Abstract
Introduction
Afatinib is an ErbB family receptor inhibitor with efficacy in head and neck squamous cell carcinoma (HNSCC). A phase I trial was conducted to determine the maximally tolerated dose (MTD) of afatinib in combination with carboplatin and paclitaxel as induction chemotherapy (IC).
Material and Methods
Patients with newly diagnosed, locally advanced HPV-negative or HPV-positive HNSCC with a significant smoking history were enrolled. Afatinib alone was given daily for two weeks as lead-in and subsequently given with carboplatin AUC 6 mg/ml*min and paclitaxel 175 mg/m2 every 21 days as IC. Afatinib was started at a dose of 20 mg daily and dose escalated using a modified Fibonacci design. After completion of IC, afatinib was discontinued and patients received concurrent cisplatin 40 mg/m2 weekly and standard radiation. Toxicity was assessed using CTCAE version 4.0.
Results
Seven of nine patients completed afatinib lead-in and IC. Five patients had partial response and two patients had stable disease after IC. Dose level 1 (afatinib 20 mg) was well tolerated with one grade 3 (ALT elevation) and one grade 4 (neutropenia) toxicities. However, dose level 2 (afatinib 30 mg) was not well tolerated with nine grade 3 (pneumonia, abdominal pain, diarrhea, pancytopenia, and UTI), two grade 4 (sepsis) and one grade 5 (death) toxicities.
Conclusions
The MTD of afatinib given with carboplatin AUC 6 mg/ml*min and paclitaxel 175 mg/m2 is 20 mg daily. Combination of afatinib at doses higher than 20 mg with carboplatin and paclitaxel should be administered with caution due to the toxicities.
Keywords: afatinib, carboplatin, paclitaxel, head and neck squamous cell carcinoma, efficacy, toxicity, ABCB1, phase I trial
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) causes a significant morbidity worldwide with the incidence of approximately 550,000 cases per year [1]. The most common risk factors are tobacco use and human papillomavirus (HPV) infection [2,3]. At diagnosis, a majority of patients present with locally advanced disease, but patients with HPV-positive HNSCC have a more favorable survival compared to patients with HPV-negative HNSCC [4–6]. However, there is a clear interaction between tobacco use and HPV-related carcinogenesis reflected by the worse survival of patients with HPV-positive HNSCC and smoking history compared to non-smokers [6,7]. While overall survival (OS) is 80–90% for HPV-positive non-smokers given concurrent chemoradiation (CRT), HPV-negative or HPV-positive smokers have a significantly lower OS ranging from 40–70% [6,8,9]. For these patients, various strategies have been explored to improve the survival such as induction chemotherapy (IC) followed by CRT [10–12]. However, these IC regimens have proven to be relatively toxic, and there is a clear need for an effective regimen that is less toxic with the potential for improved efficacy in an intermediate to high risk population.
Epidermal growth factor receptor (EGFR) has been well established as a biomarker of poor prognosis and a therapeutic target [13–16]. The most studied EGFR inhibitor in HNSCC is cetuximab which is a monoclonal antibody against EGFR and approved by Food and Drug Administration for use as a monotherapy or a combination with radiation or chemotherapy in HNSCC [17]. When cetuximab was combined with chemotherapy as a part of IC regimens, the efficacy and safety were favorable with a high response rate [18,19]. However, cetuximab may induce infusion reaction, and its weekly and intravenous administration is inconvenient for some patients [20]. Afatinib is an irreversible inhibitor of the ErbB-family tyrosine kinase receptors, EGFR (erbB1/HER1), HER2 (erbB2), and HER4 (erbB4) and administered orally with daily dosing [21,22]. In a randomized phase II trial of cetuximab or afatinib in 124 patients with recurrent and/or metastatic HNSCC, the disease control rate of afatinib was comparable to cetuximab (afatinib 50% and cetuximab 56.5%) [21]. In a randomized phase III trial, afatinib demonstrated a statistically significant improvement in progression-free survival (PFS) over methotrexate monotherapy in 483 patients with recurrent and/or metastatic HNSCC (median 2.6 months versus 1.7 months, respectively; p=0.030) [23]. In addition, current data suggest that afatinib may be more effective than methotrexate in patients with recurrent and/or metastatic p16-negative compared to p16-positive HNSCC (Median PFS p16-negative: afatinib 2.7 months vs. methotrexate 1.6 months; p16-positive: afatinib 2.0 months vs. methotrexate 2.3 months) [24].
