Abstract
BACKGROUND
In hematopoietic cell transplantation (HCT), current risk adjustment strategies are based upon clinical and disease-related variables. Though patient reported outcomes (PROs) predict mortality in multiple cancers, PROs have been less well studied within HCT. Improvements in risk adjustment strategies in HCT would inform patient selection, patient counseling, and quality reporting. Our objective was to determine whether pre-HCT PROs, in particular physical health, predict survival among patients undergoing autologous or allogeneic transplantation.
METHODS
In this secondary analysis, we studied pre-HCT PROs that were reported by 336 allogeneic and 310 autologous HCT recipients enrolled in the BMT CTN 0902 trial, a study with broad representation of patients transplanted in the US.
RESULTS
Among allogeneic HCT recipients, the pre-HCT SF-36 physical component summary score (PCS) independently predicted overall mortality (HR 1.40 per 10 point decrease, p<0.001) and performed at least as well as currently used, non-PRO risk indices. Survival probability estimates at one year for the first, second, third, and fourth quartiles of the baseline PCS were 50%, 65%, 75%, and 83%. Early post-HCT decreases in PCS were associated with higher overall and treatment-related mortality. When adjusted for patient variables included in the US Stem Cell Therapeutic Outcomes Database model for transplant center-specific reporting, the SF-36 PCS retained independent prognostic value.
CONCLUSIONS
PROs have the potential to improve prognostication in HCT. We recommend the routine collection of PROs prior to HCT, and consideration of incorporation of PROs into risk adjustment for quality reporting.
Keywords: quality of life, hematopoietic stem cell transplantation, outcome assessment, risk adjustment, physical fitness
Introduction
Accurate quantification of risk for patients undergoing hematopoietic cell transplantation (HCT) is critical for medical decision-making, stratification in clinical trials, quality reporting and comparative effectiveness research. HCT is associated with a large potential magnitude of benefit (i.e. cure in otherwise incurable patients), but high cost, and significant risk of treatment-related harm. Thus, accurate risk assessment is critical to the achievement of high-value cancer care in HCT. Quality reporting in HCT includes the legislatively-mandated, center-specific outcomes analysis, an annual public report prepared by Stem Cell Therapeutic Outcome Database (SCTOD) staff that provides risk-adjusted outcomes data for allogeneic transplants for HCT centers within the United States. This report holds significant value for administrators, regulators, payers, physicians, and patients who use this information to make decisions. Existing measures to predict HCT outcomes, including the HCT-specific Comorbidity Index (HCT-CI, 15 pre-HCT variables),1 the Disease Risk Index (DRI, combination of disease type and stage),2 and the EBMT Score (a composite index of five pre-transplant variables),3 are incomplete. Though performance status (PS) is prognostic in HCT4–6, current HCT risk assessment tools and the SCTOD model do not incorporate patient-reported functional status into their models.
Patient-reported outcomes (PROs) such as symptoms, functional status, and health-related quality of life (HR-QOL) are information provided by patients without interpretation or modification by another individual. Patient-reported outcomes predict survival in patients with and without cancer,7–20 by capturing the impact upon patients of underlying disease or prior therapy. PROs are not well-studied in the field of HCT as prognostic markers. However, patients coming to HCT have undergone prior cancer therapy and are about to experience significant additional physical stress.21–24 Thus, we hypothesized that PROs, particularly physical functional status, would predict outcomes after transplantation. We also hypothesized that early post-transplant changes in patient-reported physical function would have prognostic value, which, if true, would suggest that the tool is sensitive to clinically-meaningful treatment-related changes.
To investigate our hypotheses, we analyzed data from a large Blood and Marrow Transplant Clinical Trials Network (BMT CTN) clinical trial that was broadly representative of usual HCT clinical practice, 25 in which participants reported HR-QOL data prior to and early after autologous and allogeneic transplantation.
