Abstract
Transfection efficiency and toxicity concerns remain a challenge for gene therapy. Cell penetrating peptides (CPPs) have been broadly investigated to improve the transfection of genetic material (e.g., pDNA and siRNA). Here, a synthetic CPP (polylysine, K9 peptide) was complexed with angiotensin II type 2 receptor (AT2R) plasmid DNA (pAT2R) and complexes were condensed using calcium chloride. The resulting complexes were small (~150 nm) and showed high levels of gene expression in vitro and in vivo. This simple non-viral formulation approach showed negligible cytotoxicity in four different human cell lines (cervix, breast, kidney, and lung cell lines) and one mouse cell line (a lung cancer cell line). Additionally, this K9-pDNA-Ca2+ complex demonstrated cancer targeted gene delivery when administered via intravenous (IV) injection or intratracheal (IT) spray. The transfection efficiency was evaluated in Lewis lung carcinoma (LLC) cell lines cultured in vitro and in orthotopic cancer grafts in syngeneic mice. Immunohistochemical analysis confirmed that the complex effectively delivered pAT2R to the cancer cells, where it was expressed mainly in cancer cells along with bronchial epithelial cells. A single administration of these complexes markedly attenuated lung cancer growth offering preclinical proof of concept for a novel non-viral gene delivery method exhibiting effective lung tumor gene therapy via either IV or IT administration.
Keywords: Non-viral gene delivery, Cell penetrating peptides, Calcium chloride, Angiotensin II type 2 receptor (AT2R), Lung cancer
INTRODUCTION
Lung cancer is the third most prevalent form of cancer behind prostate and breast cancer; however, treatment options are lagging (1, 2). Even though progress has been made in early detection and prevention, mortality and morbidity associated with lung cancer is still unacceptably high (3). In 2015, the American Cancer Society reported >430,000 people in the US are living with lung cancer yielding the largest annual financial burden of any cancer type (1). Although incremental improvements have been made in the palliative care, currently available therapies have had minimal impact in reducing deaths (3). In particular, the need for completely new therapeutics to treat the most aggressive lung cancers is especially great.
Gene therapy is experiencing a renaissance in the US and around the globe. The European Union has recently approved the first gene therapy drug, Glybera®, for the treatment of Lipoprotein Lipase Deficiency (LPLD). Promising late stage clinical trials suggest the first gene therapy may be approved in the US in 2016. Most gene therapy clinical trials (64.4%) have aimed at treating cancer, and yet cancer gene therapies have been slow to advance (4, 5). Theoretical, and practical limitations still exist for gene therapy application to lung cancer. Biological barriers and toxicity continue to confound lung cancer treatment, even though the lungs may be accessed via inhalation or intravenous administration.
Gene vectors (viral and non-viral vectors) have steadily improved over the past 20 years. Viral vectors (e.g., adenoviral) are highly efficient, and facilitate strong transgene expression in different tumor tissues (6, 7). While these vectors are the most effective vectors applied in 70% of clinical trials, they continue to suffer from safety concerns (e.g., immunogenicity and pathogenicity) (8–11), rapid clearance from circulation, and production problems (11–14). For these reasons, non-viral vectors could be more promising gene carriers due to easy synthesis, low cost, and decreased immunogenicity compared to viral vectors (10, 14–18). These attributes suggest non-viral vectors could offer a safer approach for repeated dosing regimens when treating primary as well as recurrent cancers.
Nucleic acids complexed with cationic polymers (polyplexes) or cationic lipids (lipoplexes) are the most commonly used non-viral synthetic gene carriers (16, 19–21). Cationic polymers interact with cell membrane or extracellular components (e.g. glycosaminoglycans) via the positive charge of the amino acid residues (e.g. lysine and arginine) (22). The molecular weight and charge of polycations play important roles in complexing nucleic acids for delivering genetic materials (18). High molecular weight polycations often condense the genetic material (e.g., plasmid DNA (pDNA)) into small and stable complexes. On the other hand, low molecular weight polycations often produce large and unstable complexes (18, 23, 24). Unfortunately, low molecular weight polycations have historically exhibited poor transfection efficiency while high molecular weight polycations have been plagued with cytotoxicity (18).
Polylysine was one of the first polycations used for gene delivery (25). Polylysine-pDNA complexes have traditionally required polylysine chains with more than 20 residues to efficiently complex DNA, but yielding modest transfection efficiency and concerns about cytotoxicity (14). Many attempts were made to increase transfection efficiency or reduce cytotoxicity by either chemically modifying polylysine (e.g., PEGylation) or by adding excipients (26). Most research efforts focused on improving tolerability of high molecular weight polylysine (10, 27, 28), but we show that short polylysines with much lower cytotoxicity can indeed condense pDNA into small complexes when calcium is added. Such particles offer an interesting opportunity for repeat dosing to treat lung cancer if efficient transfection can be balanced with low cytotoxicity.
Here, a nine amino acid polylysine (K9) was complexed with pDNA and condensed with calcium chloride. This simple formulation (the K9-pDNA-Ca2+ complex) was explored using four different human cell lines: 1) A549 (a lung cancer cell), 2) HeLa (a cervix cancer cell), 3) MDA-MB-231 (a breast cancer cell), 4) HEK-293 (a virus-immortalized kidney cell)) and one mouse cell line, LLC (a lung cancer cell) using a luciferase reported plasmid DNA (pLUC) to assess transfection efficiency. Angiotensin II type 2 receptor (AT2R) is known to stimulate apoptosis and inhibit cell proliferation in different cell lines such as endothelial cells, cardiomyocytes, neuronal cells, prostate cancer cells and lung cancer cells (5, 16), therefore, AT2R plasmid DNA (pAT2R) was delivered to LLC tumor-bearing mice. These K9-pAT2R complexes were administered via intravenous (IV) injection and/or via intratracheal (IT) spray to determine lung cancer attenuation in LLC tumor-bearing mice.
MATERIALS AND METHODS
Materials
Plasmid DNA (pDNA) encoding firefly luciferase (pLUC, pGL3) was obtained from Promega (Madison, WI). Plasmid DNA (pDNA) encoding human AT2R (pAT2R, pcDNA3.1þ) was obtained from the UMR cDNA Resource Center (University of Missouri, Rolla, MO). K9 peptide (KKKKKKKKK; Mw = 1170.65 Da; Purity > 95%) was purchased from Biomatik Corporation (Cambridge, Ontario, Canada). Branched polyethyleneimine (PEI, 25 kDa), mouse serum albumin (MSA) and glucose were from Sigma-Aldrich (Milwaukee, WI). Calcium chloride dihydrate (CaCl2·2H2O) was obtained from Fisher Scientific (Pittsburgh). A549 (CCL-185), Lewis lung carcinoma (LLC; CRL-1642), and HeLa (CCL-2) cell line were obtained from American Type Culture Collection (ATCC; Rockville, Maryland). MDA-MB-231 and HEK-293 cell line were gifts from Dr. Nikki Cheng (University of Kansas Medical Center).