However, the objective response rate of afatinib as a monotherapy is modest at 10% in patients with recurrent and/or metastatic HNSCC [23]. Therefore, afatinib has been evaluated in combinations with commonly used chemotherapeutic agents including platinums, 5-FU, and taxanes [25]. In the phase Ib study, a treatment-related grade 5 toxicity was observed in the afatinib, cisplatin, and paclitaxel arm, but none in the afatinib, cisplatin, and 5-FU arm, suggesting the severe toxicity may be related to paclitaxel. Afatinib is known to modulate ABC transporters, ABCB1 (a.k.a. Pglycoprotein) and ABCG2 (a.k.a. BCRP), in several cancer cell lines by competitively blocking substrate transport and downregulating mRNA and protein expression of the transporters [26,27]. Paclitaxel is an ABCB1 and ABCG2 substrate, and platinum is an ABCG2 substrate [28–30]. Because our hypothesis is that patients with the ABCB1 variants who are already at risk of increased chance of paclitaxel-related toxicities may have had an even greater risk of toxicities given the combination of afatinib and paclitaxel, we evaluated the ABCB1 rs1045642 (C3435T) and rs2032582 (G2677T) and not ABCG2 variants for their association with paclitaxel-related toxicities as the literature supports this association [30].
The current study was to select newly diagnosed, locally advanced HNSCC patients with poor prognosis according to HPV status and smoking habits, in whom the need for additional therapeutic options is pressing, and demonstrate the safety of adding afatinib to the established IC regimen of carboplatin and paclitaxel.
MATERIAL AND METHODS
Patient Selection
Eligible patients had histologically confirmed diagnosis of squamous cell carcinoma, operable or inoperable tumors, stage III (T3N0-1) and IVA-B (T1-4 N2-3M0 or T4N0-1M0) of oral cavity, oropharynx, hypopharynx and larynx. For patients with oropharynx primary, either HPV negative or HPV positive with a > 10 pack year tobacco history or current smokers were eligible. HPV status was determined before the enrollment in only non-smokers with oropharynx primary by HPV in-situ hybridization and/or p16 immunostaining. Patients had measurable disease of primary, nodes or both by clinical and radiographic methods per RECIST v1.1. Patients had no prior therapy, including surgery with curative intent, chemotherapy, radiation therapy, immunotherapy, EGFR targeted therapies, or any other investigational agents. Only ECOG performance status of 0 or 1 was allowed. Patients had normal hepatic, renal and bone marrow function. Patients with a history of allergic reactions attributed to compounds of similar chemical or biological composition to afatinib, or other agents used in study were excluded (clinicaltrials.gov registration number: NCT01732640).
