Abstract
Background
Face detection, an ability to identify a visual stimulus as a face, is impaired in patients with schizophrenia. It is unclear whether impaired face processing in this psychiatric disorder results from face-specific domains or stems from more basic visual domains. In this study, we examined cortical face-sensitive N170 response in schizophrenia, taking into account deficient basic visual contrast processing.
Methods
We equalized visual contrast signals among patients (n=20) and controls (n=20) and between face and tree images, based on their individual perceptual capacities (determined using psychophysical methods). We measured N170, a putative temporal marker of face processing, during face detection and tree detection.
Results
In controls, N170 amplitudes were significantly greater for faces than trees across all three visual contrast levels tested (perceptual threshold, two times perceptual threshold and 100%). In patients, however, N170 amplitudes did not differ between faces and trees, indicating diminished face selectivity (indexed by the differential responses to face vs. tree).
Conclusion
These results indicate a lack of face-selectivity in temporal responses of brain machinery putatively responsible for face processing in schizophrenia. This neuroimaging finding suggests that face-specific processing is compromised in this psychiatric disorder.
Keywords: schizophrenia, face perception, EEG, visual detection, N170
1. Introduction
Faces are a unique class of visual object that conveys key information for social functioning. Face perception is impaired in schizophrenia (Chen 2011; Darke et al. 2014; Phillips and David 1995). Despite the importance of perceiving faces in social life, the underlying brain mechanisms of this perceptual function are not well understood in this psychiatric disorder. One outstanding question is whether face perception problems in patients are mediated by impairments in face-specific processing or impairments in general perceptual processing. This question is relevant not only for determining the brain mechanisms underlying impaired face perception but also for offering neurophysiological targets for therapeutic interventions of face-related behavioral problems in patients. Current therapeutic interventions for social dysfunction are broad and seldom target specific neurophysiological processes in the brain.
While previous behavioral studies have shown that patients with schizophrenia were deficient in face detection (Butler et al. 2008; Chen et al. 2008), facial identity discrimination (Chen et al. 2009) and recognition of facial emotion expression (Kohler et al. 2003; Norton et al. 2009), neuroimaging studies have not yet determined the brain systems mediating these aspects of face perception problems (Bortolon et al. 2015; McCleery et al. 2014).
Event related potentials (ERPs) are cortical electrophysiological responses time-locked to the presentation of a stimulus. The N170 ERP component is a right hemisphere lateralized negativity peaking around 170 ms after stimulus-onset, and a reliable marker of face detection that has been localized to the Fusiform Face Area (FFA) (Rossion et al. 2003; Watanabe et al. 1999). More specifically, the N170 is thought to reflect early representation of face configuration as a perceptual category rather than representations of individual faces (Jemel et al. 2006). The N170 is larger (i.e., more negative) for face versus non-face stimuli (Bentin et al. 1996; Rossion et al. 1999).
A pair of EEG studies has shown reduced face-selective N170 when patients performed a categorization task (face vs. building) and when passively viewing faces and non-face objects (Herrmann et al. 2004; Onitsuka et al. 2006). However, it was unclear whether the electrophysiological deficit emanated in an initial stage of face processing such as face detection. Unlike other stages of face processing, face detection is the perceptual process of identifying a visual stimulus as a face without processing detailed facial aspects such as identity or emotion expression (Chen et al. 2008; Ellis 1981). Additionally, the previous neuroimaging studies did not consider basic visual sensitivities, which are also deficient in schizophrenia (Butler et al. 2012). This left open the question of whether patients’ deficient brain responses to faces were due to impairment in face-specific processing or in basic visual processing.
To answer these questions, it is crucial to evaluate face specific responses, or face selectivity, when the different basic visual sensitivities of patients and healthy controls are taken into account. Face selectivity, or preferential response to faces over non-face visual objects, has been illustrated through N170 response in healthy people (Botzel et al. 1995). To compare face selectivity across groups, an issue that needs to be addressed is the different visual signal strengths of face and non-face objects. If visual sensitivity differs between patients and controls, which is a likely scenario (Green et al. 2009; Javitt 2009; Silverstein and Keane 2013), the yielded difference in N170 response to faces vs. non-face objects may be due to basic visual processing rather than to face-specific processing.
It is also crucial to compare face selectivity responses derived from N170 with fMRI. FMRI during face perception provides the spatial locus of cortical processing of face information, but does not provide precise temporal dynamics due to associated sluggish hemodynamic responses. Comparison of these two neuroimaging measurements provides complementary information about spatial and temporal properties of face selective responses. For example, a correlation between fMRI and N170 would be informative regarding the latency of a brain region of interest such as FFA for face processing (Sadeh et al. 2010; Yovel et al. 2014). The relationship between fMRI signal in FFA and N170 to faces has not been examined in schizophrenia.