Materials and Methods
Participants
Our investigation was a secondary analysis of BMT CTN protocol 0902, a randomized trial of self-directed exercise and stress management in HCT recipients (ClinicalTrials.gov identifier: NCT01278927, protocol available on www.bmtctn.net). The parent study was a factorial design so that participants could receive stress management training, exercise training, neither, or both. Inclusion criteria for BMT CTN 0902 were age ≥18 years, ability to exercise at low to moderate intensity, no requirement for supplemental oxygen, and autologous or allogeneic transplant planned within six weeks. The trial was a contemporary study designed to be broadly representative of the general HCT population in clinical practice. The research protocol was approved by a protocol review committee appointed by the National Heart, Lung and Blood Institute (NHLBI) and by local Institutional Review Boards or Ethics Committees. All participants provided written informed consent. Among the 711 patients enrolled in BMT CTN 0902, 709 underwent HCT (351 allogeneic and 358 autologous). All patients from BMT CTN 0902 who had completed the pre-transplant SF-36 instrument were included in the secondary analysis, which included 310 allogeneic HCT recipients and 336 autologous HCT recipients. The parent study did not show an effect of the intervention on the primary outcome, which was the SF-36 PCS and SF-36 MCS at Day +100.25 Therefore, we did not anticipate that the intervention would confound our interpretation of the prognostic ability of the SF-36 PCS and SF-36 MCS scores, and we combined all four study arms in this analysis.
Data Collection Instruments
The HR-QOL instrument studied was the Medical Outcomes Study Short Form-36 Health Survey (SF-36), a 36-item multidimensional quality of life measure that assesses patient-reported health and functioning. This instrument takes approximately five minutes to complete. From the SF-36, two summary domains are calculated, the Physical Component Summary (PCS) scale and the Mental Component Summary (MCS) scale, and there are eight subscales. The normal population mean for each summary scale is 50 with a standard deviation of 10. SF-36 questions ask patients to report information related to areas such as problems with work or daily activities, emotional distress, performance of low or high exertion physical activities, and symptoms such as fatigue, anxiety and depression. The SF-36 is thus different than a comorbidity scale or a comprehensive geriatric assessment. For this secondary analysis, the pre-HCT PCS and MCS and the SF-36 PCS and MCS change scores were analyzed. Change scores were calculated by subtracting the pre-transplant scores from the Day 100 scores. A clinically meaningful change is considered to be 0.5 standard deviation, or 5 points for the PCS and MCS.
For all participants, the pre-transplant HCT-CI, DRI, and EBMT scores were calculated.1–3 Data collection was performed through the BMT CTN and the Center for International Blood and Marrow Transplant Research (CIBMTR).25
Statistical Analyses
All analyses were performed separately for autologous and allogeneic HCT recipients. Patient demographic, disease, and transplant characteristics were summarized by transplant type (Table 1). Pre-HCT PCS and MCS as well as the SF-36 PCS and MCS change scores were modelled as continuous variables. Analysis of pre-HCT PCS and MCS included the full cohort (310 allogeneic and 336 autologous patients) while the analysis of SF-36 change scores were limited to patients alive at day 100 with completed day-100 forms.
Table 1.
Baseline characteristics of Secondary Analysis Participants
| Variable | Autologous | Allogeneic |
|---|---|---|
| Number of enrolled patients | 336 | 310 |
| Number of centers | 23 | 19 |
| Age at transplant, years, median(range) | 59 (19–76) | 54 (20–75) |
| Age at transplant, n (%) | ||
| ≤ 40 | 29 (9) | 58 (19) |
| 40-<65 | 211 (63) | 205 (66) |
| ≥ 65 | 96 (29) | 47 (15) |
| Ethnicity, n (%) | ||
| Hispanic | 20 (6) | 15 (5) |
| Non-Hispanic | 316 (94) | 295 (95) |
| Race, n (%) | ||
