Abstract
Background
A body of work focusing on brain connectivity, language dominance, and motor laterality research suggests reduced hemispheric asymmetry is a core feature in schizophrenia. However, there is little consensus about whether reduced dominance is present in those at ultrahigh risk (UHR) for psychosis.
Methods
A total of 94 demonstrated right-handed neuroleptic free participants (38 UHR and 56 matched healthy controls) were assessed with structured clinical interviews and completed an innovative handwriting task using a digital tablet computer. A laterality quotient (LQ) was calculated using kinematic variables from the participant’s left and right hands. A subset of the sample (26 UHR and 29 controls) returned after 12-months to complete clinical interviews in order to examine relationships between handwriting laterality and progression of psychosis risk symptoms.
Results
The UHR group showed decreased dextrality compared to healthy controls. At the 12-month follow-up, decreased dextrality accounted for 8% of the variance in worsened positive symptoms within the UHR group.
Conclusion
The current results suggest that disrupted cerebral dominance is also present in the ultrahigh risk period and that decreased dextrality may serve as a novel biomarker for the progression of psychosis risk.
Keywords: Ultrahigh risk, psychosis, cerebral dominance, handwriting, laterality, dextrality
1. Introduction
Cerebral asymmetry has been proposed to be an evolutionary beneficial trait for humans. Functional specialization or dominance in one hemisphere frees the other hemisphere to accomplish varied tasks (i.e., to speak and use tools at the same time) (Vallortigara and Rogers, 2005). It has been proposed that decreased cerebral asymmetry may be a core feature of the abnormal neurodevelopment leading to the emergence of psychosis (Crow, 2004; Crow et al., 1989; Crow et al., 1996; Oertel-Knochel and Linden, 2011). Supportive findings from cross-sectional structural imaging studies in patients with schizophrenia note decreased volume lateralization in language and motor areas of the cortex (Barta et al., 1997; Deep-Soboslay et al., 2010; Petty et al., 1995). Functional imaging studies utilizing language tasks have described increased bilateral functional activation in schizophrenia patients compared to controls, possibly reflecting a less efficient specialization of brain areas for language function (Sommer et al., 2001; Weiss et al., 2006). Increased prevalence of non-right handedness (i.e., left handedness or mixed handedness)—characterized by decreased specialization or preference for performing manual tasks solely with the right hand—has been observed in patients with schizophrenia and proposed to be a specific sign tied to abnormal cerebral asymmetry and etiological risk factors for psychosis (Dragovic and Hammond, 2005; Satz and Green, 1999; Sommer, 2001).
Despite the strong body of work that has elucidated handedness in patients with psychosis, it remains unclear whether non-right handedness occurs in at risk individuals (Claridge et al., 1998; Erlenmeyer-Kimling et al., 2005). Archival studies of individuals who later developed schizophrenia have found evidence for mixed handedness at 7 years of age (Crow et al., 1996). However, a separate archival study found differences in eye dominance but not hand dominance (Cannon et al., 1997). In prospective and cross sectional studies involving family members who are at high genetic risk for psychosis, there has been inconclusive evidence that non-right handedness is associated with increased genetic risk (Clementz et al., 1994; Deep-Soboslay et al., 2010; Erlenmeyer-Kimling et al., 2005; Orr et al., 1999). And a very recent cross-sectional study noted that increased genetic risk for psychosis was associated with increased rather than decreased handedness lateralization during a line drawing paradigm (Manschreck et al., 2015). However, no study to date has examined handwriting lateralization in ultrahigh risk (UHR) for psychosis individuals.
One explanation for the inconclusive evidence on handedness in at risk populations may be related to the limited methodology for assessing handedness. By and large, studies in schizophrenia spectrum populations have asked participants to demonstrate manual activities that they would do with their left, right, or both hands. In some studies, left and right handedness is treated categorically, while in others, different cut-offs are used to differentiate right from non-right handed individuals (Satz and Green, 1999). While these methods provide excellent classification, they may miss subtle variations in handedness because they rely on self and observer reports. Recent efforts to understand movement abnormalities in psychosis and at risk populations using computer based handwriting measures may provide an objective method for assessing subtle abnormalities in handedness (Caligiuri et al., 2009; Caligiuri et al., 2010; Dean and Mittal, 2015; Dean et al., 2013; Docx et al., 2012).
