Abstract
Background
Individuals infected with human immunodeficiency virus (HIV) are at increased risk for severe influenza, yet immune responses to standard-dose intramuscular (IM) influenza vaccine are suboptimal in this population. Intradermal (ID) delivery of influenza vaccine might improve immune response through enhanced stimulation of dendritic cells.
Methods
We conducted a randomized, double-blind, controlled trial to compare the immunogenicity of off-label standard-dose (15 µg) ID vs standard-dose (15 µg) IM inactive influenza vaccine in HIV-infected men in Bangkok, Thailand. The primary study outcome was seroconversion (minimum titer of 1:40 and ≥4-fold rise in antibody titer) at 1 month postvaccination based on serum hemagglutination inhibition antibody titers against each vaccine strain. Adverse events (AEs) in the 7 days following vaccination were also assessed.
Results
We enrolled 400 HIV-infected participants; 200 were randomly assigned to receive IM and 200 ID vaccine. Vaccine arms were well-balanced with respect to age, CD4 cell count, HIV RNA load, and antiretroviral treatment. Percentage of seroconversion to all (ID 14% vs IM 15%; P = .8) or at least 1 (ID 69% vs IM 68%; P = .7) of the 3 vaccine strains did not differ significantly between ID vs IM vaccine recipients. A higher proportion of participants who received ID vaccine had mild injection-site AEs compared with participants who received IM vaccine (77% vs 27%).
Conclusions
There were no significant differences in the immunogenicity of standard-dose ID vs IM influenza vaccine in this HIV-infected population in Thailand. Additional strategies to enhance immune responses to influenza vaccine among HIV-infected persons are needed.
Keywords: influenza, vaccine, HIV, immunogenicity, intradermal
An estimated 35 million people live with human immunodeficiency virus (HIV) infection globally, with a disproportionate number living in lower-income countries [1]. Influenza is a common cause of respiratory illness among persons living with HIV/AIDS (PLWHA) [2], and advanced HIV is associated with higher rates of influenza hospitalization and death [3–6]. Influenza vaccination is the most effective method to prevent influenza [7]and is recommended for PLWHA by the World Health Organization [8]. While influenza vaccination is safe and moderately effective among PLWHA [7, 9–12], antibody responses to intramuscular (IM) vaccine may be impaired [13–15], with the poorest response in those with a CD4 count <200 cells/µL [15–17]. Furthermore, the durability of antibody responses to influenza vaccine may be reduced in PLWHA [18]. Thus, influenza vaccines that provide more effective and durable protection are needed for PLWHA, especially those with low CD4 cell counts.
In 2010, an intradermal (ID) influenza vaccine was licensed for use in several countries. ID delivery is thought to be more efficient than IM delivery because of the abundance of immunostimulatory cells in the dermis that act as potent antigen presenting cells [19]. Several studies have found no difference in antibody responses when comparing reduced-dose ID (3 µg hemagglutinin [HA] [20, 21] or 9 µg HA [22]) with standard-dose IM vaccines (15 µg HA) among PLWHA. Although some studies have demonstrated superior immunogenicity of equivalent-dose ID vs IM vaccine in older adults [19, 23, 24], no comparable studies have been conducted among PLWHA.
In Thailand, HIV prevalence is estimated at 1.1% among the general population [25] and is substantially higher (>20%) in certain populations such as men who have sex with men (MSM) [26]. Influenza viruses circulate perennially in Thailand, resulting in year-round opportunities for influenza transmission [27]. Thailand’s Advisory Committee on Immunization Practices recommends influenza vaccine for 8 high-risk groups, including persons with chronic diseases [28]. To determine whether ID vaccine may improve antibody responses in PLWHA, we conducted a clinical trial comparing the immunogenicity of equivalent-dose ID and IM influenza vaccines among HIV-infected MSM in Bangkok, Thailand.
METHODS
Study Design, Objectives, and Outcomes
This randomized, double-blind, controlled trial was conducted during 2011–2013 at the Silom Community Clinic (SCC), Bangkok, Thailand. SCC provides sexually transmitted infection voluntary testing and counseling services for MSM and is also the site of the Bangkok MSM Cohort Study (BMCS), a study to estimate HIV infection incidence among Thai MSM [26].
The primary study outcome was seroconversion at 1 month postvaccination based on serum hemagglutination inhibition (HI) antibody titers against each vaccine strain [29]. The secondary outcomes were seroconversion, geometric mean titer (GMT), and seroprotection, all measured by HI against each vaccine strain at 6 and 12 months postvaccination and GMT and seroprotection at 1 month postvaccination.
Enrollment, Randomization, and Blinding
Participants were recruited from SCC and other HIV clinics in Bangkok. Eligible persons were of Thai nationality, MSM, HIV infected, and 18–60 years old. Written informed consent was obtained from all eligible persons. HIV-uninfected MSM were enrolled as a laboratory control group (Supplementary Data).
At enrollment, all non-BMCS participants received confirmatory HIV testing (Supplementary Data). Participants were also tested for CD4 cell count and plasma HIV RNA load at enrollment and 1 month postvaccination.
Participants were randomly assigned in a 1:1 ratio to receive ID or IM vaccine (Supplementary Data). Randomization was initially stratified by CD4 count (≥200 or <200 cells/µL). After the first 8 months, enrollment of participants with a CD4 count <200 cells/µL lagged substantially, so stratification by CD4 cell count was dropped to ensure adequate enrollment to evaluate primary study objectives.
While study nurses were aware of vaccine assignments, all other study investigators were blinded to vaccine assignment until the conclusion of data analysis. Study participants were able to view the vaccine as they received it but were not specifically told to which vaccine group they had been assigned.