Study Design
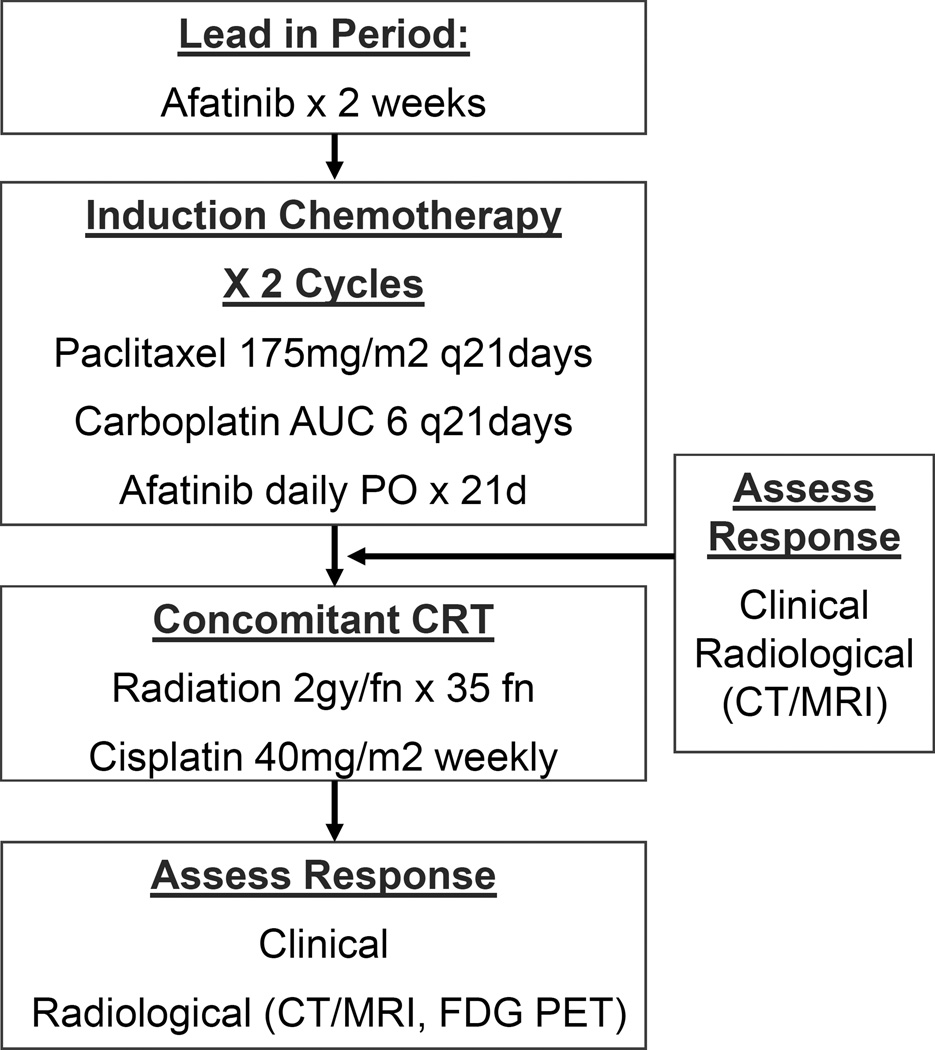
Initially this study was designed as a phase I/II trial with a planned enrollment of 6–18 patients in the phase I portion and 53 patients in the phase II portion. However, the phase II portion of the study was aborted due to the unexpected grade 4 and 5 toxicities and poor accrual. We report the phase I portion of the study alone. Eligible patients were treated with a 14-day lead-in with afatinib alone and subsequently treated with 2 cycles of IC with carboplatin AUC 6 mg/ml*min IV Day 1, paclitaxel 175 mg/m2 IV Day 1, and oral afatinib as a continuous daily dosing. Each cycle was repeated every 21 days (Figure 1). Three dose levels of afatinib were planned: 20, 30, and 40 mg. The dose escalation of the phase I portion commenced in a standard 3+3 Fibonacci design.
Figure 1.
Study Schema
Dose limiting toxicity (DLT) was defined as grade 3 or 4 neutropenia (i.e., absolute neutrophil count < 1000 cells/mm3) that was associated with a fever > 38.5°C or lasting longer than 5 days; grade 3 thrombocytopenia with bleeding or grade 4 thrombocytopenia; and any grade 3 or 4 non-hematologic toxicity per CTCAE criteria which were probably or definitely related to study therapy. During the CRT, stomatitis, pharyngitis, mucositis, or dermatitis were not considered to be a dose limiting toxicity unless it was grade 4 that did resolve to < grade 2 with a radiation treatment break (not to exceed 10 days) or with withholding chemotherapy (not to exceed 2 weekly doses). The maximally tolerated dose (MTD) was defined as the dose of afatinib in which < 2 of 6 patients experience a DLT with the next higher dose having at least 2 of up to 6 patients experiencing a DLT. No dose escalations or de-escalations are permitted within each subject’s treatment.
After completion of 2 cycles of IC, patients were assessed for response by CT/MRI and clinical exam. After the IC, all patients received CRT with weekly cisplatin 40 mg/m2 IV. The sequential CRT began 2–3 weeks after the completion of the second cycle of IC. The patients were evaluated with a MRI or CT, and FDG PET approximately 12 weeks after completion of CRT.
Statistics
The primary objective of this phase I trial was to determine the MTD or recommended phase II dose of afatinib in a combination with fixed doses of carboplatin and paclitaxel as an IC regimen. The dose escalation of the phase I commenced in a standard 3+3 design. Subjects were assigned to a dose level in the order of study entry.