| American Indian/Alaska Native | 0 | 1 (<1) |
| Asian | 5 (1) | 5 (2) |
| Hawaiian/Pacific Islander | 0 | 3 (<1) |
| Black or African American | 44 (13) | 10 (3) |
| White | 285 (85) | 284 (92) |
| More than one race | 1 (<1) | 5 (2) |
| Other/unknown | 1 (<1) | 2 (<1) |
| Recipient sex, n (%) | ||
| Male | 193 (57) | 173 (56) |
| Female | 143 (43) | 137 (44) |
| Marital status, n (%) | ||
| Married/Living with partner | 243 (72) | 242 (78) |
| Single, never married | 32 (10) | 39 (13) |
| Separated/Divorced | 47 (14) | 20 (6) |
| Widowed | 10 (3) | 7 (2) |
| Missing | 4 (1) | 2 (<1) |
| Education, n (%) | ||
| ≤ High school | 68 (20) | 60 (19) |
| College graduate | 197 (59) | 191 (62) |
| Postgraduate | 69 (21) | 58 (19) |
| Missing | 2 (<1) | 1 (<1) |
| Employment status, n (%) | ||
| No | 184 (55) | 160 (52) |
| Yes | 152 (45) | 150 (48) |
| Income, n (%) | ||
| Under $15,000 | 16 (5) | 21 (7) |
| $15,000–$24,999 | 23 (7) | 21 (7) |
| $25,000–$49,999 | 71 (21) | 52 (17) |
| $50,000–$74,999 | 71 (21) | 64 (21) |
| $75,000–$99,999 | 48 (14) | 40 (13) |
| $100,000 or above | 87 (26) | 92 (30) |
| Missing | 20 (6) | 20 (6) |
| Karnofsky score, %, n (%) | ||
| ≥90 | 192 (57) | 190 (61) |
| 70 – 80 | 139 (41) | 113 (36) |
| 50 – 60 | 5 (1) | 5 (2) |
| Missing/Not done | 0 | 2 (<1) |
| Tobacco use, n (%) | ||
| No | 306 (91) | 288 (93) |
| Yes | 28 (8) | 20 (6) |
| Unknown | 2 (<1) | 2 (<1) |
| Alcohol use, n (%) | ||
| No | 189 (56) | 190 (61) |
| Yes | 146 (43) | 120 (39) |
| Unknown | 1 (<1) | 0 |
| BMI, median (range) | 28.31 (16.10–52.27) | 27.65 (17.06–55.55) |
| BMI, n(%) | ||
| < 25 | 78 (23) | 88 (28) |
| 25–29.9 | 125 (37) | 112 (36) |
| ≥30 | 133 (40) | 110 (35) |
| Disease, n (%) | ||
| AML/ALL | 0 | 163 (53) |
| CML | 0 | 12 (4) |
| MDS/MPS | 0 | 44 (14) |
| MM/PCD | 172 (51) | 14 (5) |
| Lymphoma | 164 (49) | 57 (18) |
| CLL/SLL | 0 | 20 (6) |
| Disease status, n (%) | ||
| AML/ALL | ||
| Early | 116 (71) | |
| Intermediate | 30 (18) | |
| Late | 17 (10) | |
| CML | ||
| Early | 6 (50) | |
| Intermediate | 4 (33) | |
| Late | 2 (17) | |
| MDS/MPS | ||
| Early | 21 (48) | |
| Intermediate | 9 (20) | |
| Late | 14 (32) | |
| MM/PCD | ||
| Early | 24 (14) | 3 (21) |
| Intermediate | 135 (78) | 8 (57) |
| Late | 13 (8) | 3 (21) |
| Lymphoma | ||
| Early | 57 (35) | 3 (5) |
| Intermediate | 69 (42) | 28 (49) |
| Late | 38 (23) | 25 (44) |
| Missing | 0 | 1 (2) |
| CLL/SLL | ||
| Early | 5 (25) | |
| Intermediate | 7 (35) | |
| Late | 8 (40) | |
| Hematopoietic cell transplantation-specific comorbidity index (HCT-CI), n (%) | ||
| 0 | 109 (32) | 108 (35) |
| 1–2 | 107 (32) | 93 (30) |
| 3+ | 115 (34) | 106 (34) |
| Missing | 5 (1) | 3 (<1) |
| Disease risk index, n (%) | ||
| Low | 64 (19) | 54 (17) |
| Intermediate | 224 (67) | 144 (46) |
| High | 22 (7) | 52 (17) |
| Very high | 14 (4) | 11 (4) |
| Missing | 12 (4) | 49 (16) |
| EBMT score, n (%) | ||
| 1 | 6 (2) | 58 (19) |
| 3 | 72 (21) | 73 (24) |
| 4 | 220 (65) | 137 (44) |
| ≥5 | 38 (11) | 42 (14) |
| Prior transplant, n (%) | ||
| No | 318 (95) | 267 (86) |
| Yes | 18 (5) | 43 (14) |
| Prior cytotoxic chemotherapy, n (%) | ||
| No | 32 (10) | 43 (14) |
| Yes | 281 (84) | 250 (81) |
| Unknown | 23 (7) | 17 (5) |
| Patient CMV status, n (%) | ||
| Positive | 196 (58) | 165 (53) |
| Negative | 139 (41) | 145 (47) |
| Missing | 1 (<1) | 0 |
| In allogeneic, conditioning intensity, n (%) | ||
| MA | 135 (44) | |
| RIC/NMA | 175 (56) | |
| Graft type, n (%) | ||
| BM | 0 | 39 (13) |
| PB | 336 | 246 (79) |
| Double CB | 0 | 25 (8) |
| Baseline SF36 Physical Component Score | ||
| Median | 43 | 44 |
| IQR | 34–49 | 36–51 |
| Range | 13–64 | 13–65 |
| Baseline SF36 Mental Component Score | ||
| Median | 52 | 52 |
| IQR | 46–58 | 43–57 |
| Range | 18–74 | 7–68 |
| Median follow-up of survivors (range), months | 13 (2–36) | 23 (6–35) |