Preparation of the K9-pDNA-Ca2+ complex
For the in vitro studies, the K9-pLUC-Ca2+ complex solution was prepared by adding 15 μL K9 peptide solution (polymer nitrogen to pLUC phosphate (N/P) ratio 10) to 10 μL pDNA (0.1 μg/μL in 1 × Tris-acetate-EDTA (TAE) Buffer), followed by fast pipetting for 20 seconds. Then, 15 μL calcium chloride solution (e.g., 19, 114, and 228 mM) was added and mixed by fast pipetting. The K9-pDNA-Ca2+ complex solution was incubated at 4°C for 20–25 minutes and used for each experiment. For the intravenous (IV) administration of the K9-pAT2R-Ca2+ complex, 160 μL complex solution was mixed with 40 μL 1% mouse serum albumin (MSA) (the final volume of the complex is 200 μl, 4 μg pAT2R, and 53 μg K9 peptide). For the intratracheal (IT) administration, 40 μL the K9-pAT2R-Ca2+ complex solution was mixed with 10 μL 10% glucose for the osmolality adjustment (the final volume of the complex is 50 μl, 1 μg pAT2R, and 13 μg K9 peptide).
Preparation of the PEI-pLUC complex
The PEI-pLUC complex solution was prepared by adding 15 μL PEI solution (N/P ratio 10) to 10 μL pLUC (0.1 μg/μL) followed by fast pipetting for 20 seconds. The PEI-pLUC complex solution was incubated at 4°C for 20–25 minutes and used for each experiment. The complex solution was prepared immediately before each experiment and used as a control for all in vitro study.
Agarose gel electrophoresis
The K9-pLUC-Ca2+ complex solution was mixed with 4 μL TAE buffer. Then, 4 μL SYBR Green 1 was mixed with the complex solution, followed by incubation at 4°C for 20–25 minutes. After adding 7 μL of 6X DNA Loading Dye, the mixture solutions were loaded onto a 1 % agarose gel, and electrophoresed for 30 minutes at 110 V.
Size and zeta potential
The particle size (effective diameter (nm)) of the K9-pLUC complex with or without calcium chloride was determined by dynamic light scattering (Brookhaven Instruments, Holtsville, NY). The zeta potentials of the complexes were measured by Zeta PALS dynamic light scattering (Brookhaven Instrument). Particle size was measured after dispersing the complexes into nuclease-free water (NFW) or serum-free media (SFM). Zeta potential was measured after dispersing the complexes into 1 mM potassium chloride solution.
Cell culture
A549 cell line were grown in F-12K Nutrient Mixture media (Mediatech, Inc., Manassas, VA) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone, Logan, UT) and 1% (v/v) Penicillin/Streptomycin (MB Biomedical, LLC, Solon, OH). HeLa, MDA-MB-231, LLC, and HEK-293 cell lines were grown in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen/Life Technologies, Grand Island, NY) supplemented with 10% FBS and 1% Penicillin/Streptomycin. These cell lines were incubated at 37°C in 5% CO2 humidified the air. Cell line was authenticated by short-tandem repeat (STR) DNA profiling. The cells were maintained in low passage (<15) for this study.
Transfection efficiency of the K9-pDNA-Ca2+ complexes to cultured cells
A549, HeLa, MDA-MB-231, LLC, and HEK-293 cell (80,000 cells/well) were cultured in 96-well plates for 24 hours prior to the transfection. The cells were washed once with SFM and 100 μL transfection solution (a mixture of 20 μL of the K9-pLUC-Ca2+ complex and 80 μL of SFM, 0.5 μg pLUC/well) was added to each well. After 5 hours incubation, the transfection solution was replaced with 100 μL fresh growth medium. After 48 hour incubation, total cellular protein was collected by using BCA Protein Assay Reagent (Thermo Fisher Scientific Inc., Waltham, MA). The efficiency of the gene transfection by the complexes was determined by Luciferase Reporter Assay using Luciferase Assay System Freezer Pack (Promega). The Luciferase expression was measured by a microplate reader (SpectraMax; Molecular Devices Crope, CA). The transfection efficiency was expressed as Relative Light Units (RLUs) per milligram (mg) of cellular protein.
Cytotoxicity of K9 peptide, PEI, and calcium chloride in vitro
Cytotoxicity of K9 peptide, PEI, and calcium chloride was determined using a CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS) obtained from Promega. A549, HeLa, MDA-MB-231, LLC, and HEK-293 cells were cultured in a 96-well plate in growth media as described previously. Cells were treated with the samples (0–5 mg/ml as indicated in the Figure 4) for ~24 hours. After 24 hours of incubation, the media were replaced with a mixture of 100 μL of fresh serum medium and 20 μL of MTS. Then, the plate was incubated for 3 hours in the incubator. To determine cell viability, the absorbance of each well was measured by a microplate reader (SpectraMax) at 490 nm and normalized to untreated control cells.
Figure 4.
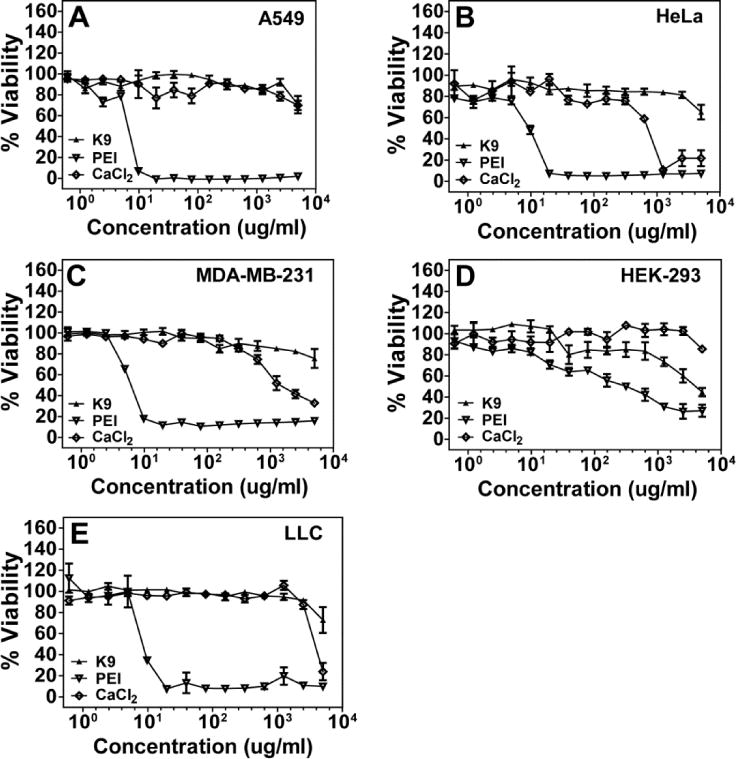
Cytotoxicity profiles of K9, PEI, and calcium chloride in A549 cells (A), HeLa cells (B), MDA-MB-231 cells (C), HEK-293 cells (D), and LLC cells (E). Cell viability was determined by MTS assay. Results are presented as mean ± SD (n = 3).