Decreased handedness has been associated with schizotypy and positive symptoms of psychosis (Badzakova-Trajkov et al., 2011; Barrantes-Vidal et al., 2013). This is particularly relevant as a neural diathesis stress model of psychosis suggests that early vulnerabilities (e.g., genetics, obstetric complications) lead to altered brain development, which in adolescence interacts with stressful life events, eventually leading to the development of psychotic symptoms (Cornblatt et al., 2003). Examining handedness may provide an important measure of abnormal brain development prior to the onset of the disorder, which is critical for understanding potential biomarkers and guiding preventive efforts in youth who are at risk for developing the disorder.
In order to investigate dextrality and associations to the progression of symptoms of risk in a group of right-handed UHR and healthy controls, participants completed clinical interviews at a baseline and a 12-month follow-up visit and a handwriting task on a computerized tablet at the baseline visit. We hypothesized that the UHR group would show decreased right-handed laterality (i.e., decreased dextrality) compared to the healthy controls, which would be characterized by a more equivalent laterality quotient. Furthermore, we hypothesized that decreased dextrality would be related to more severe positive symptoms at baseline and worsened progression of symptoms over a period of 12 months.
2. Materials and methods
2.1. Participants
Right handed adolescent and young adult UHR and healthy control participants (mean age =18.31) were recruited by Craigslist, email postings, newspaper ads, and community professional referrals. Exclusion criteria consisted of head injury, the presence of a neurological disorder, and lifetime substance dependence. The presence of an Axis I psychotic disorder (e.g., schizophrenia, schizoaffective disorder, schizophreniform) and the use of any antipsychotic medication at baseline were exclusion criteria for UHR participants. The presence of any category of Axis I disorder or a psychotic disorder in a 1st degree relative was an exclusion criterion for controls. The protocol and informed consent procedures were approved by the University Institutional Review Board. See Table 1 for the demographic characteristics of the sample.
Table 1.
UHR and healthy controls did not differ in terms of age, education, gender, and parental education. UHR participants were rated significantly higher on both positive and negative symptom domains at baseline and follow-up. UHR individuals showed significantly decreased dextrality on a measure of handwriting laterality, LQFREQ. NS indicates not significant.
| UHR | Control | Statistic | p ≤ | |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 18.69 (1.85) | 18.04 (2.79) | t(92) = 1.35 | NS |
| Gender | ||||
| Male | 21 | 24 | ||
| Female | 17 | 32 | ||
| Total | 38 | 56 | χ2(1, N = 94) = 1.40 | NS |
| Education (years) | ||||
| Mean (SD) | 12.45 (1.98) | 11.99 (2.72) | t(92) = .96 | NS |
| Parent Education | ||||
| Mean (SD) | 15.65 (2.06) | 15.98 (2.52) | t(92) = −.74 | NS |
| Baseline Symptoms | ||||
| Positive | 12.21 (4.29) | .79 (1.47) | t(42.82) = 15.56 | .001 |
| Negative | 9.85 (6.31) | .48 (1.08) | t(38.57) = 9.05 | .001 |
| Follow-Up Symptoms | ||||
| Positive | 11.73 (6.27) | .24 (.58) | t(25.38) = 9.32 | .001 |
| Negative | 9.88 (8.20) | .59 (1.50) | t(26.50) = 5.70 | .001 |
| Laterality Quotient | ||||
| Frequency Mean Rank | 55.95 | 42.46 | U = 726 | .01 |
| Frequency Mean (SD) | −.28 (.46) | −.4 (.32) | – | – |
The follow-up study is ongoing, and to date, 12 months have passed for 90 individuals who have completed a baseline assessment. Each of these individuals was invited back, and 55 participants agreed to return to complete clinical interviews. Participants did not return because they could not be contacted (UHR n=11, control n=20) or decided not to participate (control n=4). There were no baseline differences in age, gender, education or parent education between those who did and did not return for follow-up.
2.2. Clinical Interviews
At baseline, the Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., 1999) was administered to both UHR and control subjects to diagnose a UHR syndrome (the SIPS was used to rule out UHR symptoms in healthy controls). A total sum score for the positive and negative symptom domain was used as an indicator of the respective dimensions of symptomatology. The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID) (First et al., 1995) was administered to rule out a psychotic disorder diagnosis.
At follow-up, the SIPS was administered to track UHR symptom changes over 12-months and the SCID was administered to assess for possible transition to psychosis. Training of advanced doctoral student interviewers was conducted over a 2-month period, and inter-rater reliabilities exceeded the minimum study criterion of Kappa ≥ .80.