Abbreviations: BMI = Body Mass Index; AML = Acute Myeloid Leukemia; ALL = Acute Lymphoblastic Leukemia; CML = Chronic Myeloid Leukemia; MDS = Myelodysplastic Syndrome; MPS = Myeloproliferative Syndrome; MM = Multiple Myeloma; PCD = Plasma Cell Dyscrasia; CLL = Chronic Lymphocytic Leukemia; SLL = Small Lymphocytic Lymphoma; EBMT = European Group for Blood and Marrow Transplantation; CMV = Cytomegalovirus; MA = myeloablative; RIC = Reduced Intensity Conditioning; NMA = Non-myeloablative; BM = Bone Marrow; PB = Peripheral Blood; CB = Cord Blood; IQR = Interquartile Range
The primary outcome of the study was overall survival. The event for this endpoint was death from any cause. For the analysis of pre-HCT PCS and MCS, the time to this event was defined as the time from HCT to death or last follow-up. For the SF-36 change score analysis, a landmark analysis starting at day 100 was used, since that is when the post-transplant SF-36 was collected. Overall survival probabilities were estimated using the Kaplan Meier estimator.26 Treatment-related mortality (TRM) was the secondary outcome. Because of the extremely small number of TRM events in the autologous HCT cohort, TRM was only analyzed in the allogeneic HCT cohort. The event for this endpoint was death in remission, treating relapse/progression as a competing risk.27
Multivariable analyses were conducted using Cox proportional hazards models.28 All variables satisfied the proportional hazards assumption. The analysis was conducted in two stages: in the first stage, clinical covariates associated with the outcomes were selected, and in the second stage, the effects of PROs were evaluated after adjusting for clinical covariates identified in the first stage. In stage one, a stepwise variable selection procedure was used to select patient, disease, and transplant related characteristics associated with the outcome. Variables considered at this stage included age, KPS, disease, disease stage, and graft type. Additional variables considered for allogeneic recipients included conditioning regimen intensity, donor type, and Human Leukocyte Antigen-match. All variables significant at the 5% level were retained in the models for stage two. In the second stage, the additional prognostic significance of the pre-HCT SF-36 scores was evaluated by adding the SF-36 PCS, MCS, or subscales to the model. The PCS, MCS and subscales were calculated according to the published scoring algorithms. Two-way interactions between SF-36 scores and clinical factors retained in the model from stage one were also examined, and none were found statistically significant. To evaluate whether pre-HCT SF-36 scores remained independent predictors after adjusting for traditional HCT risk indices, the HCT-CI, EBMT risk score, and DRI were added to the final models. Since the DRI is a disease index, disease-related variables in the final models were excluded in the models with the DRI. The Brier score29 was calculated in the allogeneic cohort to explore whether the pre-HCT SF-36 scores have similar ability to predict OS at 6 months and at 1 year post HCT compared to the HCT-CI and the DRI. The Brier score measures the average discrepancies between the predicted and observed survival status. A Brier score can range from 0 to 1, with 0 indicating a perfect model and the maximum Brier score depending on the incidence of the outcome. In general, a lower Brier score indicated better prediction.
In a separate model, we explored whether the PCS, physical functioning or general health score provided additional prognostic information above the variables already included in the 2014 SCTOD center-specific outcomes analysis for allogeneic recipients since this model was developed in patients transplanted from 2010–2012, encompassing the years of the trial.30
Results
Participant Characteristics
Complete baseline characteristics of the 646 patients included in the secondary analysis are shown in Table 1. Because the number of patients who did not fill out the baseline SF-36 form was small, formal comparisons between those who did fill out the form and were included vs those who did not and were excluded was not possible. Forty-one percent of recipients had a Karnofsky Performance Status ≤ 90%. At the time of analysis, the median follow-up of survivors was 13 months for autologous transplant recipients and 23 months for allogeneic recipients.