Assessment of DNA accessibility to the K9-pLUC complex by SYBR Green assay
The degree of pLUC accessibility in the K9- or PEI-pLUC complex was assessed by the double-stranded-DNA-binding reagent SYBR Green. Briefly, 10 μL pLUC (0.1 mg pDNA/mL) was mixed with 15 μL of K9 peptide (N/P ratio 10), then 15 μL deionized water or 38 mM calcium chloride solution was added. After 30 min incubation at room temperature, 120 μL PBS, and 160 μL 10X SYBR Green solutions (Invitrogen) were added. And then 100 μL sample was added to one well of a 96-well cell culture plate. The fluorescence was measured using a fluorescence plate reader (SpectraMax M5; Ex., 250 nm; Em, 520 nm).
Animals
Six-week-old wild-type male C57BL/6 mice were obtained from Charles River Laboratories International, Inc. All mice were housed in a clean facility and held for 10 days to acclimatize. All animal experiments were carried out under strict adherence to the Institutional Animal Care and Use Committee (IACUC) protocols and the Institutional Biosafety Committee set by Kansas State University (Manhattan, KS).
Lung cancer graft in syngeneic mouse and treatment with the K9-pAT2R-Ca2+ complex
Seven week old C57BL/6 mice were intravenously injected with 1.2×106 LLC cells suspended in 200μl PBS via the tail vein using a 1 mL syringe with a 27G needle. The K9-pAT2R-Ca2+ complex solution was prepared immediately before injection as described above. For the intravenous (IV) administration of the K9-pAT2R-Ca2+ complex, 160 μL complex solution was mixed with 40 μL 1% mouse serum albumin (MSA). For the intratracheal (IT) administration, 40 μL the K9-pAT2R-Ca2+ complex solution was mixed with 10 μL 10% glucose for the osmolality adjustment. After 7 days of LLC injection, the mice were injected with 200 and 50 μL of the complexes with each composition via IV and IT, respectively. PBS and K9-Ca2+ solution without pAT2R were used as control. In addition, K9-pLUC-Ca2+ complex prepared as described above was also injected into LLC tumor-bearing mice via IV and IT as a non-specific gene control. Mice were sacrificed by cervical dislocation under anesthesia at 14 days after the complex treatment. The lungs were dissected, and tumor burden were analyzed.
Immunohistochemical analysis for AT2R, cell proliferation and apoptosis in LLC allografts
Fixed lung tissues were sectioned at 4 μm and stained with haematoxylin and eosin (HE) staining for histological examination. To analyze AT2R expression and cell proliferation by Ki-67 in LLC tumors, sections were deparaffinized and heat-induced epitope unmasking was performed in the citrate buffer followed by incubation with 3% H2O2/methanol for 3 minutes to block endogenous peroxidase activity. Sections were incubated with polyclonal anti-AT2R (1:100 dilution, for 18 hours at 4°C, Abcam) and polyclonal anti-Ki-67 (1:500 dilution, for 18 hours at 4°C, Abcam) antibodies. After the incubation with primary antibodies, sections were induced into reaction with a biotin-conjugated anti-rabbit IgG antibody (Vector Laboratories) at a 1:100 dilution for 1 hour at 37°C, followed by reaction with the avidin-biotin-peroxidase complex reagent (Vector Laboratories) for 40 minutes at 37°C. Reactions were developed with 3, 3′-diaminobenzidine tetrahydrochloride (Sigma) and counterstained lightly with Mayer’s hematoxylin. The cell proliferation index was assessed as the fold change of average percentage of positive cells in three randomly selected fields in the tumor nodules.
Apoptotic cells in LLC tumors were detected by a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay by using the DeadEnd Colorimetric TUNEL System (Promega), according to the manufacturer’s instructions with a slight modification (29). The apoptotic index was assessed as the fold change of average number of positive cells in three randomly selected fields in the tumor nodules.
Statistical analysis
Data were analyzed by using GraphPad software. All values were expressed as the mean ± standard error of the mean. All experiments were conducted with multiple sample determinations. A statistical evaluation comparing the significance of the difference in gene expression (RLUs/mg protein) between the means of two data sets was performed using a t-test. One-way ANOVA, Tukey post-test was used to analyze the differences when more than two data sets were compared.
RESULTS
Formation of the K9-pLUC-Ca2+ Complex
The K9-pDNA-Ca2+ and PEI-pDNA complexes were prepared by mixing pLUC or pAT2R with K9 peptide at various N/P ratios as described in the materials and methods section. In order to demonstrate complex formation, agarose gel electrophoresis assay was performed using 1% agarose gel, and electrophoresed for 30 minutes. Uncomplexed pLUC (naked pLUC) was used as a control. The K9 peptide and pLUC mixture showed ability to form complexes of pLUC and K9 peptide regardless of the presence of 38 mM calcium chloride at N/P ratios 5, 10, and 30. Since the net charges of these complexes are positive, the complexes stayed in the loading wells without migrating into the gel and no bands were observed in the electrophoresis (Figure 1A). Although lower N/P ratios (1 to 4) also showed no bands in the absence of calcium chloride, the N/P ratios lower than 0.5 showed bands (Figure 1B). In addition, mixing with calcium chloride and pLUC did not form stable complexes (Figure 1C).
Figure 1.
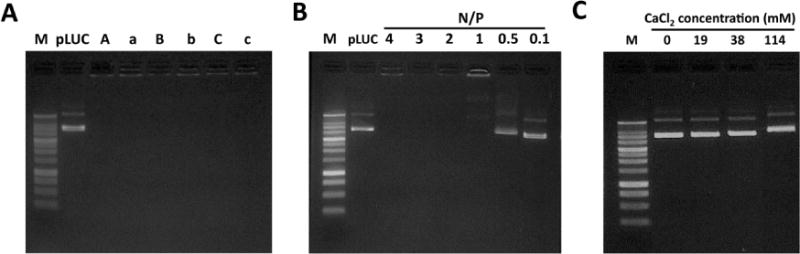
Agraose gel electrophoresis study of K9-pDNA-Ca2+ complexes at N/P ratios of 5, 10, or 30 with or without 38 mM calcium chloride (A), K9-pDNA complexes without calcium chloride at N/P ratios of 4, 3, 2, 1, 0.5, or 0.1 (B), and pLUC/calcium chloride complexes with 0, 19, 38, or 114 mM of calcium chloride (C). In the panel A, A, B and C refer to N/P 5, N/P 10, and N/P 30 in the presence of 38 mM calcium chloride, respectively, whereas a, b, and c refer to N/P 5, N/P 10, and N/P 30 in the absence of calcium chloride, respectively. “M” Refers to size marker.
Physical Characterization of the K9-pLUC-Ca2+ and the PEI-pLUC Complexes
The effect of calcium chloride concentration on the surface charge and particle size of the K9-pLUC complex was investigated. As shown in Figure 2A, addition of calcium chloride of 37.7 and 113 mM (final concentration) significantly decreased the particle size of the K9-pLUC-Ca2+ complex, with relatively narrow polydispersity (~0.1), in both nuclease-free water (NFW) and in serum-free F-12 media (SFM). The zeta potential of the K9-pLUC-Ca2+ and PEI-pLUC complexes increased significantly with increases in the concentration of calcium chloride (Figure 2B).
Figure 2.