This study had two goals. First, we evaluated the N170 response during face detection and tree detection, after the two types of visual objects were perceptually equalized across patients and controls using psychophysical methods. Our hypothesis is that face detection deficits in schizophrenia are due to impairment of face-specific processes, not simply basic visual process problems. We predict that patients will show reduced face-selective N170 responses in the right hemisphere (given the lateralization of the ERP component) even after their deficient visual sensitivities have been taken into account. Second, we compared N170 responses and fMRI responses in FFA to the same face and tree stimuli and in the same participants. The combined analysis with the fMRI data, available from a recent study (Maher et al. 2015), gives a better understanding of the relationship between spatial and temporal markers of face-specific processing in schizophrenia. A lack of face-specific processing may accompany altered spatial localization and temporal dynamics of cortical response, or a disrupted relationship between the two domains. Our hypothesis is that the N170 and the fMRI responses in FFA are associated in controls but not in patients.
2. Methods
2.1 Participants
Twenty patients and twenty healthy controls participated in this study. Patients met the DSM IV criteria for schizophrenia (n=8) or schizoaffective disorder (n=12). Diagnoses were made independently by experienced clinicians, based on a review of a structured clinical interview for DSM–IV (First et al. 1994) and by evaluating available medical records. Eighteen patients were taking antipsychotic medications (Supplement 1) (Woods 2003). Average illness duration was 23.9 years (SD: 8.5 years). All patients were stable outpatients while participating this study. Psychotic statuses of the patients were assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987) (Table 1). None of the healthy controls met DSM–IV criteria for Axis I psychiatric disorders, based on the Structured Interview (First et al. 2002). None had any family members with a history of psychosis.
Table 1.
Participant Demographics
| Group | Sex | Age (years) | Verbal IQ | PANSS+ | PANSS- | PANSSgen |
|---|---|---|---|---|---|---|
| control | n=20(f=10) | 41.35 (15.21) | 115.68(14.3) | |||
| patient | n=2D (f=9) | 47.75 (12.38) | 103.38(15.51) | 17.28(5.06) | 14.56 (3.58) | 30 (7.76) |
standard deviation in parentheses
Note: The groups do not differ significantly in sex, age or verbal IQ.
Demographic information of the sample was summarized in Table 1. All participants had no diagnosed organic brain disease and no history of substance abuse or dependence during the past two years. The verbal component of IQ (Wechsler 1981) was administered to both groups . The groups did not differ in terms of age, gender or verbal IQ. The study protocol was approved by the McLean Hospital Institutional Review Board. Written informed consent was obtained from all participants prior to testing.
2.2 Psychophysical equating perceptual stimuli for EEG recording
Prior to EEG recording, psychophysical testing was performed in order to equate visual stimuli across participants and between two types of stimulus (face and tree). For each participant, perceptual threshold was determined using a visual detection paradigm. Perceptual threshold was defined as the lowest contrast level at which a given participant could reliably (i.e., 80% correct) detect a stimulus (Chen et al. 2005).
Two visual tasks were used: face detection and tree detection. For each task, the displayed stimulus on a given trial included a face or tree line drawing embedded in scrambled line segments. The face or tree could be located on the left side or right side of the display. In each trial, participants indicated on which side of the display (left or right) the line drawing was present (Figure 1).
Figure 1.
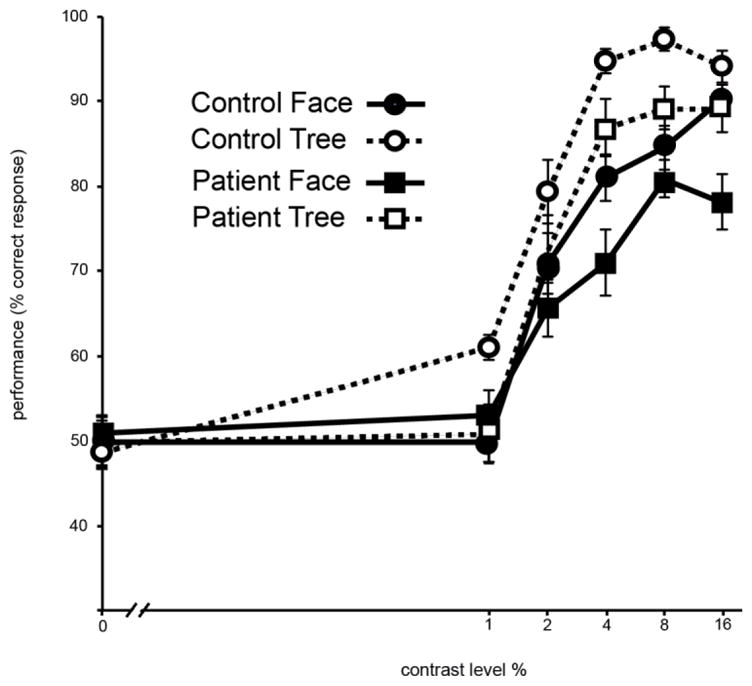
Result of pre-EEG psychophysical testing. Stimulus contrast levels (0, 1, 2, 4, 8, and 16%) are plotted in x axis. Performance accuracy (percents of correct trial) is plotted in y axis. Error bar indicate ±1 standard error.