ABCB1 single nucleotide polymorphism (SNP) genotyping
Genomic DNA was extracted from whole blood using standard methods. Samples were genotyped for ABCB1 rs1045642 (C3435T) and rs2032582 (G2677T) via Sanger sequencing using two different amplification reactions. A 251-base pair target region was amplified using 100 µM input forward (5'-TAG CAA ACT TTG GGA CAG GAA TAA T-3') and reverse (5'-AGT AAG CAG TAG GGA GTA ACA AAA TAA CAC-3') primers to determine the ABCB1 rs2032582 (G2677T) SNP allele. A 415-base pair target region was amplified using 100 µM input forward (5'-CAC AAG GAG GGT CAG GTG AT-3') and reverse (5'-TGT TTT CAG CTG CTT GAT GG-3') primers for the ABCB1 rs1045642 (C3435T) SNP allele. The reactions were amplified using 100 ng of genomic DNA for the ABCB1 rs2032582 (G2677T) amplicon and 50 ng of genomic DNA for the ABCB1 rs1045642 (C3435T) amplicon at a volume of 50 µL using 2X PCR Master Mix (Promega Corporation, Madison, WI) and water. The amplifications occurred under the following PCR cycling conditions: Initial denaturation, 94°C for 2 minutes; 35 cycles of 1 minute of cyclic denaturation at 94°C, 30 seconds of cyclic annealing at 60°C, 1 minute of cyclic extension at 72°C; final extension for 10 minutes at 72°C. The product amplicons were purified using the QuickStep™2 PCR Purification Plate and 10 µL QuickStep™2 SOPE Resin (Edge BioSystems, Gaithersburg, MD). Samples were sequenced with 3–5 µL of purified amplicon and 2 µM input of each respective forward primer using the BigDye® terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Austin, TX) under the following thermocycler conditions: Initial denaturation, 96°C for 1 minute; 25 cycles of 10 seconds of cyclic denaturation at 96°C, 5 seconds of cyclic annealing at 50°C, 4 minutes of cyclic extension at 60°C. The amplified product was purified using a QuickStep™2 PCR Purification Plate and analyzed on the Applied Biosystems 3730xl DNA Analyzer.
Determination of the HPV tumor status by immunohistochemistry (IHC) and/or in situ hybridization (ISH) for tumors from primary oropharynx site
Immunohistochemistry was performed to determine p16 expression using a p16 mouse monoclonal antibody (predilute, mtm-CINtech, E6H4) and high-risk HPV status was determined by ISH using a cocktail probe (GenPoint HPV Probe Cocktail, Dako) as previously described [6]. The p16 IHC positivity was defined as strong diffuse staining in greater than 70% of the tumor cells.
RESULTS
Patient Characteristics
From April, 2013 to July, 2014, ten patients were consented, and nine patients were enrolled on the trial from two participating institutions, Johns Hopkins University and Vanderbilt University. One patient failed the screening. Characteristics of the patients are listed in Table 1. Of nine enrolled patients, eight were male, and one was female. Median age was 58. ECOG performance status was 0 for six patients and 1 for three patients. Primary sites were one oral cavity, six oropharynx and two larynx. Within the six oropharyngeal tumors, the high-risk HPV and/or strong p16-staining status were positive in three, negative in two, and unknown in one.