2.3. Handedness
Right handedness was determined by requiring that participants normally write with their right hand in addition to demonstrated preference for using their right hand in all of the following manual tasks: deal a deck of cards, thread a needle, throw a ball, and use a tennis racket. These items are valid for establishing hand dominance and have been used in other investigations to assess handedness (Buchanan and Heinrichs, 1989).
2.4. Handwriting Samples
Handwriting samples were acquired using Neuroscript MoveAlyzer software (http://www.neuroscript.net) installed on a Fujitsu Lifebook T901 tablet computer with a non-inking pen. Participants were instructed to draw eight concentric circles continuously in either a clockwise or counterclockwise direction within a 2 cm boundary line using one hand at a time (see Figure 1). This stimulus has been used in previous studies that assess handedness and has been shown to be a sensitive measure of handwriting laterality (Henkel et al., 2001). Each trial consisted of 16 vertical strokes, which were segmented and processed for duration per stroke. Valid trials included at least 10 strokes. Kinematic variables were extracted from MoveAlyzer and imported into SPSS 22. The mean duration per stroke per trial (i.e., frequency of stroke) was calculated for each hand separately. A laterality quotient using mean frequency of stroke (LQFREQ) was calculated as follows:
Previous work suggests that this LQ can successfully classify left and right handedness (Henkel et al., 2001). More negative values on LQFREQ indicate slower duration per stroke for the left hand compared to the right hand. This would be expected given that the right hand is the preferred hand and is expected to better control pen movements. In contrast, more positive numbers (i.e., values closer to zero) indicate that the right hand was more similar to the left hand, suggesting decreased dextrality. Of note, a subset of the current participants have also completed two separate studies aimed at understanding hyperkinetic and hypokinetic movement abnormalities associated with risk for psychosis using MoveAlyzer software (Dean and Mittal, 2015; Dean et al., 2013). These studies were not focused on laterality and did not use the same kinematic variables.
Figure 1.
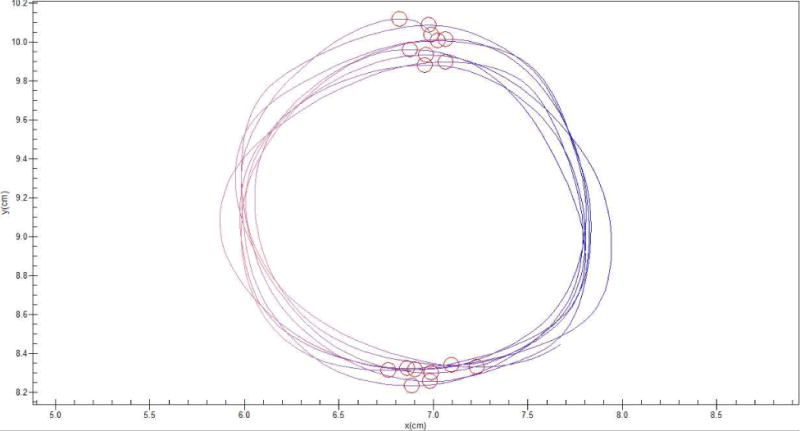
An example handwriting trial. The participants draw 8 concentric circles continuously in one direction with either their left or right hand between a 2cm boundary. Strokes (red and blue lines) were segmented by MoveAlyzer. Kinematic variables for duration per stroke were extracted for all trials for both the left and right hands.
2.5. Statistical Analyses
Independent t-tests and chi-square tests were employed to examine differences between groups in continuous and categorical demographic variables, respectively. Kolmogorov-Smirnov tests revealed that the handwriting kinematic variables and laterality quotient was normally distributed; two-tailed independent t-tests were used to examine group differences for the left and right mean frequency per stroke as well as LQFREQ. The control group showed a limited range in symptom scores and Pearson correlations were run in the UHR group alone to assess the relationship between LQFREQ and symptoms. A series of 2 hierarchical regression analyses were conducted within the UHR group alone. Positive and negative symptoms at the follow-up assessment were used as the dependent variables and the respective symptom variable for the baseline assessment was entered in the first block. In the second block, LQFREQ was entered as the predictor variable. With each analysis, the magnitude of R2 change (ΔR2) was tested for significance. This analytic approach tests the hypotheses that while controlling for the variance explained by symptoms at baseline, decreased handwriting lateralization will be associated with respective symptoms 12 months later.
