Abstract
Neutrophils are the first responders to sites of acute tissue damage and infection. Recent studies suggest that in addition to neutrophil apoptosis, resolution of neutrophil inflammation at wounds can be mediated by reverse migration from tissues and transmigration back into the vasculature. In settings of chronic inflammation, neutrophils persist in tissues, and this persistence has been associated with cancer progression. However, the role of neutrophils in the tumor microenvironment remains controversial, with evidence for both pro- and anti-tumor roles. Here we review the mechanisms that regulate neutrophil recruitment and resolution at sites of tissue damage, with a specific focus on the tumor microenvironment. We discuss the current understanding as to how neutrophils alter the tumor microenvironment to support or hinder cancer progression, and in this context outline gaps in understanding and important areas of inquiry.
Neutrophils at the crossroads of inflammation and cancer
Neutrophils are the most abundant circulating leukocyte and are the first responders to sites of infection and tissue damage. The primary function of neutrophils is to mediate host defense through multiple mechanisms including phagocytosis and intracellular killing of pathogens, release of granules containing antimicrobial peptides and proteases, and the formation of neutrophil extracellular traps (NETosis). Neutrophils are highly motile and display rapid recruitment to a variety of signals including chemokines, lipid mediators, and pathogen signals to mediate host defense (reviewed in [1-3]). However, neutrophils are not just “killing machines” but play a key role in orchestrating the innate and adaptive immune responses by releasing cytokines and chemokines and through antigen presentation [1, 2]. Indeed, neutrophils are much longer lived than initially thought and can survive for 5 or more days in the circulation [4] and may potentially live even for weeks in tissues. While it is well known that neutrophils are crucial for normal host defense and survival [5], uncontrolled neutrophil activation can contribute to chronic inflammation and tissue damage. Due to the balance needed for proper host protection and tissue homeostasis, understanding how neutrophils are recruited and subsequently resolve inflammation is an important question with broad implications including understanding the role of neutrophils in tumor progression.
The tumor microenvironment is characterized by persistent inflammation, and is often referred to as the “wound that does not heal” [6]. It has been known for some time that neutrophils are present in the tumor microenvironment; however, their role in tumor biology including tumor progression and invasion has remained controversial with both detrimental and beneficial effects reported. Several recent studies have highlighted the role of neutrophils in cancer biology using different cancer models in both mice and more recently zebrafish (see Box 1). It is known that the presence of neutrophils in tumors often correlates with poor patient outcome in humans; however whether the presence of tumor-associated neutrophils (TANs) directly contributes to disease progression is unclear. There is substantial evidence for a pro-tumor role for neutrophils in cancer progression. For example, a study by Bekes et al. showed that neutrophils produce MMP9 within the tumor microenvironment and this contributes to angiogenesis, tumor progression, and metastasis in mouse transplantation models [7]. Additionally, inhibition of myeloid cell recruitment into tumors with CXCR2 inhibition increases the efficacy of chemotherapy in breast carcinoma models, suggesting that targeting neutrophil recruitment may be beneficial [8]. By contrast, other studies have suggested that neutrophils can play an anti-tumor role by activating the immune response against tumors and promoting tumor cell clearance [9]. Indeed, neutrophils display plasticity and can be polarized into either an anti-tumoral (N1) or pro-tumoral (N2) phenotype depending on environmental factors [10]. Here we review these and other recent studies that have revealed mechanisms of neutrophil recruitment and resolution at sites of tissue damage, with a specific focus on the tumor microenvironment and how neutrophils may contribute to cancer progression.
Neutrophils and tissue damage
Neutrophil recruitment to tissue damage
There are common mechanisms that mediate neutrophil recruitment to wounds and cancer. In the case of the wound response, many signals mediate neutrophil recruitment to damaged tissues including DAMPs (Damage-Associated Molecular Pattern molecules) and chemokines (Figure 1) [11]. Wound induced recruitment signals have been elucidated using both mouse and zebrafish model systems (Box 1). In the zebrafish model, one of the earliest attractants after wounding is a hydrogen peroxide burst that is released by damaged tissue [12]. This is sensed by neutrophils through the Src family kinase, Lyn, which is required for early neutrophil response to wounds in zebrafish [13]. Other signals include the release of lipid mediators like LTB4. In mouse wounding models, LTB4 has been shown to elicit neutrophil swarming to areas of cell death in damaged tissues [14]. Neutrophils at the site of inflammation also secrete LTB4, further amplifying neutrophil recruitment. Necrotic tissue after sterile injury has also been shown to release ATP, activating the NLRP3 inflammasome via the P2X7 receptor that can mediate a chemokine gradient of CXCL2 (MIP-2) and CXCL1 [15]. A novel pathway in mouse liver injury showed that CXCL2 is regulated by TLR2 and S100A9 and mediates neutrophil recruitment [16]. Moreover, CXCL1 and G-CSF were shown to locally recruit neutrophils to areas of heart injury [17]. Additionally, chemokines like CXCL8/IL-8 recruit neutrophils via the CXCR1 and CXCR2 chemokine receptors to tissue damage [18]. Other G-protein coupled receptors are also involved in neutrophil recruitment. For example, in liver inflammation and sterile injury, the formylpeptide receptors Fpr1 and Fpr2 mediate neutrophil recruitment [15, 19]. While many pathways have been identified that mediate neutrophil recruitment to wounds, the temporal and spatial relationship between these different pathways in the context of tissue damage remains poorly understood.
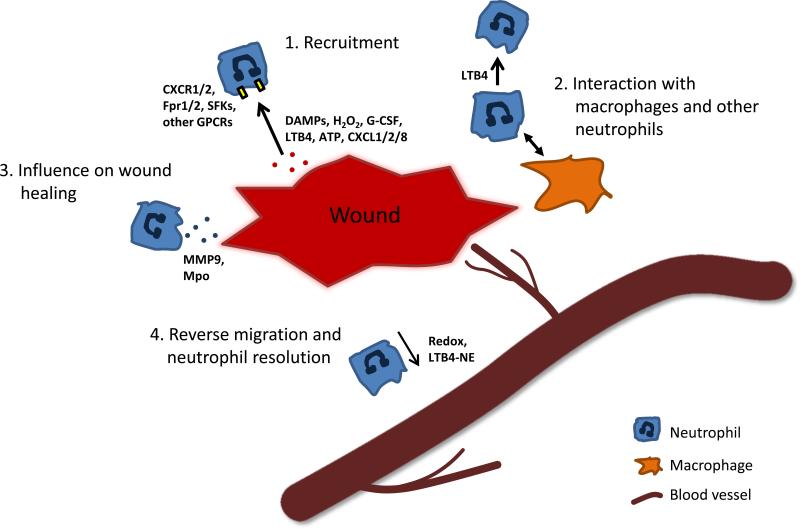
Figure 1. Neutrophils in the wound microenvironment.