Table 1.
Patient Demographic and Clinical Characteristics
| Gender | N (%) | |
| Male | 8 (88.9) | |
| Female | 1 (11.1) | |
| Age [years] | ||
| Median | 58 (48 – 68) | |
| < 60 [N (%)] | 5 (55.6) | |
| ≥ 60 [N (%)] | 4 (44.4) | |
| Race | N (%) | |
| White/Caucasian | 6 (66.7) | |
| Black/African American | 3 (33.3) | |
| ECOG Status at Baseline | N (%) | |
| 0 | 6 (66.7) | |
| 1 | 3 (33.3) | |
| Disease Site | N (%) | |
| Larynx | 2 (22.2) | |
| Oropharynx | 6 (66.7) | |
| Oral Cavity | 1 (11.1) |
Efficacy
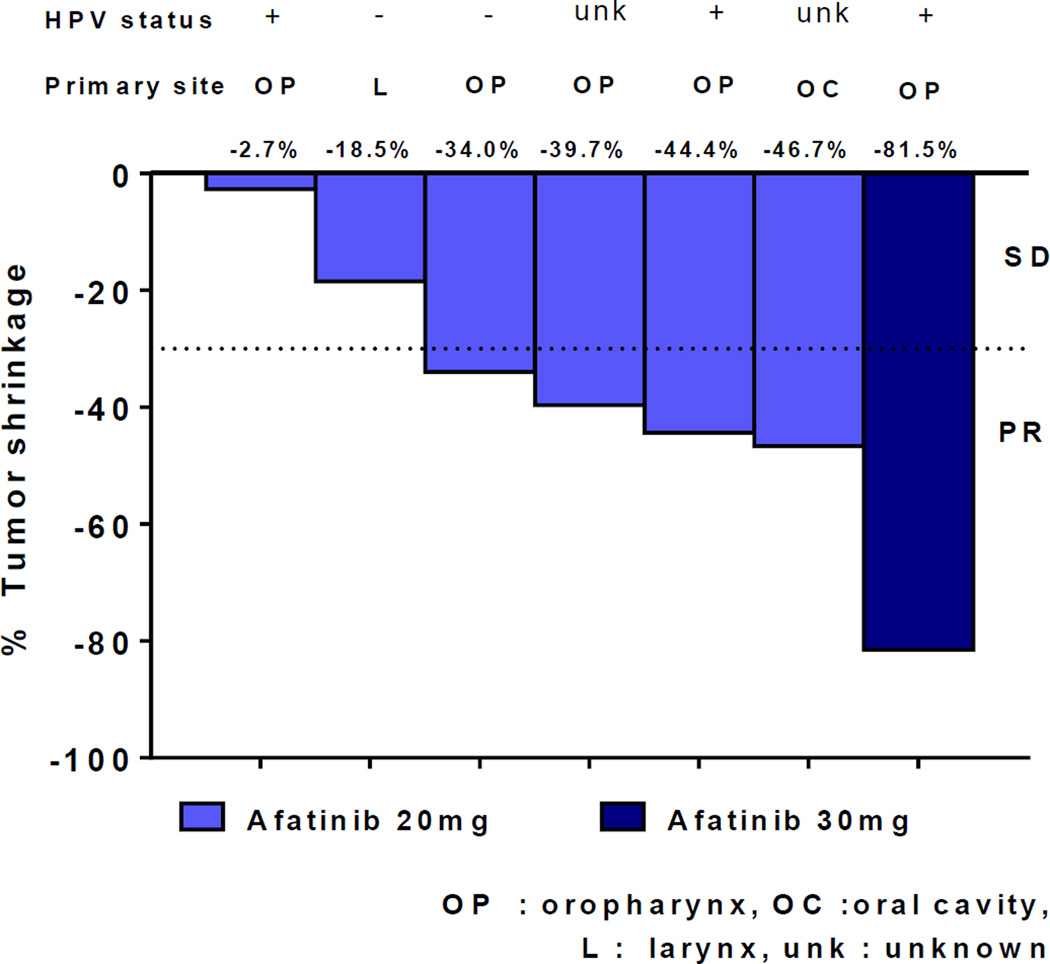
Five patients had partial response and two patients had stable disease after the completion of the IC regimen (Figure 2). One patient with the most tumor shrinkage (81.5%) received afatinib 30mg daily in the dose level 2 and had HPV-positive disease. Two other patients in the dose level 2 withdrew prior to the first planned response assessment due to toxicities and death.
Figure 2.
Waterfall plots of response rates after the induction chemotherapy; afatinib, carboplatin and paclitaxel.
Toxicity Assessment
Toxicity was considered evaluable if a patient received any therapy on the study. Six patients in the dose level 1 with afatinib 20 mg tolerated the IC regimen well with one grade 3 (ALT elevation) and one grade 4 (neutropenia) toxicities (Table 2). However, dose level 2 with afatinib 30 mg was not well tolerated in 3 patients with nine grade 3 (pneumonia, abdominal pain, diarrhea, pancytopenia, and UTI), two grade 4 (sepsis) and one grade 5 (death) toxicities. Only one of the three completed the IC regimen. The severity of profound and early onset pancytopenia seen in these two patients was unusual, particularly in newly diagnosed patients with ECOG 0 or 1 who have never been treated with chemotherapy, and these toxicities were attributed to be study drug related in combination with every 21 day carboplatin and paclitaxel.
Table 2.
Number of patients with grade 3–5 toxicity possibly, probably, or definitely attributing to afatinib by CTCAE version 4.0
| Dose Level | Adverse Event | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Dose Level 1 | ||||
| Neutropenia | 1 | |||
| Elevated ALT | 1 | |||
| Dose Level 2 | ||||
| Pneumonia | 2 | |||
| Abdominal Pain | 2 | |||
| Diarrhea | 1 | |||
| Pancytopenia | 2 | |||
| UTI | 2 | |||
| Death | 1 | |||
| Sepsis | 2 | |||
| Total | 10 | 3 | 1 |
ABCB1 Genotyping Results
DNA samples were available from nine of nine enrolled patients. One of the nine patient did not consent for research use of the collected biospecimen; therefore, eight DNA samples were tested for SNP in ABCB1 rs1045642 (C3435T) and rs2032582 (G2677T; Table 3). While the patient with grade 4 sepsis had ABCB1 rs1045642 C/C and rs2032582 G/G genotypes, the patient with grade 5 toxicity had ABCB1 rs1045642 T/T and rs2032582 T/T genotypes which have been associated with increased propensity to develop myelosuppression given paclitaxel [31].