SF-36 PCS and MCS Scores
Prior to transplantation, the median PCS for autologous transplant recipients was 43 (range 13 – 64), and for allogeneic transplant recipients the median PCS was 44 (range 13–65). Median pre-transplant MCS for autologous recipients was 52 (range of 18–74), and for allogeneic recipients the median MCS was 52 (range 7–68).
Among autologous transplant recipients with reported Day 100 SF-36 data (n=279), 25% had worsening of PCS scores by at least 5 points from pre-transplant to Day 100 after transplant; 19% had worsening of MCS scores by at least 5 points. Three patients died prior to Day 100. Change score data were missing for 14% of autologous transplant recipients. Among allogeneic transplant recipients with Day 100 SF-36 data (n=236), 39% had worsening of PCS scores by at least 5 points from pre-transplant to Day 100 after transplant; 25% had worsening of MCS scores by at least 5 points. 30 patients died prior to Day 100. Change score data were missing for 16%.
Relationship of SF-36 with Survival Outcomes: Autologous transplant recipients
Among autologous transplant recipients, no clinical covariates (including age, disease, disease stage, and graft type) were predictive of survival. Neither the pre-transplant PCS (p=0.73) nor the pre-transplant MCS (p=0.58) were predictive of post-transplant survival.
Relationship of Pre-HCT SF-36 with Survival Outcomes: Allogeneic transplant recipients
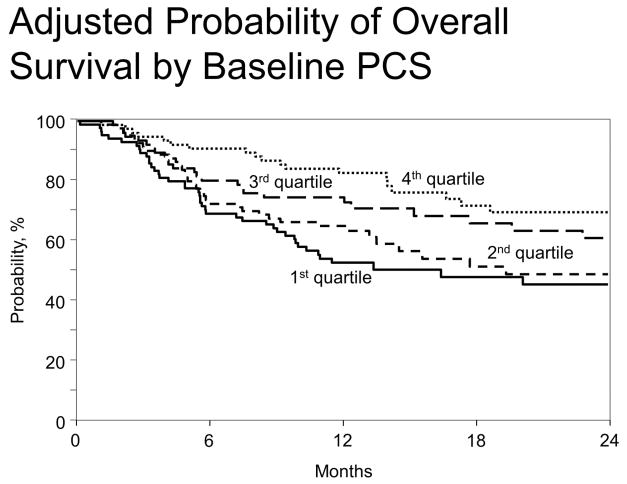
The pre-transplant PCS was correlated with the pre-transplant HCT-CI (p=0.0325), self-reported exercise, KPS (p=0.0001), and disease type, though the association with disease was driven by a few cases of multiple myeloma (p=0.0073). There were no clinical covariates (including KPS, age, conditioning intensity, donor type, graft source, disease, or disease stage) that predicted survival. The pre-HCT PCS was strongly predictive of overall survival with a median of 23 months of follow-up (HR for death of 1.40 per 10 point decrease, 95% CI 1.18–1.66, p<0.001) (Table 2). Hazard ratios remained similar after adjustment for KPS, as well as the HCT-CI, EBMT score, and DRI. Survival probability estimates for the first, second, third, and fourth quartiles of the baseline PCS were, respectively, 50%, 65%, 75%, and 83% at one year, with survival curves shown in Figure 1. Among the eight SF-36 subscales, Physical Functioning and General Health scores were strongly associated with survival. Pre-HCT PCS was also predictive for TRM (HR 1.21, 95% CI 1.01–1.47, p=0.047)). Pre-HCT PCS scores were independently predictive of overall survival after adjusting for non-PRO variables used in the SCTOD model30 for center-specific outcomes reporting (HR for death of 1.40, 95% CI 1.14 – 1.71, p = .001). The SF-36 Physical Functioning and General Health subscales likewise remained strongly associated with survival.
Table 2.
Association of pre-HCT SF-36 with mortality in allogeneic HCT patients*
| Scale | HR for 10pt decrease (95% CI) | p-value |
|---|---|---|
| PCS | 1.40 (1.18–1.66) | <0.001 |
| MCS | 1.01 (0.86–1.19) | 0.88 |
None of the clinical covariates were significant in the model that included the SF-36. Tested variables included age, KPS, disease, disease stage, graft type, conditioning regimen intensity, donor type, and Human Leukocyte Antigen-match.
Figure 1.