Four stimulus contrast levels were used for EEG testing. They were 0% contrast, perceptual threshold (Th), two times perceptual threshold (Th2), and 100% contrast (Figure 2). The Th and Th2 conditions were set for each participant individually based on their own psychophysical testing results whereas the 0% and 100% contrast conditions used the identical stimulus contrasts across participants. The 0% contrast was included as a baseline condition from which EEG acquired from the other three contrasts were subtracted.
Figure 2.
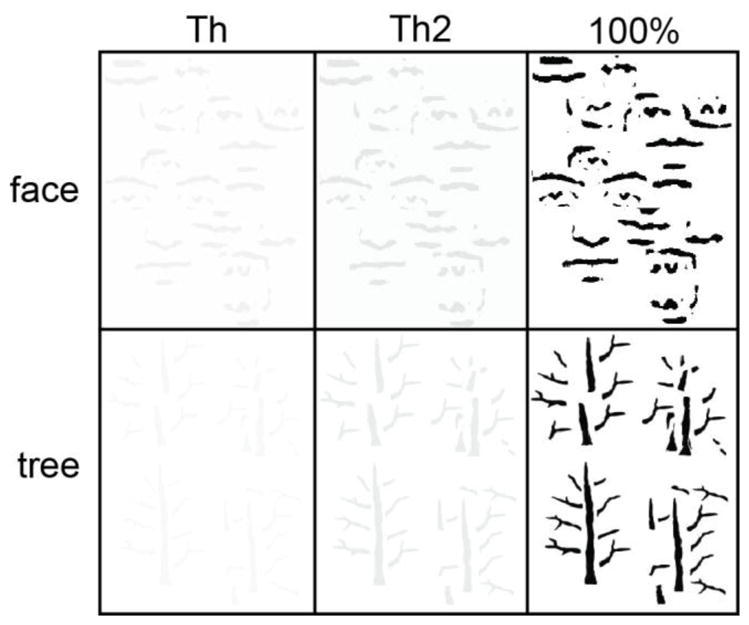
Illustration of psychophysics-determined face and tree stimuli used in EEG testing. For this illustration, the Th and Th2 conditions use average perceptual thresholds and two times average perceptual thresholds of all participants.
2.3 EEG acquisition
Two runs were used for each of the two tasks (face detection and tree detection) (Supplement 2). Each run had 192 trials (24 repeats counterbalanced across 4 contrast levels, and 2 target locations (right and left). In each trial, a stimulus was displayed within a square window (10º x 10º) for 300 ms. The average inter-stimulus interval was 1700 ms. Participants performed a face or tree detection task as described above. Face detection and tree detection runs were presented in a counterbalanced order across participants. Participants’ perceptual responses (i.e., right or left) were given using an EEG compatible button box.
2.4 Signal processing and analysis of ERP
Data processing and analysis were performed offline using ASA-Lab software (ANT Neuro, Holland). The primary ERP measures consisted of average amplitude between 120 and 220 ms at 10/20 montage right lateral electrode P8. Here the time window was extended from the one typically used for healthy population (160–200 ms after stimulus onset), which is necessary for patient studies in the event of temporal abnormality. A control analysis was also performed at P7, the analogous electrode in the left hemisphere. Average, rather than peak, was chosen because peak measurements do not necessarily reflect underlying components and may be biased to be greater for noisy conditions (Kappenman and Luck 2010).
These measures were submitted to mixed ANOVA with a between-subjects factor of group (patient and control) and within-subjects factors of stimulus type (face or tree) and contrast level (Th, Th2, 100%).