Neutrophils are recruited to areas of wounding and tissue damage via pro-inflammatory signals including DAMPs, hydrogen peroxide, lipid mediators, and chemokines (1). Neutrophils can further influence the innate immune response to wounding by recruiting additional neutrophils, as well as macrophages which in turn can phagocytose neutrophils or drive reverse migration (2). In addition, neutrophils influence the wound healing by releasing factors including MMP9 and myeloperoxidase (3). Reverse migration of neutrophils is mediated by Redox signaling and LTB4-NE signaling which promotes reverse transendothelial migration back into the blood stream (4).
The role of neutrophils at the wound, beyond clearance of pathogens and host defense, still remains unclear. Tissue damage and hypoxia can produce VEGF-A which recruits neutrophils expressing the pro-angiogenic matrix metalloproteinase MMP9, contributing to revascularization of the wounded tissue [20]. MMP9 is also important for remodeling of collagen after tissue injury to promote tissue repair and regeneration [21]. Neutrophils also recruit additional immune cells such as macrophages, which phagocytose apoptotic neutrophils and cellular debris [22]. This is important for resolution of inflammation at wounds, which is a key step in tissue repair. Neutrophils are also important for turning down the hydrogen peroxide burst through the delivery of myeloperoxidase (MPO) [23]. In chronic inflammation, neutrophils impair wound healing. A recent study in a diabetic mouse model showed that formation of neutrophil extracellular traps (NETs) impairs wound healing and that by disrupting NETosis, wound healing was enhanced [24]. On the other hand, neutrophil NETs may also limit inflammation by leading to the degradation of chemokines [25]. Further progress in characterizing the role of neutrophils at wounds will likely increase our understanding of the role of neutrophils in the tumor microenvironment.
Resolution of neutrophilic inflammation by reverse migration
Once infection and tissue debris have been cleared, it is important for neutrophil-mediated inflammation to resolve to prevent further tissue damage. It has long been known that resolution of neutrophil infiltration in tissues involves neutrophil apoptosis and their subsequent clearance by macrophages (reviewed in [22, 26]). However, more recent work has shown that neutrophils can also leave sites of tissue damage and undergo reverse migration back into the blood stream (Figure 1). Neutrophil reverse migration was first suggested in a rat model of glomerular inflammation [27] and was subsequently observed in human neutrophils co-cultured with endothelial cells in vitro [28]. The full process of neutrophil reverse migration was first visualized in vivo in the transparent zebrafish using live imaging of neutrophils after wounding [29]. In more recent studies, neutrophil reverse transmigration has also been live-imaged in mouse models [30]. Interestingly, in zebrafish, macrophages can induce reverse migration of neutrophils from a wound site by ROS-Src family kinase signaling, suggesting that macrophages may modulate resolution of neutrophil-mediated inflammation through both phagocytosis and/or a repellant mechanism [31]. In mice, recent evidence demonstrates neutrophil reverse transendothelial migration is regulated by an LTB4-neutrophil elastase signaling axis [32], suggesting that similar cues may direct both recruitment and reverse migration. Using microfluidic devices, reverse migration, or retrotaxis, of human neutrophils has also been demonstrated [33, 34]. These studies have shown that as many as 90% of human neutrophils are able to reverse migrate and that this process is enhanced in the presence of the anti-inflammatory lipid mediator, lipoxin A4, or the antioxidant compound, Tempol. There is some speculation that activated neutrophils undergoing reverse migration may lead to systemic activation of the immune response but this possibility remains to be studied. Work in zebrafish has shown that while neutrophils that have reverse migrated exhibit an activated morphology, they mount a normal response to a secondary insult [35]. This is important as neutrophils survive longer than previously thought in humans and these activated neutrophils may be able to respond to multiple tissue insults in the context of chronic inflammation or cancer.
Importantly, if neutrophils fail to resolve inflammation, acute inflammation can become chronic inflammation, leading to a cycle of tissue damage. Chronic neutrophil-mediated inflammation is observed in many diseases, including COPD, rheumatoid arthritis, diabetes, and others which are reviewed in detail elsewhere [36-40]. Importantly, the role of neutrophils in acute inflammation, as well as the transition from acute to chronic inflammation can potentially yield clues as to the role of neutrophils in cancer. Tumor progression can be considered a type of chronic inflammation where there is persistent neutrophil presence, and understanding neutrophil reverse migration and wound-healing in this context may provide a framework that is informative for cancer biology.
Neutrophils in cancer
Evidence of neutrophils’ pro-cancer role
The role of neutrophils in cancer remains controversial, and is likely to be context dependent. It has long been known that neutrophils are present in many different types of cancers. For example, neutrophils are within the tumor or surrounding tissues in many cancers including glioblastoma, renal cell carcinoma (RCC), melanoma, colorectal cancer, hepatocellular carcinoma (HCC), pancreatic ductal carcinoma, and head and neck cancer, among others [41-49]. Many of these studies, including a recent meta-analysis [50], suggest that enhanced levels of neutrophils within the tumor tissue, or tumor-associated neutrophils (TANs), correlate with poor patient prognosis. It is important to note that it is not clear whether neutrophils themselves contribute to poor patient outcomes or if they merely correlate with a more aggressive disease phenotype. Recent studies have begun to address the role of neutrophils within the tumor microenvironment, revealing both beneficial and detrimental effects.
