.844 | 1 (Ref) | - | |
|
| |||||||||
| 2001-2005 (N=16057) | Black | 1.052 (0.987 - 1.121) | 0.123 | 0.897 (0.847 - 0.951) | <.001 | 0.96 (0.911 - 1.011) | 0.124 | 0.955 (0.908 - 1.004) | 0.069 |
| Other | 0.889 (0.791 - 1) | 0.051 | 0.833 (0.751 - 0.923) | <.001 | 0.71 (0.649 - 0.778) | <.001 | 0.896 (0.819 - 0.981) | 0.017 | |
| Asian | 0.831 (0.735 - 0.939) | 0.003 | 2.287 (1.777 - 2.942) | <.001 | 1.529 (1.35 - 1.73) | <.001 | 1.018 (0.927 - 1.117) | 0.713 | |
| Hispanic | 1.141 (0.986 - 1.32) | 0.076 | NA | NA | 1.205 (1.047 - 1.388) | 0.010 | 1.184 (1.057 - 1.327) | 0.004 | |
HR (Hazard Ratio) is calculated by the multivariable Cox proportional hazards model with each race (compared to White) after adjusting for age, sex, stage, Medicare status, urban/rural, and radiation. HR for Black and Other decreases over time (p-value < 0.001). HR for Asian and Hispanic increases over time (p-value < 0.001).
Ratio is the ratio of the adjusted HR for each race (compared to White) for the diagnosis year of 1991-1995, 1996-2000 or 2001-2005 relative to the adjusted HR for each race (compared to White) for the diagnosis year of 1985-1990.
Ratio is the ratio of the adjusted HR for each race (compared to White) for the diagnosis year of 1996-2000 or 2001-2005 relative to the adjusted HR for each race (compared to White) for the diagnosis year of 1991-1995.
Ratio is the ratio of the adjusted HR for each race (compared to White) for the diagnosis year of 2001-2005 relative to the adjusted HR for each race (compared to White) for the diagnosis year of 1996-2000.
No subjects.
Propensity score-adjusted survival analysis
In order to establish the benefit of chemotherapy using comparable treated and untreated patients, survival comparisons were conducted using propensity score-adjusted analyses, which allowed us to limit the confounding effects of patient-related prognostic factors such as comorbid illnesses that could influence both the decision to administer systemic therapy as well as overall patient outcome. Propensity score adjusted analyses confirmed the superior survival in patients treated with each of the systemic therapy agents currently employed in the real-world setting over untreated patients (p <0.001; Table 4-a). In addition, there was no significant survival difference between patients treated with carboplatin or cisplatin (HR: 0.99 CI: 0.81-1.19; p=0.875) and between patients treated with platinum agent versus non-platinum containing regimens (HR: 0.98 CI: 0.86-1.12; p=0.766). The use of topotecan increased significantly over time (p<0.001; Table 4-b). Patients treated with topotecan as salvage therapy had better survival over paclitaxel (HR: 0.6; CI: 0.43-0.82; p=0.001). Patients receiving second line therapy in addition to platinum-based chemotherapy had superior survival over patients who only received platinum-based chemotherapy (HR: 0.78 CI: 0.75-0.81; p<0.001). The result was consistent when we limited this analysis to patients treated with cisplatin (HR: 0.68 CI: 0.63-0.73; p=<0.001). Patients treated with only one type of chemotherapy agent had inferior survival to those who received two or more types of chemotherapy agents (HRs: 0.88; 0.86; 0.83; p<0.001; Table 4-c).
Table 4a. Survival comparisonswith propensity score-adjusted Cox proportional hazards models.
