Abstract
Fasciola hepatica saposin-like protein-2 (FhSAP2) is a protein differentially expressed in various developmental stages of F. hepatica. Recombinant FhSAP2 has demonstrated the induction of partial protection in mice and rabbits when it is administered subcutaneously (SC) in Freund’s adjuvant. Because FhSAP2 is overexpressed in bacteria in the form of inclusion bodies (IBs), we isolated IBs expressing FhSAP2 and tested their immunogenicity when administered SC in mice emulsified in two different adjuvants: QS-21 and Montanide™ ISA720. Animals received three injections containing 20μg of protein two weeks apart and 4 weeks after the third injection, mice were infected with 10 F. hepatica metacercariae by oral route. The percentages of protection induced by FhSAP2-IBs were estimated to be between 60.0–62.5% when compared with adjuvant-vaccinated, infected controls. By determining the levels of IgG1 and IgG2a antibodies and IL-4 and IFNγ cytokines in the serum of experimental animals, it was found that both Th1 and Th2 immune responses were significantly increased in the FhSAP2-IBs vaccinated groups compared with the adjuvant-vaccinated, infected control groups. The adjuvant-vaccinated groups had significantly lower IgG1 to IgG2a ratios and lower IL-4 to IFNγ ratios than the FhSAP2-IBs vaccinated animals, which is indicative of higher levels of Th2 immune responses. Irrespective to the adjuvant used, animals vaccinated with FhSAP2-IBs exhibited significantly higher survival percentage and less liver damage than the adjuvant-control groups. This study suggests that FhSAP2 has potential as vaccine against F. hepatica and that the protection elicited by this molecule could be linked to a mechanism driven by the CD4-Th1 cells.
Keywords: Fasciola hepatica, vaccination, inclusion bodies, saposin-like proteins
Graphical abstract
Introduction
There is a need to develop a vaccine against Fasciola hepatica, a parasitic trematode distributed worldwide responsible for enormous economical losses estimated in more than 3 billion dollars yearly (Mas-Coma, 2005). Globally between 2.4 to 17 million individuals are infected with Fasciola species and more than 180 million people are at risk of infection (Mas-Coma et al., 1999a; Mas-Coma, 2005; Mas-Coma et al., 1999b). Over the last decade a large number of native and recombinant parasite proteins have been identified and tested as vaccine in a number of animals models. Some of these molecules includes leucinaminopeptidase (Maggioli et al., 2011a), cathepsin-L (Chantree et al., 2013; Golden et al., 2010; Piacenza et al., 1999), thioredoxin-glutathion reductase (Maggioli et al., 2011b), glutathione S-transferase (Sexton et al., 1990; Sexton et al., 1994), fatty acid binding protein (Casanueva et al., 2001; Martinez-Fernandez et al., 2004) and saposin-like protein-2 (Espino et al., 2010; Kueakhai et al., 2013). All these vaccine candidates have induced partial levels of protection that range between 46.9–83% in different animal models, which indicates that a vaccine against F. hepatica is not a chimera but an attainable goal. However, despite of significant advances, no vaccine candidate has yet advanced to a clinical trial phase. Much of this could be attributed to differences in the structural characteristics of the antigens (e.g. hydrophobicity, specific localization in the parasite, biological functions, etc.), the feasibility to produce it at large amount in homogeneous form, differences in the vaccination protocols (doses and route of administration); the correct selection of the adjuvant and the lack of knowledge about the correlates of protection. In the current study, we explored the effectiveness of inclusion bodies (IBs) expressing saposin-like protein-2 (FhSAP2) as a cheaper and reliable vehicle to deliver antigenic molecules in homogeneous form.
Saposin-like proteins are a family of lipid interacting proteins that binds onto the cell membrane to induce cell lysis (Bruhn, 2005). Fasciola species use these lytic proteins to cause lysis of the hosts’ erythrocytes and leuckocytes so that their contents can be digested further for the parasite’s nourishment (Espino and Hillyer, 2003). In F. hepatica, there are two isoforms (SAP1 and SAP2) (Espino and Hillyer, 2003; Reed et al., 2000). SAP1 is expressed in immature and adult stages (Reed et al., 2000), whereas SAP2 is highly expressed in newly excysted juvenile, 3-week juvenile up to adult stages and also in non-embryonated eggs (Caban-Hernandez and Espino, 2013; Espino and Hillyer, 2003). Some characteristics of the protein moiety of FhSAP2 facilitate the formation of protein aggregates as inclusion bodies (IBs) during the expression process in Escherichia coli. The primary structure of FhSAP2 consists of 101 amino acids that are rich in hydrophobic amino acids (~39.6%) some of which are exposed and available to interact with similar exposed residues on other cellular proteins. The FhSAP2 also exhibits six cysteine residues at highly conserved positions and therefore, FhSAP2 is a protein that has disulfide bonds (Espino and Hillyer, 2003). During the expression of FhSAP2 in bacteria, the exposure to the reducing environment of the bacterial cytosol may inhibit the formation of these disulfide bonds, which also strongly contributes to the IBs formation (Hartley and Kane, 1988). Because >90% of fusion protein is expressed as inclusion bodies, only relatively low yields of fusion protein (0.3–0.5mg/l of culture) could be obtained from the supernatants of bacterial lysates. This amount is inadequate for small-scale immunization experiments and is not cost-effective for commercial manufacture. Preparation of IBs is cheaper and easier than affinity purification of FhSAP2-fusion protein, which is time-consuming and tedious.
In a previous experiment, we found that purified recombinant FhSAP2 can protect mice against a liver fluke infection when administered subcutaneously in Freund’s adjuvant (Espino et al., 2010). In this study, we designed experiments to examine whether inclusion bodies (IBs) containing recombinant FhSAP2 could be as immunogenic as the purified protein when delivered subcutaneously and whether they are capable of inducing protection in a mouse model of fascioliasis. Because Freund’s complete adjuvant (FCA), an oil-base adjuvant that contain mycobacteria (Freund, 1951), produces excessive inflammation (Broderson, 1989), it is not permitted in commercial vaccine formulations for human or veterinary use. In the current study, we will use FhSAP2-IBs as a model antigen to evaluate immune responses when formulated in non-toxic adjuvants like QS-21 and Montanide™ ISA720.
2. Materials and methods
2.1. Recombinant protein expression and inclusion bodies isolation
The cDNA of FhSAP2 gene (Accession No. AF28693) was subcloned from the adult F. hepatica cDNA library (Espino and Hillyer, 2003). The FhSAP2 cDNA was cloned into pBAD His-B expression vector and transformed into Escherichia coli TOP10 (Invitrogen). The recombinant FhSAP2 expression was induced with 0.02% L-arabinose for 4 hours at 37°C. The bacterial cell pellets were suspended in a lysis buffer (500mM NaCl, 50mM Tris-HCl, 10mM EDTA, 5mM β-mercaptoethanol, 0.35 mg/ml lysozyme, pH 8.0) and incubated for 30 min at 20°C. Triton X-100 was added to the concentration of 1% and the suspension was sonicated. After sonication, the suspension was centrifuged at 12,000-x g for 30 min. The IB pellets were suspended in PBS containing 1% Triton X-100, sonicated and centrifuged at 12,000-x g for 30 min. This procedure was repeated twice and subsequently the IBs were washed two times with PBS and characterized by electrophoresis in 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The concentration of recombinant FhSAP2 in relation to all proteins expressed by E. coli were measured by densitometry scanning of a Comassie Brillian Blue G stained SDS-PAGE using GelDoc Scan Software. Proteins were electrotransferred to a nitrocellulose (NC) sheet (0.2μm; Bio-Rad) at 4°C for 2h. After blocking for 1 h in PBS containing 0.05% Tween-20 (PBST) and 5% skim milk, the NC membrane was incubated overnight in a mouse anti-Xpress™ epitope-peroxidase labeled antibody (Invitrogen) diluted 1:5,000, which recognizes a non-conformational epitope formed (Asp-Leu-Tyr-Asp-Asp-Asp-Asp-Lys) at the amino end of the fusion protein. A positive brown signal for FhSAP2-fusion protein was visualized using diaminobenzidine as a substrate.