Neutrophil recruitment to tumors
Similar to acute wound responses, tumor cells and the surrounding microenvironment produce cues that actively recruit neutrophils. These signals include chemokines and cytokines, many of which are also important for responses to acute wounding (Table 1 and Figure 2). Neutrophils express the chemokine receptors CXCR1 and CXCR2 and these receptors are important for chemotaxis [51]. Cancer cells express various ligands for these receptors that facilitate recruitment of TANs. Mouse T cell lymphoma and Lewis lung carcinoma (LLC) cells express the Liver X Receptor (LXR) ligand oxysterols which have been shown to recruit neutrophils through CXCR2 [52]. Tumors also express a host of chemokines and cytokines, including CXCL8, CXCL5 and CXCL6 among others, which are involved in neutrophil recruitment [53]. CXCL5 was found to recruit neutrophils in a mouse model of HCC, and high levels of CXCL5 correlate with poor prognosis in human HCC patients [54]. Interestingly, the CXCL5/CXCR2 axis was also found to be involved in epithelial-to-mesenchymal transition of HCC cells and contributes to tumor invasion [55], although it is not known if this is directly linked to increased neutrophil infiltration. Cytokines like TNFα have also been implicated in the recruitment or persistence of neutrophils in the tumor microenvironment, such as in a mouse skin carcinogenesis model [56]. The cytokine IL17 produced by gamma delta T cells enhances neutrophil recruitment and promotes tumor growth and metastasis in two separate mouse models of metastatic breast cancer [57, 58], suggesting that tumor-infiltrating lymphocytes can also modulate neutrophils in the tumor microenvironment. One very interesting connection between inflammation and cancer is what happens when there is chronic wounding or when wounding occurs near neoplastic cells. Using a zebrafish model of chronic wounding it was recently demonstrated that wound-induced inflammation leads to transformation of nearby melanocytes. Moreover, inflammatory cells that are recruited to a wound can influence transformed cells by increasing their proliferation [59]. This has important clinical implications as tumor biopsies are routinely used for diagnosis, and this inflammatory wound environment could have a detrimental impact on disease progression. Other work using zebrafish has demonstrated that, like in wounds, hydrogen peroxide is one of the initial signals that recruit leukocytes to transformed cells [60]. While it is known that these and many other chemotactic signals are upregulated in cancer, the direct role of these signals in recruiting neutrophils to the tumor microenvironment requires further investigation.
Table 1.
Factors that mediate neutrophil recruitment and influence on the tumor microenvironment.
| Function | Tumor process | Protein or Factor | Cancer/tissue type | Species | Reference |
|---|---|---|---|---|---|
| TAN recruitment, pro-tumor | Tumor growth, angiogenesis | Oxysterols | T cell lymphoma, LLC | mouse | 52 |
| TAN recruitment, pro-tumor | EMT/invasion, poor prognosis | CXCL5 | HCC | mouse, human | 54, 55 |
| TAN recruitment, pro-tumor | Tumor initiation | TNFα | skin | mouse | 56 |
| TAN recruitment, pro-tumor | Tumor growth, metastasis | IL17 | breast cancer | mouse | 57, 58 |
| TAN recruitment, pro-tumor | Tumor growth | H2O2 | skin | zebrafish | 60 |
| TAN recruitment, pro-tumor | Tumor invasion and migration | HGF | pulmonary adenocarcinoma, LLC | human, mouse | 66, 67 |
| TAN recruitment, pro-tumor | Tumor invasion, angiogenesis | VEGF/VEGFR | pancreatic cancer, transformed endothelial cells | mouse, zebrafish xenograft | 70, 71 |
| Pro-tumor | Angiogenesis | MMP9 | spontaneous mets model | chick | 7, 70 |
| Pro-tumor | Tumor invasion, angiogenesis | Oncostatin M | breast cancer, lung | human, mouse | 68 |
| Pro-tumor | EMT/invasion | CXCR2 | skin | zebrafish | 72 |
| Pro-tumor | Tumor cell proliferation | Prostaglandin E2 | skin | zebrafish | 73 |
| Pro-tumor | T cell inhibition | Arginase 1 | NSCLC | human, mouse | 75 |
| Pro-tumor | Metastasis, chemoresistance | CXCL1/2, CXCR2 | breast cancer | mouse | 8 |
| TAN recruitment, pro-tumor | Tumor growth, metastasis | CXCL6 | melanoma | mouse | 87 |
| Pro-tumor | Tumor growth | NE | lung | mouse | 88 |
| Pro-tumor/Anti-tumor | T cell inhibition/activation, cytotoxicity | TGF-beta | lung | mouse | 10 |
| Anti-tumor | T cell activation (CD8+, CD4+) | OX-40L, 4-1BBL | lung | human | 9 |
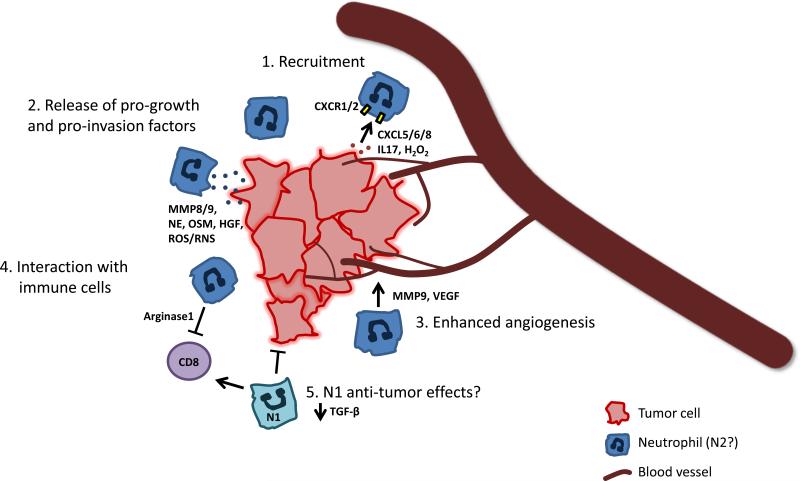
Figure 2. TAN interaction with the tumor microenvironment.
Neutrophils are recruited to tumor sites via signals produced by cells of the tumor and microenvironment, including chemokines, cytokines, and hydrogen peroxide (1). These and other signals induce TANs to release factors which can remodel the ECM in the tumor microenvironment or act directly on tumor cells themselves to enhance tumor proliferation and invasion (2). In addition, some of these TAN-produced factors stimulate angiogenesis to support tumor growth and metastasis (3). Further evidence suggests that TANs interact with other immune cells such as CD8+ T cells. Depending on their polarity, TANs can have an immunosuppressive or immunostimulatory effects (4). While the TANs represented here are likely of the N2 pro-tumoral phenotype, the role of N1 neutrophils in targeting cancer cells and the N1/N2 polarization of neutrophils in response to cancer is an area of significant interest (5). Understanding how neutrophils are recruited and the complex ways they interact with cells within the tumor microenvironment will be important for the development of new therapies to treat cancer.