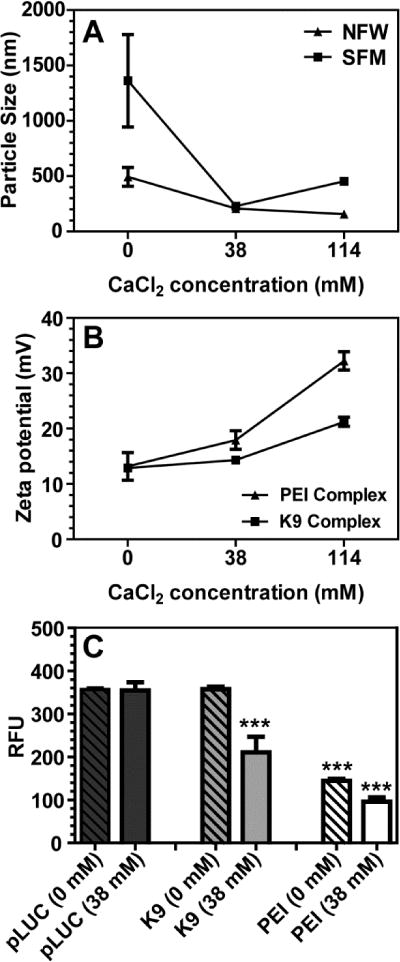
Evaluation of the particle sizes (effective diameters), zeta potentials, and SYBR Green assay of the K9-pLUC and PEI-pLUC complexes with or without calcium chloride. (A), the particle size of the K9-pLUC complexes (N/P ratio = 10) were determined by DLS in the presence of various concentrations of calcium chloride (0, 38, and 114 mM) in nuclease-free water (NFW), or serum-free F-12 media (SFM). (B), zeta potentials of the K9-pLUC and PEI-pLUC complexes (N/P ratio = 10) were determined by Zeta PALS dynamic light scattering in the presence of various concentrations of calcium chloride (0, 38, and 114 mM). (C), effect of calcium chloride concentration at 0 and 38 mM on pLUC accessibility in K9-pLUC and PEI-pLUC complexes at an N/P ratio of 10 was evaluated using the SYBR Green assay. The pLUC alone in the solution was used as a control. Results are presented as mean ± SD (n = 3). ***, P < 0.0001, one-way ANOVA, Tukey post-test comparison to pDNA.
SYBR Green assay provides a non-destructive and simple method to examine pDNA accessibility within complexes. As shown in Figure 2C, pLUC was accessible in the K9-pLUC complex, whereas pLUC accessibility in the PEI-pLUC complex was low as compared to pLUC alone. Although pLUC accessibility in the K9-pLUC complex was significantly decreased in the presence of 38 mM calcium chloride, DNA accessibility was lower in the PEI-pLUC complex. These results suggest that the addition of calcium chloride significantly decreased the size of the K9-pLUC complex, but the pLUC in the complex was more accessible than in the PEI-pLUC complex.
The K9-pLUC-Ca2+ Complex Caused Efficient Gene Transfection with Low Cytotoxicity In Vitro
The in vitro transfection efficiency of the K9-pLUC-Ca2+ and the PEI-pLUC complexes was studied using the four different human cell lines and the mouse cell line. Luciferase gene expression was evaluated 48 hours after the transfection using the K9-pLUC-Ca2+ and the PEI-pLUC complexes at N/P ratios 5, 10, 20, and 30, and at various calcium chloride concentrations during the complex formulation (Figure 3A). The K9-pLUC-Ca2+ complexes had a high level of gene expression at a calcium chloride concentration of 38 mM (N/P ratio 10) in A549 cells. Luciferase gene expression was decreased when calcium concentration was higher than 114 mM in all N/P ratios tested. This result was further confirmed in other cell lines: A549 cells (Figure 3B), HeLa cells (Figure 3C), MDA-MB-231 cells (Figure 3D), HEK-293 cells (Figure 3E), and LLC cells (Figure 3F). In all cells lines examined, the transfection efficiency of the K9-pLUC-Ca2+ with 38 mM calcium chloride was significantly higher than those with PEI-pLUC and the K9-pLUC-Ca2+ with 114 mM calcium chloride (Figure 3). Significance of including K9 peptide in pDNA transfection was further examined by comparing pLUC expression efficiency in the presence or absence of the peptide in various cell lines (Figure S1). The transfection efficiency of the pLUC-Ca2+ complex (without K9 peptide) was significantly lower than that of the K9-pLUC-Ca2+ complex at the same calcium chloride concentration.
Figure 3.
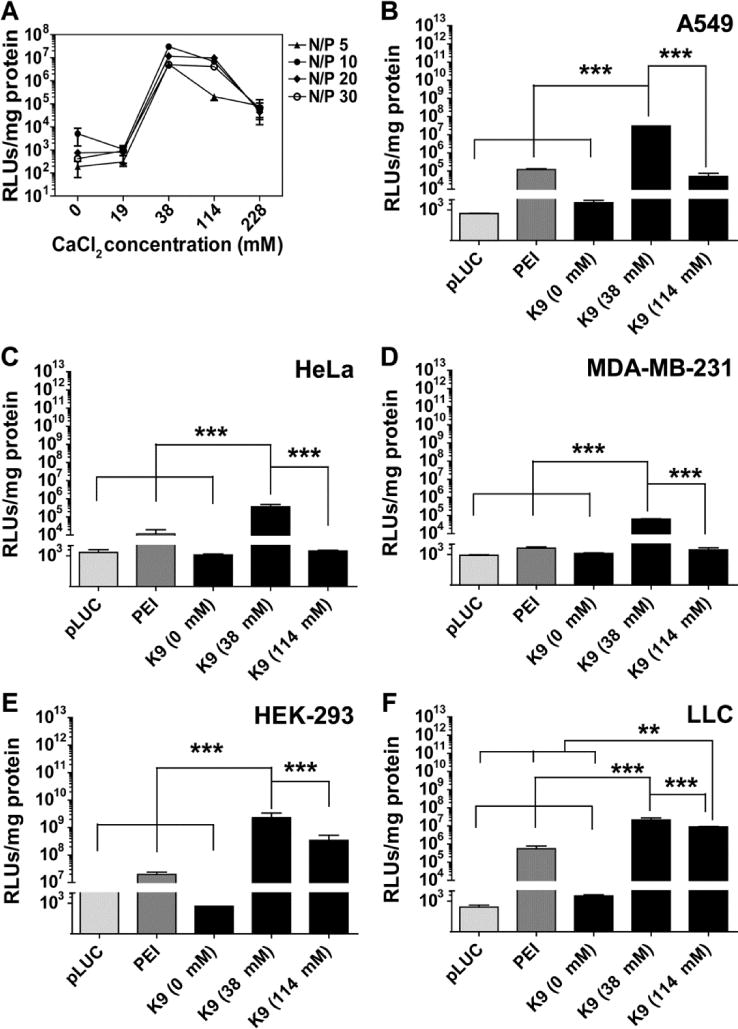
The transfection efficiencies of the K9-pLUC complex. (A), the transfection efficiencies in various concentrations of calcium chloride (0, 19, 38, 114, and 228 mM) in A549 cells at N/P ratios of 5, 10, 20 and 30. The transfection efficiencies of the K9-pLUC complexes with different concentrations of added calcium chloride (0, 38, and 114 mM) at an N/P ratio 10 in A549 cells (B), HeLa cells (C), MDA-MB-231 cells (D), HEK-293 cells (E) and LLC cells (F). RLUs refers to relative light units. Results are presented as mean ± SD (n = 4). ***, P < 0.0001; **, P < 0.001; one-way ANOVA; Tukey post test.