The participants of this study also participated in an fMRI study in which BOLD response in fusiform face area (FFA) were acquired using identical task paradigms (Maher et al. 2015). The fMRI acquisition and result are summarized in Supplement 3. The electrophysiological waveforms were divided and averaged into five time bins (24 ms each) between 100 and 219 ms to explore changes in the temporal relationship between fMRI and EEG within the N170 time window, in keeping with previous work using a similar analysis (Sadeh et al. 2010). As greater face-selective fMRI responses occur in right FFA (Kanwisher et al. 1997) and greater face-selective N170 responses occur in right hemisphere (Rossion et al. 2003), the two right lateralized neurophysiological measures were compared. The comparison between fMRI and N170 was constrained to the perceptual threshold level as the fMRI response of the two groups differed under this condition (Supplement 3). The participants’ N170 responses were compared with their fMRI responses from right FFA, using Pearson correlation coefficients.
3. Results
3.1 N170 responses
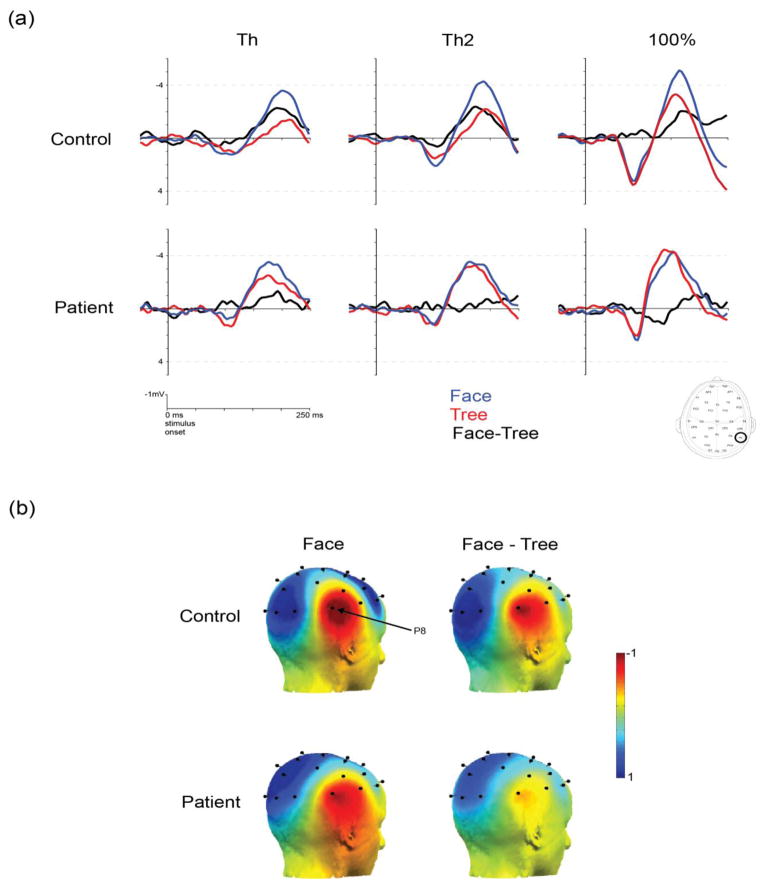
ANOVA of face-sensitive N170 amplitude at electrode P8 (right) was performed with factors of group (control vs. patient), stimulus type (face vs. tree) and contrast level (0%, Th, Th2, 100%). A significant interaction between stimulus type and group was found (F[1,38] = 4.51, p < 0.05, ηp2 =0.12) (Figure 3). Follow up analyses (one-way ANOVA for each group separately comparing responses to faces vs. trees) showed greater negativity (N170) during face detection than during tree detection in controls (F[1,19] = 25.5, p < 0.001, η p2 =1.3). In patients, however, no difference was found between face detection and tree detection (F[1,19] =0.106, p = 0.749) (Figure 3). This indicates that the putative face-sensitive N170 in patients does not selectively respond to faces.
Figure 3.
Results of N170 responses. Panel (a): Summary of average N170 waveforms during face detection and during tree detection. Tics mark 50 ms intervals. Blue traces correspond to face responses, red traces to tree responses and black traces to face responses minus tree responses. Panel (b): Scalp topography of N170 (the difference between responses to face and to trees). The time window for the measurement is from 120 to 200 ms after stimulus onset. The first column shows the N170 responses on the right from controls (the first row) and from patients (the second row). The second column shows the N170 response on the left. The face-sensitive N170 (marked in red) is maximized in the right occipitotemporal sites.
The same ANOVA of N170 amplitude was also performed at electrode P7, which is a less face sensitive left hemisphere analogue of P8 (Rossion et al. 2003). This analysis showed greater negativity on the N170 for faces than trees across both groups (F[1,38] =9.75, p = 0.003, ηp2 =0.26), but no significant main effect of group or interaction with group.