Vaccine and Vaccine Product
The IM (VAXIGRIP) and ID vaccines (Intanza/IDflu) are both inactivated, split-virion, trivalent influenza vaccines. The influenza virus strains contained in both 2011 Northern Hemisphere vaccines were A/California/7/2009(H1N1)–like virus, A/Perth/16/2009(H3N2)–like virus, and B/Brisbane/60/2008–like virus. Each 0.5-mL dose of VAXIGRIP and each 0.1-mL dose of Intanza/IDflu was formulated to contain 15 µg of HA for each strain listed. After Northern Hemisphere ID and IM vaccines expired on 31 May 2012, Southern Hemisphere vaccine was used for the remainder of the study; both vaccines had the same composition. Vaccines were approved by the Thai Food and Drug Administration. Intanza was used off-label in persons <60 years old. All vaccine products were manufactured by Sanofi Pasteur and purchased by the Thailand Ministry of Public Health (MOPH).
Influenza Serologic Testing
Participants had 10 mL of venous blood drawn prior to vaccination and at 1, 6, and 12 months postvaccination. Serum was tested by HI using guinea pig erythrocytes for antibody responses to vaccine strains. We performed 2-fold serial dilutions starting at 1:10 and ending at 1:1280; any sample positive at 1:1280 was repeated to the end dilution. Serum from all time points was tested on the same plate, and laboratory staff was blinded to randomization group and time point. Specimens were tested at the Armed Forces Research Institute of Medical Science in Bangkok, Thailand, using the standard World Health Organization protocol [30, 31].
Safety Monitoring
Participants recorded solicited and unsolicited adverse events (AEs) daily for 7 days postvaccination. AE severity was assessed using a 4-point scale [29]. AE relatedness was characterized as associated (temporally related to administration of vaccine and not explained by any other etiology) or not associated. Safety oversight involving review of all AEs was provided by a physician safety monitor who was not involved in the conduct of the study. After the first 10% of participants were enrolled, the safety monitor performed an interim analysis to determine whether stopping criteria were met (Supplementary Data).
Statistical Analysis
Seroconversion was defined as either a day 0 titer <1:10 and a day 30 titer ≥1:40 or a day 0 titer >1:10 and >4-fold rise at 1 month in serum HI titers against each vaccine strain. Seroprotection was defined as ≥1:40 antibody titer against each vaccine strain at 1 month [32]. Mean GMTs were calculated prior to vaccination (day 0) and at 1 month postvaccination (day 30); GMT ratios were calculated by dividing GMT at day 30 by GMT at day 0. To allow for calculation of GMTs, a titer of 1:5 was arbitrarily assigned to participants with undetectable (<1:10) HI antibody responses to vaccine. Missing HI titers at any visit were also assigned a titer of 1:5. We determined the percentage with 95% confidence intervals (CIs) of individuals who achieved seroconversion or seroprotection at each time point. Frequencies of seroconversion and seroprotection at 1 month were compared between study arms using χ2 or Fisher exact tests. GMTs were compared between study arms using Wilcoxon rank-sum test or Student t test. All analyses were conducted as intention-to-treat. A sensitivity analysis was performed using the “worst-case” analysis approach in which all missing outcome data were assumed to equal an undetectable immune response. Data analysts conducted all preliminary analyses with a dummy vaccine variable and were unblinded only in the final stages of analysis. All tests were 2-tailed with a level of significance of .05. In a prespecified subgroup analysis, logistic regression was used to assess for interaction between vaccine type and CD4 cell count. Analyses were conducted using SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
Sample Size
Assuming a type 1 error of 5% and type 2 error of 20%, 182 HIV-infected participants per arm would be required to demonstrate a 15% difference in the proportion of participants with seroconversion at 1 month to ID vs IM vaccine. The estimated sample size was increased to 200 participants per arm to account for 10% loss to follow-up.