High transfection efficiency and low cytotoxicity are vital attributes for non-viral gene vectors. To examine whether K9, PEI, and calcium chloride affected the viability of live cells, a membrane translocalization signal (MTS) cytotoxicity assay was conducted using five different cell lines. The five cell lines were individually incubated with up to 5 mg/mL K9, PEI, or calcium chloride for 24 hours and then MTS assay was performed (Figure 4). The K9 peptide showed no cytotoxicity to 2.5 mg/ml. Only HEK-293 cell viability was decreased at 1 mg/ml levels of K9 peptide. Calcium chloride also did not show strong cytotoxicity until 1 mg/ml. However, PEI induced significant cytotoxicity even at 10 μg/ml in the four cell lines. Although HEK-293 cells were resistant to PEI-induced cytotoxicity, their cell viability was gradually decreased at higher concentrations. This MTS assay strongly suggested that the K9-pDNA-Ca2+ complex is a low cytotoxic pDNA transfection vector.
Treatment with the K9-pAT2R-Ca2+ Complex via Intravenous Injection or Intratracheal Spray Caused Significant Growth Attenuation of Lung Tumors
The effect of the K9-pAT2R-Ca2+ complex delivering the endogenous apoptosis-inducer gene AT2R was examined using orthotopic LLC allografts in syngeneic C57BL/6 mice. To evaluate the effect of the complex on the lung tumor growth, LLC cells (1.2 × 106) were injected via the tail vein. Seven days after cancer cell injection, the mice were treated with a single dose of the K9-pAT2R-Ca2+ complexes containing 4 μg pAT2R intravenously (IV) or 1 μg pAT2R intratracheally (IT). The tumor growth attenuating the effect of these complexes was observed both macroscopically (Figure 5A and C) and microscopically (Figure 5B). Macroscopically, a large number and large size of tumor nodules were detected in PBS or K9 treated mouse lungs. Average lung weights (mg) of the K9-pAT2R-Ca2+ IT (190.6±48.3) and the K9-pAT2R-Ca2+ IV (201.6±67.0) treated groups were significantly smaller than that of the control PBS group (325.7±69.4, P<0.05, Figure 5C and Table S1). Histological examination of tumors in H&E stained lung sections also displayed only a small number and small size of LLC tumor nodules in mouse lungs treated with the K9-pAT2R-Ca2+ complexes (Figure 5B). Average numbers of tumor nodules in the lungs in the IT (6.0±4.2) and the IV (4.6±3.4) groups were significantly smaller than that of the control PBS group (17.8±6.0, P<0.01, Figure 5D and Table S2). On the other hand, treatment with K9-pLUC-Ca2+ complexes did not show any effect on average lung weight (246.8±70.3 in PBS, 213.9±52.3 in K9-pLUC-Ca2+ IV, and 267.3±6.9 in K9-pLUC-Ca2+ IT) and average numbers of tumor nodules (20.4±4.0, 20.2±11.9, and 25.7±1.5 in the same group order as the lung weights described above) in LLC tumor-bearing mouse lung (Figure S2). Both local (i.e. pulmonary) and systemic treatment with the K9-pAT2R-Ca2+ complexes were equally effective in attenuating the growth of LLC lung tumor grafts in immunocompetent mice.
Figure 5.
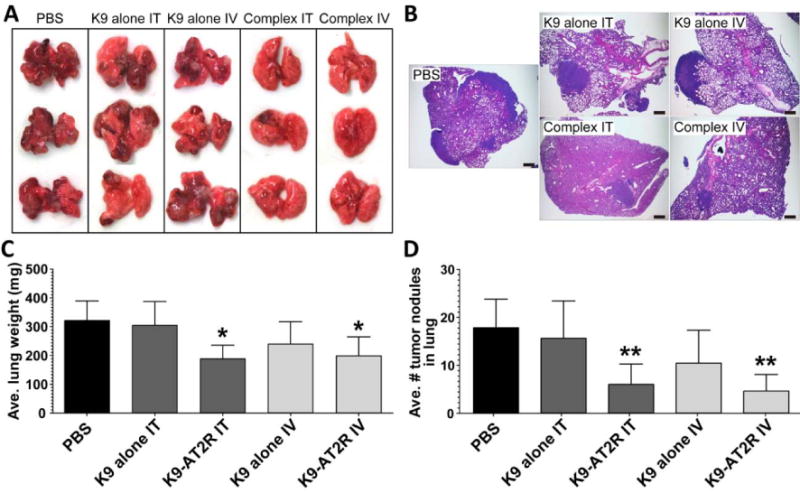
Macroscopic analysis of LLC tumors in C57BL/6 mouse lungs (A) and microscopic views (B) of the lung from PBS, K9-alone IT, K9-alone IV, K9-pAT2R-Ca2+ IT (complex IT), or K9-pAT2R-Ca2+ IV (complex IV)-treated mice (n=5). Average lung weight (C) and number of tumor nodules (D) in each treatment group were expressed in the bar graphs. Scale bars in panel B indicate 500μm. *, P < 0.05; **, P < 0.01 as compared with the level of PBS control (n=5, one-way ANOVA Tukey post-test). K9, nine amino acid polylysine; IT, intratracheal; IV, intravenous.
Immunohistochemical Analysis of AT2R Expression, Cell Proliferation and Apoptosis in LLC Grafts
The expression of AT2R gene in the lung was determined immunohistochemically (Figure 6). As shown in Figure 6A, AT2R expression was upregulated in the tumor cells in the K9-pAT2R-Ca2+ IV and IT spray groups but not in other groups. These observations suggested that the K9-pAT2R-Ca2+ administration via IV and IT significantly attenuated the lung tumor growth by expressing AT2R in the tumor cells. Immunohistochemical analysis of cell proliferation in tumors using anti-Ki-67 antibody did not show many differences (Figure 6B). Although average number of apoptotic cells detected by TUNEL assay tended to increase in the K9-pAT2R-Ca2+ treatment groups (Figure 6C), there was no statistical significance between groups due to large variations within the samples. Since the analysis was carried out 14 days after treatment with the K9-pAT2R-Ca2+ complex, these results may suggest that the treatment with the K9-pAT2R-Ca2+ complex attenuated tumor growth during an early stage of LLC tumorigenesis. Nevertheless, these results support that both IV and IT administration of the K9-pAT2R-Ca2+ offer effective modalities for lung cancer targeted non-viral gene therapy.
Figure 6.