The same analysis was also performed for patients with schizophrenia vs. patients with schizoaffective disorder as the between subject factor. This analysis produced no between subjects effects (F[1,18] =0.7, p =0.41) and no significant interactions with diagnosis (Fs >0.68, ps <0.027).
Analysis of stimulus type and contrast level as well as behavioral responses was included in Supplement 4.
3.2 Relationship between N170 responses and fMRI responses
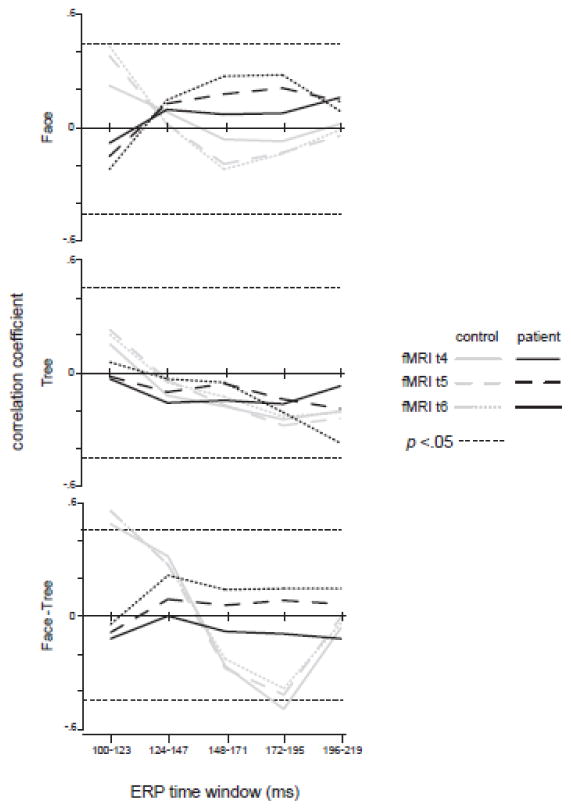
Face-selective responses (face detection minus tree detection) were significantly correlated between the ERP and BOLD responses around the time windows of 100 ms (r = 0.58) and 170 ms (r = −0.49) for controls, but not for patients (Figure 4). The lack of time-specific and physiologically meaningful correlations in patients suggests disassociations between the temporal (N170) and spatial (fMRI) markers of face-specific processing. The absence of correlation in tree detection (Figure 4 middle panel) suggests that putative face-sensitive spatial and temporal responses to non-face signals are not associated in either group.
Figure 4.
Correlations between N170 and fMRI (FFA). The x axis indicates time course of N170 responses whereas the Y axis indicates correlations between average FFA BOLD response and the N170. The two horizontal dotted lines in each panel signify significant correlations at a level of p < 0.05.
3.3 Relationship between ERP amplitudes and behavioral responses
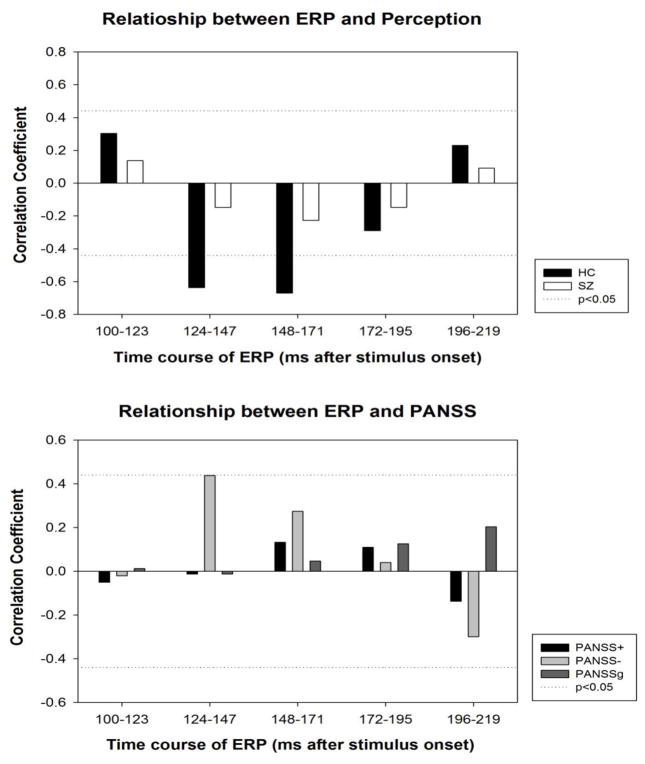
In controls, perceptual thresholds for face detection were inversely correlated with ERP response to faces between 124 and 171 ms after stimulus onset, the temporal range where N170 is presumably maximized (Figure 5). That is, the higher the perceptual threshold (worse performance), the more negative the N170 response. In patients, no correlation was found between ERP responses and perceptual thresholds. Negative PANSS scores were marginally correlated with ERP responses to faces between 124 and 147 ms (i.e. negative symptoms are associated with larger N170 amplitudes, Figure 5). In the eighteen patients taking antipsychotic medication, daily chlorpromazine equivalent dose (CPZ) was neither correlated with N170 to face or tree (between 120 and 220 ms, averaged across contrast, at electrode P8), nor with the N170 difference in response to face vs. tree.