2.2. Obtaining of adult F. hepatica excretory-secretory proteins (ES)
F. hepatica ES products were prepared by culturing live adult flukes in RPMI medium for 24 h at 4°C. The medium was centrifuged at 6,000-x g at 4°C and concentrated 10-fold using ultrafiltration membrane (YM-10) in an AMICON system. The protein concentration was estimated using the bicinchoninic acid method (BCA kit, Pearce, Inc).
2.3. Adjuvants
In the current study we analyzed two different adjuvants. QS-21 (kindly donated by Agenus Inc. Lexington, MA, USA) is an immunological adjuvant derived from a natural source: the bark of the South American tree Quijalla saponaria (QS) Molina, which has been identified as a saponin with potent adjuvant activity and low toxicity (Kensil et al., 1991). QS-21 has shown to stimulate a strong antibody response to T-dependent protein antigens in mice (Cribbs et al., 2003). Montanide™ ISA720 (Seppic, Inc. Fairfield, NJ) is a squalene-based water-in-oil emulsion that has been used as an adjuvant vaccine in a number of experimental vaccines against Leishmania (Mutiso et al., 2012), malaria (Remarque et al., 2012), HIV (Toledo et al., 2001) and cancer (Huijbers et al., 2012).
2.4. Experimental animals and F. hepatica parasites
Inbred female 6–8 week-old BALB/c mice (Harlan Laboratories) were used in the vaccination experiments. All mice were kept in steel cages in an air conditioned room at 22–25°C, with a light-dark cycle of 12:12h, and 50–50% humidity, at the Animal Resource Center of the Medical Sciences Campus, University of Puerto Rico (IACUC Protocols: #7870104, #7870106). Fresh infectious metacercariae (mc) were purchased from Lymnaea spp. snail cultures at Baldwin Aquatics, Inc. (Oregon, USA) and used immediately for infecting animals.
2.5. Vaccination and experimental infection
FhSAP2-IBs (20μg) were mixed with 20μg of QS-21 to form a simple aqueous mixture or emulsified with equal volume of Montanide™ ISA720 (vol./vol.) to form a water-in-oil depot as per manufacturer’s instructions. Animals were divided into five groups of 10 animals each. Group-1 was immunized with FhSAP2-IBs in QS-21, group-2 was immunized with FhSAP2-IBs in Montanide™ ISA720, group-3 was immunized with PBS in QS-21, group-4 was immunized with PBS in Montanide™ ISA720, and group-5 was non-immunized (negative control group). All animals received a total of three subcutaneous injections on the dorsal surface, each at 2-week intervals. Four weeks after the last injection, animals from groups 1 to 4 were orally infected with 10 mc, which resulted in the infection of 100% of animals. Mice welfare was evaluated daily according to behavior and appearance, physiological indicators and other general clinical signs. On day 45, after infected mice were humanely euthanized, necropsies were performed to collect the spleens, recover flukes from livers and score hepatic damages. Blood samples were collected from all animals before vaccination, at challenge and at euthanasia for antibody and cytokine determination.
2.6. Assessment of protection
We used three criteria to assess the protection in mice. 1) Survival rate: The survival rate percentage in infected mice was calculated as the ratio of the number of surviving experimental mice on day 45 and the total of experimental mice in each group as previously described (Espino et al., 2010). 2) Worm recovery: the livers were cut into 1-cm pieces, soaked in water at 37°C for 30 min, squeezed, and forced through 300-μm mesh sieve; the retained material was analyzed for immature or mature flukes. Total flukes were summarized for each vaccinated and control group by arithmetic means. Reduction in fluke burden in vaccinated mice compared to adjuvant-vaccinated, infected control groups was calculated as follows: Reduction (%) = [(A–B) / A] × 100 “A” represents the mean fluke recovery from the non-immunized, non-infected or adjuvant-vaccinated, infected control mice, and “B” represents the mean fluke recovery from FhSAP2-IBs vaccinated mice. 3) Reduction of liver damage: Two independent experienced observers evaluated subjectively macroscopic alterations of the organ, including color change to grayish-white, increase in size and change in consistency, dilation and thickness of bile ducts. The degree of lesions observed was summarized semi-quantitatively using plus symbols that expressed the intensity and extent of the alterations observed: “+”, mild; “++”, moderate, “+++”, intense; “++++”, severe (Espino and Rivera, 2010; Kesik et al., 2007; Wedrychowicz et al., 2007)
2.7. Splenocyte and flow cytometry analysis
Single cell suspensions were obtained from the spleen of experimental mice by mechanically squeezing the tissue with glass slides in cold PBS and filtered through a 70-μm-nylon cell strainer. Red blood cells in the filtrate were lysed by alternate washing with 1.4% NH4Cl and sterile PBS followed by incubation of 5 minutes on ice and centrifugation at 1,500 rpm. Spleen lymphocytes were then suspended in RPMI media supplemented with 2mM glutamine, 25mM HEPES, 10% fetal calf serum (FCS), 50μM 2-mercaptoethanol, penicillin (100U/ml) and streptomycin (100μg/ml). Cell counts were subsequently determined using the trypan blue exclusion test on a bright-lined Neubauer counting chamber. Cells (106 cells/well) were stimulated with 5 μg/ml of ES products and incubated for 48h at 37°C, 5% CO2 atmosphere. After incubation, cells were centrifuged, washed with PBS, and stained with monoclonal antibodies against CD69 (H1.2F3), CD8 (4D11), CD4 (H12919) and CD3 (1452C11) labeled with FITC, PE or PerCpCy5.5 (BD Pharmigen, San Diego, CA). To remove unbound antibodies, cells were washed in 2ml PBS supplemented with 10% ACD, 0.2% NaN3 and 10mM EDTA-K2 (Tliba et al., 2002). Cells were collected and analyzed using a FACSort (Becton and Dickinson, San Jose, CA).
2.8. Determination of IgG1 and IgG2a levels by indirect ELISA
Because FhSAP2 is a component of the F. hepatica ES products (Espino and Hillyer, 2003) and we have demonstrated that both, FhSAP2 and ES products show similar performances as antigens in the detection of antibodies against F. hepatica (Espino et al., 2010; Figueroa-Santiago et al., 2011) in the current study, we used the ES products to determine specific IgG1 and IgG2a antibody levels in the blood samples collected from our experimental animals. Ninety six-well plates were coated with 100μl 25μg/ml ES products in coating buffer (15mM Na2CO3, 35mM NaHCO3, pH 9.6) at 4°C, over night. The coated plates were washed three times with 0.05% PBS-Tween-20 (PBST) and non specific binding was blocked by adding 300μl per well of 3% skin milk in PBS and incubated at 37°C for 30 min. After removing the blocking solution, 100μl of each serum sample (diluted 1:100 in PBST) was added and incubated 1 h at 37°C. Plates were washed three times with PBST and incubated for 1h at 37°C with 100μl per well of sheep anti-mouse IgG1 and IgG2a (Sigma Aldrich, USA). After another washing step, 100μl of peroxidase-conjugate anti-sheep IgG was added and incubated for 1h at 37°C. Following a final washing step, 100μl of 3,3′, 5, 5′-tetramethylbenzidine (TMB) substrate (KPL, Gaithersburg, USA) was added per well and incubated for 20 min at room temperature. Finally, adding 50μl of 12.5% sulfuric acid per well stopped the enzymatic reaction. The absorbance values were measured at 492nm in a microplate ELISA reader (BioRad, USA).