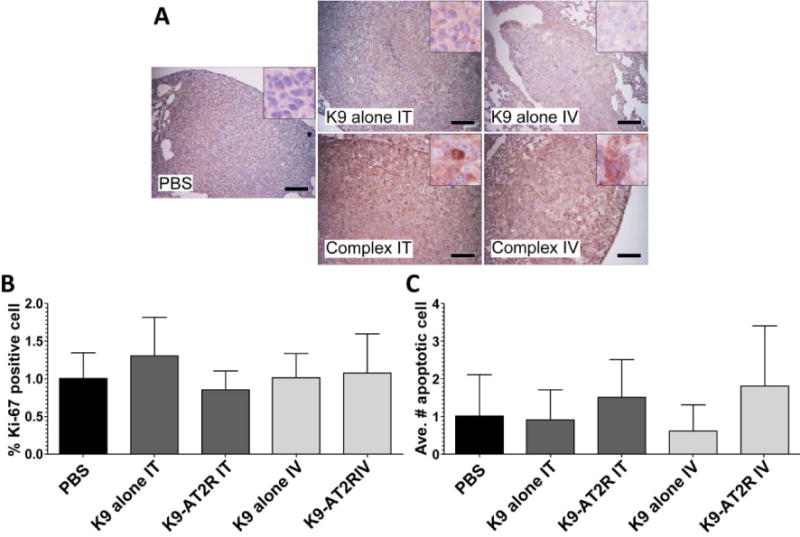
Immunohistochemical analysis of AT2R expression (A), cell proliferation (B) and apoptosis (C) in LLC graft tumors in PBS, K9-alone IT, K9-pAT2R-Ca2+ IT (complex IT), K9-alone IV or K9-pAT2R-Ca2+ IV (complex IV)-treated mice (n=5). A, Microscopic images of immunohistochemistry for AT2R expression (original magnification at 200×). AT2R expression was observed in the tumor cells from complex IT or complex IV-treated mice (insets). Scale bars in the panel A indicate 100 μm. Cell proliferation index (B) and apoptotic index (C) were evaluated by Ki-67 expressions and TUNEL assay in the tumor cells, respectively. K9, nine amino acid polylysine; IT, intratracheal; IV, intravenous.
DISCUSSION
Development in the field of gene therapy is presently hindered by the lack of robust gene delivery methods. Synthetic, non-viral gene vectors such as polycationic peptides (e.g. polylysine) are promising vectors (10, 17, 18). The toxicity of these CPPs may be minimized or eliminated by reducing their molecular size. However, the level of transfection efficiency mediated by smaller polypeptides is typically low compared to larger polypeptides (17). CPPs have been used to deliver various anti-cancer agents (e.g., small molecules, proteins, and nucleic acids) into cells in vivo and have been observed to be effective in inhibiting tumor growth in preclinical tumor models (17, 30, 31). The main goals of this work were to examine the transfection efficiency of the K9-pDNA-Ca2+ complex and to determine whether pDNA (pAT2R) can be distributed to lung cancer cells, resulting in therapeutically effective gene expression.
The in vitro transfection efficiency of the K9-pDNA-Ca2+ complex was evaluated using luciferase pDNA (pLUC) in the four different human cell lines (kidney, cervix, breast, and lung) and one mouse cancer cell line (lung). Over expression of the AT2R gene in lung cancer cells (32), or other type of CPP-pDNA-Ca2+ complex-based IT delivery of the AT2R gene was known to reduce lung cancer cell growth (16). IV injection or IT spray of the K9-pAT2R-Ca2+ complexes yielded robust gene expression, primarily in lung cancer cells, and significantly attenuated cancer growth. Therefore, this study presents an effective in vitro and in vivo gene delivery system using a low molecular weight cationic CPP (K9 peptide) for lung cancer therapy.
The formation of complexes between K9 and pDNA was observed in both the K9-pLUC-Ca2+ and K9-pLUC complexes as observed via agarose gel electrophoresis (Figure 1) when the N/P ratio is higher than 0.5. By itself, calcium chloride showed negligible ability to complex naked pLUC even at a high concentration (114 mM) (Figure 1C). However, the K9-pLUC complex without calcium chloride exhibited very low gene expression (Figure 3A), and the size of this complex was inappropriately large (500 to 1500 nm) for gene delivery (Figure 2A). The addition of calcium chloride in the K9-pLUC complex significantly decreased the complex size in water and culture medium (Figure 2A) and correspondingly increased gene transfection (Figure 3A). Therefore, calcium chloride acted as an effective condensing agent to decrease the particle size of the K9-pLUC complex and enhance transfection efficiency. These observations are in good agreement with our previous study in which calcium chloride also decreased particle sizes of CPP-pLUC complexes with other types of CPP (33). In this regard, it is of interest to note that the PEI-pLUC complex did not show a decrease in particle sizes as calcium chloride increased (17). Calcium ion-dependent increase of the total positive charge of the K9-pLUC-Ca2+ complex may also play an important role enhancing transfection efficiency by the stronger interaction with the negatively charged plasma membrane (34). In addition, accessibility of SYBER green to the pLUC in the K9-pLUC complex was significantly decreased when calcium chloride (38 mM) was added to the complex solution (Figure 2C), suggesting the K9-pLUC complex was effectively condensed, and calcium chloride played an essential role in condensation of the complex. The reduction in the particle size of the K9-pDNA-Ca2+ complexes likely led to an increase in transfection efficiency.
The effect of calcium chloride on the transfection efficiency of the K9-pDNA complex was first assessed using A549 cells. The best transfection efficiency was achieved at 38–76 mM calcium chloride (Figure 3A). Interestingly, no significant level of gene expression was detected without calcium chloride. Since calcium chloride appeared to be an essential component in the condensation of the K9-pLUC complex, yielding small complexes with optimal pLUC expression, the transfection efficiency of the K9-pLUC-Ca2+ and PEI-pLUC complexes (N/P ratio 10) was assessed across five cell lines using three different calcium chloride concentrations (0, 38, and 114 mM, Figure 3). This experiment confirmed that pLUC transfection by the K9-pLUC-Ca2+ complex was highest at 38 mM calcium chloride, and the LUC expression was significantly better than that of PEI-pLUC complex (25kDa PEI) (35).
In another study, the importance of the K9 peptide in gene expression was evaluated by comparing pLUC-Ca2+complex (without K9 peptide) and the K9-pLUC-Ca2+ complex in the same calcium concentration. The result in Figure S1 clearly shows that pLUC expression resulting from the pLUC-Ca2+complex (without K9 peptide) was significantly lower than that of the K9-pLUC-Ca2+ complex suggesting that K9 in the complex is indeed important to achieve the high gene expression from the K9-pLUC-Ca2+ complex. The PEI-pDNA complex had high transfection efficiency in the absence of calcium chloride (17, 18, 33).
A successful gene vector should be able to deliver genetic material to the target cells without influencing the viability of the host cells. The present study clearly indicated negligible cytotoxicity in vitro up to 2.5 mg/mL for K9 peptide and 1 mg/mL for calcium chloride after 24 hours for all five cell lines, whereas PEI exhibited strong cytotoxicity at low micromolar concentrations in all cell lines except for HEK-293 cells (Figure 4). Additionally, the low toxicity of the K9-pAT2R-Ca2+ complexes was also observed in the mouse study after IV and IT applications, in which all mice receiving K9-alone or the K9-pAT2R-Ca2+ complex survived during the experimental period and did not show acute inflammatory reaction or any histologically detectable abnormality. Therefore, data strongly suggested that the K9-pAT2R-Ca2+ complex represents a safe and efficient gene transfection vector.