This study was approved by the Ethical Review Committee for Research in Human Subjects of the Thai MOPH, and the Institutional Review Board of the Centers for Disease Control and Prevention, Atlanta, Georgia. Study findings are reported in accordance with the recommendations of the CONSORT (Consolidated Standards of Reporting Trials) statement.
RESULTS
Study Enrollment
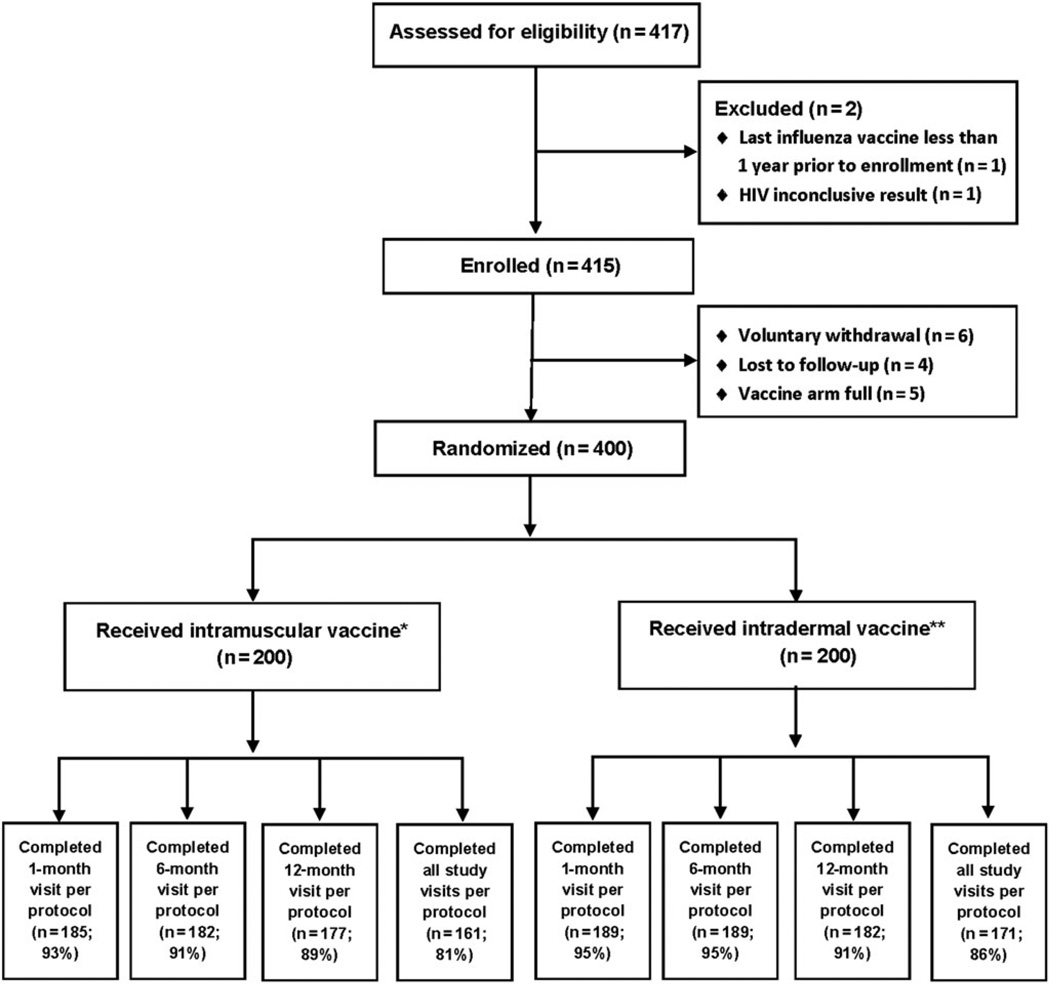
We enrolled and vaccinated 400 HIV-infected MSM (200 received IM and 200 ID vaccine; Figure 1). Among the 200 IM vaccine recipients, 185 (93%), 182 (91%), and 177 (89%), and among the 200 ID vaccine recipients, 189 (95%), 189 (95%), and 182 (91%), returned within the prespecified periods for their 1, 6, and 12 month follow-up visits, respectively (Figure 1).
Figure 1.
Enrollment and follow-up of study participants. *For intramuscular vaccine: 1 person did not complete the 1-month visit and 14 completed it outside of the acceptable window period; 11 persons did not complete the 6-month visit and 7 completed it outside the acceptable window period; 13 people did not complete the 12-month visit and 10 completed it outside the acceptable window period. **For intradermal vaccine: 1 person did not complete the 1-month visit and 10 completed it outside of the acceptable window period; 6 persons did not complete the 6-month visit and 5 completed it outside the acceptable window period; 11 people did not complete the 12-month visit and 7 completed it outside the acceptable window period. Abbreviation: HIV, human immunodeficiency virus.
Baseline Characteristics
The median age of IM and ID vaccine recipients was 29 and 30 years, respectively (Table 1). IM vs ID vaccine recipients were similar with respect to socioeconomic status, tobacco and drug use, medical comorbidities, and HIV parameters. At enrollment, 45 of 200 (23%) IM and 40 of 200 (20%) ID vaccine recipients had a CD4 count <200 cells/µL, 165 of 200 (83%) IM and 162 of 200 (81%) ID vaccine recipients had detectable HIV RNA loads, and 79 of 200 (40%) IM and 90 of 200 (45%) ID vaccine recipients were receiving antiretroviral therapy. Most participants were recently diagnosed with HIV, with a median duration of 1.7 years in both groups (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants, by Vaccine Arm
| Characteristic | Intramuscular Vaccine (n = 200) |
Intradermal Vaccine (n = 200) |
|---|---|---|
| Age, y, median (range) | 29 (20–53) | 30 (19–50) |
| BMCS cohort member | 76 (38) | 75 (38) |
| Employment status | ||
| Full time | 166 (83) | 180 (90) |
| Unemployed | 5 (3) | 4 (2) |
| Other | 29 (15) | 16 (8) |
| Education level | ||
| Less than high school | 5 (3) | 10 (5) |
| High school | 70 (35) | 63 (32) |
| Greater than high school | 125 (63) | 127 (64) |
| Income level | ||
| <10 000 Baht | 46 (23) | 43 (22) |
| 10 001–15 000 Baht | 53 (27) | 45 (23) |
| ≥15 001 Baht | 100 (50) | 111 (56) |
| Unknown | 1 (1) | 1 (1) |
| Current tobacco use | 45 (23) | 42 (21) |
| Injection drug usea | 4 (2) | 4 (2) |
| Non–injection drug useb | 65 (33) | 64 (32) |
| Medical conditions | ||
| Hepatitis B | 8 (4) | 12 (6) |
| Hepatitis C | 3 (2) | 2 (1) |
| Tuberculosis | 10 (5) | 16 (8) |
| Asthma | 5 (3) | 4 (2) |
| Diabetes | 0 (1) | 2 (1) |
| Chronic lung disease | 0 | 1 (1) |
| Cardiovascular disease | 2 (1) | 3 (2) |
| Hospitalized in month prior to vaccination |
0 (0) | 3 (2) |
| HIV infection duration, y, median (range) |
1.7 (0–20) | 1.7 (0–38) |
| Nadir CD4 count | ||
| <200 cells/µL | 82 (41) | 82 (41) |
| ≥200cells/µL | 109 (55) | 112 (56) |
| CD4 count at study enrollment | ||
| <200 cells/µL | 45 (23) | 40 (20) |
| ≥200cells/µL | 155 (78) | 160 (80) |
| Detectable HIV RNA load at study enrollment |
165 (83) | 162 (81) |
| On antiretroviral therapy | 79 (40) | 90 (45) |
| Years on antiretroviral therapy, median (range) |
1.0 (0–15) | 1.0 (0–18) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: BMCS, Bangkok Men Who Have Sex With Men Cohort Study; HIV, human immunodeficiency virus.
Use in the 4 months prior to vaccination.
Including marijuana, cocaine, ecstasy, crystal methamphetamine, γ-hydroxybutyric acid, ketamine, inhalants, or poppers.