3. Results
3.1. Participants
There were no significant differences between groups on demographic characteristics including age, education, gender, and parental education. As expected, UHR participants were rated significantly higher than controls on both SIPS symptom domains positive and negative at baseline and follow-up (see Table 1 for information about the participants). Of the participants who completed the baseline handwriting task and clinical interviews, 3 developed a psychotic disorder at follow-up.
3.2. Group Differences for Kinematic Variables and Laterality Quotient
There were no significant group differences between UHR and healthy controls for any of the mean kinematic variables, including left or right frequency. The LQFREQ for the UHR group was significantly closer to zero compared to healthy controls, suggesting that the UHR group showed a similar stroke frequency for both hands and that the UHR group was significantly less lateralized to the right hand t(92)=2.57, p≤.05.
3.3. Relationship between Baseline Symptoms and Laterality Quotient
While not significant, decreased right handedness on LQFREQ was related to elevated positive symptoms at baseline r(36)=.19, p=.24 (see Figure 2). There was not an association between LQFREQ and negative symptoms within the UHR group (p≥.5).
Figure 2.
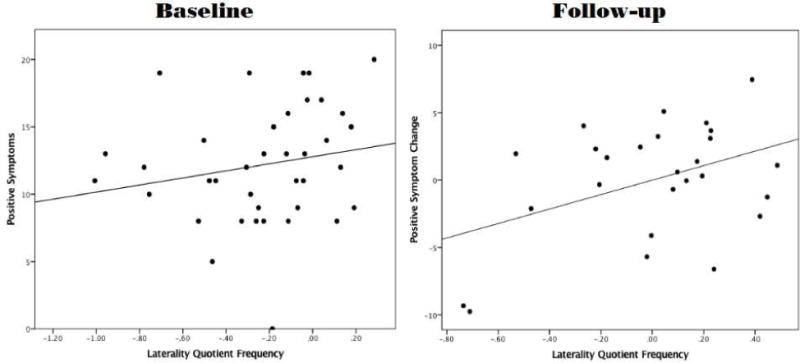
The figure shows two scatter plots: the first plot shows the relationship of LQFREQ to positive symptoms at baseline. The second plot shows the relationship between LQFREQ and positive symptom change over the 12-month follow-up period. More positive numbers on LQFREQ indicate decreased dextrality. More positive numbers on the Y-axis indicate that the total SIPS positive symptom score was higher at follow-up than at baseline.
3.4. Relationship between Follow-up Symptoms and Laterality Quotient
A hierarchical linear regression approach was used to examine the relationship between LQFREQ and the progression of positive and negative symptoms over 12-months within the UHR group. Decreased right-handedness on LQFREQ accounted for 8% of the variance in change of positive symptoms after 12-months. While a proportion of participants improved over the follow-up period, more positive values of LQFREQ (indicating decreased dextrality) were related to worsened positive symptoms at follow-up for UHR participants who did not improve (β=.29, p≤.05). There was not a significant relationship between LQFREQ and negative symptoms (see Table 2 and Figure 2).
Table 2.
Linear regression was used to evaluate if decreased dextrality using LQFREQ at baseline predicted worsening positive and negative symptoms at follow-up in the UHR group alone.
| Predicting 12-Month Variable | Block I- Baseline Symptoms | Block II- LQ FREQUENCY | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | df | F | β | P | ΔR2 | df | F | β | P | |
| Positive Symptoms | .53 | 1, 24 | 27.17 | .73 | .0001 | .08 | 1, 23 | 4.94 | .29 | .04 |
| Negative Symptoms | .43 | 1, 24 | 17.90 | .65 | .0001 | .01 | 1, 23 | .41 | .10 | .53 |
4. Discussion
Movement abnormalities tied to aberrant neurodevelopment have been proposed to be a key sign of risk for psychosis (Callaway et al., 2014; Dean and Mittal, 2015; Dean et al., 2013; Mittal et al., 2010a; Mittal et al., 2007a; Mittal et al., 2008; Mittal et al., 2007b). However, to date, our understanding of laterality during this critical period has been limited. Importantly, this is the first study to examine handedness in UHR individuals using handwriting kinematic measurements. Altered cerebral asymmetry may be indicated by decreased dextrality, and point to early neurodevelopmental changes that are associated with risk symptoms for psychosis. In this study, we examined right-handedness using an innovative instrumental measure, which allowed us to pick up decreased laterality in the UHR group compared to healthy controls using kinematic measurements for duration of stroke. Furthermore, within the UHR group, decreased laterality was associated with the progression of more severe positive symptoms over a 12-month period. These results build on previous work with inconclusive findings regarding handedness lateralization in individuals at risk for psychosis (Cannon et al., 1997; Crow et al., 1996), suggesting that subtle differences in handwriting laterality are measured on an instrumental task, and are associated with key symptoms of risk for psychosis.