The robust gene expression in various cell lines with negligible cytotoxicity compelled in vivo gene transfection studies. An endogenous apoptosis inducer gene, AT2R, was delivered using K9-pAT2R-Ca2+ complexes administered to LLC tumor-bearing mice. As shown in Figure 5, the treatment with the K9-pAT2R-Ca2+ complex via either IT or IV administrations significantly attenuated tumor growth macroscopically and microscopically, while the treatment with the non-specific control gene complex (K9-pLUC-Ca2+) did not show any effect on tumor growth attenuation effect (Figure S2). Immunohistochemical analysis of AT2R protein expression in the lung indicated that the expression of this gene was detected mainly in lung cancer cells, but not in normal lung tissues, which suggests that the K9-pAT2R-Ca2+ complex preferentially delivered the therapeutic gene into cancer cells. It has been known that an increased expression of anionic molecules in the membrane of cancer cells, such as: the phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfate, resulted in an increased net negative charge compared to the non-malignant cell membrane (36). These electric characteristics of the cancer cell’s surfaces support that poly-cationic peptide-based nanoparticles are suited for cancer-targeted gene therapy. The observed increase of apoptotic cells in the K9-pAT2R-Ca2+ complex treated groups (Figure 6) suggested AT2R expression in the tumors induced apoptosis in cancer cells. These results are consistent with earlier studies confirming that AT2R expression attenuates growth of various cancer cells including lung cancer cells (16, 32, 37–39). Therefore, AT2R gene expression is a potential treatment scheme for primary as well as metastatic lung cancers. A single IV bolus injection or IT spray of K9-alone did not attenuate cancer growth significantly as compared with the PBS controls (Figure 5). Dosing K9-alone or K9-pLUC neither exhibit any side effects such as abnormal clinical or histological signs in the native tissue of the lung nor alter the tumor growth. These results suggest that K9 peptide is a useful and safe vector for cancer gene therapy.
In the present study, K9-pDNA-Ca2+ complex-based gene delivery was found to be a useful tool for an in vivo gene delivery system for lung cancer treatment in immunocompetent mice. Since it is obvious that both cellular and humoral immune systems in the tumor microenvironment play a significant role in regulation of tumor growth (40), use of immunocompetent mice for the evaluation of the newly developed treatment regimen is appropriate. Although our study has provided first proof of principle data using murine lung carcinoma cells and syngeneic immunocompetent mouse model, further evaluation of this gene therapy using multiple types of human lung cancer cells in mouse xenograft models will solidify this discovery.
A recent study has evaluated the utilize of polylysine dendrimers for the delivery of Polo-like kinase 1 (PLK1)-specific short interfering RNA (siRNA) for the treatment of non-small cell lung cancer in vitro and in vivo (41). The apparent safety and acceptable transfection efficiency of polylysine dendrimers may support the calcium condensed polylysine complexes used for pDNA delivery in the present study. The K9-pDNA-Ca2+ complex is a simple approach, thus usefulness in future translational studies appears to be very high.
CONCLUSION
The addition of calcium chloride to nascent complexes of polylysine CPP (K9 peptide) and pDNA (pLUC and pAT2R) produced small and stable complexes. These complexes exhibited high gene expression in various human and mouse cancer cells comparable to PEI-pDNA complexes. Single IV or IT administrations of the K9-pAT2R-Ca2+ complexes significantly attenuated the growth of LLC cell allografts in mouse lungs, suggesting that K9 peptide-based gene therapy is effective and that the AT2R gene is potentially useful for lung cancer gene therapy. K9 peptide showed negligible cytotoxicity in cell culture, supporting the notion that this CPP is a safe delivery vehicle for genetic materials (e.g., siRNA and pDNA). Although, further studies are required to confirm the in vivo safety of the K9-pAT2R-Ca2+ complexes by formal pharmacokinetics, pharmacodynamics, and multispecies toxicity studies, these data showed that the K9-pDNA-Ca2+ complexes could be an effective and safe non-viral gene transfection tool.
Supplementary Material
Acknowledgments
We acknowledge financial support from the following sources: Savara Pharmaceuticals (CB and MT), and Faculty of Pharmacy of King Abdulaziz University, Jeddah, Saudi Arabia (NAA). We also thank Macromolecule and Vaccine Stabilization Center (University of Kansas) for the use of laboratory equipment. In addition, this work was also supported in part by Kansas State University Johnson Cancer Research Center (MT), NIH grants U43 CA165462 (MT), P20 GM103418 (MT), and Kansas Bioscience Authority Collaborative Cancer Research grant (MT).
Abbreviations list
- CPPs
Cell penetrating peptides
- AT2R
angiotensin II type 2 receptor
- pDNA
plasmid DNA
- pAT2R
plasmid AT2R DNA
- K9
nine amino acid polylysine
- LLC
Lewis lung carcinoma
- IV
intravenous
- IT
intratracheal
- pLUC
luciferase reported plasmid DNA
- PEI
polyethyleneimine
- MSA
mouse serum albumin
- N/P ratio
polymer nitrogen to pDNA phosphate ratio
- NFW
nuclease-free water
- SFM
serum-free media
- FBS
fetal bovine serum
- RLUs
Relative Light Units
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Disclosure of any potential Conflicts of Interest
The authors disclose no potential conflicts of interest.