Safety/Adverse Events
A higher proportion of ID vaccine recipients (153/200; 77% [95% CI, 70–82]) had at least 1 grade 1 or 2 injection-site AE compared with IM vaccine recipients (53/200; 27% [95% CI, 21–33]) including redness, swelling, and tenderness. No participant experienced grade 3 or higher injection-site AEs (Table 2). A similar proportion of participants in each group experienced systemic AEs. The most common systemic reactions included myalgia, malaise, and headache. Grade 3 or higher systemic AEs were rare, occurring in ≤4% of participants in either vaccine group.
Table 2.
Solicited Adverse Events (AEs) During the 7 Days After Receipt of Vaccine, by Vaccine Type and AE Severity Gradea
| Adverse Event | Intramuscular Vaccine (n=200), No. (%; 95% Confidence Interval) |
Intradermal Vaccine (n=200), No. (%; 95% Confidence Interval) |
||
|---|---|---|---|---|
| Grade 1–2 | Grade ≥3 | Grade 1–2 | Grade ≥3 | |
| Injection-site reactions | ||||
| Pain | 26 (13; 9–18) | 0 | 26 (13; 9–18) | 0 |
| Redness | 5 (3; 1–6) | 0 | 84 (42; 35–49) | 0 |
| Swelling | 5 (3; 1–6) | 0 | 120 (60; 53–67) | 0 |
| Tenderness | 38 (19; 14–25) | 0 | 73 (37; 30–44) | 0 |
| Any injection- site reaction |
53 (27; 21–33) | 0 | 153 (77; 70–82) | 0 |
| Systemic reactions | ||||
| Feverishness | 30 (15; 10–21) | 2 (1; 0–2) | 20 (10; 6–15) | 1 (1; 0–3) |
| Malaise | 45 (23; 17–29) | 3 (2; 0–4) | 55 (28; 21–34) | 2 (1; 0–4) |
| Myalgia | 40 (20;15–26) | 5 (3; 0–6) | 61 (31; 24–37) | 1 (1; 0–3) |
| Headache | 39 (20; 14–26) | 4 (2; 0–4) | 47 (24; 18–30) | 2 (1; 0–4) |
| Nausea | 21 (11; 7–16) | 0 | 22 (11; 7–16) | 1 (1; 0–3) |
| Itching | 16 (8; 5–13) | 2 (1; 0–4) | 20 (10; 6–15) | 0 |
| Any systemic reaction |
90 (45; 38–52) | 7 (4; 1–7) | 101 (51; 43–58) | 4 (2; 1–5) |
| Any adverse event | 112 (56; 49–63) | 7 (4; 1–7) | 169 (85; 79–89) | 4 (2; 1–5) |
Grade 1 = mild (no interference with activity); grade 2 = moderate (some interference with activity); grade 3 = severe (prevents daily activity); grade 4 = life-threatening (emergency department visit or hospitalization).
Antibody Responses at 1 Month Postvaccination
Antibody responses 1 month postvaccination did not significantly differ between vaccine groups (Table 3). Prior to vaccination, seroprotection to all 3 vaccine strains was 0% in both vaccine groups, and seroprotection to at least 1 of the 3 vaccine strains was 22% among IM and 19% among ID vaccine recipients (Table 3). At 1 month postvaccination, seroprotection to all 3 vaccine strains occurred in 39 of 200 (20%) IM vs 43 of 200 (22%) ID vaccine recipients (P = .6); seroprotection to at least 1 of the 3 vaccine strains occurred in 153 of 200 (77%) IM vs 148 of 200 (74%) ID vaccine recipients (P = .6; Table 3). Seroconversion to all 3 vaccine strains occurred in 30 of 200 (15%) IM vs 28 of 200 (14%) ID vaccine recipients (P = .8); seroconversion to at least 1 of the 3 vaccine strains occurred in 135 of 200 (68%) IM vs 138 of 200 (69%) ID vaccine recipients (P = .7).
Table 3.
Antibody Responses Prior to Vaccination and at 1 Month Postvaccination, by Vaccine Arm
| Virus | Intramuscular Vaccine (n = 200) | Intradermal Vaccine (n = 200) | P Value | ||
|---|---|---|---|---|---|
| Day 0 | Day 30 | Day 0 | Day 30 | ||
| Influenza A(H1N1) | |||||
| Seroprotection, No. (%; 95% CI) | 23 (12; 7–16) | 120 (60; 53–67) | 22 (11; 7–16) | 113 (57; 49–63) | .5 |
| Seroconversion, No. (%; 95% CI) | … | 97 (49; 41–56) | … | 94 (47; 40–54) | .8 |
| Geometric mean titer (range) | 10 (9–11) | 43 (36–52) | 10 (9–12) | 40 (33–49) | .7 |
| Geometric mean titer ratio (range) | … | 4 (4–5) | … | 4 (3–5) | .6 |
| Influenza A(H3N2) | |||||
| Seroprotection, No. (%; 95% CI) | 17 (9; 5–13) | 88 (44; 37–51) | 11 (6; 3–10) | 92 (46; 39–53) | .7 |
| Seroconversion, No. (%; 95% CI) | … | 68 (34; 27–41) | … | 83 (42; 35–49) | .1 |
| Geometric mean titer (range) | 7 (7–8) | 25 (21–30) | 7 (6–8) | 27 (22–33) | .5 |
| Geometric mean titer ratio (range) | … | 3 (3–4) | … | 4 (3–4) | .5 |
| Influenza B | |||||
| Seroprotection, No. (%; 95% CI) | 7 (4; 1–7) | 83 (42; 35–49) | 7 (4; 1–7) | 89 (45; 37–52) | .5 |
| Seroconversion, No. (%; 95% CI) | … | 73 (37; 30–44) | … | 72 (36; 29–43) | .9 |
| Geometric mean titer (range) | 7 (6–7) | 24 (20–28) | 8 (7–9) | 24 (20–28) | .9 |
| Geometric mean titer ratio (range) | … | 3 (3–4) | … | 3 (3–4) | .3 |
| All 3 influenza virus strains | |||||
| Seroprotection, No. (%; 95% CI) | 0 | 39 (20; 14–26) | 0 | 43 (22; 16–28) | .6 |
| Seroconversion, No. (%; 95% CI) | … | 30 (15; 10–21) | … | 28 (14; 10–20) | .8 |
| At least 1 of the 3 virus strains | |||||
| Seroprotection, No. (%; 95% CI) | 43 (22; 16–28) | 153 (77; 70–82) | 37 (19; 13–25) | 148 (74; 67–80) | .6 |
| Seroconversion, No. (%; 95% CI) | … | 135 (68; 61–74) | … | 138 (69; 62–75) | .7 |
Abbreviation: CI, confidence interval.