Handwriting is a highly specialized skill requiring motor coordination. Previous work in UHR samples notes that movement abnormalities typically precede the onset of psychosis and may be an early sign of abnormal brain development (Mittal et al., 2010a; Mittal et al., 2014; Mittal et al., 2011; Mittal et al., 2007a; Mittal et al., 2012; Mittal et al., 2007b; Mittal et al., 2010b; Walker et al., 1994). Archival studies suggest that early signs of abnormal fine and gross motor skill (i.e., dyspraxia) in children, also predict later development of psychosis (Schiffman et al., 2015). Handwriting analysis has emerged as an important tool for assessing movement abnormalities during the UHR period, as it is able to pick up subtle movement abnormalities that may not be visible or as severe in patients with schizophrenia (Caligiuri et al., 2010; Caligiuri et al., 2006; Docx et al., 2012; Docx et al., 2014; Morrens et al., 2014). Recent cross sectional work in UHR samples has found evidence of hyperkinetic and hypokinetic movement abnormalities using a handwriting tablet and different kinematic measurements (Dean and Mittal, 2015; Dean et al., 2013). The current study adds to this body of literature by suggesting that handwriting analysis may pick up subtle alterations in handedness in individuals identified as right-handed based on a number of demonstrated tasks, and that this is related to the progression of positive symptoms of risk for psychosis. Future work examining handwriting laterality in patients with psychosis using these instrumental measures may provide greater insight into the magnitude of these findings and further the use of instrumental measures for the assessment of movement abnormalities.
While the present results did not find a significant relationship between decreased dextrality and increased symptoms at baseline, we found a unique relationship between LQFREQ and the progression of positive symptoms over 12 months. This is in line with a large body of work in samples who may be prone to psychotic-like experiences and increased propensity for magical ideation and show increased prevalence for mixed and non-right handedness (Asai and Tanno, 2009; Barrantes-Vidal et al., 2013; Bolinskey et al., 2013; Dragovic et al., 2005; Shaw et al., 2001). In a large sample of university students, increased schizotypy is associated with decreased right-handedness (Chen and Su, 2006; van der Hoorn et al., 2010). In schizophrenia patients, first rank symptoms have been associated with increased mixed handedness using a continuous measure of hand preference on a number of different tasks (Verdoux et al., 2004). Taken together, the findings join a growing body of literature suggests that movement abnormalities may be associated with specific symptom domains in psychosis (Docx et al., 2012; Morrens et al., 2014; Morrens et al., 2008; Pappa and Dazzan, 2009; van Harten et al., 2014).
The existing understanding of the development of cerebral asymmetry and handedness is compatible with the present diathesis stress model of psychosis risk, where early genetic alterations and pre or perinatal events effect later brain development. Decreased cerebral asymmetry has been shown to be associated with genetic and epigenetic alterations as well as pre and perinatal complications (Johnston et al., 2009). The present results are in line with previous work that notes an increase prevalence of non-right-handedness in patients with psychosis (Sommer, 2001). Furthermore, these results provide support for the theory that disrupted cerebral asymmetry and altered language hemispherical dominance is a core feature of the development of psychosis (Crow, 2004; Crow et al., 1989; Crow et al., 1996; DeLisi et al., 1997). Indeed, the behavioral correlates of handedness are particularly germane to the signs and symptoms of psychosis. For example, mixed-handedness is associated with decreased cognitive abilities in several domains including arithmetic, reading and problem solving; domains also associated with risk for psychosis (Corballis et al., 2008; Wu et al., 2014). Conversely, non-right handedness is also associated with creativity in healthy individuals, an advantageous trait that has been hypothesized to promote the fitness for the genes linked to psychosis (Badzakova-Trajkov et al., 2011; Nettle and Clegg, 2006). This is particularly relevant for looking at biomarkers of risk for psychosis and the results of the present study suggest that looking at handedness lateralization may provide an easily accessible and helpful tool for assessment of risk in those showing attenuated psychotic symptoms.