Relationship of pre-HCT SF-36 PCS with overall survival in allogeneic recipients, by baseline PCS quartile. Higher quartiles represent better pre-HCT PCS scores.
Brier scores were computed for the performance of the SF-36 PCS, HCT-CI, and DRI in predicting survival among allogeneic transplant patients at six months and one year, with lower scores representing better predictive ability. For survival at six months, Brier scores were 0.169 for the pre-transplant SF-36 PCS, 0.170 for the HCT-CI and 0.177 for the DRI; at 1 year, Brier scores were 0.204 for the pre-transplant SF-36 PCS, 0.211 for the HCT-CI and 0.213 for the DRI. By this measure, the SF-36 had similar predictive ability as more commonly used prognostic scales.
Relationship of Early Post-transplant Change in SF-36 Scores with Subsequent Survival Outcomes: Allogeneic recipients
Among 236 allogeneic transplant recipients who were early survivors (Day 100 after transplant) and completed SF-36 forms at that time, 63 (27%) subsequently died at a median of 8 months post-HCT during the observation period. Of early survivors, 92% were still alive at 6 months and 83% were alive at 1 year. Early post-transplant decline in the PCS score was strongly predictive of subsequent mortality after adjusting for the pre-HCT PCS (HR 1.83 per 10 points decrease, 95% CI 1.40–2.40, p<0.001) as was the MCS change score (HR 1.43, 95% CI 1.13–1.80, p=0.003) (Table 3). Hazard ratios were similar after adjusting for the HCT-CI, EBMT score, or DRI. The early post-transplant PCS change score was also predictive for subsequent TRM (HR 3.57, 95% CI 2.13–5.88, p<0.001).
Table 3.
Relationship of early post-transplant changes in PCS and MCS scores with subsequent mortality in allogeneic recipients, adjusting for baseline SF-36 scores
| Scale | HR for 10pt worsening (95% CI) | p-value |
|---|---|---|
| Overall Mortality | ||
| PCS | 1.83 (1.40–2.40) | <0.001 |
| MCS | 1.43 (1.13–1.80) | 0.003 |
| Transplant related mortality | ||
| PCS | 3.57 (2.13–5.88) | <0.001 |
| MCS | 1.42 (0.96–2.11) | 0.079 |
Discussion
We found that pre-HCT physical HR-QOL, as measured by the SF-36 PCS, independently predicted survival and transplant-related death in allogeneic HCT recipients. Early post-transplant changes in PROs were also prognostic. The predictive strength of physical and mental function was not changed after adjusting for other non-PRO measures of comorbidity or disease risk.
While the pre-HCT SF-36 PCS was correlated with the pre-HCT clinician-assessed KPS, the KPS was not associated with survival outcomes, highlighting the strong and independent predictive ability of patient-reported functional status. The relatively healthier patient population participating in this exercise intervention trial may have helped to explain the lack of association of KPS with survival. Additionally, the KPS is a relatively insensitive measure of patient functioning; PROs such as the SF-36 are less subject to physician bias.
Pre-HCT PROs may measure physiologic reserve. For example, a Geriatric Assessment (GA) predicts toxicities and survival in older patients undergoing chemotherapy or surgery.31–33 The GA is prognostic in the setting of chemotherapy for acute myelogenous leukemia,34 and allogeneic transplant.35 In the latter of these two studies, lower pre-HCT SF-36 MCS was associated with inferior post-HCT outcomes, while pre-HCT SF-36 PCS trended in the same direction. That study reflected a smaller and older population that was less representative of the general transplant population.
It is possible that some impairments in pre-HCT HRQOL were related to concurrent comorbid illness.36 However, in our study we found that the SF-36 PCS remained independently predictive for outcome after adjustment for the HCT-CI, a transplant comorbidity index. It is also possible that HRQOL impairments may have coexisted with other prognostically relevant behavioral attributes, such as depression.37–43 Since the MCS was not predictive of survival in our sample, this potentially confounding effect is less likely.
Due to limitations in the clinical trial data, we were not able to elucidate the biologic correlates for the association of changes in the SF-36 with subsequent outcomes. It is possible that higher change scores were associated with severe acute GVHD or relapse. Regardless, the finding that early worsening in post-transplant PROs predicted subsequent death helps to validate patient-reported physical function as a marker that retains prognostic relevance throughout the transplant continuum.