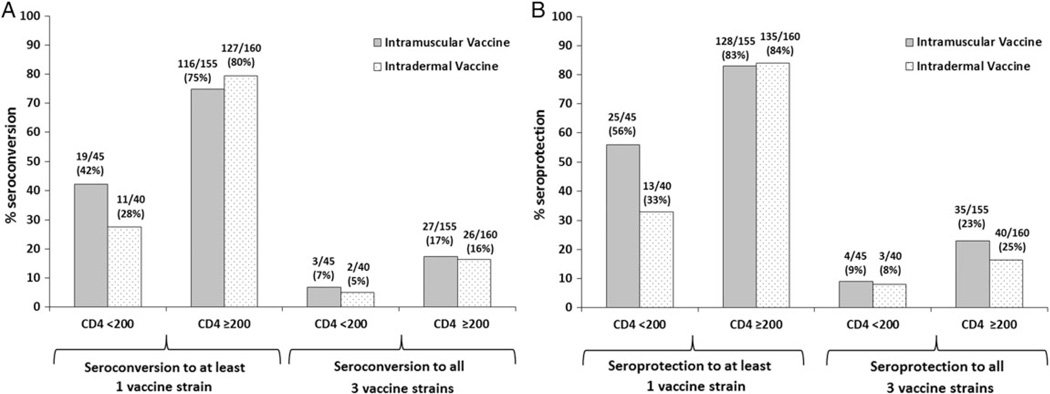
For seroconversion, among participants with a CD4 count ≥200 cells/µL, 27 of 155 (17%) and 116 of 155 (75%) IM vaccine recipients and 26 of 160 (16%) and 127 of 160 (80%) of ID vaccine recipients seroconverted to all or at least 1 of the 3 vaccine strains, respectively, at 1 month postvaccination. Among participants with a CD4 count <200 cells/µL, 3 of 45 (7%) and 19 of 45 (42%) IM vaccine recipients and 2 of 40 (5%) and 11 of 40 (28%) ID vaccine recipients seroconverted to all or at least 1 of the 3 vaccine strains, respectively, at 1 month postvaccination (Figure 2A). For seroprotection, among participants with a CD4 count ≥200 cells/µL, 35 of 155 (23%) and 128 of 155 (83%) IM vaccine recipients and 40 of 160 (25%) and 135 of 160 (84%) ID vaccine recipients had seroprotection to all or at least 1 of the 3 vaccine strains, respectively, at 1 month postvaccination. Among participants with a CD4 count <200 cells/µL, 4 of 45 (9%) and 25 of 45 (56%) IM vaccine recipients and 3 of 40 (8%) and 13 of 40 (33%) ID vaccine recipients had seroprotection to all or at least 1 of the 3 vaccine strains, respectively, at 1 month postvaccination (Figure 2B). In our prespecified subanalysis, we found a significant interaction term (P = .046) between CD4 cell count and vaccine arm for seroprotection but not seroconversion to any of the three viruses.
Figure 2.
A, Proportion of human immunodeficiency virus (HIV)-infected participants who seroconverted to at least 1 or all of the 3 vaccine strains at 1 month postvaccination, stratified by CD4 count (cells/µL) and vaccine type. B, Proportion of HIV-infected participants with seroprotection to at least 1 or all 3 of the vaccine strains at 1 month postvaccination, stratified by CD4 cell count and vaccine type.
Antibody Responses at 6 and 12 Months Postvaccination
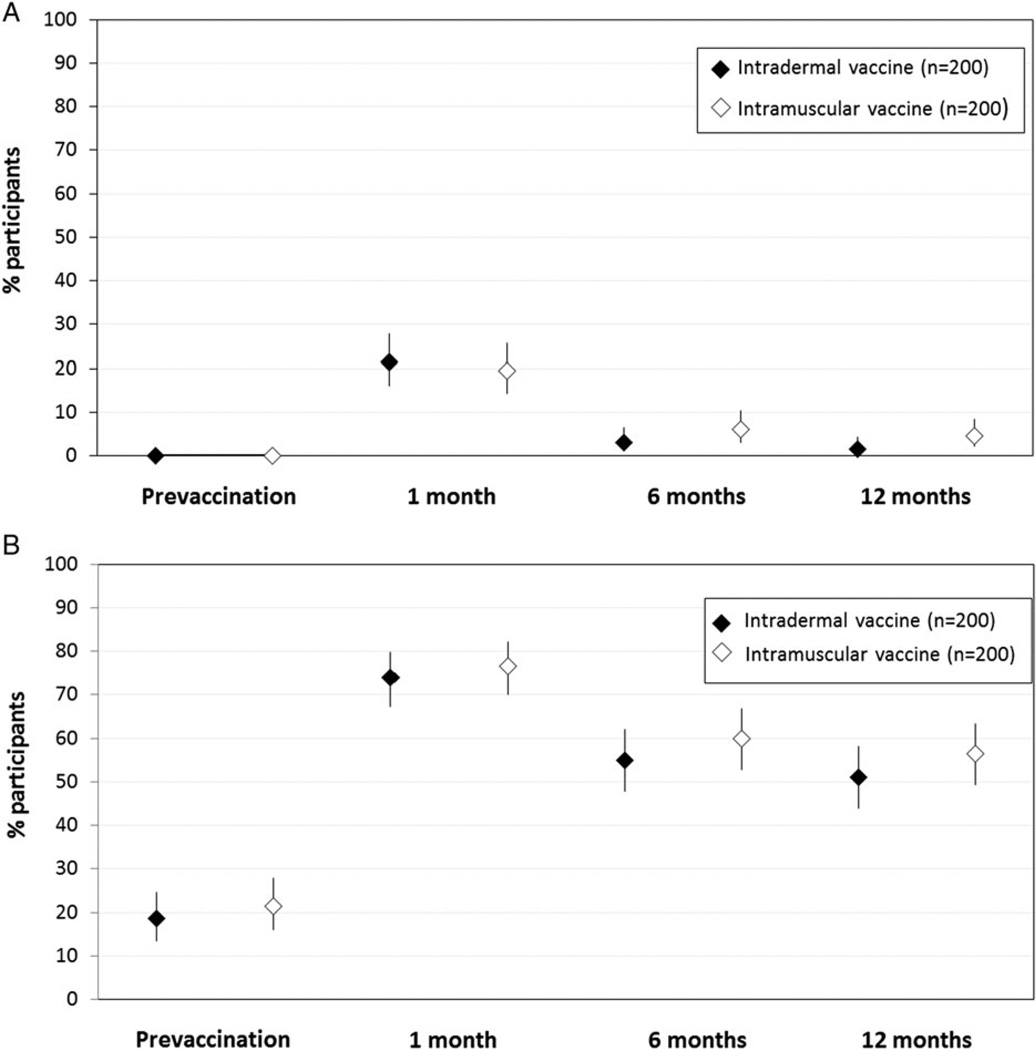
The proportion of participants with seroprotection to all (Figure 3A) or at least 1 (Figure 3B) of the 3 vaccine strains was lower at 6 months vs 1 month postvaccination for both vaccine groups. Seroprotection at 6 and 12 months was similar for both vaccine groups. Similar to the findings for seroprotection, seroconversion to all (Supplementary Figure 1A) or at least 1 (Supplementary Figure 1B) of the 3 vaccine strains was lower at 6 months vs 1 month postvaccination, and similar at 6 months vs 12 months, for both vaccine groups. GMTs to each of the 3 virus strains did not significantly differ between vaccine groups at 1, 6, and 12 months postvaccination (Supplementary Figure 2A, 2B, 2C).
Figure 3.
A, Proportion and 95% confidence interval (CI) of human immunodeficiency virus (HIV)-infected participants with seroprotection to all 3 vaccine strains prior to and at 1, 6, and 12 months postvaccination. B, Percentage and 95% CI for HIV-infected participants with seroprotection to at least 1 of the 3 vaccine strains prior to and at 1, 6, and 12 months postvaccination.
DISCUSSION