| Propensity score-adjusted Cox model | N | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|
| All patients (N=34,819) | |||
|
| |||
| Chemotherapy | 17717 | 0.691 (0.676 - 0.706) | <.001 |
| No chemotherapy | 17102 | 1 (Ref) | |
|
| |||
| Chemotherapy-treated patientsexcluding year of diagnosis of 1985 – 1990 *(N=16,386) | |||
|
| |||
| CYCLOPHOSPHAMIDE | |||
| YES | 1564 | 0.818 (0.774 - 0.864) | <.001 |
| NO | 14822 | 1 (Ref) | |
| VINCRISTINE | |||
| YES | 1323 | 0.827 (0.78 - 0.878) | <.001 |
| NO | 15063 | 1 (Ref) | |
| ETOPOSIDE | |||
| YES | 9433 | 0.762 (0.738 - 0.787) | <.001 |
| NO | 6953 | 1 (Ref) | |
| TOPOTECAN | |||
| YES | 1601 | 0.649 (0.614 - 0.686) | <.001 |
| NO | 14785 | 1 (Ref) | |
| DOXORUBICIN | |||
| YES | 1144 | 0.801 (0.753 - 0.852) | <.001 |
| NO | 15242 | 1 (Ref) | |
| GEMCITABINE | |||
| YES | 500 | 0.628 (0.571 - 0.69) | <.001 |
| NO | 15886 | 1 (Ref) | |
| PACLITAXEL | |||
| YES | 1763 | 0.74 (0.703 - 0.779) | <.001 |
| NO | 14623 | 1 (Ref) | |
| DOCETAXEL | |||
| YES | 547 | 0.73 (0.667 - 0.799) | <.001 |
| NO | 15839 | 1 (Ref) | |
| VINORELBINE | |||
| YES | 250 | 0.661 (0.581 - 0.752) | <.001 |
| NO | 16136 | 1 (Ref) | |
| CARBOPLATIN | |||
| YES | 7869 | 0.77 (0.745 - 0.796) | <.001 |
| NO | 8517 | 1 (Ref) | |
| CISPLATIN | |||
| YES | 3271 | 0.808 (0.776 - 0.841) | <.001 |
| NO | 13115 | 1 (Ref) | |
| CARBOPLATIN vs. CISPLATIN | |||
| CARBOPLATIN only | 255 | 0.985 (0.814 - 1.191) | 0.875 |
| CISPLATIN only | 267 | 1 (Ref) | |
| CARBOPLATIN/CISPLATIN + ETOPOSIDE (PE) vs. CYCLOPHOSPHAMIDE/DOXORUBICIN/VINCRINSTINE (CAV) | |||
| PE | 7746 | 0.981 (0.862 - 1.116) | 0.766 |
| CAV | 264 | 1 (Ref) | |
| CARBOPLATIN/ETOPOSIDE vs. CISPLATIN/ETOPSIDE | |||
| CARBOPLATIN DOUBLET | 5920 | 0.976 (0.923 - 1.031) | 0.381 |
| CISPLATIN DOUBLET | 1884 | 1 (Ref) | |
| TOPOTECAN vs. PACLITAXEL | |||
| TOPOTECAN only | 145 | 0.600 (0.438 - 0.821) | 0.001 |
| PACLITAXEL only | 87 | 1 (Ref) | |
| CISPLATIN DOUBLET + Second line therapy | |||
| CISPLATIN DOUBLET + Second line therapy | 1477 | 0.68 (0.629 - 0.736) | <.001 |
| CISPLATIN DOUBLET only | 1340 | 1 (Ref) | |
| CARBOPLATIN/CISPLATIN + ETOPOSIDE (PE) + Second line therapy | |||
| PE + Second line therapy | 4880 | 0.778 (0.748 - 0.81) | <.001 |
| PE only | 5801 | 1 (Ref) | |
The propensity score of receiving chemotherapy or specific chemotherapy of interest was estimated using a multivariable logistic regression model including year of diagnosis, age, sex, race, stage, Medicare status, urban/rural, and radiation in the population noted and included as a covariate in the Cox proportional hazards model.
Year of diagnosis of 1985 – 1990 was excluded as specific chemotherapy agent information is unavailable for the period.
Table 4b. Treatment with topotecan across years of diagnosis.
| Covariate | Level | TOPOTECAN | P value | |
|---|---|---|---|---|
|
| ||||
| Yes (N=1904) | No (N=18115) | |||
| Year of diagnosis | 1991-1995 | 10 (0.2) | 5009 (99.8) | <.001 |
| 1996-2000 | 516 (9.62) | 4849 (90.38) | ||
| 2001-2005 | 1378 (14.3) | 8257 (85.7) | ||
Data are presented as number of patients (%).
The p-value is calculated by Wilcoxon rank-sum test for numerical covariates.
Table 4c. Relative adjusted hazard ratio of multiple chemotherapy agents versus single chemotherapy agent across years of diagnosis.
| Period (years) | HR *(95% CI) | P-value | Ratio a (95% CI) | P-value | Ratio b (95% CI) | P-value |
|---|---|---|---|---|---|---|
| 1991-1995 (N=2123) | 0.883(0.776- 1.005) | 0.060 | 1 (Ref) | - | - | - |
| 1996-2000 (N=3083) | 0.855 (0.759- 0.964) | 0.010 | 0.968 (0.886 - 1.057) | 0.472 | 1 (Ref) | - |
| 2001-2005 (N=6537) | 0.832 (0.769 - 0.901) | <.001 | 0.942 (0.88 - 1.009)) | 0.087 | 0.973 (0.911 - 1.04) | 0.426 |
HR (Hazard Ratio) is calculated by the multivariable Cox proportional hazards model with the multiple chemotherapy agents (compared to a single chemotherapy agent) after adjusting for age, sex, race, stage, Medicare status, urban/rural, and radiation. HR decreases over time (p-value < .001).