The current study has several notable strengths and limitations. Neuroleptic medications have been shown to confound research involving movement abnormalities in patients with psychosis (van Harten et al., 2014); this confound was absent from the current UHR sample during the handwriting task. While the current study used an innovative technology to assess right-handedness, the handwriting results of this study are limited to a single time point. Future work examining handwriting laterality over multiple time points will be important for determining whether this is a stable marker of risk and related to eventual conversion to psychosis. We recruited participants who demonstrated strong right-handedness in order to measure the amount of decreased dextrality. This is applicable to a wide variety of other movement studies, which have focused solely on right-handed individuals (Willems et al., 2014). However, previous research notes that there is a higher prevalence of left-handedness in schizophrenia and it will be important to examine handwriting kinematics in a group of left handed UHR individuals to get a fuller picture of handwriting laterality.
Acknowledgments
None.
Role of Funding Source: This work was supported by National Institutes of Health Grants R01MH094650 and R21/R33MH103231 to V.A.M. J.M.O is supported by National Institutes of Health Grant F32DA034412.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
Contributors
Authors D.J.D, J.M.O, R.E.N, and V.A.M developed the study concept. V.A.M. obtained funding for the study. All authors contributed to the study design. Testing, data collection as well as data analysis and interpretation were performed by D.J.D and R.E.N under the supervision of J.M.O and V.A.M. D.J.D drafted the paper; J.M.O, R.E.N, and V.A.M provided the critical revisions. All authors approved the final version of the paper for submission.
References
- Asai T, Tanno Y. Schizotypy and handedness in Japanese participants, revisited. Laterality. 2009;14(1):86–94. doi: 10.1080/13576500802254090. [DOI] [PubMed] [Google Scholar]
- Badzakova-Trajkov G, Haberling IS, Corballis MC. Magical ideation, creativity, handedness, and cerebral asymmetries: a combined behavioural and fMRI study. Neuropsychologia. 2011;49(10):2896–2903. doi: 10.1016/j.neuropsychologia.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Barrantes-Vidal N, Gomez-de-Regil L, Navarro B, Vicens-Vilanova J, Obiols J, Kwapil T. Psychotic-like symptoms and positive schizotypy are associated with mixed and ambiguous handedness in an adolescent community sample. Psychiatry Res. 2013;206(2–3):188–194. doi: 10.1016/j.psychres.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Brill LB, Royall R, 2nd, McGilchrist IK, Pulver AE, Powers RE, Casanova MF, Tien AY, Frangou S, Petty RG. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry. 1997;154(5):661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- Bolinskey PK, Iati CA, Hunter HK, Novi JH. Season of birth, mixed-handedness, and psychometric schizotypy: preliminary results from a prospective study. Psychiatry Res. 2013;208(3):210–214. doi: 10.1016/j.psychres.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27(3):335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr J. Handwriting movement analyses for monitoring drug-induced motor side effects in schizophrenia patients treated with risperidone. Human movement science. 2009;28(5):633–642. doi: 10.1016/j.humov.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr JB. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Res. 2010;177(1–2):77–83. doi: 10.1016/j.psychres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Human movement science. 2006;25(4–5):510–522. doi: 10.1016/j.humov.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Callaway DA, Perkins DO, Woods SW, Liu L, Addington J. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Res. 2014;159(2):263–266. doi: 10.1016/j.schres.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Jones P, Murray RM, Wadsworth ME. Childhood laterality and later risk of schizophrenia in the 1946 British birth cohort. Schizophr Res. 1997;26(2–3):117–120. doi: 10.1016/s0920-9964(97)00046-7. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Su CH. Handedness and schizotypy in non-clinical populations: influence of handedness measures and age on the relationship. Laterality. 2006;11(4):331–349. doi: 10.1080/13576500600572693. [DOI] [PubMed] [Google Scholar]
- Claridge G, Clark K, Davis C, Mason O. Schizophrenia risk and handedness: a mixed picture. Laterality. 1998;3(3):209–220. doi: 10.1080/713754308. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Iacono WG, Beiser M. Handedness in first-episode psychotic patients and their first-degree biological relatives. J Abnorm Psychol. 1994;103(2):400–403. [PubMed] [Google Scholar]