2.9. Cytokines Determination
Serum IL-4, IFNγ, and TNFα were measured using commercially available Bioplex IL-4, IFNγ, and TNFα assays (Bio-Rad, USA). Briefly, serum samples were diluted 1:4 in sample diluents and ran in duplicates according to the instruction manuals. The sensitivity of the kit was 2.1pg/ml, 1.2pg/ml and 1.4pg/ml for IL-4, IFNγ, and TNFα respectively.
2.10. Statistical Analysis
The antibody and cytokine levels in serum were compared between controls and the FhSAP2-IBs-vaccinated groups using one-way ANOVA, Krustal-Walli Test. Kaplan-Meier survival analysis was performed to estimate survival of animals over time and log-rank test was performed to compare survival curves. All the statistical analysis was performed with the statistical software STATA, version 10.
3. Results
3.1. Purification of body inclusions containing FhSAP2 (FhSAP2-IBs)
The efficient expression of large amount of FhSAP2-IBs was assessed by immunoblot. Results show that a single and homogenous band of around 16.4kDa is revealed when blot was incubated with the anti-Xpress™ antibody (Fig. 1). This size comprises the 11.5kDa of the predicted amino acid sequence of FhSAP2 plus an additional portion of ~4.9kDa that include the 6x (His)-tag plus the Xpress epitope at the amino terminus of the protein moiety. In addition, a very weak protein band of ~31kDa was also observed, which could represent dimeric forms of FhSAP2, a characteristic that has been previously observed for this protein when is over-expressed in bacteria (Espino and Hillyer, 2003).
Figure-1. Western blot analysis of inclusion bodies containing FhSAP2.
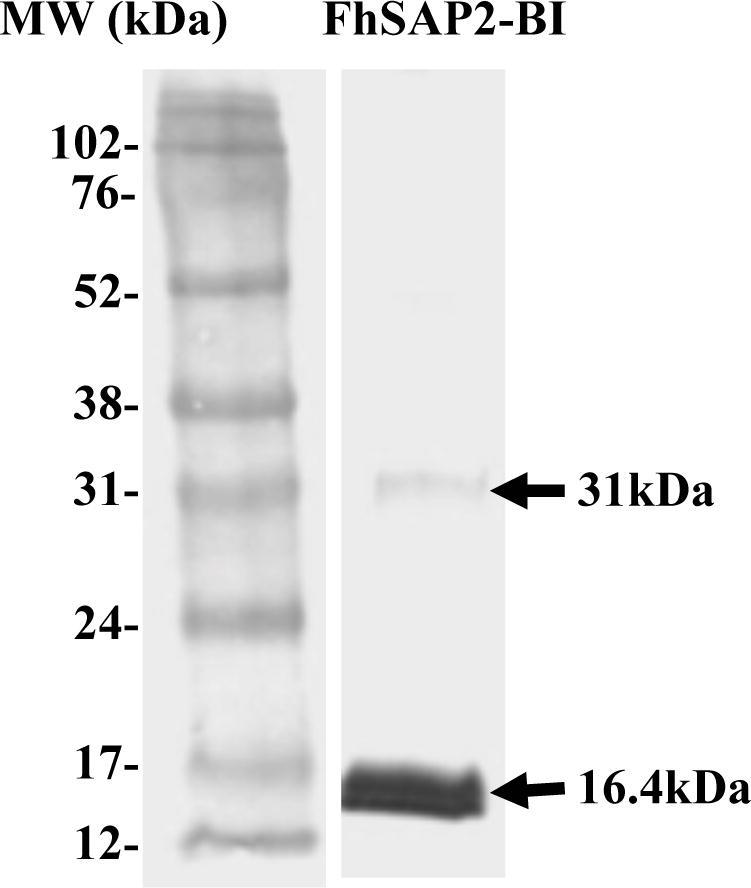
After expression inclusion bodies were isolated and analyzed by 15% SDS-PAGE. Proteins were electrotransferred to a nitrocellulose (NC) sheet and after blocking were probed with anti-Xpress™ epitope-peroxidase labeled antibody. A strong major band of ~16.4kDa was revealed, which represent the predicted 11.5kDa polypeptide band of FhSAP2 plus 4.9kDa that include the 6x (His)-tag plus the Xpress epitope at the amino terminus of the protein moiety. In addition, a very weak protein band of ~31kDa was also observed, which could represent dimeric forms of FhSAP2.
3.2. In vivo protection assessment
Following the challenge, infected mice were monitored for mortality during 45-days. Vaccination of mice with FhSAP2-IBs in QS-21 or Montanide™ ISA720 significantly enhanced their survival rate. Most mice in the adjuvant control groups died between days 15 to 21-post infection. The few animals that survived in the adjuvant-control groups were humanely euthanized at days 40–45 because they exhibited evident signs of severe pain and suffering. The survival rate in the group vaccinated with FhSAP2-IBs in QS-21 was 90%, whereas in the group vaccinated with FhSAP2-IBs in Montanide™ ISA720 the survival rate was 80%. According to Kaplan-Meier the survival rate of both FhSAP2-IBs vaccinated groups was significantly different from those observed in the QS-21 or Montanide™ ISA720-control groups (p<0.005). No statistical difference was found between the survival rates of groups vaccinated with FhSAP2-IBs in QS-21 or FhSAP2-IBs in Montanide™ ISA720 and neither between the groups vaccinated only with QS-21 or Montanide™ ISA720 (Fig. 2).
Figure-2. Survival analysis.
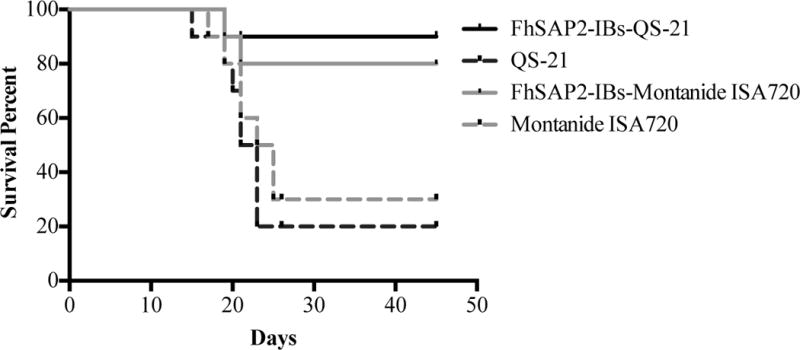
Kapplan-Meier analysis to estimate the survival probability over the time after challenge in mice immunized with FhSAP2-IBs in QS-21 or Montanide™ ISA720 compared to mice vaccinated with E. coli proteins in QS-21 or Montanide™ ISA720 adjuvants. Log-rank test determined that there are statistical differences (p <0.05) between survivals probabilities of FhSAP2-IBs vaccinated groups compared to their corresponding adjuvant-vaccinated, infected control group.