Ratio is the ratio of the adjusted HR for multiple chemotherapy agents (compared to single chemotherapy agent) for the diagnosis year of 1996-2000or 2001-2005 relative to the adjusted HR for multiple chemotherapy agents (compared to single chemotherapy agent) for the diagnosis year of 1991-1995.
Ratio is the ratio of the adjusted HR for multiple chemotherapy agents (compared to single chemotherapy agent) for the diagnosis year of 2001-2005 relative to the adjusted HR for multiple chemotherapy agents (compared to single chemotherapy agent) for the diagnosis year of 1996-2000.
Discussion
We analyzed the quality-assured MEDICARE-SEER database to determine the trends in the use of FDA-approved systemic chemotherapy agents for the treatment of SCLC patients in the US. Our analysis included data from more than 47,000 patients diagnosed between 1985 and 2005. The majority of the patients (84%) were 65 years of age or older. This is in part due to the fact that our study population consisted of MEDICARE eligible patients, the majority of whom qualified based on age. Nonetheless, the age distribution was not too dissimilar to the general lung cancer patient population, where the median age at diagnosis is 65-70 years.
Similar to prior reports from our group and others, approximately 50% of the patients did not receive any systemic treatment.15, 16 This proportion was higher than the rate observed in non-small cell lung cancer patients, where about a third of the patients were not treated.15 While we could not specifically define the factors responsible for this low rate of chemotherapy usage in SCLC, we can reasonably speculate that SCLC patients are generally sicker and may suffer from other tobacco-related disease that compromised their suitability for active therapy. Moreover, the general nihilism associated with SCLC by medical oncologists and other physicians as an incurable cancer could also have contributed to this low rate of chemotherapy usage. It is reassuring that the rate of chemotherapy use in this population seemed to have increased in the more recent period covered by our study over the baseline period. While the higher rate of usage in younger patients, Asians and female patients is consistent with prior reports,17 the higher rate of chemotherapy use in rural over urban/metro patients is an intriguing observation that is not easily explained and deserves to be further studied. One possible reason for this disparity may be the increased likelihood of rural population to die in the acute hospital setting in part due to limited access to appropriate end-of-life palliative care program in the rural communities.18, 19
The efficacy of platinum agents and topotecan is supported by data from prospective randomized phase III studies.20-22 We observed a greater than 10-fold increase in the proportion of patients treated with topotecan in the late 1990s and early 2000s as compared to the early 1990s. This uptick in topotecan use coincided with published data of efficacy of topotecan in relapsed SCLC and the subsequent approval by FDA.23, 24 This data suggests that clinical trial result is a strong driver of adoption of new therapies in the real world setting. Various other agents employed for the treatment of SCLC, especially in the salvage setting, were adopted based on limited evidence generally adduced from small phase II studies.25 The demonstration in this study that agents such as paclitaxel, docetaxel and gemcitabine confer survival advantage in the real world setting is therefore important and provides additional support for this strategy that is part of current treatment guidelines. Consistent with data from clinical trials, we did not observe any survival difference between patients treated with a platinum-containing chemotherapy and those non-platinum regimens such as CAV chemotherapy.22 Although there has been no prospective comparative study of cisplatin versus carboplatin in SCLC, meta-analysis of data from prospective clinical trials of non-small cell lung cancer showed that the two agents are comparable in terms of survival.26, 27 The comparable survival between carboplatin and cisplatin observed in our study provides additional validation that either of these agents is appropriate for the treatment of SCLC patients.