- Corballis MC, Hattie J, Fletcher R. Handedness and intellectual achievement: an even-handed look. Neuropsychologia. 2008;46(1):374–378. doi: 10.1016/j.neuropsychologia.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29(4):633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Cerebral asymmetry and the lateralization of language: core deficits in schizophrenia as pointers to the gene. Current Opinion in Psychiatry. 2004;17(2):97–106. [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989;46(12):1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Done DJ, Sacker A. Cerebral lateralization is delayed in children who later develop schizophrenia. Schizophr Res. 1996;22(3):181–185. doi: 10.1016/s0920-9964(96)00068-0. [DOI] [PubMed] [Google Scholar]
- Dean DJ, Mittal VA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. npj Schizophrenia 1. 2015 doi: 10.1038/npjschz.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Teulings HL, Caligiuri M, Mittal VA. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naive adolescents at high risk for psychosis. J Vis Exp. 2013;81:e50852. doi: 10.3791/50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep-Soboslay A, Hyde TM, Callicott JP, Lener MS, Verchinski BA, Apud JA, Weinberger DR, Elvevag B. Handedness, heritability, neurocognition and brain asymmetry in schizophrenia. Brain. 2010;133(10):3113–3122. doi: 10.1093/brain/awq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Kushner M, Finer DL, Hoff AL, Crow TJ. Anomalous cerebral asymmetry and language processing in schizophrenia. Schizophr Bull. 1997;23(2):255–271. doi: 10.1093/schbul/23.2.255. [DOI] [PubMed] [Google Scholar]
- Docx L, Morrens M, Bervoets C, Hulstijn W, Fransen E, De Hert M, Baeken C, Audenaert K, Sabbe B. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126(4):256–265. doi: 10.1111/j.1600-0447.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- Docx L, Sabbe B, Fransen E, Bervoets C, Hulstijn W, Van Den Bossche MJ, Vermeylen S, Temmerman A, Morsel A, Morrens M. Longitudinal Evaluation of the Psychomotor Syndrome in Schizophrenia. J Neuropsychiatry Clin Neurosci. 2014;26(4):359–368. doi: 10.1176/appi.neuropsych.13020027. [DOI] [PubMed] [Google Scholar]
- Dragovic M, Hammond G. Handedness in schizophrenia: a quantitative review of evidence. Acta Psychiatr Scand. 2005;111(6):410–419. doi: 10.1111/j.1600-0447.2005.00519.x. [DOI] [PubMed] [Google Scholar]
- Dragovic M, Hammond G, Jablensky A. Schizotypy and mixed-handedness revisited. Psychiatry Res. 2005;136(2–3):143–152. doi: 10.1016/j.psychres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Hans S, Ingraham L, Marcus J, Wynne L, Rehman A, Roberts SA, Auerbach J. Handedness in children of schizophrenic parents: data from three high-risk studies. Behavior Genetics. 2005;35(3):351–358. doi: 10.1007/s10519-005-3227-y. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Patient Edition, January 1995 FINAL SCID-I/P Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Henkel V, Mergl R, Juckel G, Rujescu D, Mavrogiorgou P, Giegling I, Moller H, Hegerl U. Assessment of handedness using a digitizing tablet: a new method. Neuropsychologia. 2001;39(11):1158–1166. doi: 10.1016/s0028-3932(01)00043-4. [DOI] [PubMed] [Google Scholar]
- Johnston DW, Nicholls ME, Shah M, Shields MA. Nature’s experiment? Handedness and early childhood development. Demography. 2009;46(2):281–301. doi: 10.1353/dem.0.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschreck T, Chun J, Merrill A, Maher B, Boshes R, Glatt S, Faraone S, Tsuang M, Seidman L. Impaired motor performance in adolescents at familial high-risk for schizophrenia. Schizophr Res. 2015 doi: 10.1016/j.schres.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Daley M, Shiode MF, Bearden CE, O’Neill J, Cannon TD. Striatal volumes and dyskinetic movements in youth at high-risk for psychosis. Schizophr Res. 2010a;123(1):68–70. doi: 10.1016/j.schres.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Carol EE, Gupta T, Turner J, Leopold DR, Robustelli BL, Millman ZB. Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull. 2014;40(6):1204–1215. doi: 10.1093/schbul/sbt199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A, Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr Res. 2011;132(2–3):194–196. doi: 10.1016/j.schres.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007a;61(10):1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65(2):165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Smolen A, Dean DJ, Pelletier AL, Lunsford-Avery J, Smith A. BDNF Val66Met and spontaneous dyskinesias in non-clinical psychosis. Schizophr Res. 2012;140(1–3):65–70. doi: 10.1016/j.schres.