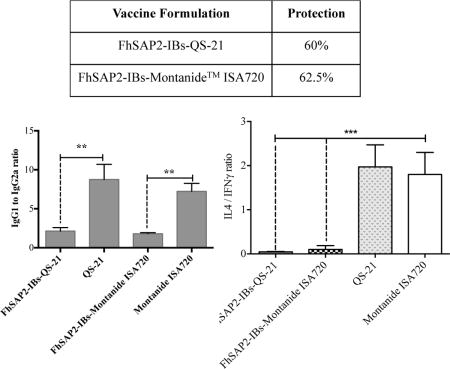
Concerning liver damage score, mice vaccinated with FhSAP2-IBs in QS-21 or Montanide™ ISA720 demonstrated less damage than controls. A total of 2 flukes were recovered from mice vaccinated with FhSAP2-IBs in QS-21 and 3 flukes were recovered from livers of mice vaccinated with FhSAP2-IBs in Montanide™ ISA720. The number of flukes collected from the QS-21 and Montanide™ ISA720 control groups was 5 and 8, respectively. Thus, the fluke reduction of FhSAP2-IBs vaccinated groups compared to the controls ranged from 60–62.5% (Table-1).
Table-1.
Fluke recoveries and percentage of fluke reduction in mice immunized with inclusion bodies containing FhSAP2 challenged with 10 F. hepatica metacercariae.
| Groups | No. of mice* | Fluke Recovery | Total of flukes | Fluke Reduction (%) | Index of liver damage |
|---|---|---|---|---|---|
| FhSAP2-IBs-QS-21 | 9 | 1, 0, 0, 0, 1, 0, 0, 0, 0 | 2 | 60.0 | + |
| FhSAP2-IBs-Montanide™ ISA720 | 8 | 1, 1, 0, 0, 1, 0, 0, 0 | 3 | 62.5 | + |
| E. coli proteins + QS-21 | 2 | 3, 2 | 5 | − | ++++ |
| E. coli proteins + Montanide™ ISA720 | 3 | 3, 2, 3 | 8 | − | +++ |
Animals that survived to the challenge infection and were euthanized 45 days after the challenge infection.
3.3. The IgG1 and IgG2a levels
Indirect ELISA was used to analyze the IgG1 and IgG2a levels in the four experimental groups of mice at three time points (prior initiate experiment, at challenge and at necropsy) as previously mentioned. Prior to initiate the vaccinations, the absorbance at 490 nm of F. hepatica ES products-specific IgG1 and IgG2a were found at background level in sera, which demonstrated the absence of previous exposition to F. hepatica infection in the experimental animals. After receiving three injections with PBS in QS-21 or Montanide™ ISA720, the control animals did not have any detectable amount of antibody to F. hepatica ES products, which indicated that the adjuvants alone do not elicit specific antibody response to parasite antigens. However, at necropsy, which occurred 45-days after challenge, specific antibody levels were detectable in the serum of these animals, which is a clear indicative of F. hepatica infection. The IgG1 was the antibody isotype that significantly predominated in the serum of all control animals with IgG2a amounts close to the background level (p<0.0001) (Fig. 3A). In contrast, after receiving three SC injections all animals that were vaccinated with FhSAP2-IBs in QS-21 or Montanide™ ISA720 had high specific antibody levels to F. hepatica ES products in serum characterized by higher levels of IgG1 than IgG2a (p<0.0001). After challenge a similar antibody pattern was observed in the serum of these animals, which indicated that the infection does not modified the antibody response pattern established by the vaccination. The FhSAP2-IBs formulation prepared in QS-21 elicited the highest levels of both antibody isotypes. Although, the animals vaccinated with FhSAP2-IBs in Montanide™ ISA720 had IgG1 levels that were similar in magnitude to those developed by the adjuvant-control groups, they had detectable IgG2a, whereas the control-group did not. Thus, when the IgG1 to IgG2a ratios were analyzed, it was observed that the group vaccinated with FhSAP2-IBs in QS-21 or in Montanide™ ISA720 had 1.87-fold and 1.94-fold more IgG1 than IgG2a, respectively, whereas the IgG1 to IgG2a ratio in both adjuvant-control groups was 7-fold more. These observations indicate that whereas the FhSAP2-IBs formulations elicited a more balanced Th1 / Th2 antibody response, the adjuvant-control groups developed a polarized Th2 antibody response (Fig. 3B).
Figure-3. Antibody response to F. hepatica ES products in animals immunized with FhSAP2-IBs after the challenge infection.
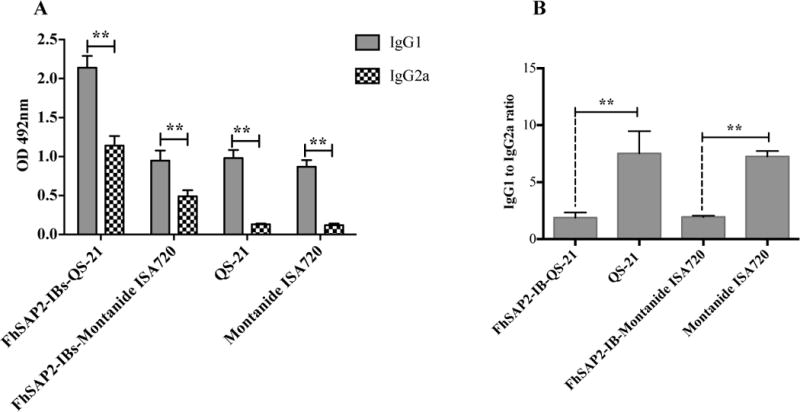
(A) Specific anti-ES products IgG1 and IgG2a antibody levels elicited in mice vaccinated with FhSAP2-IBs in QS-21 or Montanide™ ISA720 were measured at euthanasia, which occurred 45-days after the challenge infection. ** Indicate significant differences (p<0.0001) between the levels of IgG1 and IgG2a of each FhSAP2-IBs vaccinated or control group. Values represent the mean ± SD of triplicate ELISA readings of each experimental group. (B) ** Differences highly significant (p<0.0001) were found between the IgG1 to IgG2a ratios between FhSAP2-IBs vaccinated groups compared with their corresponding control group.
3.4. T-cell activation and serum cytokine levels
The activation status of T-cells was assessed prior to the challenge infection in the groups vaccinated with FhSAP2-IBs in QS-21 or Montanide™ ISA720. Results show that in both formulations, ~51.89% and 88.33% of the activated T-cells belong to the CD4+ T phenotype, which is significantly higher (p<0.001) than the activation observed in the CD8 T cells (Fig. 4).
Figure-4. Activation status of spleen lymphocytes from FhSAP2-IBs immunized animals prior to the challenge infection.