Figure 5.
Relationship between ERP and behavioral variables. Top panel: Correlations between face-selective ERPs (face detection minus tree detection) and perceptual threshold for face detection. Perceptual threshold was defined as the lowest contrast level at which a given participant can adequately (i.e., 80% correct) detect a face. Bottom panel: Correlations between face-selective ERPs and PANSS scores in patients.
4. Discussion
This study showed that the putative face-sensitive N170 did not differ between face detection and tree detection in patients. This diminished face-selective electrophysiological response persisted even after patients’ deficient basic visual sensitivities were taken into account. Unlike controls, patients’ N170 was not correlated with their fMRI response from FFA.
4.1 Face-specific processing and basic visual processing
It was not clear whether reduced N170 responses for faces relative to non-face objects observed in schizophrenia patients (Herrmann et al., 2004; Onitsuka et al, 2006) was attributable to face-specific processing per se or to impairments of basic visual processing. It has been shown that face perception deficits in schizophrenia are associated with basic visual contrast detection (Butler et al. 2008). By equating visual contrast signals between patients and controls, this study demonstrated reduced N170 effect in schizophrenia independent of basic visual sensitivities. The presence of significant N170 interaction between group and stimulus type in the face-sensitive right hemisphere, but not in the left hemisphere, suggests impaired face-specific processing as a brain mechanism underlying face detection problems associated with patients.
Previous ERP studies showed somewhat mixed results in terms of whether the N170 is reduced in patients with schizophrenia. As described above, reduced N170 was found when patients viewed face vs. non-face objects such as buildings (Herrmann et al. 2004) or cars/hands (Onitsuka et al. 2006). However, for a face gender discrimination task, one study showed comparable N170 between patients and controls (Wynn et al. 2008). One reason for the discrepancy is the use of different types (line-drawn vs. photographic) and display times of face images in the studies. In the present study, a line-drawn face was used that contains only basic configural facial information whereas the previous studies used a photographic face image that contains more detailed facial information. It is possible that additional facial information from the photographs helped compensate for deficient configural processing in patients and yielded comparable N170. Also, stimulus display time was longer in the previous study (Wynn et al. 2008) (500 ms) than in the present study (300 ms). It was shown that the difference between patients and controls in the face inversion task (a hallmark of face-specific processing) was reduced as stimulus duration increased (Butler et al. 2008; Chen et al. 2008).
4.2 Association of spatial and temporal processing of face information: fMRI and N170
The N170 is likely generated in occipitotemporal cortex and posterior fusiform gyrus (Botzel et al. 1995; Rossion et al. 2003) and the N170 response to faces is associated with fMRI response from FFA ((Iidaka et al. 2006; Sadeh et al. 2010; Yovel et al. 2014), Figure 4 of the present study), suggesting a close link between the electrophysiological marker and the BOLD response for face processing. The present study found that the ERP and fMRI correlations for distinct ERP latencies (around 170 ms after stimulus onset) were not present in patients (Figure 4). This temporally-specific disassociation suggests that in schizophrenia, face-sensitive N170 response is not linked to the activation of the putative face processing region - FFA. Given that multiple neural sources may contribute to the generation of the N170, it is possible that patients’ N170 response is linked to activations of other cortical regions that are not as specifically devoted to face processing as FFA.
4.3 Relationships between N170 and behavioral responses and clinical implications
This study found contrasting relationships between perceptual capacities and N170 responses between the two groups of participants. In controls, perceptual thresholds for face detection were selectively correlated with N170 responses but not with other ERP components (Figure 5), suggesting that face detection is constrained by the face-sensitive electrophysiological response. In patients, however, the correlation was lacking (Figure 5), suggesting that their face detection is not mediated through the face-sensitive electrophysiological activity.