TABLE 3.
ABCB1 single nucleotide polymorphism (SNP) genotyping
| Sample ID | HPV Status | ABCB1 RS1045642 (3435C>T) |
ABCB1 RS2032582 (2677GT>A) |
|---|---|---|---|
| 01001 | negative | C/T | G/T |
| 01002 | positive | C/T | Inconclusive |
| 01004* | N/A | T/T | T/T |
| 02001 | N/A | C/T | Inconclusive |
| 02002# | N/A | C/C | G/G |
| 02003 | positive | C/C | G/T |
| 02004 | positive | T/T | G/T |
| 02005 | positive | T/T | G/T |
Grade 5 toxicity
Grade 4 toxicity
DISCUSSION
The combination of afatinib with carboplatin and paclitaxel as an IC regimen was well tolerated at the dose level of 1 (20 mg) in patients with newly diagnosed, locally advanced HNSCC. The 20 mg daily dosing is consistent with the phase II dosing determined by a phase Ib trial of afatinib in a combination with cisplatin plus paclitaxel or cisplatin plus 5-fluorouracil (5-FU) in patients with advanced solid tumors which included patients with unresectable and/or metastatic cancers in gastrointestinal, head and neck, gynecologic, skin, lung, and other disease sites [25]. During the dose escalation of the arm with afatinib, cisplatin, and paclitaxel, two of the five patients experienced DLT at the 30mg dose, and the phase II dose of afatinib 20 mg with cisplatin 75 mg/m2 and paclitaxel 175 mg/m2 was determined. During the dose escalation of the arm with afatinib, cisplatin and 5-FU, two of the three patients experienced DLT in the afatinib 30 mg, cisplatin 100 mg/m2, and 5-FU 1000 mg/m2 group and the afatinib 40 mg, cisplatin 75 mg/m2, and 5-FU 750 mg/m2 group resulting the phase II dose of afatinib to be 30 mg with cisplatin 75 mg/m2 and 5-FU 750 mg/m2. This prior study and our data suggest that the combination of afatinib with a paclitaxel-containing regimen may be more toxic.
In close assessment of our patients who experienced DLTs, both patients experienced profound early onset pancytopenia during the first cycle considering they had been chemotherapy naive. The first patient with the DLT was a 48-year old woman with T4N0M0 laryngeal primary disease. She tolerated two weeks of afatinib lead-in treatment well at a dose of 30 mg. After the first cycle of the IC regimen, she was admitted to the hospital for severe abdominal pain and diarrhea on Day 4 and discharged with oral antibiotics on Day 6. She was hospitalized again on Day 8 with intractable abdominal pain, diarrhea, weakness, hypotension and tachycardia. She was admitted to medical intensive care unit for bilateral pneumonia, sepsis and pancytopenia (white blood cell count of 0.2 K/cu mm, hemoglobin 7.5 g/dL and platelet count 17 K/cu mm). She was discharged from the hospital on Day 18.
The second patient with the DLT was a 58-year old man with history of hypertension, heavy smoking, heavy drinking, and HPV-negative T4bN2cM0 base of tongue primary disease. He tolerated two weeks of afatinib lead-in treatment at a dose of 30 mg. After the first cycle of the IC, he developed shortness of breath with cough, fatigue, low grade fever and abdominal pain with diarrhea on Day 3 evening. He developed excruciating pain, diarrhea and shortness of breath on Day 4 morning. He was instructed to go to the Emergency Department (ED). While he was walking down stairs to go to ED, he collapsed and lost consciousness. When the emergency response service arrived, he was pulseless. His cardiac rhythm showed v-fibrillation which turned into pulseless electrical activity after a shock. Paramedics successfully resuscitated him to normal sinus rhythm. He was brought into ED and placed on a mechanical ventilator. At this point, white blood cell count was 0.25 K/cu mm, hemoglobin was 11 g/dL, and platelet count was 69 K/cu mm. His chest X-ray showed diffuse consolidation throughout the right lung. The patient’s family discussed prognosis with an ED physician and decided to withdraw care. The patient expired.