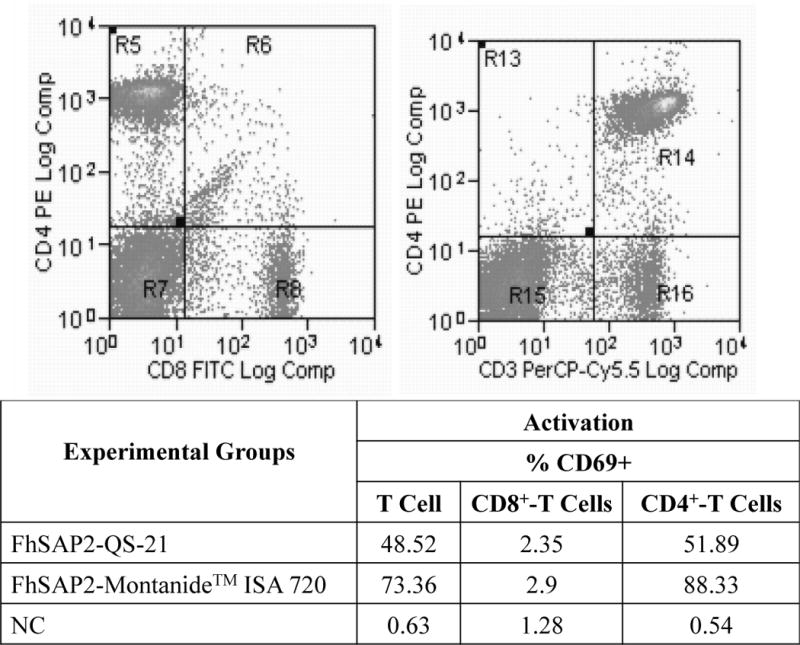
Spleen lymphocytes from FhSAP2-IBs immunized animals were analyzed by flow cytometry. CD69+ was used as marker of activation. Results demonstrate that both formulations significantly activate significantly higher CD4+ T-cells than CD8+ T-cells population.
At necropsy, animals vaccinated with FhSAP2-IBs in QS-21 had concentrations of serum IFNγ (mean 99.78 ± 20.73) that were not significantly different from those elicited by the vaccination with FhSAP2-IBs in Montanide™ ISA720 (mean 78.68 ± 37.15) and neither were different from those found in serum from both control groups collectively (mean 67.96 ± 3.73). In contrast, the control groups had notably higher concentrations of serum IL-4 (134.0 ± 24.0) than the groups vaccinated with FhSAP2-IBs in QS-21 (mean 4.91 ± 1.24) or FhSAP2-IBs in Montanide™ ISA720 (mean 6.74 ± 4.63) (Fig. 5A). When the IL-4 to IFNγ ratio was analyzed, it was found that irrespective to the adjuvant used, the control groups had 1.97-fold more IL-4 than IFNγ, which is a clear indicative of a polarized Th2 immune response. In contrast, both FhSAP2-IBs in QS21 or Montanide™ ISA720 formulations had very low IL-4 to IFNγ ratios indicating a dominant Th1-immune response (Fig. 5B). TNFα was also detected at very high concentration in serum of FhSAP2-IBs in QS-21 (mean 1,134.0 ± 100 pg/ml) or Montanide™ ISA720 (360 ± 45 pg/ml), whereas in the controls groups the amount of TNFα in serum was significantly lower (p<0.0001) (Fig. 5C).
Figure-5. Production of cytokines in serum from animals immunized with FhSAP2-IBs after the challenge infection.
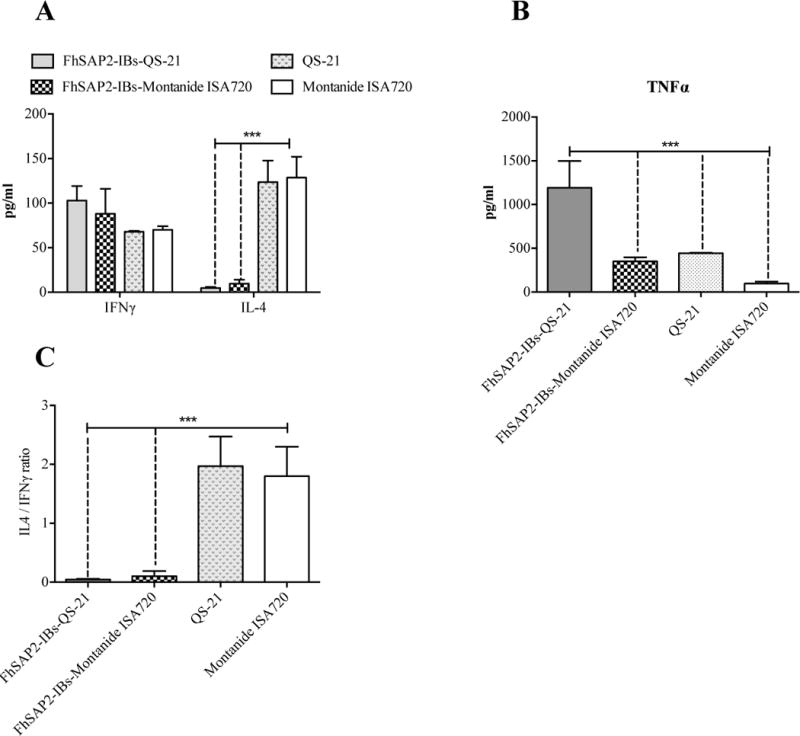
We used BioPlex for determining levels of IFNγ, IL-4 (A) and TNFα (B) cytokine in serum of animals vaccinated with FhSAP2-IBs in QS-21 or Montanide™ ISA720 that were euthanized 45-days after the challenge infection. Results are compared to those obtained in the control groups. *** QS-21 or Montanide™ ISA720-control groups had significantly more amount of IL-4 than FhSAP2-IBs vaccinated groups. The highest IL-4 to IFNγ ratios were found in the controls, which indicate polarized Th2 immune responses. The highest levels of serum TNFα were found in animals from groups vaccinated with FhSAP2-IBs in QS-21 (p=0.0016) compared to those immunized with FhSAP2-IBs in Montanide™ ISA720 or control animals.
4. Discussion
The primary aim of vaccination is to prevent or reduce the infection of the host. In the present study, mice were immunized with inclusion bodies containing FhSAP2 emulsified in the adjuvants, QS-21 and Montanide™ ISA720. As reported earlier, FhSAP2 is the initial agent that causes lysis of plasma membrane of the host’s erythrocytes and leukocytes (Espino and Hillyer, 2003), which is followed by digestion of the lyzed cells’ products with the parasite’s endo and exopeptidases (Collins et al., 2004). Therefore, blocking FhSAP2 activity by vaccination may also block these parasite’s activities leading to their death due to dysfunction in the nutrient digestion. Adjuvants improve the immunogenicity of antigens and can enhance the function of antigen presenting cells by enhancing systemic immune responses and influencing T-helper cell polarization creating bias towards Th1 or Th2 immune responses (Baldridge et al., 2000; Guy, 2007; McKee et al., 2010). The QS-21 and Montanide™ ISA720 adjuvants have shown to stimulate strong humoral and cellular responses (Kensil and Kammer, 1998; Kensil et al., 1991; Kensil et al., 1998; Pye et al., 1997). Both, adjuvants have been tested in human vaccines against malaria and viral diseases (Hui and Hashimoto, 2008; Mbawuike et al., 2007; Toledo et al., 2001; Vandepapeliere et al., 2008; Xue et al., 2010) but to our knowledge, these adjuvants have not been yet assayed in vaccines against parasitic helminths. The levels of protection obtained in this study (60–62.5%) by subcutaneous injection of mice with FhSAP2-IBs in QS-21 or Montanide™ ISA720 were similar to those observed in vaccination trials of mice that employed the purified protein from bacterial lysate in FA (Espino et al., 2010). These results are consistent with earlier studies performed with other F. hepatica antigens (Kesik et al., 2007; Wedrychowicz et al., 2007) and antigens from other parasitic organisms (Dempster et al., 1996; Malgorzata, 2004), which have also demonstrated that inclusion bodies constitute a stable and effective vehicle of antigen delivery with poor differences in immunogenicity compared to the purified antigens. However, the protection induced by FhSAP2-IBs in mice was lower than those observed in the same animal species (74.6–78.5%) with the homolog protein of F. gigantica (FgSAP2) administered subcutaneously in FA (Kueakhai et al., 2013) and lower than those previously reported for the rabbit model of fascioliasis using TiterMax (Espino and Hillyer, 2004) or FA (Espino and Rivera, 2010). These observations confirm the notion that the vaccine potential of FhSAP2 is highly depending of the animal species and the experimental conditions. Trials on a number of vaccine candidates, glutathione S-transferase (Morrison et al., 1996; Sexton et al., 1990; Sexton et al., 1994), cathepsin L- proteases (Mulcahy et al., 1999; Villa-Mancera et al., 2014) and fatty acid binding proteins (Casanueva et al., 2001; Lopez-Aban et al., 2007; Martinez-Fernandez et al., 2004) have also shown a lack of consistency in the degree of protection obtained, which may be due, in part, to differences in the experimental design, for example route of immunization (injection or oral), form of antigen, presence of adjuvants in the vaccine and differences in the animal models (van Milligen et al., 2000; Vercruysse et al., 2004; Wedrychowicz et al., 2003).