We acknowledge limitations to our study. Our data set was taken from a single prospective trial. The mechanism for the relationship between the SF-36 and outcomes is also unclear. Other measurements of physical function might perform even better than the SF-36, such as an alternative PRO instrument (e.g. FACT BMT, PROMIS), or direct measurements of physical fitness.44,45
Because of its significant predictive ability and ease of use, the pre-transplant SF-36 PCS could be a useful tool for risk stratification within hematopoietic cell transplantation. In contrast to currently used, more complex indices, the SF-36 is readily scalable to general practice without anticipated variation in data collection and interpretation across different centers. Because it can be collected and scored before patient visits, the SF-36 may also be more clinic-friendly than other measures. Based upon our analysis of the SCTOD model, PROs may serve an important role in risk adjustment for HCT quality reporting. However, further work will be needed to inform the optimal integration of PROs into routine clinical care and quality reporting. If a patient has a poor SF-36 PCS at the time of transplant consultation, it is not known whether a “prehabilitative” intervention could optimize physical function and improve clinical outcomes. Within the domain of quality reporting, additional effort will be required to inform the hierarchical ordering of prognostic pre-HCT variables, such as baseline disease risk, comorbidity, and PROs.
In conclusion, our findings suggest that patient-reported data may have a critical role in improving HCT treatment risk stratification with its accompanying effects on publicly released data, clinical trial interpretation, and comparative effectiveness studies. To date, HCT prognostic models have been limited largely to variables or indices derived from medical records data abstraction, and PROs represent a promising opportunity to incorporate the patient perspective into formal tools for risk stratification and treatment planning. Further work is needed to support the integration of PROs into HCT clinical practice and quality reporting.
Footnotes
Authorship statement: All authors designed the study, collected and analyzed data, and wrote the manuscript. JL performed the statistical analysis and edited the manuscript. All authors critically revised the manuscript for important intellectual content and approved the manuscript for publication.
Conflict of interest statement: There are no conflicts of interest to report.
Financial disclosure: This work was supported by the National Heart, Lung, and Blood Institute and the National Cancer Institute (Grant U10HL069294).
References
- 1.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905–913. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gratwohl A. The EBMT Risk Score. Bone Marrow Transplantation. 2012;47:749–756. doi: 10.1038/bmt.2011.110. [DOI] [PubMed] [Google Scholar]
- 4.Guilfoyle R, Demers A, Bredeson C, et al. Performance status, but not the hematopoietic cell transplantation comorbidity index (HCT-CI), predicts mortality at a Canadian transplant center. BMT. 2009;43:133–139. doi: 10.1038/bmt.2008.300. [DOI] [PubMed] [Google Scholar]
- 5.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 6.Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. BBMT. 2006;12:954–964. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan MS, Berthelot JM, Feeny D, McFarland BH, Khan S, Orpana H. The predictive validity of health-related quality of life measures: mortality in a longitudinal population-based study. Qual Life Res. 2007;16(9):1539–1546. doi: 10.1007/s11136-007-9256-7. [DOI] [PubMed] [Google Scholar]
- 8.Han PK, Lee M, Reeve BB, et al. Development of a prognostic model for six-month mortality in older adults with declining health. J Pain Symptom Manage. 2012;43(3):527–539. doi: 10.1016/j.jpainsymman.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ried LD, Tueth MJ, Handberg E, Nyanteh H. Validating a self-report measure of global subjective well-being to predict adverse clinical outcomes. Qual Life Res. 2006;15(4):675–686. doi: 10.1007/s11136-005-3515-2. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4(5):496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 12.Kleefstra N, Landman GW, Houweling ST, et al. Prediction of mortality in type 2 diabetes from health-related quality of life (ZODIAC-4) Diabetes Care. 2008;31(5):932–933. doi: 10.2337/dc07-2072. [DOI] [PubMed] [Google Scholar]
- 13.Wisloff F, Hjorth M. Health-related quality of life assessed before and during chemotherapy predicts for survival in multiple myeloma. Nordic Myeloma Study Group. Br J Haematol. 1997;97(1):29–37. doi: 10.1046/j.1365-2141.1997.222667.x. [DOI] [PubMed] [Google Scholar]