In our population of HIV-infected MSM in Bangkok, standarddose ID influenza vaccine was safe but was not more immunogenic at 1 month postvaccination compared with standard-dose IM vaccine. Seroconversion was low for both vaccine groups and ranged from 14% to all vaccine strains to 77% to at least 1 vaccine strain. Antibody responses were reduced at 6 and 12 months compared with 1 month postvaccination and were similar in both vaccine groups. Vaccine responses may have been modified by immune status. Among participants with a CD4 count <200 cells/µL, seroconversion and seroprotection was higher at 1 month among IM compared to ID vaccine recipients, although only the difference in seroprotection was statistically significant. Overall, regardless of vaccine group, antibody responses were higher for participants with a CD4 count >200 cells/µL.
While influenza vaccination is recommended for all PLWHA, the efficacy of standard-dose IM vaccine is suboptimal [9, 10, 12]. Because vaccine efficacy studies with symptomatic influenza as the endpoint require substantial resources, serologic responses to vaccine are often used as a proxy for efficacy. Many studies have found suboptimal immunogenicity of influenza vaccine among PLWHA, consistent with our findings [13– 17]. Strategies for boosting antibody responses to influenza vaccine, including use of higher-dose vaccines [33, 34], multidose regimens [33, 35, 36], and vaccine adjuvants [36–39] have demonstrated mixed results.
ID delivery of influenza vaccine is a promising approach to increasing immunogenicity in older adults and immunocompromised populations [40]. Studies among non-HIV-infected older adults, who may have decreased immune function, have found standard-dose ID vaccine to be more immunogenic than conventional IM vaccine [23, 24]. However, few studies in PLWHA have examined antibody responses to vaccine delivered by an ID vs IM route, and none have compared standard-dose ID to standard-dose IM vaccine. Studies comparing low-dose ID to standard-dose IM vaccine have found noninferiority of low-dose ID vaccine [20–22]. Given previous findings, it was somewhat surprising that ID vaccine did not elicit higher HI responses 1 month postvaccination in our study. One contributing factor may be that the majority of participants had detectable HIV RNA loads despite having CD4 counts >200 cells/µL. HIV virologic suppression has been associated with increased seroconversion rates postvaccination [41, 42]. Our study was not designed to examine associations between viral suppression and antibody responses to vaccination.
Although overall antibody responses to both vaccine types at 1 month were relatively low, responses were higher among those with CD4 >200 cells/µL, consistent with findings from multiple other studies [15–17]. Although our study was not powered to look at antibody responses to vaccine by CD4 cell count, we did observe a trend toward lower antibody responses as measured by seroconversion and seroprotection at 1 month following ID vs IM vaccination among those with CD4 counts <200 cells/µL. While there was an initial drop in seroconversion and seroprotection between 1 and 6 months postvaccination, antibody responses generally remained stable between 6 and 12 months. Data on antibody persistence in PLWHA after influenza vaccination are limited. Similar to our findings, Crum-Cianflone and colleagues found a decline in seroprotection after a single dose of monovalent 2009 influenza A(H1N1) vaccine at 6 months postvaccination [18]. In countries such as Thailand, where influenza circulates year-round, additional strategies are needed to boost the durability of immune responses to influenza vaccine.