Role of neutrophils in tumor progression, angiogenesis, and invasion
There has been recent progress to identify potential mechanisms for how neutrophils contribute to cancer progression. It is likely that TANs play a role in cancer progression by releasing factors that modulate the extracellular matrix (ECM) and inflammation in the tumor microenvironment. More recent evidence also suggests that neutrophils may have more direct effects on cancer cell proliferation and invasion (Figure 2). Neutrophils release granules containing neutrophil elastase (NE), neutrophil collagenase (MMP8), and gelatinase B (MMP9), factors that remodel the ECM and affect cancer progression [61]. For example, MMP9 is produced by neutrophils and affects keratinocyte proliferation and invasion in skin cancer models [62]. While it is important to note that tumor-associated macrophages are also likely a source of MMP9 within the tumor microenvironment, it has been demonstrated that inhibition of neutrophil infiltration in a chick spontaneous metastasis model decreases tumor angiogenesis and dissemination, and the effect was rescued by neutrophil production of MMP9 [7]. Neutrophils can also enhance tumorigenesis through the release of reactive oxygen species (ROS) and reactive nitrogen species (RNS) which likely contribute to DNA damage and genetic instability in chemically induced carcinogenesis models [63-65]. Importantly, neutrophils produce growth factors like hepatocyte growth factor (HGF), which influences the invasion of human pulmonary adenocarcinoma cells [66]. Interestingly, HGF signaling through the Met receptor also can recruit neutrophils that have anti-tumor effects [67] suggesting a potential feedback loop mediated by HGF signaling. Neutrophils have also been shown to release cytokines like Oncostatin M, a member of the IL-6 family, which induces VEGF production and increases angiogenesis and tumor cell invasion in breast cancer [68], as well as affecting infiltrating neutrophils in lung cancer [69]. Moreover, it was reported that neutrophil release of MMP9 increases VEGF, while inhibiting neutrophils impairs the VEGF-induced angiogenic switch in a mouse pancreatic cancer model [70]. In a zebrafish xenograft model, VEGFR inhibition reduces tumor vascularization but also enhances neutrophil infiltration and promotes tumor invasion, potentially through effects on collagen remodeling [71]. In another zebrafish model of oncogene-transformed cells, the presence of neutrophils was observed to enhance epithelial-to-mesenchymal transition of keratinocytes [72] at least in part through CXCR2 signaling, suggesting that neutrophils may directly affect the invasive behavior of transformed cells. Neutrophils also produce prostaglandin E2 (PGE2) which supports proliferation of early transformed cells [73], supporting the beneficial effects of targeting COX2-PGE2 in cancer prevention. In an in vitro co-culture model of RCC cells, neutrophils enhance the migration and invasion of cancer cells by upregulating estrogen receptor β, VEGFα, and HIF-2 pathways, providing further insight into how neutrophils affect cancer progression [74]. Although there has been recent interest and progress, there remain significant gaps in understanding how neutrophils affect cancer cell invasion and progression, in part because these effects are likely dependent on factors such as tumor type and stage of progression.
Neutrophils are also involved in tumor immunity by orchestrating the activity of other components of the immune response. In addition to proinflammatory factors, neutrophils produce factors that suppress anti-tumor immunity, similar to the effects described for granulocytic myeloid-derived suppressor cells (G-MDSCs, see Box 2 for further discussion). One such factor is arginase 1, an inhibitor of T cell function, which is produced by neutrophils in response to CXCL8 signaling [75]. Accordingly, depletion of neutrophils in mouse lung tumors results in increased activation of CD8+ T cells and decreased tumor growth that can be mediated by TGF-β [10]. However, in some contexts, such as early-stage human lung cancer, TANs are not necessarily inhibitory to T cell function but can instead promote T cell mediated immunity through the production of OX-40L and 4-1BBL costimulatory molecules which enhance proliferation of CD4+ and CD8+ T cells and increase their cytotoxic abilities in early stage lung cancer [9], further demonstrating the complexity of TAN crosstalk within the tumor microenvironment. It is an intriguing idea that TANs may differentially affect anti-tumor immunity depending on the stage or type of cancer. An additional element is the possibility that neutrophils may act as antigen presenting cells to promote the targeting of cancer cells by cytotoxic T cells, similar to what has been reported in response to microbial environments [76] or in the presence of chemokines (recently reviewed here [77]).
One new avenue of research focuses on the role of neutrophil extracellular traps (NETs) in cancer progression (recently reviewed in [78]). There is evidence that the presence of NETs within tumors correlates with poor prognosis [79]. In various mouse models of cancer, it has been seen that tumor cells can increase NET production by TANs, and potentially circulating neutrophils as well [80]. NETs appear to have a pro-tumor effect both by enhancing proliferation of cancer cells and by inhibiting apoptosis [79-82], however the precise mechanism by which NETs enhance tumor progression is unclear. One potential mechanism is that the release of NETs is associated with the release of pro-tumor factors including MMP9, NE, and cathepsin G [78]. As NETs are traditionally involved in the capture of pathogens, it has also been suggested that NETs may enhance adhesion and metastasis of escaped circulating tumor cells. Importantly, inhibition of NETs has been shown to decrease adhesion of lung carcinoma cells and formation of metastases [83]. By contrast, a recent study of chronic inflammation in mice suggests that the formation of NETs by high density neutrophils can degrade pro-inflammatory cytokines and chemokines and alleviate inflammation [25]. While this role has not yet been addressed in the context of cancer, it suggests once again that neutrophils can play a dual role in inflammation and cancer. A caveat to the study of NETs’ role in tumor progression is that it can be difficult to distinguish NETs, which are frequently identified by DNA stains or histone labeling, from the DNA of other dead cells including neutrophils themselves. However, co-labeling with proteins such as NE can help to clarify the presence of NETs. As live-imaging techniques advance and other models, including zebrafish (Box 1) are developed, it may be possible to better identify NET producing neutrophils within the tumor microenvironment through observation of their behavior and NET dynamics.