- 14.Sloan JA, Zhao X, Novotny PJ, et al. Relationship between deficits in overall quality of life and non-small-cell lung cancer survival. J Clin Oncol. 2012;30(13):1498–1504. doi: 10.1200/JCO.2010.33.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival; a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10(9):865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 16.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 17.Trajokovic-Vidakovic M, De Graeff A, Voest EE, Teunissen S. Crit Rev Oncol Hematol. 2012;84(1):130–48. doi: 10.1016/j.critrevonc.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang XS, Shi Q, Lu C, et al. Prognostic value of symptom burden for overall survival in patients receiving chemotherapy for advanced nonsmall cell lung cancer. Cancer. 2010;116:137–145. doi: 10.1002/cncr.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viala M, Bhakar AL, de la Loge C, et al. Patient-reported outcomes helped predict survival in multiple myeloma using partial least squares analysis. Journal of Clinical Epidemiology. 2007;60:670–679. doi: 10.1016/j.jclinepi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Dubois D, Dhawan R, van de Velde H. Descriptive and prognostic value of patient-reported outcomes: the bortezomib experience in relapsed and refractory multiple myeloma. Journal of Clinical Oncology. 2006;24(6):976–982. doi: 10.1200/JCO.2005.04.0824. [DOI] [PubMed] [Google Scholar]
- 21.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114:7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Fairclough D, Parsons SK, et al. Recovery after stem-cell transplantation for hematological disease. J Clin Oncol. 2001;19:242–252. doi: 10.1200/JCO.2001.19.1.242. [DOI] [PubMed] [Google Scholar]
- 23.Syrjala KL, Chapko MK, Vitaliano PP, et al. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant. 1993;11:319–327. [PubMed] [Google Scholar]
- 24.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen PB, Le Rademacher J, Jim H. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20(10):1530–1536. doi: 10.1016/j.bbmt.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan EI, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 27.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimates. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Cox DR. Regression Models and Life Tables (with Discussion) Journal of the Royal Statistical Society. 1972:187–220. Series B 34. [Google Scholar]
- 29.Graf E, Schmoor C, Sauerbrei W, Schumacher M. Assessment and comparison of prognostic classification schemes for survival data. Statistics in Medicine. 1999;18:2529–2545. doi: 10.1002/(sici)1097-0258(19990915/30)18:17/18<2529::aid-sim274>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.http://www.cibmtr.org/ReferenceCenter/SlidesReports/USStats/Documents/CIBMTR_HCT_Center_Survival_Report_Methodology.pdf
- 31.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16(11):1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 32.Repetto L, Fratinio L, Audisio RA, et al. Comprehensive geriatric assessment addsd information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 33.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 34.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogenic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373–1379. doi: 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorror ML. Comorbidities and hematopoietic cell transplantation outcomes. Hematol Am Soc Hematol Educ Program. 2010;2010:237–247. doi: 10.1182/asheducation-2010.1.237. [DOI] [PubMed] [Google Scholar]
- 37.Hoodin F, Uberti JP, Lynch TJ, Steel P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38:255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- 38.Costanzo ES, Juckett MB, Coe CL. Biobehavioral influences on recovery following hematopoietic stem cell transplantation. Brain Behav Immun. 2013;30 (Suppl):S68–74. doi: 10.1016/j.bbi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grulke N, Larbig W, Kachele H, Bailer H. Pre-transplant depression as risk factor for survival of patients undergoing allogeneic haematopoietic stem cell transplantation. Psychooncology. 2008;17(5):480–7. doi: 10.1002/pon.1261. [DOI] [PubMed] [Google Scholar]
- 40.Prieto JM, Atala J, Blanch J, Carreras E, Rovira M, Cirera E, Espinal A, Gasto C. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. Journal of Clinical Oncology. 2005;23(25):6063–71. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 41.Andrykowski MA, Brady MJ, Henslee-Downey PJ. Psychosocial factors predictive of survival after allogeneic bone marrow transplantation for leukemia. Psychosomatic Medicine. 1994;56(5):432–439. doi: 10.1097/00006842-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Loberiza FR, Rizzo JD, Bredeson CN, Antin JH, Horowitz MM, Weeks JC, Lee SJ. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. Journal of Clinical Oncology. 2002;20(8):2118–26. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- 43.Knight JM, Lyness JM, Sahler OJZ, Liesveld JL, Moynihan JA. Psychosocial factors and hematopoietic cell transplantation: potential biobehavioral pathways. Psychoneuroendocrinology. 2013;38(11):2383–2393. doi: 10.1016/j.psyneuen.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood WA, Deal AM, Reeve BB, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant. 2013;48:1342–9. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 45.Kelsey CR, Scott JM, Lane A. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone Marrow Transplant. 2014;49:1330–6. doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]