The outcome of an immunological response to infection or immunization can be determined by the degree of activation of CD4+ and CD8+. CD4+ cells are T helper cells that, upon activated, produce a number of different cytokines and play a role in the activation and proliferation of B-cells, cytotoxic T lymphocytes, and macrophages (Zhang et al., 2009). In our study, activation status of CD4+ T-cells collected from FhSAP2-IBs vaccinated animals clearly indicates that both formulations elicited T-helper responses. This is an expected finding since FhSAP2 is a protein derived from F. hepatica that is an extracellular organism that typically induces T-helper immune responses, whereas the activation of CD8+ T-cells and CTL-responses are typically elicited by intracellular organisms. Infections with F. hepatica drives the host immune system towards an immunomodulatory response leading to the activation of Th2 immune response within 7 days of infection, with the concurrent production of high titers of IgG1 antibodies without IgG2a (Donnelly et al., 2005; Donnelly et al., 2008), and a downregulation of Th1 (Brady et al., 1999; O’Neill et al., 2000). These studies indicate that while the parasite induce host hepatic damage, it has the ability to limit the extent of pro-inflammatory Th1 driven protective immune response and promote anti-inflammatory healing mechanisms, involving a Th2-mediated immune response (Donnelly et al., 2005; Donnelly et al., 2008; Mulcahy et al., 1998; Wongkham et al., 2005). Mice are highly susceptible to F. hepatica infection and consequently die secondary to high-level infection due to severe liver damage caused by the migrating fluke, which provoke organ failure. This explains why in our study a large number of mice that were vaccinated only with the adjuvant died secondary to the challenge infection exhibiting the most severe hepatic damage. In contrast, in the groups vaccinated with FhSAP2-IBs, few adult flukes were recovered, their mortality rate and liver damage was significantly reduced, indicating that these animals acquired partial immunity against the challenge infection. In the adjuvant-vaccinated, infected animals, the IgG1 was the dominant antibody isotype detected whereas the IgG2a was detected at very low levels. In contrast, in the FhSAP2-IBs vaccinated animals enhanced levels of both IgG1 and IgG2a antibodies were detected, implicating mixed Th1/Th2 antibody response associated to the protection. Although is not clear the role of antibodies in the protection against F. hepatica this type of mixed humoral response has been reported by other vaccination studies in both F. hepatica and F. gigantica (Chantree et al., 2013; Golden et al., 2010; Kueakhai et al., 2013; Preyavichyapugdee et al., 2008). Moreover, the observation that FhSAP2-IBs vaccinated animals developed significantly higher levels of IgG2a, companied with high levels of serum IFNγ and TNFα, and significantly lower levels of IL-4 than controls support the hypothesis that the Th1-response could be necessary to induce protection against fascioliasis, which has been also suggested by other authors (Hacariz et al., 2009). Since FhSAP2-IBs formulated in QS-21 and Montanide™ ISA720 induced partial immunity in the mouse model of fascioliasis, it will be necessary in the future to test these formulations in livestock or sheep. Perhaps, an FhSAP2-IBs formulation that includes QS-21 as immunostimulator and a then emulsified as a water-in-oil emulsion with Montanide™ ISA720 could be more efficient and maximize the protection.
In conclusion, the results of this study suggest that inclusion bodies containing FhSAP2 formulated in QS-21 or Montanide™ ISA720 adjuvants induce partial immunity against a challenge F. hepatica infection in mice, which was associated to significant increasing of Th1 immune responses. Further work will be required to pinpoint the putative role of Th1 immune response in the protection against F. hepatica and establish the correlates of protection in mice and other animal species.
Highlights.
Inclusion bodies (IBs) containing FhSAP2 were tested in a mouse model of fascioliasis
FhSAP2-IBs emulsified in Montanide™ ISA720 or QS-21 induces partial immunity in mice
FhSAP2-IBs in Montanide™ ISA720 or QS-21 induces high levels of IgG1 and IgG2a antibodies
FhSAP2-IBs in Montanide™ ISA720 or QS-21 induces higher levels of IFNγ than IL-4
Acknowledgments
The authors thank Lorna Cruz for her technical assistant in preparation of some inclusion bodies batches and Vasti Aguayo for editing the manuscript. This research was supported by grants from the NIH 1SC1AI096108-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldridge JR, Yorgensen Y, Ward JR, Ulrich JT. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine. 2000;18:2416–2425. doi: 10.1016/s0264-410x(99)00572-1. [DOI] [PubMed] [Google Scholar]
- Brady MT, O’Neill SM, Dalton JP, Mills KH. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Inf Immu. 1999;67:5372–5378. doi: 10.1128/iai.67.10.5372-5378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderson JR. A retrospective review of lesions associated with the use of Freund’s adjuvant. Lab Animal Sci. 1989;39:400–405. [PubMed] [Google Scholar]
- Bruhn H. A short guided tour through functional and structural features of saposin-like proteins. Biochem J. 2005;389:249–257. doi: 10.1042/BJ20050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caban-Hernandez K, Espino AM. Differential expression and localization of saposin-like protein 2 of Fasciola hepatica. Acta Trop. 2013;128:591–597. doi: 10.1016/j.actatropica.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanueva R, Hillyer GV, Ramajo V, Oleaga A, Espinoza EY, Muro A. Immunoprophylaxis against Fasciola hepatica in rabbits using a recombinant Fh15 fatty acid-binding protein. J Parasitol. 2001;87:697–700. doi: 10.1645/0022-3395(2001)087[0697:IAFHIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Chantree P, Phatsara M, Meemon K, Chaichanasak P, Changklungmoa N, Kueakhai P, Lorsuwannarat N, Sangpairoj K, Songkoomkrong S, Wanichanon C, Itagaki T, Sobhon P. Vaccine potential of recombinant cathepsin B against Fasciola gigantica. Exp Parasitol. 2013;135:102–109. doi: 10.1016/j.exppara.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Collins PR, Stack CM, O’Neill SM, Doyle S, Ryan T, Brennan GP, Mousley A, Stewart M, Maule AG, Dalton JP, Donnelly S. Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: propetide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. J Biol Chem. 2004;279:17038–17046. doi: 10.1074/jbc.M308831200. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster RP, Robinson CM, Harrison GB. Parasite vaccine development: large-scale recovery of immunogenic Taenia ovis fusion protein GST-45W(B/X) from Escherichia coli inclusion bodies. Parasitol Res. 1996;82:291–296. doi: 10.1007/s004360050116. [DOI] [PubMed] [Google Scholar]