References
- 1.Howlader N, Noone A, Krapcho M. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; Apr, 2012. seer cancer gov/csr. 2014. [Google Scholar]
- 2.Hensing T, Chawla A, Batra R, Salgia R. A personalized treatment for lung cancer: molecular pathways, targeted therapies, and genomic characterization. Systems Analysis of Human Multigene Disorders: Springer. 2014:85–117. doi: 10.1007/978-1-4614-8778-4_5. [DOI] [PubMed] [Google Scholar]
- 3.Cai K, Sham M, Tam P, Lam WK, Xu R. Lung cancer gene therapy. Gene Ther Mol Biol. 2003;7:255–72. [Google Scholar]
- 4.Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. Journal of Clinical Investigation. 1999;104:925–35. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du H, Liang Z, Zhang Y, Jie F, Li J, Fei Y, et al. Effects of Angiotensin II Type 2 Receptor Overexpression on the Growth of Hepatocellular Carcinoma Cells In Vitro and In Vivo. PloS one. 2013;8:e83754. doi: 10.1371/journal.pone.0083754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Current Opinion in Biotechnology. 1999;10:440–7. doi: 10.1016/s0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 7.Simons M, Beinroth S, Gleichmann M, Liston P, Korneluk R, MacKenzie A, et al. Adenovirus-Mediated Gene Transfer of Inhibitors of Apoptosis Proteins Delays Apoptosis in Cerebellar Granule Neurons. Journal of neurochemistry. 1999;72:292–301. doi: 10.1046/j.1471-4159.1999.0720292.x. [DOI] [PubMed] [Google Scholar]
- 8.Karagiannis ED, Alabi CA, Anderson DG. Rationally Designed Tumor-Penetrating Nanocomplexes. ACS nano. 2012;6:8484–7. doi: 10.1021/nn304707b. [DOI] [PubMed] [Google Scholar]
- 9.Khondee S, Baoum A, Siahaan TJ, Berkland C. Calcium condensed LABL-TAT complexes effectively target gene delivery to ICAM-1 expressing cells. Molecular pharmaceutics. 2011;8:788–98. doi: 10.1021/mp100393j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhakamy NA, Nigatu AS, Berkland CJ, Ramsey JD. Noncovalently associated cell-penetrating peptides for gene delivery applications. Therapeutic delivery. 2013;4:741–57. doi: 10.4155/tde.13.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng J, Gao N, Wang Y, Yi H, Fang S, Ma Y, et al. Self-assembled cationic micelles based on PEG-PLL-PLLeu hybrid polypeptides as highly effective gene vectors. Biomacromolecules. 2012;13:3795–804. doi: 10.1021/bm3012538. [DOI] [PubMed] [Google Scholar]
- 12.Bennett J. Immune response following intraocular delivery of recombinant viral vectors. Gene therapy. 2003;10:977–82. doi: 10.1038/sj.gt.3302030. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, Muruve D. Molecular basis of the inflammatory response to adenovirus vectors. Gene therapy. 2003;10:935–40. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 14.Kwoh DY, Coffin CC, Lollo CP, Jovenal J, Banaszczyk MG, Mullen P, et al. Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression. 1999;1444:171–90. doi: 10.1016/s0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene therapy. 2000;7:31–4. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 16.Kawabata A, Baoum A, Ohta N, Jacquez S, Seo G-M, Berkland C, et al. Intratracheal administration of a nanoparticle-based therapy with the angiotensin II type 2 receptor gene attenuates lung cancer growth. Cancer research. 2012;72:2057–67. doi: 10.1158/0008-5472.CAN-11-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baoum A, Xie S-X, Fakhari A, Berkland C. “Soft” Calcium Crosslinks Enable Highly Efficient Gene Transfection Using TAT Peptide. Pharmaceutical research. 2009;26:2619–29. doi: 10.1007/s11095-009-9976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhakamy NA, Berkland CJ. Polyarginine molecular weight determines transfection efficiency of calcium condensed complexes. Molecular pharmaceutics. 2013;10:1940–8. doi: 10.1021/mp3007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang MX, Redemann CT, Szoka FC. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjugate chemistry. 1996;7:703–14. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 21.Hofland HE, Nagy D, Liu J-J, Spratt K, Lee Y-L, Danos O, et al. In vivo gene transfer by intravenous administration of stable cationic lipid/DNA complex. Pharmaceutical research. 1997;14:742–9. doi: 10.1023/a:1012146305040. [DOI] [PubMed] [Google Scholar]
- 22.Dennison SR, Phoenix AJ, Phoenix DA. Effect of salt on the interaction of Hal18 with lipid membranes. European Biophysics Journal. 2012;41:769–76. doi: 10.1007/s00249-012-0840-6. [DOI] [PubMed] [Google Scholar]
- 23.Koo H, Kang H, Lee Y. Analysis of the relationship between the molecular weight and transfection efficiency/cytotoxicity of Poly-L-arginine on a mammalian cell line. Bull Korean Chem Soc. 2009;30:927–30. [Google Scholar]
- 24.de Raad M, Teunissen EA, Lelieveld D, Egan DA, Mastrobattista E. High-content screening of peptide-based non-viral gene delivery systems. Journal of Controlled Release. 2012;158:433–42. doi: 10.1016/j.jconrel.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Oulad-Abdelghani M, Zelkin AN, Wang Y, Haîkel Y, Mainard D, et al. Poly (L-lysine) nanostructured particles for gene delivery and hormone stimulation. Biomaterials. 2010;31:1699–706. doi: 10.1016/j.biomaterials.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW. Polylysine Copolymers for Gene Delivery. Cold Spring Harbor Protocols. 2012;2012 doi: 10.1101/pdb.ip068619. pdb. ip068619. [DOI] [PubMed] [Google Scholar]
- 27.Ward CM, Read ML, Seymour LW. Systemic circulation of poly (L-lysine)/DNA vectors is influenced by polycation molecular weight and type of DNA: differential circulation in mice and rats and the implications for human gene therapy. Blood. 2001;97:2221–9. doi: 10.1182/blood.v97.8.2221. [DOI] [PubMed] [Google Scholar]
- 28.Toncheva V, Wolfert MA, Dash PR, Oupicky D, Ulbrich K, Seymour LW, et al. Novel vectors for gene delivery formed by self-assembly of DNA with poly (L-lysine) grafted with hydrophilic polymers. Biochimica et Biophysica Acta (BBA)-General Subjects. 1998;1380:354–68. doi: 10.1016/s0304-4165(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A, et al. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer. 2010;70:28–36. doi: 10.1016/j.lungcan.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindsay MA. Peptide-mediated cell delivery: application in protein target validation. Current opinion in pharmacology. 2002;2:587–94. doi: 10.1016/s1471-4892(02)00199-6. [DOI] [PubMed] [Google Scholar]
- 31.Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharmaceutical research. 2004;21:389–93. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- 32.Pickel L, Matsuzuka T, Doi C, Ayuzawa R, Maurya DK, Xie S-X, et al. Over-expression of angiotensin II type 2 receptor gene induces cell death in lung adenocarcinoma cells. Cancer biology & therapy. 2010;9:277–85. doi: 10.4161/cbt.9.4.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baoum AA, Berkland C. Calcium condensation of DNA complexed with cell-penetrating peptides offers efficient, noncytotoxic gene delivery. Journal of pharmaceutical sciences. 2011;100:1637–42. doi: 10.1002/jps.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam HY, Kim J, Kim S, Yockman JW, Kim SW, Bull DA. Cell penetrating peptide conjugated bioreducible polymer for siRNA delivery. Biomaterials. 2011;32:5213–22. doi: 10.1016/j.biomaterials.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee W-S, Kim Y-K, Zhang Q, Park T-E, Kang S-K, Kim D-W, et al. Polyxylitol-based gene carrier improves the efficiency of gene transfer through enhanced endosomal osmolysis. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:525–34. doi: 10.1016/j.nano.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190–4. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. Journal of Clinical Investigation. 1995;95:651. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proceedings of the National Academy of Sciences. 1996;93:156–60. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miura Si, Karnik SS. Ligand-independent signals from angiotensin II type 2 receptor induce apoptosis. The EMBO journal. 2000;19:4026–35. doi: 10.1093/emboj/19.15.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. [Google Scholar]
- 41.McCarroll J, Dwarte T, Baigude H, Dang J, Yang L, Erlich R, et al. Therapeutic targeting of polo-like kinase 1 using RNA-interfering nanoparticles (iNOPs) for the treatment of non-small cell lung cancer. Oncotarget. 2014 doi: 10.18632/oncotarget.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