Our experience raises a concern for a drug interaction among afatinib, carboplatin and paclitaxel although there was no systemic alteration of paclitaxel pharmacokinetics noted on a small number of patients in the phase Ib trial of afatinib, cisplatin, and paclitaxel [25]. Because of the small sample size in the previous study and difference in the study design (our study had 2 weeks of lead-in period with afatinib alone), an interaction cannot be ruled out. While no plasma or intratumoral pharmacokinetics or pharmacodynamics were performed to evaluate direct modulation of ABCB1 and ABCG2 by afatinib in our trial, an interaction at the drug transporter level is a plausible explanation for the increased toxicity observed at 30 mg of afatinib. Paclitaxel is a substrate of both transporters with an association between ABCB1 3435C>T and ABCB1 2677G>T genotypes and neutropenia [29,31,32]. Of note, ABCB1 is very important in myeloid stem cells [33]. Thus, afatinib may be inhibiting the myeloid stem cell’s ability to efflux paclitaxel resulting in a high intracellular paclitaxel concentration and subsequently causing the early onset and profound pancytopenia as we have observed. We speculate whether the lead-in with afatinib had saturated the ABCB1 transporter when paclitaxel was infused worsening the toxicity. Indeed, the patient with grade 5 toxicity had the variant ABCB1 genotypes which may have predisposed to more profound pancytopenia. Carboplatin and cisplatin are not substrates of ABCB1 but are of ABCG2 [28]. While association between the ABCG2 variant (rs2231142, C421A) and improved median PFS in ovarian cancer patients treated with platinum and taxanebased chemotherapy has been reported, data are not available assessing increased toxicities [34].
Furthermore, our study opens a question whether addition of afatinib 20 mg daily which is only 50% of the recommended monotherapy dose to chemotherapy would be sufficient to exert anti-tumor activity or render synergistic activity compared to delivering chemotherapy alone. Even though afatinb 40 mg daily is the recommended dose, the majority of patients require dose reductions to 30 mg (>50%) or 20 mg (17%) due to long-term tolerability issues [35]. In a recent study, the dose reductions did not appear to compromise clinical activity in EGFR mutant-positive non-small cell lung cancer patients suggesting that it may be acceptable to indivudalize therapy based on tolerability [36]. Therefore, evaluation of afatinib 20 mg daily in combination with chemotherapy may warrant further evaluation for efficacy beyond the toxicity evaluation in newly diagnosed patients and in a combination with established treatments in HNSCC such as chemotherapy and radiation therapy. In addition, future development should consider the HPV/p16 status in the clinical trial design considering less clinical efficacy was observed in patients with recurrent and/or metastatic p16-positive compared to p16-negative HNSCC given afatinib monotherapy [24]. Further development of afatinib in combination regimens will require additional studies to identify the appropriate dose and dosing schedule.
While our data are limited, it sufficiently supports that the MTD of afatinib given with carboplatin AUC 6 mg/ml*min and paclitaxel 175mg/m2 every 21 day is 20 mg daily, and the combination of afatinib with paclitaxel-containing chemotherapy regimens should be administered with caution due to the toxicities potentially related to paclitaxel clearance. An alternative would be to consider evaluating daily afatinib with weekly doses of carboplatin and paclitaxel instead of every 21 day doses. Further studies are required to delineate the role of afatinib in management of newly diagnosed HNSCC.
Highlights.
Maximum tolerated dose of afatinib with carboplatin/paclitaxel is 20 mg daily.
Afatinib at doses >20 mg with carboplatin/paclitaxel should be used with caution.
Afatinib needs further evaluation in management of newly diagnosed HNSCC.
Acknowledgments
Grant Funding: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). These studies were supported by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI) through National Comprehensive Cancer Network Oncology Research Program (ORP). BIPI had no role in the design, analysis or interpretation of the results in this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations. The project described was also supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 TR 001079). Grant Number UL1 TR 001079 is from the National Center for Advancing Translational Sciences (NCATS) a component of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
Christine H. Chung received research funding from Boehringer Ingelheim for preclinical research. Sarah Bonerigo received honorarium from Boehringer Ingelheim for serving in a focus group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All other authors have no conflict of interest.
Contributor Information
Christine H. Chung, Email: christine.chung@moffitt.org.
Michelle A. Rudek, Email: mrudek2@jhmi.edu.
Hyunseok Kang, Email: hkang30@jhmi.edu.
Shanthi Marur, Email: smarur1@jhmi.edu.
Pritish John, Email: pjohn5@jhmi.edu.
Nancy Tsottles, Email: tsottna@jhmi.edu.
Sarah Bonerigo, Email: sboneri1@jhmi.edu.
Andy Veasey, Email: Andy.Veasey@cgix.com.
Ana Kiess, Email: akiess1@jhmi.edu.
Harry Quon, Email: hquon2@jhmi.edu.