- Donnelly S, O’Neill SM, Sekiya M, Mulcahy G, Dalton JP. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Inf Immu. 2005;73:166–173. doi: 10.1128/IAI.73.1.166-173.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly S, Stack CM, O’Neill SM, Sayed AA, Williams DL, Dalton JP. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008;22:4022–4032. doi: 10.1096/fj.08-106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino AM, Hillyer GV. Molecular cloning of a member of the Fasciola hepatica saposin-like protein family. J Parasitol. 2003;89:545–552. doi: 10.1645/GE-3113. [DOI] [PubMed] [Google Scholar]
- Espino AM, Hillyer GV. A novel Fasciola hepatica saposin-like recombinant protein with immunoprophylactic potential. J Parasitol. 2004;90:876–879. doi: 10.1645/GE-215R. [DOI] [PubMed] [Google Scholar]
- Espino AM, Morales A, Delgado B, Rivera FM, Figueroa O, Suarez E. Partial immunity to Fasciola hepatica in mice after vaccination with FhSAP2 delivered as recombinant protein or DNA construct. Ethn Dis. 2010;20:S1-17-23. [PMC free article] [PubMed] [Google Scholar]
- Espino AM, Rivera F. Quantitation of cytokine mRNA by real-time RT-PCR during a vaccination trial in a rabbit model of fascioliasis. Vet Parasitol. 2010;169:82–92. doi: 10.1016/j.vetpar.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Santiago O, Delgado B, Espino AM. Fasciola hepatica saposin-like protein-2-based ELISA for the serodiagnosis of chronic human fascioliasis. Diag Microbiol Inf Dis. 2011;70:355–361. doi: 10.1016/j.diagmicrobio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J. The effect of paraffin oil and mycobacteria on antibody formation and sensitization; a review. Am J Clin Pathol. 1951;21:645–656. doi: 10.1093/ajcp/21.7.645. [DOI] [PubMed] [Google Scholar]
- Golden O, Flynn RJ, Read C, Sekiya M, Donnelly SM, Stack C, Dalton JP, Mulcahy G. Protection of cattle against a natural infection of Fasciola hepatica by vaccination with recombinant cathepsin L1 (rFhCL1) Vaccine. 2010;28:5551–5557. doi: 10.1016/j.vaccine.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Guy B. The perfect mix: recent progress in adjuvant research. Nature Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- Hacariz O, Sayers G, McCullough M, Garrett M, O’Donovan J, Mulcahy G. The effect of Quil A adjuvant on the course of experimental Fasciola hepatica infection in sheep. Vaccine. 2009;27:45–50. doi: 10.1016/j.vaccine.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Hartley DL, Kane JF. Properties of inclusion bodies from recombinant Escherichia coli. Biochem Soc Trans. 1988;16:101–102. doi: 10.1042/bst0160101. [DOI] [PubMed] [Google Scholar]
- Hui GS, Hashimoto CN. Adjuvant formulations possess differing efficacy in the potentiation of antibody and cell mediated responses to a human malaria vaccine under selective immune genes knockout environment. Int Immunopharmacol. 2008;8:1012–1022. doi: 10.1016/j.intimp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers EJ, Femel J, Andersson K, Bjorkelund H, Hellman L, Olsson AK. The non-toxic and biodegradable adjuvant Montanide ISA 720/CpG can replace Freund’s in a cancer vaccine targeting ED-B–a prerequisite for clinical development. Vaccine. 2012;30:225–230. doi: 10.1016/j.vaccine.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Kensil CR, Kammer R. QS-21: a water-soluble triterpene glycoside adjuvant. Expert Opinion Inv Drugs. 1998;7:1475–1482. doi: 10.1517/13543784.7.9.1475. [DOI] [PubMed] [Google Scholar]
- Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- Kensil CR, Wu JY, Anderson CA, Wheeler DA, Amsden J. QS-21 and QS-7: purified saponin adjuvants. Dev Biol Stand. 1998;92:41–47. [PubMed] [Google Scholar]
- Kesik M, Jedlina-Panasiuk L, Kozak-Cieszczyk M, Plucienniczak A, Wedrychowicz H. Enteral vaccination of rats against Fasciola hepatica using recombinant cysteine proteinase (cathepsin L1) Vaccine. 2007;25:3619–3628. doi: 10.1016/j.vaccine.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Kueakhai P, Changklungmoa N, Riengrojpitak S, Chaichanasak P, Meemon K, Chaithirayanon K, Chantree P, Sansri V, Itagaki T, Sobhon P. Vaccine potential of recombinant saposin-like protein 2 against Fasciolosis gigantica in mice. Vaccine. 2013;31:5518–5523. doi: 10.1016/j.vaccine.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Lopez-Aban J, Casanueva P, Nogal J, Arias M, Morrondo P, Diez-Banos P, Hillyer GV, Martinez-Fernandez AR, Muro A. Progress in the development of Fasciola hepatica vaccine using recombinant fatty acid binding protein with the adjuvant adaptation system ADAD. Vet Parasitol. 2007;145:287–296. doi: 10.1016/j.vetpar.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Maggioli G, Acosta D, Silveira F, Rossi S, Giacaman S, Basika T, Gayo V, Rosadilla D, Roche L, Tort J, Carmona C. The recombinant gut-associated M17 leucine aminopeptidase in combination with different adjuvants confers a high level of protection against Fasciola hepatica infection in sheep. Vaccine. 2011a;29:9057–9063. doi: 10.1016/j.vaccine.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Maggioli G, Silveira F, Martin-Alonso JM, Salinas G, Carmona C, Parra F. A recombinant thioredoxin-glutathione reductase from Fasciola hepatica induces a protective response in rabbits. Exp Parasitol. 2011b;129:323–330. doi: 10.1016/j.exppara.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Malgorzata K. Inclusion bodies from recombinant bacteria as a novel system for delivery of vaccine antigen by the oral route. Immunol Letters. 2004;91:197–204. doi: 10.1016/j.imlet.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Martinez-Fernandez AR, Nogal-Ruiz JJ, Lopez-Aban J, Ramajo V, Oleaga A, Manga-Gonzalez Y, Hillyer GV, Muro A. Vaccination of mice and sheep with Fh12 FABP from Fasciola hepatica using the new adjuvant/immunomodulator system ADAD. Vet Parasitol. 2004;126:287–298. doi: 10.1016/j.vetpar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull W H O. 1999a;77:340–346. [PMC free article] [PubMed] [Google Scholar]
- Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol. 2005;79:207–216. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Angles R, Esteban JG, Bargues MD, Buchon P, Franken M, Strauss W. The Northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Trop Med Int Health. 1999b;4:454–467. doi: 10.1046/j.1365-3156.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- Mbawuike I, Zang Y, Couch RB. Humoral and cell-mediated immune responses of humans to inactivated influenza vaccine with or without QS-21 adjuvant. Vaccine. 2007;25:3263–3269. doi: 10.1016/j.vaccine.2007.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AS, MacLeod MK, Kappler JW, Marrack P. Immune mechanisms of protection: can adjuvants rise to the challenge? BMC Biol. 2010;8:37. doi: 10.1186/1741-7007-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CA, Colin T, Sexton JL, Bowen F, Wicker J, Friedel T, Spithill TW. Protection of cattle against Fasciola hepatica infection by vaccination with glutathione S-transferase. Vaccine. 1996;14:1603–1612. doi: 10.1016/s0264-410x(96)00147-8. [DOI] [PubMed] [Google Scholar]