As has been previously described [19], ID vaccination did result in a higher number of low-grade injection-site reactions compared with IM vaccination. However, neither vaccine elicited high-grade local reactions, and both had similar systemic AE profiles. Although the increase in local injection-site reactions is a potential deterrent to widespread use of ID vaccine, studies have shown that ID vaccine is highly acceptable because of the use of smaller needles with a decreased depth of penetration and decreased pain at the time of injection [43, 44]. Cost may also be a potential deterrent in low- and middle-income countries, as newer technologies for delivery of ID vaccine, including microinjection systems and disposable-syringe jet injectors, are more expensive than traditional IM delivery. Both cost and AE profiles will need to be considered as ID vaccine strategies are investigated for use in PLWHA in low- to middle-income countries.
Several limitations to this study should be noted. Immunogenicity may not be directly translatable to clinical disease prevention strategies. However, a recent randomized controlled trial by Madhi and colleagues of trivalent inactivated influenza vaccine vs placebo among PLWHA found higher clinical efficacy against confirmed influenza illness than predicted by corresponding immunologic outcomes [9], supporting the use of antibodies as a reasonable proxy for clinical vaccine efficacy. Second, given that the Thai MSM included in this study were relatively homogenous with respect to age and HIV indicators, our ability to generalize these results to other populations may be limited. Third, while vaccine response may have been modified by CD4 cell count, our study was not powered to look at antibody responses stratified by CD4 count. A major strength of this study was the high retention rate, which allowed us to evaluate our primary outcome of seroconversion at 1 month postvaccination as well as secondary seroconversion and seroprotection outcomes at 6 and 12 months postvaccination.
Our data support the safety of ID influenza vaccine among PLWHA and also demonstrate comparable immunogenicity of standard-dose ID and IM vaccine. As the majority of participants in our study had relatively preserved immune function, additional studies are needed to specifically examine the immunogenicity of ID vaccine among persons with CD4 counts <200 cells/µL. Although ongoing strategies are needed to optimize immune responses to influenza vaccine among PLWHA, currently available vaccines are still moderately efficacious in reducing the risk of influenza illness and should be administered yearly in this vulnerable population.
Supplementary Material
Acknowledgments
The authors thank all study participants, as well as Charung Muangchana, Chawetsan Namwat, Waranya Yommakhot, Ratchadaporn Unrueam, Pornchai Sornsathapornkul, Supaporn Chaikummao, and the staff of the Silom Clinic for their support of this project.
Financial support. This work was supported by the CDC (cooperative agreement 5U01GH000152).
Footnotes
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC), the Thailand Ministry of Public Health, or the Department of Defense.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Clinical Trials Registration. NCT01538940.
References
- 1.World Health Organization. [Accessed 8 December 2014];World health statistics. Available at: http://apps.who.int/iris/bitstream/10665/82058/1/WHO_HIS_HSI_13.1_eng.pdf?ua=1&ua=1.
- 2.Klein MB, Lu Y, DelBalso L, Cote S, Boivin G. Influenza virus infection is a primary cause of febrile respiratory illness in HIV-infected adults, despite vaccination. Clin Infect Dis. 2007;45:234–240. doi: 10.1086/518986. [DOI] [PubMed] [Google Scholar]
- 3.Cohen C, Moyes J, Tempia S, et al. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis. 2013;19:1766–1774. doi: 10.3201/eid1911.130546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med. 2001;161:441–446. doi: 10.1001/archinte.161.3.441. [DOI] [PubMed] [Google Scholar]
- 5.Neuzil KM, Reed GW, Mitchel EF, Jr, Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–907. doi: 10.1001/jama.281.10.901. [DOI] [PubMed] [Google Scholar]
- 6.Ormsby CE, de la Rosa-Zamboni D, Vazquez-Perez J, et al. Severe 2009 pandemic influenza A (H1N1) infection and increased mortality in patients with late and advanced HIV disease. AIDS. 2011;25:435–439. doi: 10.1097/QAD.0b013e3283434844. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep. 2013;62:1–43. (RR-07) [PubMed] [Google Scholar]
- 8.World Health Organization. [Accessed 9 December 2014];Vaccines against influenza: WHO position paper. Available at: http://www.who.int/wer/2012/wer8747.pdf?ua=1.