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, Trottman HD, Esterberg M, Dhrub SH, Simeonova DI, McMillan AL, Murphy E, Saczawa ME, Walker EF. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. J Abnorm Psychol. 2007b;116(2):260–267. doi: 10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, Bearden CE, Walder D, Trottman H, Daley M, Simone A, Cannon TD. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010b;68(1):93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M, Docx L, Walther S. Beyond boundaries: in search of an integrative view on motor symptoms in schizophrenia. Frontiers in psychiatry. 2014;5:145. doi: 10.3389/fpsyt.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, Lewi P, Sabbe B. Bleuler revisited: psychomotor slowing in schizophrenia as part of a catatonic symptom cluster. Psychiatry Res. 2008;161(1):121–125. doi: 10.1016/j.psychres.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Nettle D, Clegg H. Schizotypy, creativity and mating success in humans. Proceedings Biological sciences/The Royal Society. 2006;273(1586):611–615. doi: 10.1098/rspb.2005.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V, Linden DE. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17(5):456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- Orr KG, Cannon M, Gilvarry CM, Jones PB, Murray RM. Schizophrenic patients and their first-degree relatives show an excess of mixed-handedness. Schizophr Res. 1999;39(3):167–176. doi: 10.1016/s0920-9964(99)00071-7. [DOI] [PubMed] [Google Scholar]
- Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med. 2009;39(7):1065–1076. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- Petty RG, Barta PE, Pearlson GD, McGilchrist IK, Lewis RW, Tien AY, Pulver A, Vaughn DD, Casanova MF, Powers RE. Reversal of asymmetry of the planum temporale in schizophrenia. Am J Psychiatry. 1995;152(5):715–721. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- Satz P, Green MF. Atypical handedness in schizophrenia: some methodological and theoretical issues. Schizophr Bull. 1999;25(1):63–78. doi: 10.1093/oxfordjournals.schbul.a033367. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Mittal V, Kline E, Mortensen EL, Michelsen N, Ekstrom M, Millman ZB, Mednick SA, Sorensen HJ. Childhood dyspraxia predicts adult-onset nonaffective-psychosis-spectrum disorder. Dev Psychopathol. 2015:1–8. doi: 10.1017/S0954579414001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J, Claridge G, Clark K. Schizotypy and the shift from dextrality: a study of handedness in a large non-clinical sample. Schizophr Res. 2001;50(3):181–189. doi: 10.1016/s0920-9964(00)00167-5. [DOI] [PubMed] [Google Scholar]
- Sommer I. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: Meta-analysis. The British Journal of Psychiatry. 2001;178(4):344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 2001;52(1–2):57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci. 2005;28(4):575–589. doi: 10.1017/S0140525X05000105. discussion 589–633. [DOI] [PubMed] [Google Scholar]
- van der Hoorn A, Oldehinkel AJ, Ormel J, Bruggeman R, Uiterwaal CS, Burger H. Non-right-handedness and mental health problems among adolescents from the general population: The Trails Study. Laterality. 2010;15(3):304–316. doi: 10.1080/13576500902746839. [DOI] [PubMed] [Google Scholar]
- van Harten PN, Bakker PR, Mentzel CL, Tijssen MA, Tenback DE. Movement disorders and psychosis, a complex marriage. Front Psychiatry. 2014;5:190. doi: 10.3389/fpsyt.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoux H, Liraud F, Droulout T, Theillay G, Parrot M, Franck N. Is the intensity of Schneiderian symptoms related to handedness and speech disorder in subjects with psychosis? Schizophr Res. 2004;67(2–3):167–173. doi: 10.1016/j.schres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20(3):441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Weiss E, Hofer A, Golaszewski S, Siedentopf C, Felber S, Fleischhacker WW. Language lateralization in unmedicated patients during an acute episode of schizophrenia: A functional MRI study. Psychiatry Research: Neuroimaging. 2006;146:185–190. doi: 10.1016/j.pscychresns.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Willems RM, Van der Haegen L, Fisher SE, Francks C. On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nat Rev Neurosci. 2014;15(3):193–201. doi: 10.1038/nrn3679. [DOI] [PubMed] [Google Scholar]
- Wu SS, Mittal V, Pennington B, Willcutt EG. Mathematics achievement scores and early psychosis in school-aged children. Schizophr Res. 2014;156(1):133–134. doi: 10.1016/j.schres.2014.03.027. [DOI] [PubMed] [Google Scholar]