The modest correlation between N170 response of patients (between 124 to 147 ms) and negative PANSS scores (Figure 5) is consistent with the notion that social withdrawal issues (as measured in negative PANSS scores) are related to the brain’s response to faces. Such a notion would be best evaluated in first-episode patients. Unlike clinically stabilized chronic patients who participated in this study, first-episode patients typically have a greater spectrum of psychosis (more varied PANSS scores). A previous study noted that N170 was related to the nature of patients’ clinical symptoms in that those who had more delusions presented with lower N170 amplitudes (Turetsky et al. 2007). The relationship between N170 and psychotic symptoms merits further investigation.
4.4 Limitations
First, although this study eliminated effects of group difference in perceptual sensitivities to visual contrast, one of the most important precursors for perceptual and cognitive processing of faces, other factors such as spatial frequency also need to be considered for patients with schizophrenia (Lee et al. 2010; McBain et al. 2010). Second, although comparisons between separately recorded N170 and fMRI response yielded group differences between patients and controls in the present study, simultaneous measurement of the two types of brain response may provide more precise temporal dynamics and spatial locus of impaired face-specific processing in schizophrenia. Third, the present study evaluated ERP during face detection, but other aspects of face perception, such as facial identity discrimination, facial emotion perception and gaze detection (Tso et al. 2015), can also be evaluated using the approach developed in the present study. Such studies may further reveal face-specific processing problems in this psychiatric disorder. Fourth, dividing ERP components into multiple time bins and comparing them with other responses (such as fMRI and behaviors) may allow determining if the evaluated relationships are temporally specific (Calvo and Nummenmaa 2011; Hietanen et al. 2008; Sadeh et al. 2010). Yet, this division increases statistical chances of finding existing effects. Thus, the yielded results should be taken with this caveat in mind.
4.5 Conclusion
By isolating out basic visual sensitivity and evaluating the face-sensitive N170 marker and its relationship to FFA fMRI response, this study shows the existence of deficient face-specific processing in schizophrenia. This study also suggests that this electrophysiological marker can be adapted to characterize functional properties of face-specific processing in schizophrenia and potentially assess efficacies of therapeutic interventions targeting face perception domains.
Supplementary Material
Acknowledgments
We thank Dr. Dost Ongur for supervising clinical assessment and Dr. George Trksak for supporting EEG recordings during the initial phase of the present study.
Footnotes
Contributions
Chen Y and Maher S designed the study. Ekstrom T recruited subjects and evaluated their clinical statues. Maher S and Mashhoon Y collected and analyzed data. Chen Y, Maher S Mashhoon Y and Lukas S wrote the manuscript. All authors revised and approved the paper.
Conflict of interest
All authors do not have conflict of interest.
Role of funding source
This study was funded by grants (R01 MH 096793 to YC; K01 DA 034028 to YM) from the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological Studies of Face Perception in Humans. J Cogn Neurosci. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolon C, Capdevielle D, Raffard S. Face recognition in schizophrenia disorder: A comprehensive review of behavioral, neuroimaging and neurophysiological studies. Neurosci Biobehav Rev. 2015;53:79–107. doi: 10.1016/j.neubiorev.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Botzel K, Schulze S, Stodieck SR. Scalp topography and analysis of intracranial sources of face-evoked potentials. Exp Brain Res. 1995;104(1):135–43. doi: 10.1007/BF00229863. [DOI] [PubMed] [Google Scholar]
- Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr Bull. 2012;38(1):81–91. doi: 10.1093/schbul/sbr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Tambini A, Yovel G, Jalbrzikowski M, Ziwich R, Silipo G, Kanwisher N, Javitt DC. What's in a face? Effects of stimulus duration and inversion on face processing in schizophrenia. Schizophr Res. 2008;103(1–3):283–292. doi: 10.1016/j.schres.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Nummenmaa L. Time course of discrimination between emotional facial expressions: the role of visual saliency. Vision Res. 2011;51(15):1751–9. doi: 10.1016/j.visres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Chen Y. Face perception in schizophrenia spectrum disorders: interface between cognitive and social cognitive functioning. In: Ritsner M, editor. Handbook of Schizophrenia Spectrum Disorders. New York: Springer; 2011. pp. 111–120. [Google Scholar]
- Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr Res. 2005;74(2–3):271–81. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Norton D, McBain R, Ongur D, Heckers S. Visual and cognitive processing of face information in schizophrenia: detection, discrimination and working memory. Schizophr Res. 2009;107(1):92–8. doi: 10.1016/j.schres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Norton D, Ongur D, Heckers S. Inefficient face detection in schizophrenia. Schizophr Bull. 2008;34(2):367–74. doi: 10.1093/schbul/sbm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke H, Peterman JS, Park S, Sundram S, Carter O. Are patients with schizophrenia impaired in processing non-emotional features of human faces? Front Psychol. 2014;4:529. doi: 10.3389/fpsyg.2013.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. Theoretical aspects of face recognition. In: AWY, editor. Functions of the right hemisphere. London: Academic Press; 1981. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JB. Structure Clinical Interview for DSM -IV-TR Axis I Disorders - Non-patient Edition (SCID-I/NP, 11/2002 revision) New York, NY: Biometric Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Disorders (SCID) Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception Measurement in Clinical Trials of Schizophrenia: Promising Paradigms From CNTRICS. Schizophr Bull. 2009;35(1):163–81. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Ellgring H, Fallgatter AJ. Early-stage face processing dysfunction in patients with schizophrenia. Am J Psychiatry. 2004;161(5):915–7. doi: 10.1176/appi.ajp.161.5.915. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Leppanen JM, Nummenmaa L, Astikainen P. Visuospatial attention shifts by gaze and arrow cues: an ERP study. Brain Res. 2008;1215:123–36. doi: 10.1016/j.brainres.2008.03.091. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Haneda K, Okada T, Sadato N. Hemodynamic and electrophysiological relationship involved in human face processing: evidence from a combined fMRI-ERP study. Brain Cogn. 2006;60(2):176–86. doi: 10.1016/j.bandc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–75. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: fact or artifact? J Autism Dev Disord. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology. 2010;47(5):888–904. doi: 10.1111/j.1469-8986.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–74. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Lee J, Gosselin F, Wynn JK, Green MF. How Do Schizophrenia Patients Use Visual Information to Decode Facial Emotion? Schizophr Bull. 2010 doi: 10.1093/schbul/sbq006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher S, Ekstrom T, Holt D, Ongur D, Chen Y. The core brain region for face processing in schizophrenia lacks face selectivity. Schizophrenia Bulletin. 2015 doi: 10.1093/schbul/sbv140. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain R, Norton D, Chen Y. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophr Res. 2010;122(1–3):151–5. doi: 10.1016/j.schres.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery A, Lee J, Joshi A, Wynn JK, Hellemann GS, Green MF. Meta-analysis of face processing event-related potentials in schizophrenia. Biol Psychiatry. 2014;77(2):116–26. doi: 10.1016/j.biopsych.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biol Psychiatry. 2009;65(12):1094–8. doi: 10.1016/j.biopsych.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Niznikiewicz MA, Spencer KM, Frumin M, Kuroki N, Lucia LC, Shenton ME, McCarley RW. Functional and structural deficits in brain regions subserving face perception in schizophrenia. Am J Psychiatry. 2006;163(3):455–62. doi: 10.1176/appi.ajp.163.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, David AS. Facial processing in schizophrenia and delusional misidentification: cognitive neuropsychiatric approaches. Schizophr Res. 1995;17(1):109–14. doi: 10.1016/0920-9964(95)00035-k. [DOI] [PubMed] [Google Scholar]
- Rossion B, Campanella S, Gomez CM, Delinte A, Debatisse D, Liard L, Dubois S, Bruyer R, Crommelinck M, Guerit JM. Task modulation of brain activity related to familiar and unfamiliar face processing: an ERP study. Clin Neurophysiol. 1999;110(3):449–62. doi: 10.1016/s1388-2457(98)00037-6. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20(3):1609–24. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Sadeh B, Podlipsky I, Zhdanov A, Yovel G. Event-related potential and functional MRI measures of face-selectivity are highly correlated: a simultaneous ERP-fMRI investigation. Hum Brain Mapp. 2010;31(10):1490–501. doi: 10.1002/hbm.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. Vision science and schizophrenia research: toward a re-view of the disorder. Editors' introduction to special section. Schizophr Bull. 2013;37(4):681–9. doi: 10.1093/schbul/sbr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso IF, Calwas AM, Chun J, Mueller SA, Taylor SF, Deldin PJ. Altered attentional and perceptual processes as indexed by N170 during gaze perception in schizophrenia: Relationship with perceived threat and paranoid delusions. J Abnorm Psychol. 2015;124(3):519–31. doi: 10.1037/abn0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94(1–3):253–63. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kakigi R, Koyama S, Kirino E. Human face perception traced by magneto- and electro-encephalography. Brain Res Cogn Brain Res. 1999;8(2):125–42. doi: 10.1016/s0926-6410(99)00013-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Lee J, Horan WP, Green MF. Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophr Bull. 2008;34(4):679–87. doi: 10.1093/schbul/sbn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, Wilmer JB, Duchaine B. What can individual differences reveal about face processing? Front Hum Neurosci. 2014;8:562. doi: 10.3389/fnhum.2014.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.