To the best of our knowledge, this is the largest and most comprehensive study of clinical benefit of chemotherapy in SCLC patients treated in the real-world setting. While these findings are relevant for patient management and future research, several limitations need to be acknowledged and given adequate consideration. The retrospective nature of the analysis is an important drawback due to inability to fully control for important prognostic variables such as co-morbidities, overall disease burden, and performance status between the comparator groups. This weakness is somewhat ameliorated by the replication of the survival benefit associated with chemotherapy in propensity-matched patient subgroups. Also, the 5-year periods defined for trend analysis were arbitrarily chosen without any recourse to specific shifts in management paradigm. Since there were no newly available agents corresponding to the temporal periods, the improved outcome over time probably reflected both increased use of chemotherapy as well as potential improvement in supportive care over time. The improved survival over time within the treated and untreated patient subgroups as well as the slight reduction in the benefit of chemotherapy when comparing treated to untreated patients provides further indirect support for this assertion. Finally, the use of prophylactic cranial irradiation (PCI) for SCLC became an established intervention during the latter part of the period covered by this study.28 It is conceivable that patients treated with chemotherapy also received PCI, which could have magnified the potential benefit of systemic therapy although the survival benefit of PCI has been called into question by the preliminary report of a more recent study from Japan. Indeed, patients treated with chemotherapy in this population were more likely to also receive radiation to the CNS (54% vs. 46%). However, we were unable to establish whether the CNS radiation was prophylactic or for established brain metastasis. We observed an inferior survival in chemotherapy-treated patients who also received CNS radiation.
In conclusion, we observed clinical benefit of chemotherapy in SCLC patients treated in the real world. Both platinum-containing and salvage chemotherapy were associated with survival benefit. While the overall rate of chemotherapy use was very low, we observed a modest but significant increase in the proportion of patients treated with chemotherapy over time.
Supplementary Material
Acknowledgments
We would like to thank Dr.Anthea Hammond for editorial assistance and proofreading the manuscript.
Funding: This work was supported in part by NCI-1K23CA164015 and the Georgia Cancer Coalition awards to TKO and by the Biostatistics & Bioinformatics Shared Resource of Winship Cancer Institute of Emory University under NIH/NCI award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Footnotes
Presented in part at the 2014 Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL USA.
Conflict of Interest Disclosures: Dr.Ramalingam has been on the advisory boards and received honorarium from AstraZeneca, Abbvie, Boehringer Ingelheim, Bristol-Myers-Squibb, Celgene, Novartis, Lilly, Genentech and Merck.
References
- 1.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110:1532–1541. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Morabito A, Carillio G, Daniele G, et al. Treatment of small cell lung cancer. Crit Rev Oncol Hematol. 2014 doi: 10.1016/j.critrevonc.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 6.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 7.Kato Y, Ferguson TB, Bennett DE, Burford TH. Oat cell carcinoma of the lung. A review of 138 cases. Cancer. 1969;23:517–524. doi: 10.1002/1097-0142(196903)23:3<517::aid-cncr2820230301>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 9.Pillai RN, Owonikoko TK. Small cell lung cancer: therapies and targets. Semin Oncol. 2014;41:133–142. doi: 10.1053/j.seminoncol.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11:78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharjee Y. Medicine. Drug bestows radiation resistance on mice and monkeys. Science. 2008;320:163. doi: 10.1126/science.320.5873.163. [DOI] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Cox DR. Regression Models and Life Tables. J Royal Stat Society. 1972;B34:187–220. [Google Scholar]
- 14.RL KJaP . The statistical analysis of failure time data. New York: Wiley; 1980. [Google Scholar]
- 15.Owonikoko TK, Ragin C, Chen Z, et al. Real-world effectiveness of systemic agents approved for advanced non-small cell lung cancer: a SEER-Medicare analysis. Oncologist. 2013;18:600–610. doi: 10.1634/theoncologist.2012-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 17.Caprario LC, Kent DM, Strauss GM. Effects of chemotherapy on survival of elderly patients with small-cell lung cancer: analysis of the SEER-medicare database. J Thorac Oncol. 2013;8:1272–1281. doi: 10.1097/JTO.0b013e3182a007ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health. 2010;10:1349. [PubMed] [Google Scholar]
- 19.Menec VH, Nowicki S, Kalischuk A. Transfers to acute care hospitals at the end of life: do rural/remote regions differ from urban regions? Rural Remote Health. 2010;10:1281. [PubMed] [Google Scholar]
- 20.O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 21.Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 22.Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20:4665–4672. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 23.Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol. 1997;15:2090–2096. doi: 10.1200/JCO.1997.15.5.2090. [DOI] [PubMed] [Google Scholar]
- 24.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 25.Pelayo Alvarez M, Westeel V, Cortes-Jofre M, Bonfill Cosp X. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2013;11:CD001990. doi: 10.1002/14651858.CD001990.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 27.de Castria TB, da Silva EM, Gois AF, Riera R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2013;8:CD009256. doi: 10.1002/14651858.CD009256.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