Targeting neutrophil-mediated inflammation in the tumor microenvironment
There is increasing interest in the idea that targeting neutrophils may be a useful therapeutic approach to affect cancer progression. For example, inhibition of CXCL8-CXCR1/2 signaling by CXCL8 antibodies [7] or small-molecules targeting CXCR1 and/or CXCR2 [84-86] has been shown to decrease tumor growth and progression in mouse models of cancer. Importantly, CXCR2 inhibition in a mouse metastatic breast cancer model enhanced response of both tumor and micrometastases to chemotherapy treatment, which was attributed at least in part to the dampening of the chemoresistant, pro-myeloid and granulocyte recruitment effects mediated by CXCL1/2 signaling [8]. Additionally, anti-CXCL6 antibodies impaired neutrophil recruitment to melanoma in mouse models and affected tumor growth [87]. Inhibitors of NE are also being tested and have shown some promise in mouse models of lung cancer [88], however in some cases such as the K14-HPV16 mouse model of squamous cell carcinoma, knockdown of NE had no effect on tumor development [89]. However, targeting neutrophils can be associated with side effects as neutrophils are critical for host defense against infection. An intriguing target for future studies is the idea that neutrophil resolution of inflammation could be induced within the tumor microenvironment. In particular, the idea that neutrophilic inflammation resolves by neutrophil reverse migration provides an avenue to promote resolution rather than blocking recruitment to sites of infection or tissue damage. Moreover, reverse migrated TANs may provide a mechanism for systemic activatation of the immune response to cancer, either by direct stimulation of peripheral cytotoxic T cells or antigen presentation. The identification of drugs that promote neutrophil reverse migration from wounds [90] raises the idea that drugs that promote neutrophil reverse migration and resolution in the tumor microenvironment may be developed. Another intriguing possibility is the use of nanoparticles to target TANs. This has recently been described in a mouse model of inflammation to block neutrophil adhesion [91]. In addition, since wound associated macrophages can have neutrophil repelling functions [31], it is an interesting idea that macrophages could be engineered to promote resolution of neutrophil inflammation in the tumor microenvironment.
A key caveat in blocking neutrophils in the tumor microenviroment is that not all neutrophils have pro-tumor effects. Recent studies suggest that neutrophils exhibit substantial plasticity and can be polarized to an N1 anti-tumoral or N2 pro-tumoral phenotype in response to the microenvironment, reminiscent of the M1/M2 polarization of macrophages [10, 92]. Tumor-associated N2 neutrophils are characterized by high expression of CXCR4, VEGF, and gelatinase B/MMP9 and can be induced upon exposure to high TGF-β levels. By contrast, N1 neutrophils are induced upon TGF-β blockade and express immunoactivating cytokines and chemokines, low levels of arginase, and are able to kill cancer cells [10]. Additionally, there is recent evidence suggesting that neutrophils from certain healthy donors are able to kill cancer cells and onocogene-transformed cells in a cell-specific manner [93], and that neutrophil killing of cancer cells may be enhanced by β-glucan treatment [94] making neutrophils a compelling candidate for cancer immunotherapy. Indeed, several studies that induce neutrophilia via prolonged G-CSF treatment in tumors demonstrate a shift from a chronic to acute inflammatory environment and an anti-cancer effect [95]. Understanding how neutrophils are polarized to a pro- or anti-tumor phenotype, and if and how they can be reprogrammed to switch this fate, will be crucial to developing successful cancer therapies.
Additionally, neutrophils are now being used to aid in the diagnosis of cancer and to help guide therapeutic decisions. Blood counts such as the neutrophil-to-lymphocyte ratio (NLR) predict patient outcome and can be used to measure response to treatment, where high NLRs correlate with poor prognosis and failure to respond to treatment [96]. Recently, NLRs have also been used to help differentiate primary breast carcinoma from benign proliferative breast disease [97]. NLR has also recently been applied for early detection and as a prognostic marker in gastric cancer [98], early diagnosis of ovarian cancer [99], and prognosis and survival prediction in colon cancer [100] and HCC [101], among others. The use of NLRs and other systemic inflammation markers could help not only aid in treatment choices and prognostic predictions, but also potentially enhance early detection of cancer. Additionally, some of the factors released by neutrophils in the tumor microenvironment can be used as biomarkers during early cancer detection. For example, neutrophil gelatinase-associated lipocalin (NGAL) is a strong biomarker for early pancreatic cancer [102] and ovarian cancer detection [103]. Identification of further TAN proteins could identify other biomarkers for the early diagnosis and characterization of cancer.
Concluding Remarks
There has been recent progress in defining the role of neutrophils in cancer progression, however gaps remain in understanding neutrophil plasticity and the switch between pro- and anti-tumor effects (Outstanding Questions). Recent studies have provided clues as to how neutrophils are recruited to the tumor microenvironment and what roles they play there, including remodeling of the ECM, promoting angiogenesis, and mediating interactions with other cell types including epithelia, stroma, and immune cells. It is clear that neutrophils often play a pro-inflammatory, pro-tumoral role within the tumor microenvironment. As the presence of neutrophils is a well-characterized phenotype of aggressive disease, it will be important to understand how neutrophil inflammation is resolved following tissue damage and how failure to resolve in conditions of chronic inflammation contributes to cancer initiation and progression. In addition, identification of factors that influence neutrophil recruitment to target tissues and drive neutrophil reverse migration or resolution could lead to novel therapies to reduce neutrophil-mediated inflammation in tumors. However, methods to engineer neutrophils to an anti-tumor role in cancer are also an interesting avenue for future research. In particular, as cancer immunotherapy comes to the forefront of cancer treatments, a better understanding of how neutrophils regulate other immune cells within the tumor microenvironment and how this can influence prognosis will be needed. It is evident from recent work that neutrophils play a far more complex role than previously appreciated, and that further study of the role of neutrophils in wounding and inflammation will provide clues and guide research on the role of neutrophils in tumor progression with the hope of aiding and advancing cancer diagnosis and treatment.
Box 1: Zebrafish as an emerging model to study neutrophils and cancer.