- 9.Madhi SA, Maskew M, Koen A, et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis. 2011;52:128–137. doi: 10.1093/cid/ciq004. [DOI] [PubMed] [Google Scholar]
- 10.Tasker SA, Treanor JJ, Paxton WB, Wallace MR. Efficacy of influenza vaccination in HIV-infected persons: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:430–433. doi: 10.7326/0003-4819-131-6-199909210-00006. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka H, Teruya K, Tanaka M, et al. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39:167–173. [PubMed] [Google Scholar]
- 12.Remschmidt C, Wichmann O, Harder T. Influenza vaccination in HIV-infected individuals: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness and safety. Vaccine. 2014;32:5585–5592. doi: 10.1016/j.vaccine.2014.07.101. [DOI] [PubMed] [Google Scholar]
- 13.Crum-Cianflone NF, Eberly LE, Duplessis C, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis. 2011;52:138–146. doi: 10.1093/cid/ciq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson KE, Clements ML, Miotti P, Cohn S, Polk BF. The influence of human immunodeficiency virus (HIV) infection on antibody responses to influenza vaccines. Ann Intern Med. 1988;109:383–388. doi: 10.7326/0003-4819-109-5-383. [DOI] [PubMed] [Google Scholar]
- 15.Tiu CT, Lin YS, Pagala M, et al. Antibody response to inactivated influenza A (H1N1) 2009 monovalent vaccine in patients with and without HIV. J Acquir Immune Defic Syndr. 2011;58:e99–e102. doi: 10.1097/QAI.0b013e318232b50e. [DOI] [PubMed] [Google Scholar]
- 16.Fuller JD, Craven DE, Steger KA, Cox N, Heeren TC, Chernoff D. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 17.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine. 2000;18:3040–3049. doi: 10.1016/s0264-410x(00)00079-7. [DOI] [PubMed] [Google Scholar]
- 18.Crum-Cianflone NF, Iverson E, Defang G, et al. Durability of antibody responses after receipt of the monovalent 2009 pandemic influenza A (H1N1) vaccine among HIV-infected and HIV-uninfected adults. Vaccine. 2011;29:3183–3191. doi: 10.1016/j.vaccine.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respi Viruses. 2013;7:584–603. doi: 10.1111/irv.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelinck LB, van den Bemt BJ, Marijt WA, et al. Intradermal influenza vaccination in immunocompromized patients is immunogenic and feasible. Vaccine. 2009;27:2469–2474. doi: 10.1016/j.vaccine.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Khanlou H, Sanchez S, Babaie M, et al. The safety and efficacy of dose-sparing in-tradermal administration of influenza vaccine in human immunodeficiency virus-positive patients. Arch Intern Med. 2006;166:1417. doi: 10.1001/archinte.166.13.1417. [DOI] [PubMed] [Google Scholar]
- 22.Ansaldi F, Valle L, de Florentiis D, et al. Phase 4 randomized trial of intradermal low-antigen-content inactivated influenza vaccine versus standard-dose intramuscular vaccine in HIV-1-infected adults. Human Vaccin Immunother. 2012;8:1048–1052. doi: 10.4161/hv.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 25.Joint United Nations Programme on HIV/AIDS. [Accessed 9 December 2014];HIV and AIDS estimates. Available at: http://www.unaids.org/en/regionscountries/countries/thailand.
- 26.van Griensven F, Thienkrua W, McNicholl J, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS. 2013;27:825–832. doi: 10.1097/QAD.0b013e32835c546e. [DOI] [PubMed] [Google Scholar]
- 27.Chittaganpitch M, Supawat K, Olsen SJ, et al. Influenza viruses in Thailand: 7 years of sentinel surveillance data, 2004–2010. Influenza Other Respir Viruses. 2012;6:276–283. doi: 10.1111/j.1750-2659.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owusu JT, Prapasiri P, Ditsungnoen D, et al. Seasonal influenza vaccine coverage among high-risk populations in Thailand, 2010–2012. Vaccine. 2014;33:742–747. doi: 10.1016/j.vaccine.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. [Accessed 16 December 2014];Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm.
- 30.World Health Organization. [Accessed 10 December 2015];The 2011–2012 WHO influenza reagent kit for identification of influenza isolates. Available at: http://www.influenzareagentresource.org/Portals/6/PDFS/WHO%20Insert%202011–2012.pdf.
- 31.World Health Organization. [Accessed 31 March 2015];WHO global influenza surveillance network manual for the laboratory diagnosis and virological surveillance of influenza. Available at: http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf.
- 32.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 33.El Sahly HM, Davis C, Kotloff K, et al. Higher antigen content improves the immune response to 2009 H1N1 influenza vaccine in HIV-infected adults: a randomized clinical trial. J Infect Dis. 2012;205:703–712. doi: 10.1093/infdis/jir837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med. 2013;158:19–26. doi: 10.7326/0003-4819-158-1-201301010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Bickel M, von Hentig N, Wieters I, et al. Immune response after two doses of the novel split virion, adjuvanted pandemic H1N1 influenza A vaccine in HIV-1-infected patients. Clin Infect Dis. 2011;52:122–127. doi: 10.1093/cid/ciq003. [DOI] [PubMed] [Google Scholar]
- 36.Cooper C, Klein M, Walmsley S, et al. High-level immunogenicity is achieved vaccine with adjuvanted pandemic H1N1(2009) and improved with booster dosing in a randomized trial of HIV-infected adults. HIV Clin Trials. 2012;13:23–32. doi: 10.1310/hct1301-023. [DOI] [PubMed] [Google Scholar]
- 37.Bickel M, Wieters I, Khaykin P, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–F35. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 38.Kajaste-Rudnitski A, Galli L, Nozza S, et al. Induction of protective antibody response by MF59-adjuvanted 2009 pandemic A/H1N1v influenza vaccine in HIV-1-infected individuals. AIDS. 2011;25:177–183. doi: 10.1097/QAD.0b013e328341afa8. [DOI] [PubMed] [Google Scholar]
- 39.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza A H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52:248–256. doi: 10.1093/cid/ciq104. [DOI] [PubMed] [Google Scholar]
- 40.Durando P, Iudici R, Alicino C, et al. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Human Vaccin. 2011;7(suppl):29–40. doi: 10.4161/hv.7.0.14560. [DOI] [PubMed] [Google Scholar]
- 41.Leahy TR, Goode M, Lynam P, Gavin PJ, Butler KM. HIV virological suppression influences response to the AS03-adjuvanted monovalent pandemic influenza A H1N1 vaccine in HIV-infected children. Influenza Other Respir Viruses. 2014;8:360–366. doi: 10.1111/irv.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maruszak H, Jeganathan S, Smith DE, Robertson P, Barnes T, Furner V. Improved serological response to H1N1 monovalent vaccine associated with viral suppression among HIV-1-infected patients during the 2009 influenza (H1N1) pandemic in the southern hemisphere. HIV Med. 2012;13:352–357. doi: 10.1111/j.1468-1293.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- 43.Arnou R, Frank M, Hagel T, Prebet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther. 2011;28:555–565. doi: 10.1007/s12325-011-0035-z. [DOI] [PubMed] [Google Scholar]
- 44.Dhont PA, Albert A, Brenders P, et al. Acceptability of Intanza(R) 15 mug intra-dermal influenza vaccine in Belgium during the 2010–2011 influenza season. Adv Ther. 2012;29:562–577. doi: 10.1007/s12325-012-0025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.