- Mulcahy G, O’Connor F, Clery D, Hogan SF, Dowd AJ, Andrews SJ, Dalton JP. Immune responses of cattle to experimental anti-Fasciola hepatica vaccines. Res Vet Science. 1999;67:27–33. doi: 10.1053/rvsc.1998.0270. [DOI] [PubMed] [Google Scholar]
- Mulcahy G, O’Connor F, McGonigle S, Dowd A, Clery DG, Andrews SJ, Dalton JP. Correlation of specific antibody titre and avidity with protection in cattle immunized against Fasciola hepatica. Vaccine. 1998;16:932–939. doi: 10.1016/s0264-410x(97)00289-2. [DOI] [PubMed] [Google Scholar]
- Mutiso JM, Macharia JC, Gicheru MM. Immunization with Leishmania vaccine-alum-BCG and montanide ISA 720 adjuvants induces low-grade type 2 cytokines and high levels of IgG2 subclass antibodies in the vervet monkey (Chlorocebus aethiops) model. Scand J Immunol. 2012;76:471–477. doi: 10.1111/j.1365-3083.2012.02764.x. [DOI] [PubMed] [Google Scholar]
- O’Neill SM, Brady MT, Callanan JJ, Mulcahy G, Joyce P, Mills KH, Dalton JP. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 2000;22:147–155. doi: 10.1046/j.1365-3024.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- Piacenza L, Acosta D, Basmadjian I, Dalton JP, Carmona C. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Inf Immu. 1999;67:1954–1961. doi: 10.1128/iai.67.4.1954-1961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preyavichyapugdee N, Sahaphong S, Riengrojpitak S, Grams R, Viyanant V, Sobhon P. Fasciola gigantica and Schistosoma mansoni: vaccine potential of recombinant glutathione S-transferase (rFgGST26) against infections in mice. Exp Parasitol. 2008;119:229–237. doi: 10.1016/j.exppara.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Pye D, Vandenberg KL, Dyer SL, Irving DO, Goss NH, Woodrow GC, Saul A, Alving CR, Richards RL, Ballou WR, Wu MJ, Skoff K, Anders RF. Selection of an adjuvant for vaccination with the malaria antigen, MSA-2. Vaccine. 1997;15:1017–1023. doi: 10.1016/s0264-410x(96)00289-7. [DOI] [PubMed] [Google Scholar]
- Reed MB, Strugnell RA, Panaccio M, Spithill TW. A novel member of the NK-lysin protein family is developmentally regulated and secreted by Fasciola hepatica. Mol Biochem Parasitol. 2000;105:297–303. doi: 10.1016/s0166-6851(99)00185-1. [DOI] [PubMed] [Google Scholar]
- Remarque EJ, Roestenberg M, Younis S, Walraven V, van der Werff N, Faber BW, Leroy O, Sauerwein R, Kocken CH, Thomas AW. Humoral immune responses to a single allele PfAMA1 vaccine in healthy malaria-naive adults. PLoS One. 2012;7:e38898. doi: 10.1371/journal.pone.0038898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JL, Milner AR, Panaccio M, Waddington J, Wijffels G, Chandler D, Thompson C, Wilson L, Spithill TW, Mitchell GF, et al. Glutathione S-transferase. Novel vaccine against Fasciola hepatica infection in sheep. J Immunol. 1990;145:3905–3910. [PubMed] [Google Scholar]
- Sexton JL, Wilce MC, Colin T, Wijffels GL, Salvatore L, Feil S, Parker MW, Spithill TW, Morrison CA. Vaccination of sheep against Fasciola hepatica with glutathione S-transferase. Identification and mapping of antibody epitopes on a three-dimensional model of the antigen. J Immunol. 1994;152:1861–1872. [PubMed] [Google Scholar]
- Tliba O, Sibille P, Boulard C, Chauvin A. Early hepatic cytokine mRNA expression in experimental rat fasciolosis. Vet Parasitol. 2002;103:237–249. doi: 10.1016/s0304-4017(01)00584-2. [DOI] [PubMed] [Google Scholar]
- Toledo H, Baly A, Castro O, Resik S, Laferte J, Rolo F, Navea L, Lobaina L, Cruz O, Miguez J, Serrano T, Sierra B, Perez L, Ricardo ME, Dubed M, Lubian AL, Blanco M, Millan JC, Ortega A, Iglesias E, Penton E, Martin Z, Perez J, Diaz M, Duarte CA. A phase I clinical trial of a multi-epitope polypeptide TAB9 combined with Montanide ISA 720 adjuvant in non-HIV-1 infected human volunteers. Vaccine. 2001;19:4328–4336. doi: 10.1016/s0264-410x(01)00111-6. [DOI] [PubMed] [Google Scholar]
- van Milligen FJ, Cornelissen JB, Bokhout BA. Fasciola hepatica: an antigen fraction derived from newly excysted juveniles, containing an immunoreactive 32-kDa protein, induces strong protective immunity in rats. Exp Parasitol. 2000;94:163–171. doi: 10.1006/expr.1999.4476. [DOI] [PubMed] [Google Scholar]
- Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, Van Belle P, Clement F, Hanon E, Wettendorff M, Garcon N, Leroux-Roels G. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26:1375–1386. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Vercruysse J, Knox DP, Schetters TP, Willadsen P. Veterinary parasitic vaccines: pitfalls and future directions. Trends Parasitol. 2004;20:488–492. doi: 10.1016/j.pt.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Villa-Mancera A, Reynoso-Palomar A, Utrera-Quintana F, Carreon-Luna L. Cathepsin L1 mimotopes with adjuvant Quil A induces a Th1/Th2 immune response and confers significant protection against Fasciola hepatica infection in goats. Parasitol Res. 2014;113:243–250. doi: 10.1007/s00436-013-3650-6. [DOI] [PubMed] [Google Scholar]
- Wedrychowicz H, Kesik M, Kaliniak M, Kozak-Cieszczyk M, Jedlina-Panasiuk L, Jaros S, Plucienniczak A. Vaccine potential of inclusion bodies containing cysteine proteinase of Fasciola hepatica in calves and lambs experimentally challenged with metacercariae of the fluke. Vet Parasitol. 2007;147:77–88. doi: 10.1016/j.vetpar.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Wedrychowicz H, Lamparska M, Kesik M, Kotomski G, Mieszczanek J, Jedlina-Panasiuk L, Plucienniczak A. The immune response of rats to vaccination with the cDNA or protein forms of the cysteine proteinase of Fasciola hepatica. Vet Immunol Immunopathol. 2003;94:83–93. doi: 10.1016/s0165-2427(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Wongkham C, Tantrawatpan C, Intapan PM, Maleewong W, Wongkham S, Nakashima K. Evaluation of immunoglobulin G subclass antibodies against recombinant Fasciola gigantica cathepsin L1 in an enzyme-linked immunosorbent assay for serodiagnosis of human fasciolosis. Clin Diag Lab Immunol. 2005;12:1152–1156. doi: 10.1128/CDLI.12.10.1152-1156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Ding F, Zhang Q, Pan X, Qu L, Pan W. Stability and potency of the Plasmodium falciparum MSP1-19/AMA-1(III) chimeric vaccine candidate with Montanide ISA720 adjuvant. Vaccine. 2010;28:3152–3158. doi: 10.1016/j.vaccine.2010.02.054. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang H, Zhao J. The role of CD4 T cell help for CD8 CTL activation. Biochem Bioph Res Commu. 2009;384:405–408. doi: 10.1016/j.bbrc.2009.04.134. [DOI] [PubMed] [Google Scholar]