With the emergence of new evidence that neutrophils play a role in cancer progression, researchers are identifying novel tools to gain more insight into the interaction between neutrophils and tumor cells. One of these tools is the larval zebrafish. While the zebrafish model system has traditionally been used to study developmental processes, the optical clarity of zebrafish embryos and larvae allow for unparalleled imaging of cellular processes and cell-cell interactions in an in vivo model. Many studies have taken advantage of easy and rapid genetic manipulations to drive oncogenic transformation of various cell types or to create mutants in tumor suppressor genes and generate cancer models. In addition, xenografts of human tumor cells into an immunocompetent zebrafish larval host may also be performed due to the delayed development of the zebrafish adaptive immune system (the use of zebrafish as a cancer model is further reviewed here [104, 105]). Xenografted cells can be labeled and tracked within a zebrafish larva over long periods of time allowing for visualization of tumor cell invasion [71, 106]. Additionally, primary human tumor cells can be readily transplanted in zebrafish tissues, allowing for robust, patient-specific studies of cancer cells within a host microenvironment [107]. With the generation of fluorescently labeled transgenic lines, high resolution live-imaging of neutrophil recruitment and interactions with cancer or transformed cells has allowed researchers to begin to uncover the molecular mechanisms underlying neutrophils’ regulation of tumor progression. Zebrafish are an especially useful tool for analyzing neutrophil and other immune cell responses to areas of initial cell transformation that are not easily accessible in a mouse. Several key studies have demonstrated that neutrophils are specifically recruited to early transformed cells within a matter of days following expression of an oncogene and that neutrophils dynamically interact with these cells and promote EMT and invasion [60, 72, 73]. Importantly, the zebrafish system is ideal for large-scale, high-throughput drug screening which may help identify new therapies for cancer [108] and new anti-inflammatory drugs that may inhibit neutrophil recruitment to tumors and/or promote their resolution [90]. Using xenografts of patient-derived tumor cells in zebrafish larvae, it is becoming increasingly feasible to perform patient-specific drug screens and analyze tumor growth and proliferation, invasion, metastasis, and immune cell infiltration to help guide patient treatment in personalized medicine.
Box 2: Neutrophils and G-MDSCs.
Myeloid derived suppressor cells (MDSCs) were initially described in 2007 and comprise a heterogeneous population of immature myeloid cells with immunosuppressive phenotypes [109]. There are two subpopulations of MDSC, the monocytic M-MDSCs, and the granulocytic G-MDSCs. Due to similarities in cell surface markers used to distinguish neutrophils and G-MDSCs, there is some confusion and controversy in the field as to whether these are in fact two separate populations of cells or are two different phenotypes or polarities of the same cell type. While this subject is discussed extensively in several recent reviews [110-112], it is important to note some of the key studies focused on tumor-associated granulocytes. Neutrophils and G-MDSC populations are routinely identified in mice using the same markers, for example Gr-1, CD11b, and Ly-6G, making direct comparison of the two populations challenging. In addition, there are several similarities in behavior and function for neutrophils and G-MDSCs, including production of ROS, antigen presentation, and immune suppression. A study directly comparing neutrophils and G-MDSCs in mouse models with and without tumors described neutrophils as mature, highly phagocytic cells that express high levels of proteases and TNF-α, and G-MDSCs as immunosuppressive cells that express high levels of ROS, arginase, and MPO [113]. Additionally, comparison of gene expression has suggested that neutrophils from tumor-free mice and G-MDSCs from tumor-bearing mice are more closely related to each other than to TANs [114], suggesting that the tumor microenvironment may influence TANs to adopt a unique phenotype. Indeed, recent analysis by Sagiv et al. using circulating blood neutrophils from both mouse mammary tumor models and human lung and breast cancer patients demonstrated that there are phenotypically diverse subpopulations of neutrophils that can be separated based on density [115]. The authors describe a population of mature, high density neutrophils which appear to switch to a higher proportion of low density neutrophils, characterized by immune suppression and pro-tumor properties, as cancer progresses. This switch is TGF-β dependent and is reminiscent of N1/N2 polarization, which is also regulated by TGF-β expression in mouse lung cancer models [10]. Sagiv et al. describe G-MDSCs as immature neutrophils; however, it is also likely that G-MDSCs may simply be an intermediary for N1/N2 phenotypic switching. Further defining G-MDSCs, especially in the context of N1/N2 polarity, is needed to provide consensus and clarity moving forward.
Trends Box.
Neutrophils are recruited to wounds and sites of tissue damage by signals including hydrogen peroxide, chemokines, and cytokines, many of which also recruit neutrophils to the tumor microenvironment.
Neutrophils reverse migrate from target tissues through interactions with macrophages and other cell types, thus contributing to resolution of inflammation and promoting wound healing.
Tumor-associated neutrophils contribute to tumor progression, invasion, and angiogenesis; however, there is evidence that neutrophils can play both pro- and anti-tumor roles and that they may be polarized to either phenotype based on external cues.
Targeting neutrophils through blockade of pro-recruitment signals or driving reverse migration of neutrophils from tumors to promote an anti-inflammatory environment could provide therapeutic avenues for the treatment of cancer.
Outstanding Questions.
What are the signals driving neutrophil reverse migration and reverse transendothelial migration? Can these signals be used to exogenously drive reverse migration of neutrophils away from tumor tissues and/or areas of chronic inflammation?
What mechanisms drive polarization of neutrophils to an N1 or N2 phenotype? Are these mechanisms cell or non-cell autonomous, i.e. are they purely extracellular signals or do neutrophils regulate their own polarity?
Is TAN recruitment and polarity dependent on tumor type? Are certain tissues more prone to pro-inflammatory neutrophil infiltration?
Does treatment with currently available anti-inflammatory drugs reduce tumor progression in cancer?
Does neutrophil infiltration affect tumors differently at different stages of tumor development? Does neutrophil polarity change throughout the course of tumor progression?
What are the mechanisms of neutrophil communication with other immune cells including tumor-associated macrophages and cytotoxic T cells? As macrophages have a demonstrated role in driving reverse migration of neutrophils during wound response, can tumor-associated macrophages similarly drive reverse migration of TANs?