Anthony Cmelak, Email: Anthony.cmelak@vanderbilt.edu.
Barbara A. Murphy, Email: barbara.murphy@Vanderbilt.Edu.
Jill Gilbert, Email: jill.gilbert@vanderbilt.edu.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Maier H, Dietz A, Gewelke U, et al. Tobacco and alcohol and the risk of head and neck cancer. Clin Investig. 1992;70(3–4):320–327. doi: 10.1007/BF00184668. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere A, Koch W, Trotti A, et al. Head and neck cancer. N Engl J Med. 2001;345(26):1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28(27):4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22(5):1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 11.Cohen EE, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad R, O'Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 13.Grandis J, Melhem M, Gooding W, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90(11):824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 14.Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24(25):4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 15.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 16.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 17.Erbitux® (cetuximab) prescribing information. ImClone Systems, Inc. 2006, New York, NY 10014 and Bristol-Myers Squibb Company, Princeton, NJ 08543; October 2007.
- 18.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 28(1):8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28(36):5294–5300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seiwert TY, Fayette J, Cupissol D, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25(9):1813–1820. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Useros J, Garcia-Foncillas J. The challenge of blocking a wider family members of EGFR against head and neck squamous cell carcinomas. Oral Oncol. 2015;51(5):423–430. doi: 10.1016/j.oraloncology.2015.02.092. [DOI] [PubMed] [Google Scholar]
- 23.Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583–594. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 24.Cohen EEW, Licitra LF, Fayette J, et al. Biomarker analysis in recurrent and/or metastatic head and neck squamous cell carcinoma (R/M HNSCC) patients (pts) treated with second-line afatinib versus methotrexate (MTX): LUX-Head & Neck 1 (LUXH& N1) Journal of Clinical Oncology. 2015;33(15) Abtr 6023. [Google Scholar]
- 25.Vermorken JB, Rottey S, Ehrnrooth E, et al. A phase Ib, open-label study to assess the safety of continuous oral treatment with afatinib in combination with two chemotherapy regimens: cisplatin plus paclitaxel and cisplatin plus 5-fluorouracil, in patients with advanced solid tumors. Ann Oncol. 2013;24(5):1392–1400. doi: 10.1093/annonc/mds633. [DOI] [PubMed] [Google Scholar]
- 26.Wang SQ, Liu ST, Zhao BX, et al. Afatinib reverses multidrug resistance in ovarian cancer via dually inhibiting ATP binding cassette subfamily B member 1. Oncotarget. 2015;6(28):26142–26160. doi: 10.18632/oncotarget.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XK, To KK, Huang LY, et al. Afatinib circumvents multidrug resistance via dually inhibiting ATP binding cassette subfamily G member 2 in vitro and in vivo. Oncotarget. 2014;5(23):11971–11985. doi: 10.18632/oncotarget.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh S, McLeod H, Dolan E, et al. Platinum pathway. Pharmacogenet Genomics. 2009;19(7):563–564. doi: 10.1097/FPC.0b013e32832e0ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshiro C, Marsh S, McLeod H, et al. Taxane pathway. Pharmacogenet Genomics. 2009;19(12):979–983. doi: 10.1097/FPC.0b013e3283335277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frederiks CN, Lam SW, Guchelaar HJ, et al. Genetic polymorphisms and paclitaxel- or docetaxel-induced toxicities: A systematic review. Cancer Treat Rev. 2015 doi: 10.1016/j.ctrv.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Bergmann TK, Brasch-Andersen C, Green H, et al. Impact of ABCB1 variants on neutrophil depression: a pharmacogenomic study of paclitaxel in 92 women with ovarian cancer. Basic Clin Pharmacol Toxicol. 2012;110(2):199–204. doi: 10.1111/j.1742-7843.2011.00802.x. [DOI] [PubMed] [Google Scholar]
- 32.Sissung TM, Mross K, Steinberg SM, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42(17):2893–2896. doi: 10.1016/j.ejca.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunting KD, Zhou S, Lu T, et al. Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood. 2000;96(3):902–909. [PubMed] [Google Scholar]
- 34.Tian C, Ambrosone CB, Darcy KM, et al. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;124(3):575–581. doi: 10.1016/j.ygyno.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US prescribing information for GILOTRIF (afatinib) tablets. https://www.gilotrif.com. [Google Scholar]
- 36.Yang JC, Ahn M, Dickgreber NJ, et al. Influence of dose adjustment on afatinib safety and efficacy in patients (pts) with advanced EGFR mutation-positive (EGFRm+) non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33(8073) [Google Scholar]