Acknowledgments
The authors thank Sofia de Oliveira for insightful reading of the manuscript. D.R.P. is supported by the Cancer Research Institute Irvington Postdoctoral Fellowship award and A.H. is supported by NIH/NCI R01 CA085862.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kruger P, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 3.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 4.Pillay J, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 5.Lakshman R, Finn A. Neutrophil disorders and their management. Journal of clinical pathology. 2001;54:7–19. doi: 10.1136/jcp.54.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 7.Bekes EM, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. The American journal of pathology. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acharyya S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eruslanov EB, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. The Journal of clinical investigation. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niethammer P, et al. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo SK, et al. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 16.Moles A, et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. Journal of hepatology. 2014;60:782–791. doi: 10.1016/j.jhep.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anzai A, et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circulation research. 2015;116:612–623. doi: 10.1161/CIRCRESAHA.116.304918. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira S, et al. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. Journal of immunology. 2013;190:4349–4359. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, et al. Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. PloS one. 2014;9:e90613. doi: 10.1371/journal.pone.0090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christoffersson G, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–4662. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBert DC, et al. Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development. 2015;142:2136–2146. doi: 10.1242/dev.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. The American journal of pathology. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pase L, et al. Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Current biology : CB. 2012;22:1818–1824. doi: 10.1016/j.cub.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 24.Wong SL, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nature medicine. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauer C, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 26.Silva MT. Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. Journal of leukocyte biology. 2011;89:675–683. doi: 10.1189/jlb.0910536. [DOI] [PubMed] [Google Scholar]
- 27.Hughes J, et al. Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. The American journal of pathology. 1997;150:223–234. [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley CD, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. Journal of leukocyte biology. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 29.Mathias JR, et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of leukocyte biology. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 30.Woodfin A, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nature immunology. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauzin S, et al. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. The Journal of cell biology. 2014;207:589–598. doi: 10.1083/jcb.201408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colom B, et al. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity. 2015;42:1075–1086. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamza B, Irimia D. Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab on a chip. 2015;15:2625–2633. doi: 10.1039/c5lc00245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamza B, et al. Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integrative biology : quantitative biosciences from nano to macro. 2014;6:175–183. doi: 10.1039/c3ib40175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellett F, et al. Defining the phenotype of neutrophils following reverse migration in zebrafish. Journal of leukocyte biology. 2015 doi: 10.1189/jlb.3MA0315-105R. doi: 10.1189/jlb.1183MA0315-1105R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stockley JA, et al. Aberrant neutrophil functions in stable chronic obstructive pulmonary disease: the neutrophil as an immunotherapeutic target. International immunopharmacology. 2013;17:1211–1217. doi: 10.1016/j.intimp.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 37.Rao X, et al. The heterogenic properties of monocytes/macrophages and neutrophils in inflammatory response in diabetes. Life sciences. 2014;116:59–66. doi: 10.1016/j.lfs.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caielli S, et al. Neutrophils come of age in chronic inflammation. Current opinion in immunology. 2012;24:671–677. doi: 10.1016/j.coi.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battaglia M. Neutrophils and type 1 autoimmune diabetes. Current opinion in hematology. 2014;21:8–15. doi: 10.1097/MOH.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 40.Wright HL, et al. The multifactorial role of neutrophils in rheumatoid arthritis. Nature reviews. Rheumatology. 2014;10:593–601. doi: 10.1038/nrrheum.2014.80. [DOI] [PubMed] [Google Scholar]
- 41.Jensen HK, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 42.Kuang DM, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. Journal of hepatology. 2011;54:948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Li YW, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. Journal of hepatology. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 44.Ilie M, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–1737. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 45.Jensen TO, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118:2476–2485. doi: 10.1002/cncr.26511. [DOI] [PubMed] [Google Scholar]
- 46.Trellakis S, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International journal of cancer. Journal international du cancer. 2011;129:2183–2193. doi: 10.1002/ijc.25892. [DOI] [PubMed] [Google Scholar]
- 47.Fossati G, et al. Neutrophil infiltration into human gliomas. Acta neuropathologica. 1999;98:349–354. doi: 10.1007/s004010051093. [DOI] [PubMed] [Google Scholar]
- 48.Rao HL, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PloS one. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid MD, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen M, et al. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PloS one. 2014;9:e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy PM. Neutrophil receptors for interleukin-8 and related CXC chemokines. Seminars in hematology. 1997;34:311–318. [PubMed] [Google Scholar]
- 52.Raccosta L, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. The Journal of experimental medicine. 2013;210:1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viola A, et al. The pros and cons of chemokines in tumor immunology. Trends in immunology. 2012;33:496–504. doi: 10.1016/j.it.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Zhou SL, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- 55.Zhou SL, et al. CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3beta/Snail signaling. Cancer letters. 2015;358:124–135. doi: 10.1016/j.canlet.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 56.Moore RJ, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nature medicine. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 57.Coffelt SB, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benevides L, et al. IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment. Cancer research. 2015;75:3788–3799. doi: 10.1158/0008-5472.CAN-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antonio N, et al. The wound inflammatory response exacerbates growth of pre neoplastic cells and progression to cancer. The EMBO journal. 2015;34:2219–2236. doi: 10.15252/embj.201490147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng Y, et al. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS biology. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dumitru CA, et al. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Seminars in cancer biology. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Coussens LM, et al. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandhu JK, et al. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. The American journal of pathology. 2000;156:509–518. doi: 10.1016/S0002-9440(10)64755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gungor N, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010;25:149–154. doi: 10.1093/mutage/gep053. [DOI] [PubMed] [Google Scholar]
- 65.Tazawa H, et al. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. The American journal of pathology. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wislez M, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer research. 2003;63:1405–1412. [PubMed] [Google Scholar]
- 67.Finisguerra V, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349–353. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Queen MM, et al. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer research. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 69.Lauber S, et al. Novel function of Oncostatin M as a potent tumour-promoting agent in lung. International journal of cancer. Journal international du cancer. 2015;136:831–843. doi: 10.1002/ijc.29055. [DOI] [PubMed] [Google Scholar]
- 70.Nozawa H, et al. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He S, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. The Journal of pathology. 2012;227:431–445. doi: 10.1002/path.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freisinger CM, Huttenlocher A. Live Imaging and Gene Expression Analysis in Zebrafish Identifies a Link between Neutrophils and Epithelial to Mesenchymal Transition. PloS one. 2014;9:e112183. doi: 10.1371/journal.pone.0112183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng Y, et al. Live imaging of tumor initiation in zebrafish larvae reveals a trophic role for leukocyte-derived PGE(2). Current biology : CB. 2012;22:1253–1259. doi: 10.1016/j.cub.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song W, et al. Infiltrating neutrophils promote renal cell carcinoma progression via VEGFa/HIF2alpha and estrogen receptor beta signals. Oncotarget. 2015;6:19290–19304. doi: 10.18632/oncotarget.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rotondo R, et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. International journal of cancer. Journal international du cancer. 2009;125:887–893. doi: 10.1002/ijc.24448. [DOI] [PubMed] [Google Scholar]
- 76.Davey MS, et al. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. Journal of immunology. 2014;193:3704–3716. doi: 10.4049/jimmunol.1401018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takashima A, Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell. Journal of leukocyte biology. 2015;98:489–496. doi: 10.1189/jlb.1MR1014-502R. [DOI] [PubMed] [Google Scholar]
- 78.Cools-Lartigue J, et al. Neutrophil extracellular traps in cancer progression. Cellular and molecular life sciences : CMLS. 2014;71:4179–4194. doi: 10.1007/s00018-014-1683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berger-Achituv S, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Frontiers in immunology. 2013;4:48. doi: 10.3389/fimmu.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demers M, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sangaletti S, et al. Defective stromal remodeling and neutrophil extracellular traps in lymphoid tissues favor the transition from autoimmunity to lymphoma. Cancer discovery. 2014;4:110–129. doi: 10.1158/2159-8290.CD-13-0276. [DOI] [PubMed] [Google Scholar]
- 82.Mishalian I, et al. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer immunology, immunotherapy : CII. 2013;62:1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cools-Lartigue J, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. The Journal of clinical investigation. 2013 doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jamieson T, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. The Journal of clinical investigation. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ginestier C, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. The Journal of clinical investigation. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan MN, et al. CXCR1/2 antagonism with CXCL8/Interleukin-8 analogue CXCL8(3-72)K11R/G31P restricts lung cancer growth by inhibiting tumor cell proliferation and suppressing angiogenesis. Oncotarget. 2015;6:21315–21327. doi: 10.18632/oncotarget.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verbeke H, et al. Isotypic neutralizing antibodies against mouse GCP-2/CXCL6 inhibit melanoma growth and metastasis. Cancer letters. 2011;302:54–62. doi: 10.1016/j.canlet.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 88.Houghton AM, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nature medicine. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruffell B, et al. Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes & development. 2013;27:2086–2098. doi: 10.1101/gad.224899.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robertson AL, et al. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Science translational medicine. 2014;6:225ra229. doi: 10.1126/scitranslmed.3007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z, et al. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nature nanotechnology. 2014;9:204–210. doi: 10.1038/nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piccard H, et al. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Critical reviews in oncology/hematology. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 93.Yan J, et al. Human polymorphonuclear neutrophils specifically recognize and kill cancerous cells. Oncoimmunology. 2014;3:e950163. doi: 10.4161/15384101.2014.950163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li B, et al. Yeast beta-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. Journal of immunology. 2006;177:1661–1669. doi: 10.4049/jimmunol.177.3.1661. [DOI] [PubMed] [Google Scholar]
- 95.Souto JC, et al. Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Medicinal research reviews. 2011;31:311–363. doi: 10.1002/med.20185. [DOI] [PubMed] [Google Scholar]
- 96.Clarke SJ, et al. Use of inflammatory markers to guide cancer treatment. Clinical pharmacology and therapeutics. 2011;90:475–478. doi: 10.1038/clpt.2011.122. [DOI] [PubMed] [Google Scholar]
- 97.Ozyalvacli G, et al. Diagnostic and prognostic importance of the neutrophil lymphocyte ratio in breast cancer. Asian Pacific journal of cancer prevention : APJCP. 2014;15:10363–10366. doi: 10.7314/apjcp.2014.15.23.10363. [DOI] [PubMed] [Google Scholar]
- 98.Lian L, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer biomarkers : section A of Disease markers. 2015 doi: 10.3233/CBM-150534. [DOI] [PubMed] [Google Scholar]
- 99.Yildirim MA, et al. Roles of neutrophil/lymphocyte and platelet/lymphocyte ratios in the early diagnosis of malignant ovarian masses. Asian Pacific journal of cancer prevention : APJCP. 2014;15:6881–6885. doi: 10.7314/apjcp.2014.15.16.6881. [DOI] [PubMed] [Google Scholar]
- 100.Azab B, et al. The value of the pretreatment neutrophil lymphocyte ratio vs. platelet lymphocyte ratio in predicting the long-term survival in colorectal cancer. Cancer biomarkers : section A of Disease markers. 2014;14:303–312. doi: 10.3233/CBM-140416. [DOI] [PubMed] [Google Scholar]
- 101.Gao F, et al. Pretreatment neutrophil-lymphocyte ratio: an independent predictor of survival in patients with hepatocellular carcinoma. Medicine. 2015;94:e639. doi: 10.1097/MD.0000000000000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moniaux N, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. British journal of cancer. 2008;98:1540–1547. doi: 10.1038/sj.bjc.6604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lim R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. International journal of cancer. Journal international du cancer. 2007;120:2426–2434. doi: 10.1002/ijc.22352. [DOI] [PubMed] [Google Scholar]
- 104.Yen J, et al. Zebrafish models of cancer: progress and future challenges. Current opinion in genetics & development. 2014;24:38–45. doi: 10.1016/j.gde.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blackburn JS, Langenau DM. Zebrafish as a model to assess cancer heterogeneity, progression and relapse. Disease models & mechanisms. 2014;7:755–762. doi: 10.1242/dmm.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teng Y, et al. Evaluating human cancer cell metastasis in zebrafish. BMC cancer. 2013;13:453. doi: 10.1186/1471-2407-13-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marques IJ, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veinotte CJ, et al. Hooking the big one: the potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Disease models & mechanisms. 2014;7:745–754. doi: 10.1242/dmm.015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer research. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brandau S, et al. Protumor and antitumor functions of neutrophil granulocytes. Seminars in immunopathology. 2013;35:163–176. doi: 10.1007/s00281-012-0344-6. [DOI] [PubMed] [Google Scholar]
- 111.Dumitru CA, et al. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer immunology, immunotherapy : CII. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pillay J, et al. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cellular and molecular life sciences : CMLS. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Youn JI, et al. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Journal of leukocyte biology. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fridlender ZG, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PloS one. 2012;7:e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sagiv JY, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell reports. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]